Abstract
OBJECTIVE:
Epidemiologic evidence indicates that prenatal vitamin supplementation reduces risk for some childhood cancers; however, a systematic evaluation of population-based childhood cancer incidence trends after fortification of enriched grain products with folic acid in the United States in 1996–1998 has not been previously reported. Here we describe temporal trends in childhood cancer incidence in association with US folic acid fortification.
METHODS:
Using Surveillance, Epidemiology, and End Results program data (1986–2008), we calculated incidence rate ratios and 95% confidence intervals to compare pre- and postfortification cancer incidence rates in children aged 0 to 4 years. Incidence trends were also evaluated by using joinpoint and loess regression models.
RESULTS:
From 1986 through 2008, 8829 children aged 0 to 4 years were diagnosed with malignancies, including 3790 and 3299 in utero during the pre- and postfortification periods, respectively. Pre- and postfortification incidence rates were similar for all cancers combined and for most specific cancer types. Rates of Wilms tumor (WT), primitive neuroectodermal tumors (PNETs), and ependymomas were significantly lower postfortification. Joinpoint regression models detected increasing WT incidence from 1986 through 1997 followed by a sizable decline from 1997 through 2008, and increasing PNET incidence from 1986 through 1993 followed by a sharp decrease from 1993 through 2008. Loess curves indicated similar patterns.
CONCLUSIONS:
These results provide support for a decrease in WT and possibly PNET incidence, but not other childhood cancers, after US folic acid fortification.
KEY WORDS: child, cancer, epidemiology, folic acid, incidence, trends
What’s Known on This Subject:
The hypothesis that maternal prenatal folic acid lowers risk for childhood malignancy in offspring is supported by experimental and epidemiologic evidence, including 2 Canadian ecologic studies that showed inverse associations for some cancer types in the very young.
What This Study Adds:
Examining Surveillance Epidemiology and End Results Program data, a decrease in the incidence of some childhood cancers (Wilms tumor, primitive neuroectodermal tumors) was observed in those <5 years after mandatory US folic acid fortification, with stronger effects detected in infants.
Folate is critical for embryonic development because of its contributions to DNA biosynthesis, cell proliferation, and DNA methylation.1 Randomized controlled trials and observational studies have provided strong evidence that sufficient maternal prenatal consumption of folic acid significantly reduces the incidence of offspring neural tube defects and some congenital abnormalities.2,3 Accordingly, the US Public Health Service recommended a minimum daily folic acid intake of 400 μg for women of childbearing age in 1992,4 and the US Food and Drug Administration mandated in March 1996 that all enriched grain products be fortified with folic acid by January 1, 1998.5 Fortified grains contribute ∼140 μg of folic acid per day to the US adult diet, whereas the contributions of prepared cereals and vitamin supplements are more variable (range: 14–107 μg and 42–392 μg, respectively).6 A comparison of mean serum folate concentrations among women aged 15 to 44 years participating in NHANES pre- and postfortification showed a significant increase between NHANES III (1988–1991) and NHANES 1999 (6.3 vs 16.2 ng/mL, respectively); similar results were observed for red blood cell folate concentrations (181 vs 315 ng/mL, respectively).7 Accordingly, a 31% reduction in neural tube defect prevalence was observed after fortification.8
Epidemiological evidence also supports the hypothesis that maternal prenatal folic acid supplementation lowers risk for childhood cancer in offspring. For example, 2 Canadian ecological studies reported reductions in neuroblastoma and Wilms tumor (WT) incidence in young children in association with mandatory folic acid fortification in 1997.9,10 Additionally, results of several case-control studies have supported a protective role for maternal prenatal multivitamin or folic acid supplementation.11–23
Given the epidemiological evidence and the idea that several childhood malignancies are thought to be initiated in utero,24–29 we examined temporal trends in childhood cancer incidence in the United States in association with mandated fortification to elucidate further the effect of maternal prenatal folic acid exposure on childhood cancer occurrence.
Methods
Incidence data were acquired from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program.30 Since 1973, SEER has actively collected data on all cancer cases (excluding nonmelanoma skin cancers) in 5 US states (Connecticut, Hawaii, Iowa, New Mexico, and Utah) and in the metropolitan areas of Detroit, San Francisco-Oakland, Seattle-Puget Sound (since 1974), and Atlanta (since 1975). These 9 registries represent ∼9.5% of the US population.28 With an estimated ascertainment rate of 98%,31 SEER captures information on a number of variables including age, diagnosis year, and tumor site and morphology.
We included first malignancies diagnosed during 1986–2008 among children <5 years of age within the SEER 9 registries. Approximately 94% of diagnoses were confirmed by histology. Annual US population estimates were obtained from the Census Bureau by SEER. In addition to childhood cancers overall, 14 categories and subcategories of malignancies most likely to have prenatal origins, due to compelling evidence of in utero initiation,24–27 the early age at onset,28 the premature cell type involved,29 or a combination of these factors, were included and were defined by SEER International Classification of Childhood Cancer32 site recode extended International Classification of Diseases for Oncology, Third Edition (ICD-O-3) categories. These categories included any leukemia (I), lymphoid leukemia (Ia, primarily comprising acute lymphoblastic leukemia [ALL], 99.7%), acute myeloid leukemia (AML, Ib), any central nervous system (CNS) tumor (III), ependymoma (IIIa.1), astrocytoma (IIIb), medulloblastoma (IIIc.1), primitive neuroectodermal tumors (PNET, IIIc.2), neuroblastoma and ganglioneuroblastoma (IVa), retinoblastoma (V), nephroblastoma or Wilms tumor (WT, VIa.1), hepatoblastoma (VIIa), rhabdomyosarcoma (IXa), and germ cell tumors (X).
