Abstract
The proneural genes are fundamental regulators of neuronal development in all metazoans. A critical role of the fly proneural factor Atonal (AtoDm) is to induce photoreceptor neuron formation in Drosophila, whereas its murine homolog, Atonal7Mm (aka Ath5) is essential for the development of the ganglion cells of the vertebrate eye. Here, we identify the Bombyx mori ato homolog (atoBm). In a pattern strikingly reminiscent of atoDm, the atoBm mRNA is expressed as a stripe in the silkworm eye disc. Its DNA-binding and protein-protein interaction domain is highly homologous to the AtoDm bHLH. Targeted expression of AtoBm in the endogenous atoDm pattern rescues the eyeless phenotype of the fly ato1 mutant and its ectopic expression induces similar gain-of-function phenotypes as AtoDm. Rescue experiments with chimeric proteins show that the non-bHLH portion of AtoBm (N-region) can effectively substitute for the corresponding region of the fly transcription factor, even though no apparent conservation can be found at the amino acid level. On the contrary, the highly similar bHLH domain of AtoBm cannot similarly substitute for the corresponding region of AtoDm. Thus, the bHLHBm domain requires the AtoBm N-region to function effectively, whereas the bHLHDm domain can operate well with either N-region. These findings suggest a role for the non-bHLH portion of Ato proteins in modulating the function of the bHLH domain in eye neurogenesis and implicate specific aa residues of the bHLH in this process.
Keywords: proneural factor, bHLH, neurogenesis
Introduction
Proneural genes play critical roles during neurogenesis in all metazoans. In Drosophila, nervous system development is largely controlled by two proneural gene families, the achaete-scute complex which includes achaete (ac), scute (sc), lethal of scute (l’sc) and asense (ase), and the atonal family which includes atonal (ato), absent MD neurons and olfactory sensilla (amos) and cousin of atonal (cato) (Bertrand et al., 2002; Gonzalez et al., 1989; Goulding et al., 2000a; Goulding et al., 2000b; Huang et al., 2000; Jarman et al., 1993; Villares and Cabrera, 1987).
The ato family controls formation of several types of peripheral sensory organs mediating visual, olfactory and mechano-sensation. During development of these sensory organs, ato family proneural factors play a dual role. They confer neuronal potential as well as specify the neuronal progenitor type. First expressed in clusters of cells, each proneural factor soon becomes restricted to one cell (or a few) out of the proneural cluster. This cell develops into a founder neuron, which in turn recruits surrounding cells to also develop as neurons (Brand et al., 1993; Campuzano and Modolell, 1992; Goulding et al., 2000b; Jarman et al., 1993).
The proneural gene ato is dedicated to the formation of photoreceptor neurons in all three visual systems of the fly (adult eye, ocelli and larval eye) as well as chordotonal organs (stretch receptors) and a few olfactory sensilla (Jarman et al., 1993; Jarman et al., 1994). As other proneural proteins, Ato contains a basic domain for DNA binding and a helix-loop-helix domain for protein-protein interactions (bHLH motif) (Massari and Murre, 2000). In the developing fly eye, Atonal is critical to the specification of the R8 founder photoreceptor-neuron. Thus, in the ato1 mutant (either homozygous or hemizygous over Df(3R)p13, very few, if any, R8 photoreceptors develop and consequently none of the other R1-R7 neurons form as well. This results in a nearly complete lack of ommatidia or single eyes in the adult (Jarman et al., 1994).
Bombyx mori is an important model organism, which belongs to the Lepidoptera order, the second largest order of Insecta with ~200,000 species (Nardi, 1995). Thanks to its accessible development, the availability of mutants and the completion of the Silkworm Genome Project (Duan et al., 2010; Xia et al., 2004), Bombyx mori has become a useful tool in insect genomics and genetic studies. The compound eyes of the adult are comprised of ~3,000 ommatidia and the developing eye epithelium can be easily dissected at the late larval stage. Based on the evolutionary relationship between Lepidoptera and Diptera as well as genetic studies of developmentally related genes (Nakao, 2010; Zhou et al., 2009), we expect critical players in Drosophila neurogenesis to play a conserved role in the development of the Bombyx nervous system.
In this paper, we report the identification and cloning of the ato homologue from Bombyx mori (atoBm), and a comparative functional analysis of the AtoBm and AtoDm proteins using transgenic tools generated in Drosophila.
Results
Identification and conservation of the atonal gene in Bombyx mori
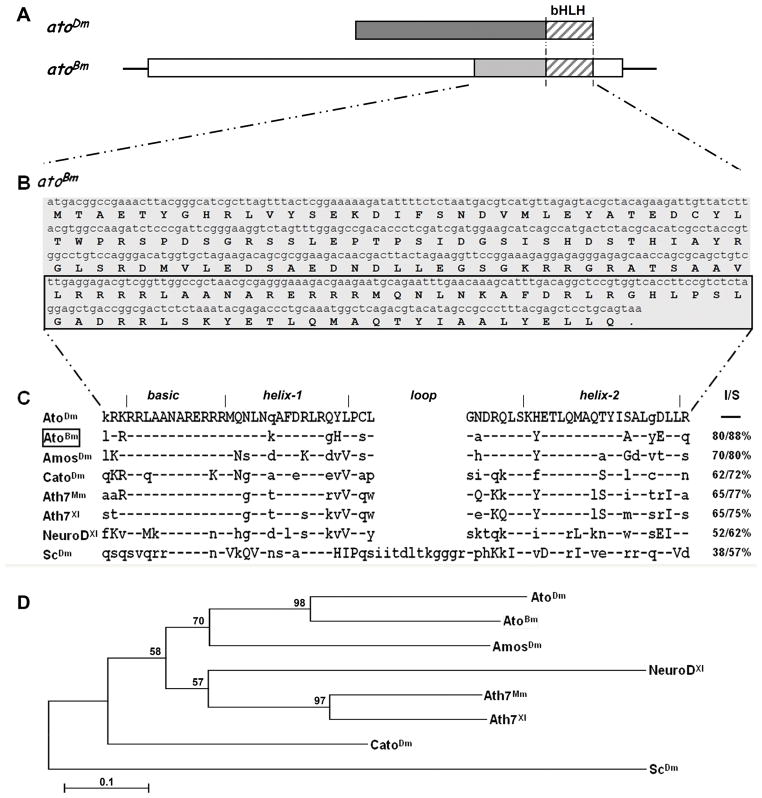
Genome sequencing projects greatly facilitate the identification and comparison of genes across diverse species. As our interest lay in the ato gene, we searched the Bombyx mori genome database by tBLASTn using the full length AtoDm protein sequence. Using this approach, we found a single genomic region (Genbank AADK01015472.1) that encoded a translation product with high similarity to AtoDm within the bHLH region and that in turn identified a single related cDNA (EST BP120230). A search of the Silkworm Genome Database (http://silkworm.genomics.org.cn/) with this EST led us to the annotated gene BGIBMGA013643. Through RT-PCR and 5′ RACE experiments on total larval head RNA (5th instar – day 4), we were able to confirm the existence of a full-length open reading frame (ORF) of 480 bp identical to the genomic DNAand encoded by a putative transcription unit of ~2.6 kb, which we named atoBm (Fig. 1A; see Fig. S1 and legend for details).
