Abstract
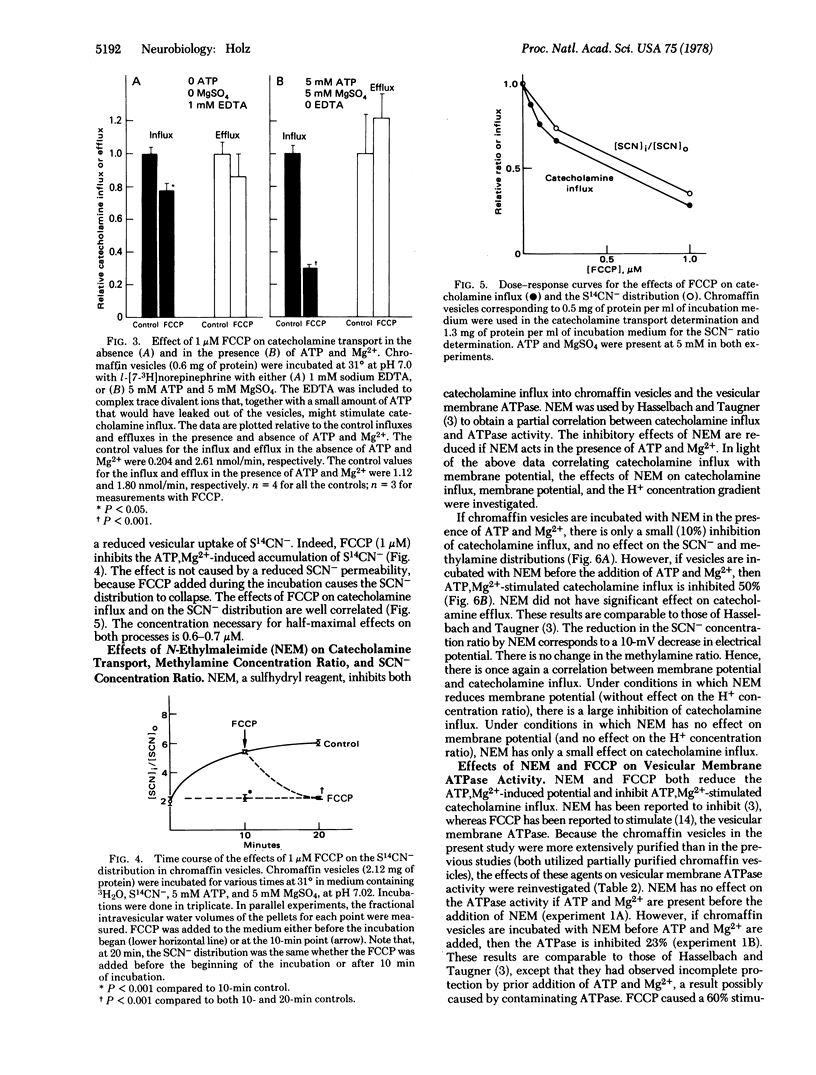
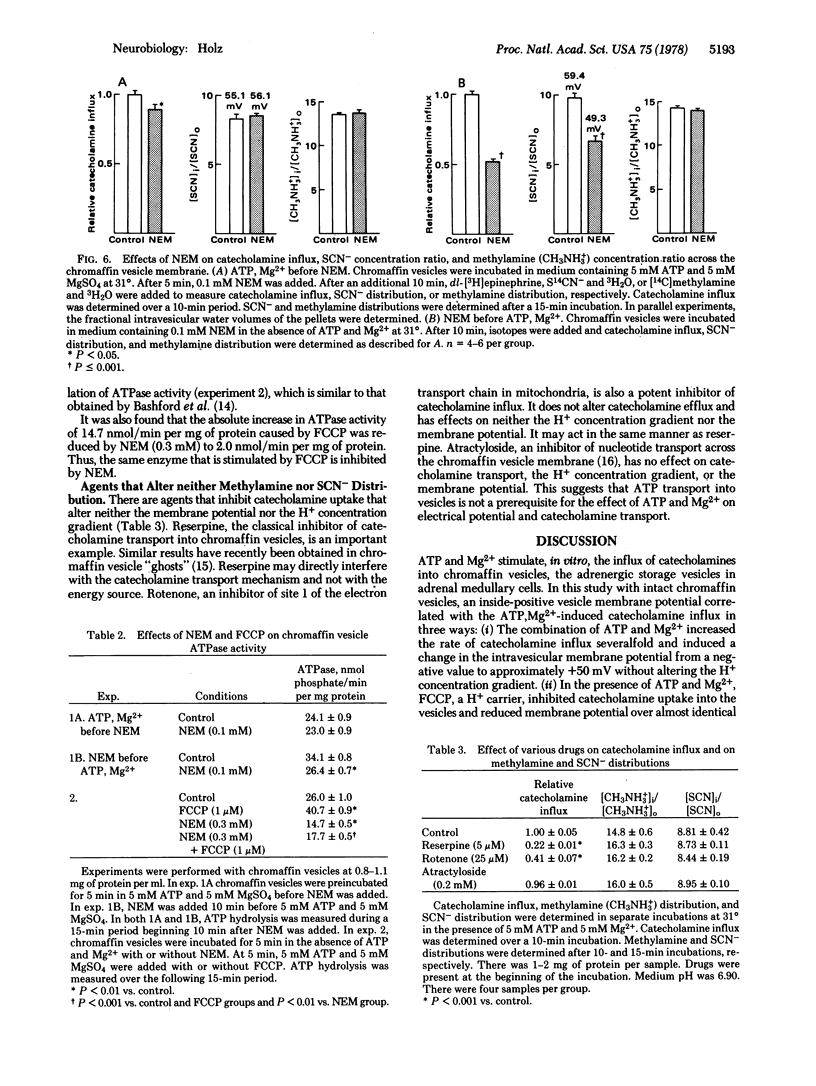
The effects of ATP, Mg2+, and various agents on pH gradient, membrane potential, and catecholamine transport across membranes of intact bovine chromaffin vesicles were investigated. Methylamine and thiocyanate (SCN-) distributions across the vesicle membrane were used to estimate the H+ concentration gradient and membrane potential, respectively. The H+ concentration ratio (intravesiculanmedium) equals 16 when the medium pH is 6.9 and is unaltered by ATP and Mg2+. In the absence of ATP and Mg2+, the steady-state intravesicular S14CN- concentration is lower than the medium concentration. ATP and Mg2+ cause an increased influx and a decreased efflux of SCN- that results in SCN- being concentrated in the vesicles 6- to 8-fold over the medium. The findings are consistent with an ATP,Mg2+-induced potential of approximately 50 mV (intravesicular side positive). Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), a H+ translocater, and N-ethylmaleimide (NEM), a sulfhydryl reagent, decrease the SCN- ratio and, thus, the membrane potential in the presence of ATP and Mg2+. They have no effect on the H+ concentration gradient. The rate of catecholamine uptake into vesicles is increased 4- to 6-fold by ATP and Mg2+. The ATP,Mg2+-stimulated uptake is inhibited by FCCP and NEM over the same concentration ranges that reduce the SCN- distribution (membrane potential). FCCP increases and NEM decreases vesicular membrane ATPase activity. Thus, catecholamine uptake is correlated to an inside-positive membrane potential, and not to ATPase activity. If catecholamine uptake is coupled to membrane potential, then a charged species must be involved in the transport mechanism. Reserpine and rotenone inhibit catecholamine influx but have no effect on the H+ electrochemical gradient; they probably act at a step before coupling to the membrane potential (or the H+ electrochemical gradient). Atractyloside, an inhibitor of nucleotide transport, has no effects on catecholamine transport or the H+ electrochemical gradient.
Keywords: secretory vesicles, neurotransmitter, H+ electrochemical gradient
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Casey R. P., Radda G. K., Ritchie G. A. Energy-coupling in adrenal chromaffin granules. Neuroscience. 1976;1(5):399–412. doi: 10.1016/0306-4522(76)90133-0. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Casey R. P., Radda G. K., Ritchie G. A. The effect of uncouplers on catecholamine incorporation by vesicles of chromaffin granules. Biochem J. 1975 Apr;148(1):153–155. doi: 10.1042/bj1480153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Radda G. K., Ritchie G. A. Energy-linked activities of the chromaffin granule membrane. FEBS Lett. 1975 Jan 15;50(1):21–24. doi: 10.1016/0014-5793(75)81031-3. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Njus D., Radda G. K., Sehr P. A. Adenosine triphosphate-evoked catecholamine release in chromatin granules. Osmotic lysis as a consequence of proton translocation. Biochem J. 1976 Sep 15;158(3):583–588. doi: 10.1042/bj1580583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W., Taugner G. The effect of a cross-bridging thiol reagent on the catecholamine fluxes of adrenal medulla vesicles. Biochem J. 1970 Sep;119(2):265–271. doi: 10.1042/bj1190265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Carlson N. J., Scarpa A. deltapH and catecholamine distribution in isolated chromaffin granules. J Biol Chem. 1978 Mar 10;253(5):1512–1521. [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Internal pH of isolated chromaffin vesicles. J Biol Chem. 1976 Apr 10;251(7):2189–2191. [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Ion permeability of isolated chromaffin granules. J Gen Physiol. 1976 Dec;68(6):601–631. doi: 10.1085/jgp.68.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSHNER N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. J Biol Chem. 1962 Jul;237:2311–2317. [PubMed] [Google Scholar]
- Kostron H., Winkler H., Peer L. J., König P. Uptake of adenosine triphosphate by isolated adrenal chromaffin granules: a carrier-mediated transport. Neuroscience. 1977;2(1):159–166. doi: 10.1016/0306-4522(77)90077-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Phillips J. H., Allison V. P. Proton translocation of the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):661–672. doi: 10.1042/bj1700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Hydroxytryptamine transport by the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):673–679. doi: 10.1042/bj1700673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H. B., Zinder O., Hoffman P. G., Nikodejevic O. Regulation of the transmembrane potential of isolated chromaffin granules by ATP, ATP analogs, and external pH. J Biol Chem. 1976 Aug 10;251(15):4544–4550. [PubMed] [Google Scholar]
- Taugner G., Hasselbach W. Uber den Mechanismus der Catecholamin-Speicherung in den "chromaffinen Granula" des Nebennierenmarks. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;255(3):266–286. [PubMed] [Google Scholar]
- Taugner G. The membrane of catecholamine storage vesicles of adrenal medulla. Uptake and release of noradrenaline in relation to the pH and the concentration and steric configuration of the amine present in the medium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(3):299–314. doi: 10.1007/BF00501939. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Dworkind J. A new and simple method for isolation of adrenal chromaffin granules by means of an isotonic density gradient. Anal Biochem. 1970 Apr;34(2):403–412. doi: 10.1016/0003-2697(70)90125-9. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- von Euler U. S., Lishajko F. Effects of some metabolic co-factors and inhibitors on transmitter release and uptake in isolated adrenergic nerve granules. Acta Physiol Scand. 1969 Nov;77(3):298–307. doi: 10.1111/j.1748-1716.1969.tb04574.x. [DOI] [PubMed] [Google Scholar]