We used several analytic strategies to examine whether mandatory folic acid fortification was associated with childhood cancer incidence. First, we compared incidence rates for those estimated to be in utero before fortification to those estimated to be in utero after fortification. We excluded those born during 1996–1998 as the fortification mandate was being implemented during the years that these children were in utero. Individuals born before fortification (ie, “unexposed” individuals) were defined as those who were <1 year in 1986–1995, 1 to <2 years in 1987–1996, 2 to <3 years in 1988–1997, 3 to <4 years in 1989–1998, and 4 to <5 years in 1990–1999 (SUPPLEMENTAL Table 3). Individuals born postfortification (ie, “exposed” individuals) were defined as those aged <1 year in 1999–2008, 1 to <2 years in 2000–2008, 2 to <3 years in 2001–2008, 3 to <4 years in 2002–2007, and 4 to <5 years in 2003–2008. We used SEER*Stat software (version 7.0.4) to calculate age-adjusted incidence rates (IRs), incidence rate ratios (IRRs), and 95% confidence intervals (CIs) for comparison of pre- and postfortification incidence rates.33 We also restricted the analysis to infants (<1 year in 1986–1995 vs 1999–2008) to determine if the effect of fortification was stronger for cancers diagnosed most immediately after in utero exposure.
Second, we examined trends in childhood cancer incidence in 0- to 4-year-olds during 1986–2008 (no exclusions) by 2 forms of regression in which calendar year was the independent variable, and annual incidence rate (or its natural logarithm) was the dependent variable. By using JoinPoint Regression Software (version 3.5.0),34 we evaluated whether incidence trends changed in magnitude and/or direction, allowing a maximum of 1 inflection point, or joinpoint, to be fit. The software program then selected the simplest model based on significance tests by using a Monte Carlo permutation method.35
Local regression, or loess, was also used to examine childhood cancer incidence patterns. Loess is a nonparametric regression method that fits points to a curve by applying a local smoothing parameter and is robust to outliers but makes no assumptions about the distribution of the underlying data.36 Loess was performed via Statistical Analysis Software (version 9.2, SAS Inc., Cary, NC) program, PROC LOESS.
Results
From 1986 to 2008, 8829 children between 0 and 4 years of age were diagnosed with malignancies in the SEER 9-registry catchment areas, including 3790 and 3299 estimated to be in utero during the pre- and postfortification periods, respectively (Table 1). In comparing the pre- and postfortification periods, incidence rates were similar for all cancers combined (IRR = 1.01, 95% CI: 0.96–1.06) and for most cancer types examined (Table 1). Incidence rates of WT (nephroblastoma), PNETs, and ependymomas, were significantly lower postfortification (IRRWT = 0.80, 95% CI: 0.68–0.95; IRRPNET = 0.56, 95% CI: 0.37–0.84; IRRependymoma = 0.70, 95% CI: 0.51–0.97), however. Upon restricting the analysis to infants, a greater reduction in postfortification incidence was observed for the first 2 cancer subtypes (IRRWT = 0.61, 95% CI: 0.40–0.90; IRRPNET = 0.30, 95% CI: 0.11 to 0.73; Supplemental Table 4), while the rate of infant AML increased (IRR = 1.51, 95% CI: 1.03–2.25). Results of posterior analysis do not show differences in the rate distributions by age in the pre- and postfortification periods for any of the cancer types.
TABLE 1.