Figure 1. Identification of the atonal gene in Bombyx mori.
A) Schematic drawing of the transcription unit for atoBm . Open boxes represent 5′ and 3′ UTR, shaded boxes represent ORF with the stripes marking the region encoding the bHLH. Structure of the atoDm transcript is shown above for comparison. The two proteins are 80% identical and 88% conserved in the bHLH region (striped boxes), but not conserved elsewhere. See Supplementary Figure 1 for more details. B) Full length ORF and translation of atoBm. The bHLH domain is marked by the box. C) Sequence alignment of the bHLH domains of selected proneural proteins (Mm=Mus musculus; Xl=Xenopus laevis). Conservation is given as I=Identities (%identical aa) and S=Similarity (% identical + similar aa) as compared to AtoDm. Residues identical to AtoDm are marked by a hyphen (−), conserved changes are in upper case, nonconserved changes are in lower case. GenBank accession numbers are as follows: AtoDm = NP731223; AtoBm = HQ888870; AmosDm = NP477446; CatoDm = NP477344; Ato7Mm = NP058560; Ato7Xl = NP001079289; NeuroDXl = 1096595; ScDm = NP476803. D) Phylogenetic tree showing the relationship of atonal homologs from different species. The bootstrap 50% majority-rule consensus trees were made with the maximum parsimony method (PAUP, v.4.0b10) using multiple alignments of amino acid sequences. Statistical support (percentage) for each node was evaluated by bootstrap analysis with 1,000 replicates.
The predicted transcription unit of AtoBm spans ~2.6 kb, including a 1967 bp 5′UTR, a 480 bp ORF, and a ~170 bp 3′UTR (Figs. 1A). The putative atoBm ORF encodes a 159 amino acids (aa) protein that is considerably shorter than AtoDm (312 aa) (Fig. 1A and B). Similarly to AtoDm, the bHLH domain is located at the very end of the aa chain (C-terminus) and the transcription unit does not appear to have any introns. Comparative sequence analysis of the Bombyx and Drosophila proteins shows that within the bHLH region AtoBm shares 80% amino acid identity and 88% similarity with AtoDm (Fig. 1C), whereas outside the bHLH, there is no obvious conservation.
We also compared AtoBm with a number of other proneural proteins from the fly and vertebrates including the Mus musculus Atonal7Mm(Tautz, 1989), Xenopus laevis Atonal7Xl (Sommer and Tautz, 1989), the ato-family membersAmosDm, CatoDm, and NeuroDXl, as well as the more distantly related proneural factor ScDm (Fig. 1C). The protein sequence alignment showed that none of the proteins are conserved outside the bHLH domain (not shown). As expected, conservation of the bHLH with Ato family members from vertebrate species, i.e. Atonal7Mm and Atonal7Xl, is higher than conservation with the more distant fly proneural factor ScDm (Fig. 1C).
Phylogenetic analysis and direct sequence comparison of the bHLH domains confirms that AtoBm is most closely related to AtoDm than other ato family members (Fig. 1D). In particular, AtoBm is more closely related to AtoDm than AmosDm in aa sequence conservation. The Bombyx protein is also most similar to AtoDm in that its bHLH domain is located at the very end of the protein, whereas additional aa follow the second helix in other ato family factors, from just a few in Amos to extensive C-terminal region in other factors.
In conclusion, we believe to have identified the ortholog of Ato in Bombyx mori.
Expression pattern of atoBm in the eye disc of Bombyx
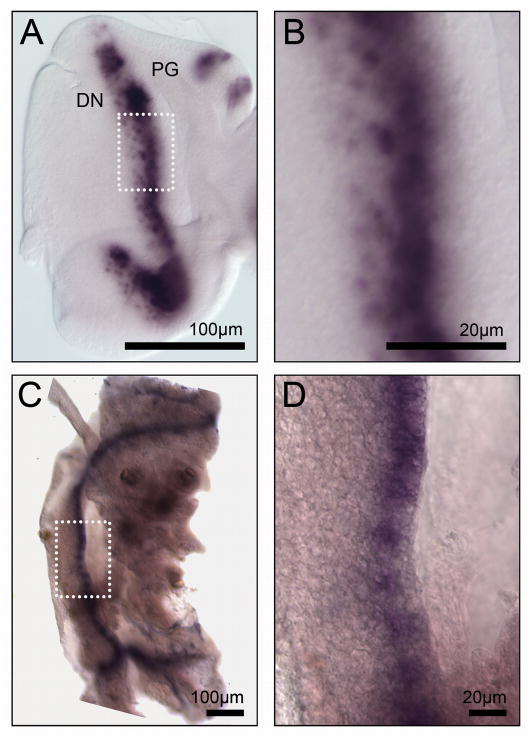
In the developing Drosophila eye, the ato gene is expressed soon after eye progenitor cells cease to divide. Thus, initial expression occurs in a stripe that bisects the disc into an eye progenitor region, anterior to the stripe, and a developing neuronal field, posterior to it (Fig. 2A) (Jarman et al., 1994; Jarman et al., 1995). Whereas initially transcribed in all eye precursor cells ready to undergo morphogenesis, ato expression soon resolves into evenly spaced clusters of 10–15 cells (intermediate groups) and thereafter down to a single expressing cell, that is thus selected as the founder R8 photoreceptor neuron (Fig. 2B) (Jarman et al., 1994; Jarman et al., 1995). The other R neurons emerge around R8 during the following hours in an Ato-independent fashion.
Figure 2. The atoBm mRNA is expressed as a stripe in the silkworm eye disc.
In all figures, panels showing fly discs or adult eyes are oriented posterior to the left. A,B) In situ hybrization to atoDm mRNA in the wt L3 eye disc of Drosophila. atoDm is expressed in a stripe marking neuronal progenitor cells. C, D) In situ hybrization to atoBm mRNA in the wt eye disc (L5 day 4) of Bombyx. Strikignly, atoBm is also expressed in a stripe. Although eye morphogenesis in B.mori is not well understood, this pattern of atoBm expression suggests that neurogenesis may proceed in a similar fashion as in the fruit fly. Identification of the eye discs was based on position within the larval head region relative to the larval ocelli (which are unrelated to the fruit fly ocelli, being already fully differentiated and visible externally at the larva stage in this species).