Pre- and Postfortification Frequencies and Age-Adjusted Incidence Rates, As Well As Incidence Rate Ratios Comparing the Periods, for Childhood Cancers Diagnosed in Children Aged 0 to 4 Years in SEER 9 Catchment Areas
| Cancer | Prefortificationa | Postfortificationb | Post- vs Prefortification | |||||
|---|---|---|---|---|---|---|---|---|
| Count | IRc | 95% CI | Count | IRc | 95% CI | IRR | 95% CI | |
| All cancers | 3790 | 205.3 | 198.8–211.9 | 3299 | 207.4 | 200.3–214.8 | 1.01 | 0.96–1.06 |
| I Leukemias; myeloproliferative and myelodysplastic diseases | 1329 | 72.1 | 68.2–76.0 | 1133 | 75.0 | 70.6–79.6 | 1.04 | 0.96–1.13 |
| I(a) Lymphoid leukemias | 1093 | 59.3 | 55.8–62.9 | 888 | 60.4 | 56.5–64.6 | 1.02 | 0.93–1.12 |
| I(b) Acute myeloid leukemias | 177 | 9.6 | 8.2–11.1 | 191 | 11.3 | 9.8–13.1 | 1.18 | 0.96–1.46 |
| III CNS and miscellaneous intracranial and intraspinal neoplasms | 703 | 38.1 | 35.3–41.0 | 594 | 38.6 | 35.5–41.8 | 1.01 | 0.90–1.13 |
| III(a.1) Ependymomas | 108 | 5.8 | 4.8–7.1 | 65 | 4.1 | 3.1–5.2 | 0.70d | 0.51–0.97d |
| III(b) Astrocytomas | 292 | 15.8 | 14.1–17.7 | 266 | 17.5 | 15.4–19.8 | 1.10 | 0.93–1.31 |
| III(c.1) Medulloblastomas | 96 | 5.2 | 4.2–6.4 | 86 | 5.7 | 4.6–7.1 | 1.10 | 0.81–1.49 |
| III(c.2) PNET | 77 | 4.2 | 3.3–5.2 | 37 | 2.3 | 1.6–3.2 | 0.56d | 0.37–0.84d |
| IV(a) Neuroblastoma and ganglioneuroblastoma | 554 | 30.0 | 27.5–32.6 | 511 | 29.5 | 27.0–32.2 | 0.98 | 0.87–1.11 |
| V Retinoblastoma | 231 | 12.5 | 10.9–14.2 | 200 | 11.3 | 9.7–13.0 | 0.90 | 0.74–1.10 |
| VI(a.1) Nephroblastoma (Wilms tumor) | 340 | 18.4 | 16.5–20.5 | 231 | 14.8 | 12.9–16.9 | 0.80d | 0.68–0.95d |
| VII(a) Hepatoblastoma | 84 | 4.5 | 3.6–5.6 | 98 | 5.6 | 4.5–6.8 | 1.23 | 0.91–1.67 |
| IX(a) Rhabdomyosarcomas | 127 | 6.9 | 5.7–8.2 | 99 | 6.5 | 5.2–7.9 | 0.94 | 0.71–1.24 |
| X Germ cell and trophoblastic tumors; neoplasms of gonads | 127 | 6.9 | 5.7–8.2 | 133 | 7.2 | 6.0–8.6 | 1.05 | 0.82–1.36 |
| Population | 18 454 775 | 15 578 427 | ||||||
Includes children diagnosed in the following age/year categories: <1 y in 1986–1995, 1 to <2 y in 1987–1996, 2 to <3 y in 1988–1997, 3 to <4 y in 1989–1998, 4 to <5 y in 1990–1999.
Includes children diagnosed in the following age/year categories: <1 y in 1999–2008, 1 to <2 y in 2000–2008, 2 to <3 y in 2001–2008, 3 to <4 y in 2002–2008, 4 to <5 y in 2003–2008.
IRs are calculated per 1 000 000 person-years and are age-adjusted to the 2000 US Standard Population via direct age-standardization.
Pre- and postfortification rates are statistically significantly different (P < .05).
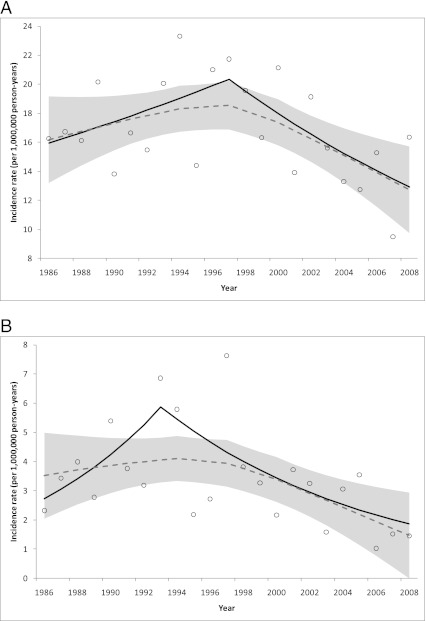
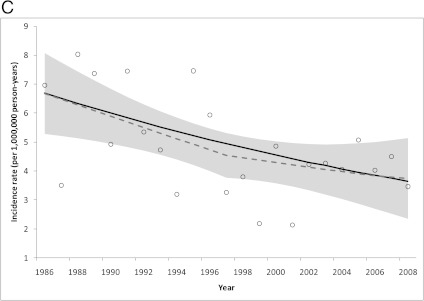
Joinpoint regression models (Fig 1, results shown as black lines) detected a nonsignificant increase in WT incidence from 1986 to 1997 (annual percent change [APC] = 2.2, 95% CI: −1.2 to 5.8; Fig 1A; Table 2) followed by a sizable decline from 1997 to 2008 (APC = −4.0%, 95% CI: −7.3 to −0.6), and a nonsignificant increase in PNET incidence from 1986 to 1993 (APC = 11.5, 95% CI: −5.0 to 31.0; Fig 1B) followed by a sharp decrease from 1993 to 2008 (APC = −7.4%, 95% CI: −12.1 to −2.4). Although no inflection point was identified in the ependymoma trend, a significant decrease in annual incidence rate was detected from 1986 to 2008 (APC = −2.7, 95% CI: −4.5 to −0.9; Fig 1C). In contrast, a positive trend was observed for hepatoblastoma over the same time period (APC = 2.1, 95% CI: 0.2–4.0).
FIGURE 1.
Observed incidence rates (open circles) and trends modeled using joinpoint (solid line) and local regression (dashed line; shaded area is 95% CI): A, WT; B, PNETs; and C, ependymomas diagnosed in children aged 0 to 4 years in SEER 9 catchment areas in 1986–2008.
TABLE 2.
Annual Percent Change From Joinpoint Regression Models for Childhood Cancers Diagnosed in Children Aged 0 to 4 Years in SEER 9 Catchment Areas in 1986–2008
| Cancer | Years | APC1a | 95% CI | Years | APC2a | 95% CI |
|---|---|---|---|---|---|---|
| All cancers | 1986–2008 | 0.2 | −0.2 to 0.7 | |||
| I Leukemias; myeloproliferative and myelodysplastic diseases | 1986–2008 | 0.4 | −0.2 to 1.1 | |||
| I(a) Lymphoid leukemias | 1986–2008 | 0.3 | −0.3 to 1.0 | |||
| I(b) Acute myeloid leukemias | 1986–2008 | 1.7 | −0.2 to 3.6 | |||
| III CNS and miscellaneous intracranial and intraspinal neoplasms | 1986–2008 | 0.1 | −0.5 to 0.7 | |||
| III(a.1) Ependymomas | 1986–2008 | −2.7b | −4.5 to −0.9b | |||
| III(b) Astrocytomas | 1986–2008 | 0.5 | −0.8 to 1.8 | |||
| III(c.1) Medulloblastomas | 1986–2008 | 0.5 | −1.6 to 2.7 | |||
| III(c.2) PNET | 1986–1993 | 11.5 | −5.0 to 31.0 | 1993–2008 | −7.4b | −12.1 to −2.4b |
| IV(a) Neuroblastoma and ganglioneuroblastoma | 1986–2008 | 0.4 | −0.7 to 1.4 | |||
| V Retinoblastoma | 1986–2008 | −0.8 | −2.1 to 0.6 | |||
| VI(a.1) Nephroblastoma (Wilms tumor) | 1986–1997 | 2.2 | −1.2 to 5.8 | 1997–2008 | −4.0b | −7.3 to −0.6b |
| VII(a) Hepatoblastoma | 1986–2008 | 2.1b | 0.2 to 4.0b | |||
| IX(a) Rhabdomyosarcomas | 1986–2008 | −0.1 | −1.8 to 1.7 | |||
| X Germ cell and trophoblastic tumors; neoplasms of gonads | 1986–2008 | 0.7 | −1.2 to 2.6 |
APCs calculated via weighted least-squares regression.
Trend is statistically significant (P < .05).
Similarly, the loess curves showed evidence of inflection points for WT and PNETs in 1994–1997, such that the incidence trend increased before and decreased after those years (Fig 1, curves shown as dashed gray lines and 95% CIs shown as shaded areas). The loess curve for ependymomas also appeared to change in magnitude in 1997; however, the decreasing slope in 1997–2008 was less steep than in 1986–1997. In addition, loess curves for childhood cancers overall, medulloblastoma, and neuroblastoma increased before 1997 and leveled off thereafter, whereas the retinoblastoma curve appeared flat before 1997 and subsequently decreased (data not shown).
Discussion
Evidence from both observational9–23 and experimental37,38 research provides strong empirical support for a link between low folate levels and increased risk for malignancy. In the current ecological study using SEER data, we observed a decreased incidence of some childhood cancers (WT, PNET, ependymoma) after US mandatory folic acid fortification with stronger results for infants for WT and PNET. Notably, trend analyses revealed a reversal in the direction of incidence rate trends for WT and PNET approximately coinciding with fortification.
An ad hoc sensitivity analysis was conducted in which the IR for each year was omitted in turn to determine the robustness of the WT and PNET results from joinpoint regression. The WT trend was quite consistent with that described earlier except for the iterations in which years 1996 and 1997 were omitted, respectively, where nonsignificant downward trends of ∼0.9% per year were observed with no inflection points. These results suggest that the years in which fortification was implemented are important in defining the trend. For PNET, results were more variable; the inflection point was situated in 1993 for about half of the iterations, 1997 for one-quarter, and was not present in the remainder, which parallels the 2 inflection points suggested in the loess curve.
A causal relationship cannot be inferred with this type of analysis, however, and at least four potential scenarios can be envisioned to explain these results. First, it is possible that observed reductions are attributable to concurrent increases of other preventive factors (or to parallel declines in harmful exposures). This scenario may apply to ependymoma rates in which trend analyses revealed a notable decrease throughout the timeframe examined. Of note, it would be difficult to disentangle any effects of the temporal increase in prenatal supplement use in recent decades from the results of folic acid fortification. A minimum daily folic acid intake of 400 μg was first formally recommended for women of childbearing age in the United States in 1992,4 and daily consumption of folic acid–containing supplements increased in this group from 1995 (28%) to 2007 (40%) with some annual variation.39 Thus, the inflection point observed in 1993 for PNETs may reflect the effects of that recommendation or may reflect the cumulative effect of the recommendation plus fortification.