Similarly to Drosophila, the larger compound eye of Bombyx (3,000 ommatidia) also forms from a larval eye disc. Unlike the fruit fly, however, development of the eye disc follows a different pattern. Studies of eye disc formation in Lepidoptera are limited to B. mori and Manduca sexta (Koyama and Tanaka, 1954; Koyama, 1958; Allee et al., 2006). In these species, adult eye primordium cells are initially part of the epidermis of the larval head. In fact, like other epidermal cells, they produce cuticle. By day 1-day 2 of L5, cuticle production in these cells is down-regulated, the cells detach from the cuticle, initiate rapid cell divisions and begin to invaginate to form the inverted eye imaginal disc. Morphogenesis of the eye would presumably begin soon after and may continue partly into pupation. Thus, B. mori eye discs were dissected on day 4 of L5 and in situ hybridization was carried out to detect atoBm expression.
Interestingly, in spite of these differences in disc development, the expression pattern of atoBm in the L5 eye disc was reminiscent of the pattern observed in the Drosophila eye epithelium. We detected the atoBm mRNA in a stripe, and within the stripe there were groups of more highly expressing cells, reminiscent of the intermediate groups seen in the fly (Fig. 2C, D). Noticeably, we did not observe rows of single expressing cells similar to the R8 cells of the fly eye disc; only few single cells were occasionally detected nearby some clusters. It is unclear whether this discrepancy reflects a significant difference in the way ommatidial neuronal clusters assemble in the two species or, alternatively, if it results from the lower sensitivity of the in situ hybridization protocol in Bombyx. It may well be the latter case, because even in the fly, detection of atoDm expression in single cells is achieved only under optimal conditions.
Due to our very limited understanding of eye disc development and neurogenesis in Bombyx, the relationship between the atoBm mRNA stripe and developmental events in the eye disc is unclear. However, the striking similarities with atoDm expression in the fruit fly suggest that atoBm may play an analogous role in the formation of the ommatidial array during development of the moth eye.
The putative AtoBm can induce Ato-like gain-of-function effects in Drosophila
Depending on biological context, ectopic AtoDm expression has been shown to induce formation of supernumerary R8 neurons or chordotonal organs (Chien et al., 1996; Jarman et al., 1993; Sun et al., 2000). Hence, we sought to establish whether the putative AtoBm homologue could also induce such effects.
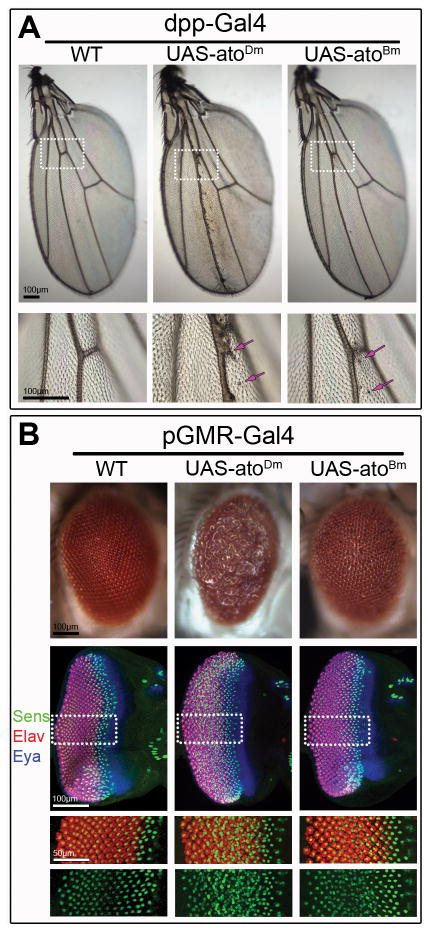
In the developing wing disc, ectopic gene expression was induced using the dpp-Gal4 driver along the anterior-posterior compartments boundary (Fig. 3A). As expected expression of AtoDm led to formation of ectopic chordotonal organs along the third wing vein that were clearly visible on the wing blade of the adult fly. Analogous expression of AtoBm also resulted in formation of a few ectopic chordotonal organs nearby vein 3 (Fig. 3A, arrows).
Figure 3. Mis-expression of AtoDm and AtoBm induces related phenotypes in the wing and the eye.
A) Mis-expression AtoDm or AtoBm in the developing wing disc under the control of dpp-Gal4 induces formation of ectopic chordotonal organs (arrow) along the 3rd wing vein. Lower panel shows high magnification of region marked in upper panel. B) Mis-expression AtoDm or AtoBm under the control of pGMR-Gal4 disrupts eye development leading to the formation of a ‘rough’ adult eye in which the spatial arrangement of the ommatidia is disrupted. Developing disc corresponding to the adult eyes shown above were stained for Sens to mark R8 neurons, the pan-neural marker Elav to highlight all photoreceptors and the retina-identity determinant Eya to mark the developing eye disc. Single channels for Sens and Elav are shown below. Misexpression of AtoDm or AtoBm results in the formation of supernumerary R8 neurons (Sens-positive). In both wing and eye, AtoDm has a stronger effect than AtoBm but the resulting phenotypes are consistent with a similar activity of the two proteins in these experiments.
In the developing eye epithelium, broad over-expression of either AtoBm or the AtoDm within and posterior to the MF was induced using the pGMR-Gal4 driver (Fig. 3B). The adult eyes of pGMR-Gal4 UAS-atoDm flies were “rough” that is the ordered packing of ommatidia was disrupted. In the developing eye discs, it was clear that AtoDm induces many extra R8 neurons, identified using the R8-specific marker Senseless (Sens) (Fig. 3B). In addition, staining with the pan-neural marker Elav showed that extra neurons in general, many not of the R8 type, were also present (Fig. 3B). Given the large number of R8 cells induced and the inductive role R8 plays in recruiting additional R-cells within the clusters (Carthew, 2007), this result was as expected for misexpression of AtoDm.
The adult eyes of pGMR-Gal4 UAS-atoBm flies were also “rough,” but mildly compared to UAS-atoDm-expressing eyes. In the developing eye discs, it was nonetheless clear that AtoBm could induce supernumerary R8 neurons, though not to such an extent to lead to a dramatic increase in total R cells (Fig. 3B).
These results strongly suggest that AtoBm functions in a similar way as AtoDm when ectopically expressed in the fruit fly, although its activity appears to be somewhat lower than the Drosophila factor.