Second, folic acid may impart a protective effect for other childhood cancers in addition to WT and PNET but reductions in incidence were not detected here because of the effects of other, opposing secular trends, such as increasing birth weight that has been linked to increased risk for a number of childhood cancers.40–44 Third, prenatal folic acid exposure may reduce risk of malignancy through pathways relevant to WT and PNET but may have either no effect or increase risk for other types of childhood cancers. Recent murine studies provide plausibility for this concept because differential effects of gestational folic acid supplementation on risk of offspring malignancy were observed, depending on the type of malignancy induced.45,46 The significant increases in hepatoblastoma and infant AML incidence observed herein suggest that either prenatal folic acid is protective for these cancers but the adverse effects of other risk factors have overwhelmed this protective effect (second scenario), or that folic acid is etiologically neutral or possibly contributes to the development of these cancers (third scenario). Improved survival of very low birth weight infants has been suggested as an explanation for secular increases in hepatoblastoma, for example.47
Lastly, shifting classification schemes,32,48,49 coupled with improvements in tumor classification,29 require consideration. Although the World Health Organization CNS tumor classification system was substantially modified in 1993, 2000, and 2007,50 the revisions expanded the definition of ependymomas with no change in ICD-O morphology codes,51–53 which should not contribute to incidence declines. Because PNETs were previously grouped under medulloblastomas,32,48,49 the ICD-O morphology code for PNET (9473) was not introduced until 1990,52 and atypical teratoid/rhabdoid tumors were more recently recognized as a separate entity,32,53 we examined changes in rates for medulloblastomas (IIIc.1) and PNETs combined and obtained similar results, although somewhat attenuated, compared with those for PNET alone (data not shown). Changes in WT classification also occurred within the study period, including the assignment of renal rhabdoid tumors and clear cell sarcomas to distinct ICD-O codes32,48,49,54; some misclassification of these tumors persists because of diagnosis difficulties.29 The small percentage of cases likely affected seems insufficient to account for the notable decrease in incidence, however, and comparable, but attenuated, results were observed for WT, renal rhabdoid tumors (VIa.2), and kidney sarcomas (VIa.3) combined compared with those for WT alone (data not shown).
Mandatory folic acid fortification has been associated with declines in childhood neuroblastoma and WT incidence in Ontario, Canada.9,10 No incidence differences were observed for ALL, brain cancers, hepatoblastoma, or other embryonal tumors in children 0 to 4 years of age relative to the prefortification period, however.9,10 In contrast to the Canadian study,10 we did not find evidence of a temporal decrease in neuroblastoma incidence in the postfortification period; the Canadian study included 37 neuroblastoma cases postfortification, whereas our study included >500. Similar to the Canadian results,9 we also found a significant reduction in WT incidence after fortification (26% reduction in Canada vs 20% reduction in the United States).
Some countries do not mandate folic acid fortification, in part because of concerns about the potential for enhancing cancer development.55 This presents a potential research opportunity because similar analyses conducted within countries with no or limited voluntary fortification might be informative. For example, a concomitant downward shift in WT incidence in European countries without fortification would indicate that fortification is an unlikely explanation for North American WT trends.
Apart from the ecologic studies described here, evidence for folic acid’s protective effect on carcinogenesis in children is sparse with most studies examining the effect of maternal prenatal vitamin supplementation. For WT, a German case-control study observed an inverse association (odds ratio = 0.66, 95% CI: 0.45–0.95) for maternal vitamin and/or iron supplementation during pregnancy.23 With respect to brain tumors, 7 of 9 case-control studies13–15,17,18,22,23,56,57 that included cases born before fortification or cases diagnosed in populations without fortification programs reported inverse associations between prenatal vitamin use and CNS tumors overall,15,17,18,23 astrocytoma,14 and medulloblastoma/PNET.13,22 An initial examination of prenatal folate from dietary sources revealed an inverse association with medulloblastoma/PNET,13 whereas associations for astrocytoma14 or medulloblastoma/PNET22 were not detected in subsequent studies.
For childhood ALL, 4 of 6 studies15,19,20,23,58,59 conducted in populations without fortification programs indicated a protective effect for maternal prenatal supplementation,15,19,20,23 including the largest study (1842 cases and 1986 controls) that reported an odds ratio of 0.7 (95% CI: 0.5–1.0).20 No associations have been reported for childhood AML23,60 or infant leukemia, however.20,61 A handful of case-control studies have examined associations for maternal vitamin supplementation for other tumors examined herein, including 2 neuroblastoma studies that described protective effects16,21 and 1 showing increased risk,23 a hepatoblastoma study that did not observe a significant association,62 a retinoblastoma investigation that found a 60% reduction in risk,12 and germ cell tumor studies that yielded inconsistent results.63,64
Two mechanisms have been suggested to explain the association between folate deficiency and cancer (reviewed in Duthie65 and Kim66). The first concerns the diminished synthesis of thymine in a state of folate deficiency, causing uracil to be inserted into DNA instead. Chronic folate deficiency with repeated uracil misincorporation and excision is thought to lead to a “catastrophic” cycle of DNA repair, resulting in chromosomal damage and malignancy.65 The second relates to potential epigenetic effects, wherein folate deficiency reduces the available pool of S-adenosylmethionine, leading to DNA hypomethylation.65 Global hypomethylation may cause genetic instability, whereas hypomethylation of promoter regions may result in proto-oncogene activation; either mechanism may lead to malignant transformation.67 Conversely, adequate or high folate levels may promote the malignant process by silencing tumor suppressor genes via methylation of their promoters or by uncontrolled cell proliferation due to greater production of nucleotide precursors.45 Although a relationship between in utero folic acid and risk of childhood cancers is biologically plausible, there is currently no direct evidence supporting a specific mechanism.
A strength of our study is the high rate of case ascertainment of the SEER 9 registries that capture ∼98% of diagnoses within 9.5% of the US population under active surveillance.28,31 The racial and ethnic distributions of the SEER 9 and US populations are similar, although SEER 9 included somewhat fewer white (SEER 9: 71.1% vs US: 75.1%) and Hispanic (10.3% vs 12.5%) and slightly more Asian (3.6% vs 7.4%) persons.68 A similar comparison of 16 SEER registries and the US population reveals comparable socioeconomic indicators.69 Additional strengths include the restriction of the initial analysis to those in utero either before or after fortification and the additional restriction to infants, in whom any true effect of folic acid would be expected to be greater.
A primary limitation of ecologic studies is that individual-level conclusions cannot be derived from group-level data. However, because folic acid supplementation of enriched grain products is ubiquitous, the ecologic fallacy is less of a concern. Moreover, 10 years of postfortification data are now available, and any substantial rate changes attributable to fortification should be evident. Nevertheless, childhood cancers are rare outcomes, and there is limited power to assess rate differences due to random annual variation. In addition, it is difficult to draw firm conclusions regarding the isolated effects of folic acid because other temporal trends were occurring during the study period, as described earlier. It is also possible that the inherent cancer susceptibility or distribution of risk factors in the 9.5% of the US population under surveillance in the SEER 9 registries is different from the 90.5% not followed simply by chance, leading to spurious associations. To partially address this concern, we compared our SEER postfortification incidence rates with those from the National Program of Cancer Registries (1999–2008), which collects cancer surveillance data on 96% of the US population, and found that rates were quite similar.70 Importantly, these results must be interpreted cautiously because they are not adjusted for multiple comparisons, and we would expect statistically significant associations just by chance in ∼5% of malignancies using α = .05.
Conclusions
We did not observe reductions in the incidence of most childhood cancers after mandatory folic acid fortification of enriched grain products in the United States in 1996–1998. However, declines in WT and PNET incidence were detected by multiple analytic strategies. Alternative study designs, such as prenatal feeding experiments in animal models, are required to rule out other explanations, confirm causal relationships, and elucidate mechanisms.
Supplementary Material
Glossary
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- APC
annual percent change
- CI
confidence interval
- CNS
central nervous system
- ICD-O-3
International Classification of Diseases for Oncology, Third Edition
- IR
incidence rate
- IRR
incidence rate ratio
- PNET
primitive neuroectodermal tumors
- SEER
Surveillance, Epidemiology, and End Results
- WT
Wilms tumor
Footnotes
Drs Linabery and Johnson contributed equally to this work.
Drs Linabery, Johnson, and Ross each made substantial contributions to the study concept and design, analysis, or interpretation of data, and drafting or revising the manuscript for important intellectual content; and each author provided final approval of the manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Research supported by NIH grants T32 CA099936 and K05 CA157439 and the Children’s Cancer Research Fund, Minneapolis, MN. Funded by the National Institutes of Health (NIH).
References
- 1.Morrison K, Papapetrou C, Hol FA, et al. Susceptibility to spina bifida; an association study of five candidate genes. Ann Hum Genet. 1998;62(pt 5):379–396 [DOI] [PubMed] [Google Scholar]
- 2.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137 [PubMed] [Google Scholar]
- 3.Czeizel AE, Dobo M, Vargha P. Hungarian cohort-controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth Defects Res 2004;70(11):853–861 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992;41(RR-14):1–7 [PubMed] [Google Scholar]
- 5.Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–8797 [Google Scholar]
- 6.Yeung L, Yang Q, Berry RJ. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA. 2008;300(21):2486–2487 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control Folate status in women of childbearing age—United States, 1999. MMWR Morb Mortal Wkly Rep 2000;49(42):962–965 [PubMed] [Google Scholar]
- 8.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66(1):33–39 [DOI] [PubMed] [Google Scholar]
- 9.Grupp SG, Greenberg ML, Ray JG, et al. Pediatric cancer rates after universal folic acid flour fortification in Ontario. J Clin Pharmacol. 2011;51(1):60–65 [DOI] [PubMed] [Google Scholar]
- 10.French AE, Grant R, Weitzman S, et al. Folic acid food fortification is associated with a decline in neuroblastoma. Clin Pharmacol Ther. 2003;74(3):288–294 [DOI] [PubMed] [Google Scholar]
- 11.Goh YI, Koren G. Prenatal supplementation with multivitamins and the incidence of pediatric cancers: clinical and methodological considerations. Pediatr Blood Cancer. 2008;50(suppl 2):487–489, discussion 498 [DOI] [PubMed] [Google Scholar]
- 12.Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD. Pre- and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res. 1989;49(20):5730–5735 [PubMed] [Google Scholar]