AtoBm can rescue ato loss-of-function in Drosophila
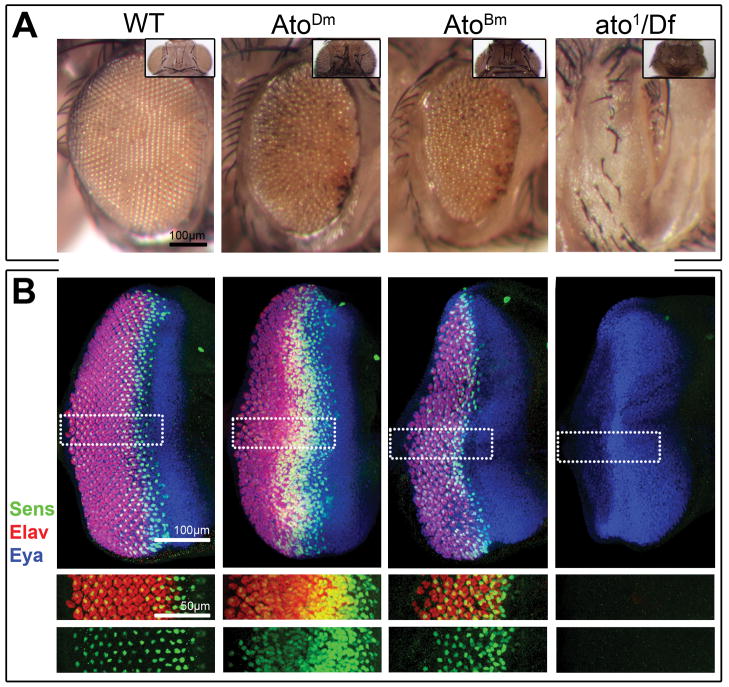
To further explore the functional conservation between AtoBm and AtoDm, we developed a rescue assay in the fly based on the restoration of the adult eye in the ato loss-of-function. The ato1 allele is a semi-lethal recessive mutant allele that shows extensive loss of Ato-dependent sensory organs, including the eye, at larval and adult stages. Hemizygous ato1/Df(3R)p13 escapers are nearly or completely eyeless, displaying only a few ommatidia when eye tissue is present (Fig. 4).
Figure 4. AtoBm can rescue the loss of eye of the ato1 mutant.
A) Adult compound eyes of wt, AtoDm-rescued, AtoBm-rescued and ato1-mutant flies (left to right). Inset shows dorsal views of corresponding fly genotypes. Quantitative data are shown in Fig. 5C. Control ‘wt’ genotype is ato5′EYE3′-Gal4 ato1/+; rescued flies genotypes are UAS-atoX/+ ; ato5′EYE3′-Gal4 ato1/Df(3R)p13; ato mutant genotype is ato5′EYE3′-Gal4 ato1/Df(3R)p13. B) L3 eye discs of wt, AtoDm-rescued, AtoBm-rescued and ato1-mutant flies (left to right). Discs are stained for Sens to mark R8 neurons, the pan-neural marker Elav to highlight all photoreceptors and the retina-identity determinant Eya to mark the developing eye disc. Single channels for Sens and Elav are shown below.
Previous rescue experiments in this mutant background made use of various Gal4 drivers with broad expression quite different from the very restricted and transient pattern of the endogenous atoDm gene in the L3 eye disc (Jarman et al., 1994; Maung and Jarman, 2007; Sun et al., 2003). In addition, the UAS-cDNA transgenes used to express bHLH factors were generated at a time when site-directed transgenesis was not available (Bischof et al., 2007). Thus, UAS transgenes displayed differences in expression levels due largely to their random sites of integration within the fly genome. In these cases, rescue results were variable and interpretable only when differences in the extent of rescue were very large. As a consequence, the findings cannot always be reconciled with aa conservation (Jarman et al., 1994; Maung and Jarman, 2007; Sun et al., 2003).
To set up an improved rescue assay, we generated new UAS transgenic lines in which each transgene was always inserted at the same site (51C on chromosome II; (Bischof et al., 2007). This approach allowed us to avoid variability in expression levels due to insertion-site effects. Moreover, in order to reproduce the endogenous atoDm pattern as closely as possible, we generated a novel Gal4 driver (ato5′EYE3′-Gal4) based on the eye enhancer elements of the fly gene (Supplementary Figure 2).
Using these tools, a robust rescue of the loss-of-eye phenotype in ato1/Df(3R)p13 flies was obtained with UAS-atoDm (Figs. 4A). Expression of UAS-atoBm also resulted in a dramatic rescue of the adult and developing eye (Figs. 4 and 5C). However, in both cases, the ordered ommatidial packing was not restored suggesting that neuronal patterning in the L3 discs was somewhat disrupted. Staining of developing eye discs for Sens (R8) and Elav (all neurons) showed that supernumerary R8 cells were visible in the developing neuronal fields, though more so when AtoDm was expressed (Fig. 4B). This is likely due to higher than wt levels of Ato proteins’ expression through the Gal4/UAS binary method. Interestingly, this effect was stronger with AtoDm than AtoBm, suggesting once again that the D. melanogaster protein is somewhat more ‘effective’ than the B. mori homologue.
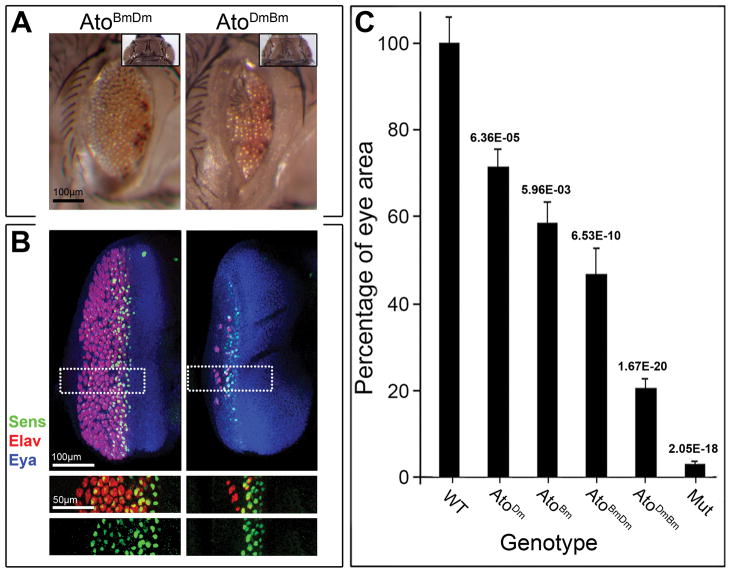
Figure 5. The N-regions but not the bHLH domains can effectively substitute for each other in the rescue assay.