- 13.Bunin GR, Kuijten RR, Buckley JD, Rorke LB, Meadows AT. Relation between maternal diet and subsequent primitive neuroectodermal brain tumors in young children. N Engl J Med. 1993;329(8):536–541 [DOI] [PubMed] [Google Scholar]
- 14.Bunin GR, Kuijten RR, Boesel CP, Buckley JD, Meadows AT. Maternal diet and risk of astrocytic glioma in children: a report from the Childrens Cancer Group (United States and Canada). Cancer Causes Control. 1994;5(2):177–187 [DOI] [PubMed] [Google Scholar]
- 15.Sarasua S, Savitz DA. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States). Cancer Causes Control. 1994;5(2):141–148 [DOI] [PubMed] [Google Scholar]
- 16.Michalek AM, Buck GM, Nasca PC, Freedman AN, Baptiste MS, Mahoney MC. Gravid health status, medication use, and risk of neuroblastoma. Am J Epidemiol. 1996;143(10):996–1001 [DOI] [PubMed] [Google Scholar]
- 17.Preston-Martin S, Pogoda JM, Mueller BA, Holly EA, Lijinsky W, Davis RL. Maternal consumption of cured meats and vitamins in relation to pediatric brain tumors. Cancer Epidemiol Biomarkers Prev. 1996;5(8):599–605 [PubMed] [Google Scholar]
- 18.Preston-Martin S, Pogoda JM, Mueller BA, et al. Prenatal vitamin supplementation and risk of childhood brain tumors. Int J Cancer Suppl. 1998;11:17–22 [PubMed] [Google Scholar]
- 19.Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case-control study. Lancet. 2001;358(9297):1935–1940 [DOI] [PubMed] [Google Scholar]
- 20.Wen W, Shu XO, Potter JD, et al. Parental medication use and risk of childhood acute lymphoblastic leukemia. Cancer. 2002;95(8):1786–1794 [DOI] [PubMed] [Google Scholar]
- 21.Olshan AF, Smith JC, Bondy ML, Neglia JP, Pollock BH. Maternal vitamin use and reduced risk of neuroblastoma. Epidemiology. 2002;13(5):575–580 [DOI] [PubMed] [Google Scholar]
- 22.Bunin GR, Gallagher PR, Rorke-Adams LB, Robison LL, Cnaan A. Maternal supplement, micronutrient, and cured meat intake during pregnancy and risk of medulloblastoma during childhood: a Children’s Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1660–1667 [DOI] [PubMed] [Google Scholar]
- 23.Schüz J, Weihkopf T, Kaatsch P. Medication use during pregnancy and the risk of childhood cancer in the offspring. Eur J Pediatr. 2007;166(5):433–441 [DOI] [PubMed] [Google Scholar]
- 24.Gale KB, Ford AM, Repp R, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci USA. 1997;94(25):13950–13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taub JW, Ge Y. The prenatal origin of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2004;45(1):19–25 [DOI] [PubMed] [Google Scholar]
- 26.Greaves MF, Maia AT, Wiemels JL, Ford AM. Leukemia in twins: lessons in natural history. Blood. 2003;102(7):2321–2333 [DOI] [PubMed] [Google Scholar]
- 27.Woodward PJ, Sohaey R, Kennedy A, Koeller KK. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics. 2005;25(1):215–242 [DOI] [PubMed] [Google Scholar]
- 28.Howlader N, Noone AM, Krapcho M, et al, eds. SEER cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute, based on November 2010 SEER data submission; 2011. Available at: http://seer.cancer.gov/csr/1975_2008. Accessed June 8, 2011
- 29.Pizzo PA, Poplack DG. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006 [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results Program (SEER). SEER*Stat database: incidence—SEER 9 Regs Research Data, November 2010 Sub (1973–2008) [Single Ages to 85+, Katrina/Rita Population Adjustment]—linked to county attributes—total US, 1969–2009 counties. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission. Available at www.seer.cancer.gov. Accessed January 12, 2012
- 31.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343–2350 [DOI] [PubMed] [Google Scholar]
- 32.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467 [DOI] [PubMed] [Google Scholar]
- 33.SEER*Stat software version 7.0.4. Bethesda, MD: Surveillance Research Program, National Cancer Institute. 2011. Available at: www.seer.cancer.gov/seerstat Accessed October 17, 2011
- 34.Joinpoint Regression Program. Version 3.5.0. Bethesda, MD: Statistical Research and Applications Branch, National Cancer Institute; April 2011. Available at: http://surveillance.cancer.gov/joinpoint Accessed May 27, 2011
- 35.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351 [DOI] [PubMed] [Google Scholar]
- 36.Cohen RA. An Introduction to PROC LOESS for local regression. Proceedings of the 24th SAS Users Group International Conference, Miami, FL, April 1999; Paper 273. Cary, NC: SAS Institute, Inc.; 1999. [Google Scholar]
- 37.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA. 1997;94(7):3290–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YI. Folic acid supplementation and cancer risk: point. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2220–2225 [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Use of supplements containing folic acid among women of childbearing age—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(1):5–8 [PubMed] [Google Scholar]
- 40.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124(11):2658–2670 [DOI] [PubMed] [Google Scholar]
- 41.Harder T, Plagemann A, Harder A. Birth weight and subsequent risk of childhood primary brain tumors: a meta-analysis. Am J Epidemiol. 2008;168(4):366–373 [DOI] [PubMed] [Google Scholar]
- 42.Schüz J, Schmidt LS, Kogner P, et al. Birth characteristics and Wilms tumors in children in the Nordic countries: a register-based case-control study. Int J Cancer. 2011;128(9):2166–2173 [DOI] [PubMed] [Google Scholar]
- 43.Puumala SE, Soler JT, Johnson KJ, Spector LG. Birth characteristics and Wilms tumor in Minnesota. Int J Cancer. 2008;122(6):1368–1373 [DOI] [PubMed] [Google Scholar]
- 44.Ognjanovic S, Carozza SE, Chow EJ, et al. Birth characteristics and the risk of childhood rhabdomyosarcoma based on histological subtype. Br J Cancer. 2010;102(1):227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ly A, Lee H, Chen J, et al. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Res. 2011;71(3):988–997 [DOI] [PubMed] [Google Scholar]
- 46.Sie KK, Medline A, van Weel J, et al. Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut 2011;60(12):1687–1694 [DOI] [PubMed]
- 47.Spector LG, Johnson KJ, Soler JT, Puumala SE. Perinatal risk factors for hepatoblastoma. Br J Cancer. 2008;98(9):1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birch JM, Marsden HB. A classification scheme for childhood cancer. Int J Cancer. 1987;40(5):620–624 [DOI] [PubMed] [Google Scholar]
- 49.Kramárová E, Stiller CA. The international classification of childhood cancer. Int J Cancer. 1996;68(6):759–765 [DOI] [PubMed] [Google Scholar]
- 50.Scheithauer BW. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol. 2009;19(4):551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization International Classification of Diseases for Oncology . Geneva, Switzerland: World Health Organization; 1976 [Google Scholar]
- 52.Percy C, Van Holten V, et al; World Health Organization. International Classification of Diseases for Oncology. 2nd ed. Geneva, Switzerland: World Health Organization; 1990 [Google Scholar]
- 53.Fritz AG; World Health Organization. International Classification of Diseases for Oncology: ICD-O. 3rd ed. Geneva, Switzerland: World Health Organization; 2000 [Google Scholar]
- 54.Delemarre JF, Sandstedt B, Harms D, Boccon-Gibod L, Vujanić GM, International Society of Paediatric Oncology The new SIOP (Stockholm) working classification of renal tumours of childhood. Med Pediatr Oncol. 1996;26(2):145–146 [DOI] [PubMed] [Google Scholar]
- 55.Wright AJ, Dainty JR, Finglas PM. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br J Nutr. 2007;98(4):667–675 [DOI] [PubMed] [Google Scholar]
- 56.Preston-Martin S, Yu MC, Benton B, Henderson BE. N-Nitroso compounds and childhood brain tumors: a case-control study. Cancer Res. 1982;42(12):5240–5245 [PubMed] [Google Scholar]
- 57.Lubin F, Farbstein H, Chetrit A, et al. The role of nutritional habits during gestation and child life in pediatric brain tumor etiology. Int J Cancer. 2000;86(1):139–143 [DOI] [PubMed] [Google Scholar]
- 58.Dockerty JD, Herbison P, Skegg DC, Elwood M. Vitamin and mineral supplements in pregnancy and the risk of childhood acute lymphoblastic leukaemia: a case-control study. BMC Public Health. 2007;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milne E, Royle JA, Miller M, et al. Maternal folate and other vitamin supplementation during pregnancy and risk of acute lymphoblastic leukemia in the offspring. Int J Cancer. 2010;126(11):2690–2699 [DOI] [PubMed] [Google Scholar]
- 60.Robison LL, Buckley JD, Daigle AE, et al. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group). Cancer. 1989;63(10):1904–1911 [PubMed] [Google Scholar]
- 61.Pombo-de-Oliveira MS, Koifman S, Brazilian Collaborative Study Group of Infant Acute Leukemia Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2336–2341 [DOI] [PubMed] [Google Scholar]
- 62.Buckley JD, Sather H, Ruccione K, et al. A case-control study of risk factors for hepatoblastoma. A report from the Childrens Cancer Study Group. Cancer. 1989;64(5):1169–1176 [DOI] [PubMed] [Google Scholar]
- 63.Shu XO, Nesbit ME, Buckley JD, Krailo MD, Robinson LL. An exploratory analysis of risk factors for childhood malignant germ-cell tumors: report from the Childrens Cancer Group (Canada, United States). Cancer Causes Control. 1995;6(3):187–198 [DOI] [PubMed] [Google Scholar]
- 64.Johnson KJ, Poynter JN, Ross JA, Robison LL, Shu XO. Pediatric germ cell tumors and maternal vitamin supplementation: a Children’s Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2661–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull. 1999;55(3):578–592 [DOI] [PubMed] [Google Scholar]
- 66.Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10(2):66–88 [DOI] [PubMed] [Google Scholar]
- 67.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775(1):138–162 [DOI] [PubMed] [Google Scholar]
- 68.Surveillance, Epidemiology, and End Results (SEER) Program. Number of persons by race and hispanic ethnicity for SEER participants (2000 US Census data). Available at: http://seer.cancer.gov/registries/data.html Accessed January 10, 2012
- 69.Surveillance, Epidemiology, and End Results (SEER) Program. Population characteristics, characteristics of the SEER population compared with the total United States population (2000 US Census data). Available at: http://seer.cancer.gov/registries/characteristics.html Accessed January 10, 2012
- 70.National Program of Cancer Registries. 1999–2008 Incidence, WONDER On-line Database. Washington, DC: US Department of Health and Human Services; Atlanta, GA: Centers for Disease Control and Prevention; Washington, DC: National Cancer Institute; 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.