A) Adult compound eyes of AtoBmDm-rescued, AtoDmBm-rescued ato1-mutant flies (left to right). Inset shows dorsal views of corresponding fly genotypes. Compare to wt, AtoDm-rescued, AtoBm-rescued and ato1-mutant in Fig. 4 for extent of rescue. Quantitative data are shown in Fig. 5C. Rescued flies genotypes are UAS-atoX/+; ato5′EYE3′-Gal4 ato1/Df(3R)p13. B) L3 eye discs of eyes of AtoBmDm-rescued, AtoDmBm-rescued ato1-mutant flies (left to right). Discs are stained for Sens to mark R8 neurons, the pan-neural marker Elav to highlight all photoreceptors and the retina-identity determinant Eya to mark the developing eye disc. Single channels for Sens and Elav are shown below. C) Quantitative data comparing the eye size of wt, AtoDm-rescued, AtoBm-rescued, AtoBmDm-rescued, AtoDmBm-rescued and ato1-mutant flies (N=10, 15, 40, 35, 25, 10 respectively). Columns show relative eye size compared to wt; bars show standard deviation; numbers on the top of each column indicate the p value from t-test between each sample and the one to the left. See Supplementary Figure 3 for details of data collection, analysis and males-females breakdown.
Nonetheless, these findings confirm that the AtoBm factor behaves similarly to AtoDm in rescue experiments and thus closely mimics AtoDm function in the fruit fly eye.
Differential activity of the highly conserved AtoBm and AtoDm bHLH domains
As shown above (Fig. 1C), the conservation between AtoBm and AtoDm is very high within the bHLH domain. The few distinct aa within their respective bHLH domains do not map to any residues of known significance for protein folding, DNA-protein or protein-protein interactions (Ellenberger et al., 1994; Ma et al., 1994). The similar rescue of ato1/Df(3R)p13 eyes by AtoDm and AtoBm is consistent with a minimal effect of these aa changes on bHLH function. Thus, one might predict that the two bHLH domains would be nearly interchangeable such that a chimeric AtoDmBm protein, encoding a fusion of the N-terminus from atoDm to the bHLH from atoBm (NDm+bHLHBm = UAS-atoDmBm), would substitute quite well for the wt AtoDm factor. Once again, this transgene was inserted in the 51C site to prevent insertion site effects.
Surprisingly, using the same Gal4 driver (ato5′EYE3′-Gal4), AtoDmBm-expressing mutant flies consistently displayed eyes much smaller than those of either atoDm or atoBm-rescued animals (compare Figs. 5A to 4A; Figs. 5C and S2). The extent of rescue in the discs of L3 wandering-stage larvae (Fig. 5B) also correlated well with the adult phenotypes. Thus, in spite of the extensive conservation between the AtoBm and AtoDm bHLHs, these domains are not interchangeable.
Interchangeable activity of the highly divergent AtoBm and AtoDm N-fragments
On the contrary, no conservation can be detected between AtoBm and AtoDm outside the bHLH domains (N-regions). Thus, the N-regions may not be expected to effectively substitute for one another. The significantly less effective rescue by the AtoDmBm chimera than by the AtoBm protein is consistent with a necessary coupling of N-regions and bHLH domains.
To further test this hypothesis, we also compared the rescue of the ato1/Df(3R)p13 phenotype by AtoDm to that by a chimeric AtoBmDm protein, in which the AtoBm N-region was linked to the AtoDm bHLH domain (NDm+bHLHBm = UAS-atoDmBm). Using the ato5′EYE3′-Gal4 driver, AtoBmDm-expressing mutant flies consistently displayed eyes only a little smaller than AtoDm eyes and considerably larger than eyes expressing AtoDmBm (compare Figs. 5A to 4; and Fig. 5C and S2). As before, the extent of rescue in the discs (Fig. 5B) correlated well with the adult phenotypes.
In conclusion, the N-region of AtoBm can robustly substitute for the corresponding region of the AtoDm transcription factor despite the lack of evolutionary conservation at the aa level.
Discussion
We report here the cloning of the ato gene of Bombyx mori and show that it is highly similar to fly Ato both in aa sequence of its bHLH domain and apparently also in its expression pattern (mRNA) in the developing eye disc of the silkworm. Based on these striking similarities, it is likely that AtoBm plays an analogous role to AtoDm in the formation of the ommatidial array during development of the Bombyx eye.
As mentioned above, the conservation between atoBm and atoDm is very high within the bHLH domain, whereas no obvious similarities can be found on the N-terminal side (Fig. 1C). This is also true for Ato homologs from other, non-Drosophila species. Interestingly, the 3 members that have been tested for ato1 rescue - AmosDm, Atonal7Mm and Atonal7Xl - all have robustly and similarly conserved bHLH domains (48/60, 47/60, 46/60) (Fig. 1C). Nonetheless, as previously shown, they each rescued ato1 to surprisingly different extent as compared to each other and AtoDm (Sun et al., 2003). The reason for this may reside in the few distinct aa within their respective bHLH domains; or it may result from the activity of their very divergent N-terminal regions.
We sought to investigate these alternatives using the AtoBm protein. On one hand, based on the high conservation with AtoDm within the bHLH, we might have expected a nearly perfect rescue of the ato1 phenotype by AtoBm. On the other hand, considering the lack of conservation on the N-terminal side, a poor rescue could also have been expected. As shown here, we found that UAS-atoDm and UAS-atoBm can both rescue the loss of eye phenotype of the ato1 mutant to a similar extent, with the fly factor doing so a little better than the Bombyx one.
Given the high conservation between the bHLH domains of these proteins, such result may have simply reflected the proposed preeminence of the bHLH motif in defining the function of bHLH factors. In this scenario the contribution made by the N-termini to protein activity would be minor. The robust rescue induced by a chimeric protein composed of the Bombyx N-terminal region and the Drosophila bHLH domain, AtoBmDm, initially appeared consistent with this view (Fig. 5). However, in this case, we would have also expected that the Bombyx bHLH domain induce a similar level of rescue when provided with the N-terminal region of AtoDm. Surprisingly, this was not the case. The AtoDmBm fusion rescued poorly, at a much lower level that AtoBm itself or the AtoBmDm chimera (Fig. 4 and 5). Thus, whereas the bHLHBm domain is nearly as effective as the bHLHDm when in the context of the full length AtoBm protein, it is not so when associated with the AtoDm N-region. In short, the bHLHBm domain requires the AtoBm N-region to function effectively, whereas the bHLHDm domain can operate sufficiently well in the context of a profoundly different N-sequence.
These findings strongly suggest that, in the eye, bHLH function is modulated by non-bHLH sequences, notwithstanding the proposed critical role of the bHLH motif in defining the function of bHLH factors (Chien et al., 1996; Nakada et al., 2003; Quan et al., 2004; Maung and Jarman, 2007). Given the high conservation in the bHLH, the small number of residues changed between the bHLHDm and bHLHBm appear to be significant in mediating interactions with the N-region. All 5 non-conserved changes (Fig. 1C) occur at positions that have not been associated with particular functions (folding, dimerization or DNA contact) (Ellenberger et al., 1994; Ma et al., 1994). None occur within the DNA-binding basic region, two occur in helix 1, two in the loop, and one in helix 2. The helices, in particular, are known mediators of protein-protein binding suggesting a potential role for some of these residues in modulating interactions between the bHLH and the rest of the protein, either directly or indirectly.
Further analyses of modified AtoDmBm proteins using the tools presented here and other approaches will permit a detailed investigation of how this effect is mediated at the molecular level.
Methods
Insect stock and transgenic lines
The silkworm strain Dazao was reared on an artificial diet at 25°C and 70%–80% relative humidity in the Zhang lab. Transgenic Drosophila lines were generated by site-specific transformation using the 51C or 68E insertion sites (Bischof et al., 2007). UAS-atoBm and UAS-atoDm expression constructs were generated by cloning the full-length ORFs of atoBm and atoDm into the pUAST-attB transformation vector. The chimeric trangenes UAS-atoBmDm and UAS-atoDmBm were generated by fusing aa 1–85 of atoBm to aa 238–312 of atoDm, and aa 1–237 of atoDm to aa 86–159 of atoBm, respectively. The ato5′EYE3′-Gal4 transgene was constructed in the site-specific transformation vector pattB (Venken et al., 2006). 1.1 kb of the genomic DNA around the AtoDm promoter was first cloned in the pattB MCS; the 2.6 kb 5′ eye enhancer region was then inserted upstream of the 1.1 Kb; and lastly the 6.8 kb 3′ enhancer region was inserted downstream of the Gal4 ORF. The transgenic flies were generated by insertion at 68E. Other fly lines used were dpp-Gal4 (Staehling-Hampton et al., 1994), pGMR-Gal4 (Freeman, 1996), ato5′EYE3′-Gal4 (Zhou and Pignoni, submitted), ato1 (Jarman et al., 1994) and Df(3R)p13.
Molecular biology and Bioinformatics
The bHLH domain of the atoBm gene was identified by tBLASTn search (NCBI) of the Bombyx mori genome and EST databases using the full length AtoDm protein sequence. Gene-specific primers for 5′ RACE and RT-PCR were designed based on the genomic sequence (Fig. S1), Total RNA was extracted from the heads of 5th instar larvae using a Trizol-based protocol (Chomczynski, 1993). One microgram of total RNA was DNAse (Promega, US) treated and then used as template for 1st strand cDNA synthesis using PrimeScript Reverse Transcriptase (Takara, Dallian, China). 5′-RACE was performed using the SMART™ RACE cDNA Amplification Kit (Clontech, USA) to extend ORF sequence into 5′UTR. Second strand PCR amplification was then performed in a total volume of 25 ul composed of 1× PCR buffer, 0.2 mM dNTP mix, 1.5 mM MgCl2, 1 U of Taq polymerase (Takara), 0.4 mM each primer, and 0.5 ml of the cDNA template. PCR products were analyzed by electrophoresis, gel-purified, cloned into pMD18-T (Takara), and sequenced commercially. The atoBm sequence has been deposited in GenBank (HQ888870). Protein sequences alignments and conservation analyses were done with ClustalX (Thompson et al., 1997) and GeneDoc (Thompson et al., 2002). Phylogenetic analysis was carried out by PAUP software (v.4.0b10) (Wilgenbusch and Swofford, 2003), with the maximum parsimony method and bootstrapping sampled for 1,000 times (Wilgenbusch and Swofford, 2003). Figure 1D was generated with TreeView application (Page RD, 1996).
Whole-mount in situ hybridization
In situ hybridization was performed on the L5 day 4 larval eye discs of the silkworm and L3 eye discs of the fly with DIG-labeled RNA probes (Sullivan et al., 2000). Sense and antisense digoxigenin-labeled RNA probes were prepared using the cloned RT-PCR atoBm product; atoDm probe was prepared from a ORFs amplified from the genome and subcloned. Digoxigenin RNA labeling kit (Roche, USA). AP-conjugated anti-digoxigenin Fab fragments (Roche, USA) were used at 1:2000. Tissue was mounted in 80% glycerol.
Immunohistochemistry
Staining of Drosophila eye disc was carried out by standard protocols (Sullivan et al., 2000) with the following antibodies: rat mAb anti-Elav (DSHB), mouse mAb anti-Eya (DSHB), guinea pig Ab anti-Sens (Nolo et al., 2000), rabbit anti-β-gal (1:1000) (Cappell) and secondary antibodies (anti-mouse, anti-rat, anti-rabbit, and anti-guinea pig) conjugated to Cy2, Cy3, and Cy5 (Jackson ImmunoResearch Laboratories). Tissue was mounted in 80% glycerol. All Ab stainings shown are images from confocal microscopy. Images were obtained with a Leica DM5500Q confocal system and processed with Adobe-Photoshop.
Supplementary Material
A Bombyx mori genomic sequence that encoded a translation product with high similarity to the AtoDm bHLH domain was first identified by tBLASTn using the full length AtoDm protein sequence. A BLASTn search using the genomic sequence (part of contig AADK01015472.1) identified a cDNA clone (EST # BP120230) isolated from an eye-tissue mixed-stage cDNA library (5th instar or L5 larvae and pupae). This cDNAencoded a 473 bp ORF lacking the starting methionine (Met) but followed by a 3′UTR that included the start of a poly(A) tail. Expression of this ORF was confirmed by RT-PCR on total larval head RNA (L5 – day 4) using the sequence specific primers F1 and R1; the amplification product was cloned in T-vector and sequenced. In order to identify the start of the ORF, we carried out 5′RACE and the resulting amplification product was similarly cloned and sequenced. It overlapped extensively with the cDNA but also included a 1967 bp 5′UTR before a 480bp ORF. In this clone, the ORF included 7 additional bp on the 5′ side, up to a starting Met which was preceded by multiple stop codons in all three frames. The sequence of the ORF was identical in all cases (EST, RT-PCR product, 5′ RACE product and genome database). The predicted transcription unit of AtoBm spans ~2.6 kb, including a 1967 bp 5′UTR, a 480 bp ORF, and a ~170 bp 3′UTR. Sequences of all gene specific primers in shown below. UPM and NUP are Universal primers from the SMART™ RACE cDNA Amplification Kit (Clontech, USA).
A) Map of the ato5′EYE3′-Gal4 transgene and its relationship to the genomic regulatory region of atoDm. A schematic of the wt atoDm expression pattern color-coded to relate it to the relevant regulatory fragments is shown to the right. Open boxes indicate transcription units; yellow, black and blue boxes show regions of the ato genomic DNA included in the construct; asterisk marks a hsp70 basal promoter carried over from the pattB Gal4 vector (see also materials and methods for details). B,C,D) L3 Drosophila eye discs stained for endogenous AtoDm (red) and the Gal4-driven β-Galactosidase protein (green). Cells in the disc transiently express AtoDm (B) as neuronal development proceeds from the posterior (right) towards the anterior (left) of the epithelium. Green staining in the developing ommatidial field, to the right of the AtoDm domain, is due to persistence of the β-Galactosidase protein (as confirmed by the mRNA pattern shown in E). E) In situ hybridization showing expression of the lacZ mRNA driven by ato5′EYE3′-Gal4. Expression of the mRNA occurs in a stripe and mimics endogenous atoDm transcription.
Breakdown of a portion of the data shown in Figure 5C by sex. Quantitative comparison of the eye size of wt, AtoDm-rescued, AtoBm-rescued, AtoBmDm-rescued, AtoDmBm-rescued and ato1-mutant flies. Photos of fly eyes were taken using a stereomicroscope at 66X magnification. Size of the eye region was calculated in Adobe Photoshop CS4 (unit as pixel). The average size of the wt was set as 100%; other columns show the relative eye size of each genotype compared to wt; bars show standard deviation. The sample size N (=number of eyes) is reported above each column. The p values from t-test are shown below. Number of ato1/Df(3R)p13 and rescued flies is low due to the semi-lethal phenotype of ato1 and represent escapers. Viability of the mutant is affected by a requirement for Ato in non-eye tissue. The ato5′EYE3′-Gal4 driver reproduces faithfully the pattern of ato expression in the L3 eye disc but not in the embryo or other larval tissues.
Acknowledgments
We thank members of the Pignoni, Zuber and Viczian labs for stimulating discussion, C. Pina for helpful comments on the manuscript, D. Money for assistance with manuscript preparation, the IDSHB and Dr. H. Bellen for antibodies, and the Bloomington stock center for wt and attA fly stocks. This work is supported by NEI grant #R01EY013167 (FP); an RPB unrestricted grant and the Lions of CNY (Upstate Medical University, Dept. of Ophthalmology); and grant #2010CB126205 from the National Basic Research Program of China (CZ).
References
- Allee JP, Pelletier CL, Fergusson EK, Champlin DT. Early events in adult eye development of the moth, Manduca sexta. J Insect Physiol. 2006;52:450–460. doi: 10.1016/j.jinsphys.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Jarman AP, Jan LY, Jan YN. asense is a Drosophila neural precursor gene and is capable of initiating sense organ formation. Development. 1993;119:1–17. doi: 10.1242/dev.119.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Campuzano S, Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992;8:202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Carthew RW. Pattern formation in the Drosophila eye. Curr Opin Genet Dev. 2007;17:309–313. doi: 10.1016/j.gde.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CT, Hsiao CD, Jan LY, Jan YN. Neuronal type information encoded in the basic-helix-loop-helix domain of proneural genes. Proc Natl Acad Sci U S A. 1996;93:13239–13244. doi: 10.1073/pnas.93.23.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- Duan J, Li R, Cheng D, Fan W, Zha X, Cheng T, Wu Y, Wang J, Mita K, Xiang Z, Xia Q. SilkDB v2.0: a platform for silkworm (Bombyx mori ) genome biology. Nucleic Acids Res. 2010;38:D453–456. doi: 10.1093/nar/gkp801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T, Fass D, Arnaud M, Harrison SC. Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 1994;8:970–980. doi: 10.1101/gad.8.8.970. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Romani S, Cubas P, Modolell J, Campuzano S. Molecular analysis of the asense gene, a member of the achaete-scute complex of Drosophila melanogaster, and its novel role in optic lobe development. EMBO J. 1989;8:3553–3562. doi: 10.1002/j.1460-2075.1989.tb08527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SE, White NM, Jarman AP. cato encodes a basic helix-loop-helix transcription factor implicated in the correct differentiation of Drosophila sense organs. Dev Biol. 2000a;221:120–131. doi: 10.1006/dbio.2000.9677. [DOI] [PubMed] [Google Scholar]
- Goulding SE, zur Lage P, Jarman AP. amos, a proneural gene for Drosophila olfactory sense organs that is regulated by lozenge. Neuron. 2000b;25:69–78. doi: 10.1016/s0896-6273(00)80872-7. [DOI] [PubMed] [Google Scholar]
- Huang ML, Hsu CH, Chien CT. The proneural gene amos promotes multiple dendritic neuron formation in the Drosophila peripheral nervous system. Neuron. 2000;25:57–67. doi: 10.1016/s0896-6273(00)80871-5. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Koyama N. Studies on the morphogenesis of mosaic compound eye in the silkworm moth. Journal of the Faculty of Textiles and Sericulture. Shinshu University. Series A, Biology. 1958;8:1–18. [Google Scholar]
- Koyama N, Tanaka S. Studies on the morphogenesis of mosaic compound eye in the silkworm moth. Faculty of Textiles and Sericulture. Shinshu University. Series A, Biology. 1956;4:50–55. [Google Scholar]
- Ma PC, Rould MA, Weintraub H, Pabo CO. Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell. 1994;77:451–9. doi: 10.1016/0092-8674(94)90159-7. [DOI] [PubMed] [Google Scholar]
- MacWhinnie SG, Allee JP, Nelson CA, Riddiford LM, Truman JW, Champlin DT. The role of nutrition in creation of the eye imaginal disc and initiation of metamorphosis in Manduca sexta. Dev Biol. 2005;285:285–297. doi: 10.1016/j.ydbio.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung SM, Jarman AP. Functional distinctness of closely related transcription factors: a comparison of the Atonal and Amos proneural factors. Mech Dev. 2007;124:647–656. doi: 10.1016/j.mod.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao H. Characterization of Bombyx embryo segmentation process: expression profiles of engrailed, even-skipped, caudal, and wnt1/wingless homologues. J Exp Zool B Mol Dev Evol. 2010;314:224–231. doi: 10.1002/jez.b.21328. [DOI] [PubMed] [Google Scholar]
- Nardi JB. Molecular model systems in the lepidoptera. Science. 1995;269:1743. doi: 10.1126/science.269.5231.1743. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Sommer R, Tautz D. Minimal homology requirements for PCR primers. Nucleic Acids Res. 1989;17:6749. doi: 10.1093/nar/17.16.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K, Jackson PD, Clark MJ, Brand AH, Hoffmann FM. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 1994;5:585–593. [PubMed] [Google Scholar]
- Sullivan W, Ashburner M, Hawley RS. Drosophila Protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Sun Y, Jan LY, Jan YN. Ectopic scute induces Drosophila ommatidia development without R8 founder photoreceptors. Proc Natl Acad Sci U S A. 2000;97:6815–6819. doi: 10.1073/pnas.110154497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Kanekar SL, Vetter ML, Gorski S, Jan YN, Glaser T, Brown NL. Conserved and divergent functions of Drosophila atonal, amphibian, and mammalian Ath5 genes. Evol Dev. 2003;5:532–541. doi: 10.1046/j.1525-142x.2003.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svacha P. What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola) Dev Biol. 1992;154:101–117. doi: 10.1016/0012-1606(92)90052-i. [DOI] [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989;17:6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2002;Chapter 2(Unit 2):3. doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R, Cabrera CV. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987;50:415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Wilgenbusch JC, Swofford D. Inferring evolutionary trees with PAUP*. Curr Protoc Bioinformatics. 2003;Chapter 6(Unit 6):4. doi: 10.1002/0471250953.bi0604s00. [DOI] [PubMed] [Google Scholar]
- Xia Q, Zhou Z, Lu C, Cheng D, Dai F, Li B, Zhao P, Zha X, Cheng T, Chai C, Pan G, Xu J, Liu C, Lin Y, Qian J, Hou Y, Wu Z, Li G, Pan M, Li C, Shen Y, Lan X, Yuan L, Li T, Xu H, Yang G, Wan Y, Zhu Y, Yu M, Shen W, Wu D, Xiang Z, Yu J, Wang J, Li R, Shi J, Li H, Su J, Wang X, Zhang Z, Wu Q, Li J, Zhang Q, Wei N, Sun H, Dong L, Liu D, Zhao S, Zhao X, Meng Q, Lan F, Huang X, Li Y, Fang L, Li D, Sun Y, Yang Z, Huang Y, Xi Y, Qi Q, He D, Huang H, Zhang X, Wang Z, Li W, Cao Y, Yu Y, Yu H, Ye J, Chen H, Zhou Y, Liu B, Ji H, Li S, Ni P, Zhang J, Zhang Y, Zheng H, Mao B, Wang W, Ye C, Wong GK, Yang H. A draft sequence for the genome of the domesticated silkworm (Bombyx mori) Science. 2004;306:1937–1940. doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Yu L, Shen X, Li Y, Xu W, Yi Y, Zhang Z. Homology of dipteran bristles and lepidopteran scales: requirement for the Bombyx mori achaete-scute homologue ASH2. Genetics. 2009;183:619–627. 611SI–613SI. doi: 10.1534/genetics.109.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Bombyx mori genomic sequence that encoded a translation product with high similarity to the AtoDm bHLH domain was first identified by tBLASTn using the full length AtoDm protein sequence. A BLASTn search using the genomic sequence (part of contig AADK01015472.1) identified a cDNA clone (EST # BP120230) isolated from an eye-tissue mixed-stage cDNA library (5th instar or L5 larvae and pupae). This cDNAencoded a 473 bp ORF lacking the starting methionine (Met) but followed by a 3′UTR that included the start of a poly(A) tail. Expression of this ORF was confirmed by RT-PCR on total larval head RNA (L5 – day 4) using the sequence specific primers F1 and R1; the amplification product was cloned in T-vector and sequenced. In order to identify the start of the ORF, we carried out 5′RACE and the resulting amplification product was similarly cloned and sequenced. It overlapped extensively with the cDNA but also included a 1967 bp 5′UTR before a 480bp ORF. In this clone, the ORF included 7 additional bp on the 5′ side, up to a starting Met which was preceded by multiple stop codons in all three frames. The sequence of the ORF was identical in all cases (EST, RT-PCR product, 5′ RACE product and genome database). The predicted transcription unit of AtoBm spans ~2.6 kb, including a 1967 bp 5′UTR, a 480 bp ORF, and a ~170 bp 3′UTR. Sequences of all gene specific primers in shown below. UPM and NUP are Universal primers from the SMART™ RACE cDNA Amplification Kit (Clontech, USA).
A) Map of the ato5′EYE3′-Gal4 transgene and its relationship to the genomic regulatory region of atoDm. A schematic of the wt atoDm expression pattern color-coded to relate it to the relevant regulatory fragments is shown to the right. Open boxes indicate transcription units; yellow, black and blue boxes show regions of the ato genomic DNA included in the construct; asterisk marks a hsp70 basal promoter carried over from the pattB Gal4 vector (see also materials and methods for details). B,C,D) L3 Drosophila eye discs stained for endogenous AtoDm (red) and the Gal4-driven β-Galactosidase protein (green). Cells in the disc transiently express AtoDm (B) as neuronal development proceeds from the posterior (right) towards the anterior (left) of the epithelium. Green staining in the developing ommatidial field, to the right of the AtoDm domain, is due to persistence of the β-Galactosidase protein (as confirmed by the mRNA pattern shown in E). E) In situ hybridization showing expression of the lacZ mRNA driven by ato5′EYE3′-Gal4. Expression of the mRNA occurs in a stripe and mimics endogenous atoDm transcription.
Breakdown of a portion of the data shown in Figure 5C by sex. Quantitative comparison of the eye size of wt, AtoDm-rescued, AtoBm-rescued, AtoBmDm-rescued, AtoDmBm-rescued and ato1-mutant flies. Photos of fly eyes were taken using a stereomicroscope at 66X magnification. Size of the eye region was calculated in Adobe Photoshop CS4 (unit as pixel). The average size of the wt was set as 100%; other columns show the relative eye size of each genotype compared to wt; bars show standard deviation. The sample size N (=number of eyes) is reported above each column. The p values from t-test are shown below. Number of ato1/Df(3R)p13 and rescued flies is low due to the semi-lethal phenotype of ato1 and represent escapers. Viability of the mutant is affected by a requirement for Ato in non-eye tissue. The ato5′EYE3′-Gal4 driver reproduces faithfully the pattern of ato expression in the L3 eye disc but not in the embryo or other larval tissues.