Abstract
The study examined the interaction between early maturational timing [as measured by premature adrenarche (PA)] and executive functioning and cortisol reactivity on symptoms of psychopathology. The study included 76 girls aged 6 through 8 years (mean = 7.50; SD = .85) with PA (n = 40) and on-time adrenarche (n = 36). Girls completed a battery of psychological and neuropsychological tests and blood sampling for cortisol. Parents completed the Child Behavior Checklist. Results demonstrated that girls with PA with lower levels of executive functioning had higher externalizing and anxious symptoms compared to other girls. Additionally, girls with PA who demonstrated increases in serum cortisol had higher externalizing symptoms than those with stable patterns. Finally, girls with PA who demonstrated decreases in cortisol reported higher depressive symptoms. Findings from this study provide important information concerning the impact of cognitive functioning and stress reactivity on adjustment to early maturation in girls with PA. Results of this research may inform screening and intervention efforts for girls who may be at greatest risk for emotional and behavioral problems as a result of early maturation.
Researchers attempting to understand what factors place children and adolescents at risk for the development of psychopathology have long been interested in the short- and long-term impact of pubertal timing (i.e., developing earlier, at the same time, or later than same-age peers). Numerous studies report that early maturation in girls is linked to a variety of emotional and behavioral problems including symptoms of depression, anxiety, and aggression in both psychiatric or endocrine-related clinical and non-clinical samples (e.g., Caspi, Lynam, Moffitt, & Silva, 1993; Dorn, Hitt, & Rotenstein, 1999; Ehrhardt & Meyer-Bahlburg, 1986; Ge, Brody, Conger, Simons, & Murry, 2002; Graber, Seeley, Brooks-Gunn, & Lewinsohn, 2004). These findings have fostered strong interest in understanding why early maturation confers risk for psychopathology, particularly for girls.
One prominent theory explaining the link between pubertal timing and psychopathology is the early maturation or early timing hypothesis (e.g., Brooks-Gunn, Petersen, & Eichorn, 1985; Petersen & Taylor, 1980). It is suggested that early maturation is particularly disadvantageous because individuals who develop earlier than same-age peers bypass the opportunity to complete the normative developmental tasks of late childhood and early adolescence that facilitate more advanced cognitive and emotion regulation skills. These missed opportunities may negatively affect the individual’s ability to navigate the transitions occurring during puberty and cope with the associated psychosocial demands (Susman & Dorn, 2009). Inherent to the early maturation hypothesis is the mismatch between physical development and cognitive and emotional resources that ultimately yields emotional and behavioral problems. Moreover, this mismatch may be most salient for early maturing girls, as maturation occurs at an age when cognitive and emotional resources are significantly underdeveloped for managing the complex demands of puberty.
Premature Adrenarche as a Case of Early Maturation
Premature adrenarche (PA) is defined as the early activation of the hypothalamic-pituitary-adrenal (HPA) axis through which the concentrations of adrenal androgens [i.e., dehydroepiandrosterone, its sulfate (DHEAS), and androstenedione] increase beyond what is usually seen in age-matched peers (Dorn & Rotenstein, 2004). PA is characterized by the appearance of pubic hair and sometimes axillary hair, body odor, and increased skeletal maturation with onset by age 8 in girls and 9.5 in boys (Ibáñez, Díaz, López-Bermejo, & Marcos, 2009; Reiter & Saenger, 1997 for review). Importantly, at the onset of PA there is no gonadal axis activation and thus no thelarche (onset of breast development) or menarche. Generally, the etiology of PA is unclear. Some research has shown an association between PA and being small for gestational age or low birth weight (Ibáñez, Dimartino-Nadri, Potau, & Saenger, 2000; Meriq, 2006). Further, although they are often referred to as similar, PA and premature gonadarche (i.e., clinically early activation of the reproductive axis and subsequent increases in gonadal steroid concentrations) are not highly correlated and seem to represent two separate maturational processes (Sklar, Kaplan, & Grumbach, 1980). In fact, it is rare for PA children to develop precocious puberty. More commonly, timing of gonadarche and entry into puberty tends to be normal after PA (Ibáñez et al., 2000).
Until the last decade or so, PA was considered a benign process representing a variation of normal pubertal maturation (Ibáñez et al., 2009). Recently, studies have shown that girls with PA may be at increased risk for exaggerated adrenal and ovarian androgen biosynthesis and, later in the reproductive years, an increased incidence of polycystic ovarian syndrome (PCOS; de Zegher & Ibáñez, 2009; Ibáñez et al., 2000). Girls with PA may also be at increased risk for mood and behavior problems (Dorn et al., 1999; Dorn et al., 2008). However, causal association between PA and symptoms of psychopathology remains unknown. To date, only one study has specifically examined the occurrence of psychosocial problems in girls with PA. These investigators reported that, in comparison to age-matched community controls with on-time adrenarche, girls with PA demonstrated higher levels of internalizing and externalizing behavior problems, as well as higher psychiatric syndrome scores as measured by a diagnostic interview (Dorn et al., 2008). It is possible that the association between PA and symptoms of psychopathology was attributable to increased concentrations of DHEAS and androstenedione; however, because not all girls with PA manifested elevated levels of symptoms and disorders it is likely that contextual and individual factors also played key roles. Hence, a more thorough understanding of risk and protective factors related to the emotional and behavioral development of girls with PA is needed.
Extant research evaluating early maturation and associated risk factors for emotional and behavioral problems have focused exclusively on gonadarche, rather than adrenarche. With gonadarche, external physical changes (e.g., breast development) signal to others (e.g., parents, teachers, and peers) that the child is maturing. Traditionally, the physical changes of PA (e.g., body hair, acne, body odor) are considered less evident to others, suggesting that PA may not have the same social signaling effect as gonadarche. Although some of these physical changes of PA may not be obvious to peers (i.e., pubic hair), parents are often aware of these changes and recognize them as being commensurate with adolescence (Dorn, 2007). In turn, some parents may expect behavioral and intellectual functioning that is normative for a teenager, even if the child is only 6 to 8 years old. Additionally, other physical changes may be evident to peers (e.g., increased acne, body odor, axillary hair growth, increased height), which may result in peer expectations that are reflective of being perceived as older and certainly “different” than same aged peers. As outlined in the early maturation hypothesis, girls with PA may be unprepared, cognitively and emotionally, to cope with these expectations and their associated demands. For this reason, examination of PA is essential because it provides information about the impact of clinically early maturation on adjustment and clarifies whether risk for psychopathology emerges prior to the appearance of more obvious physical changes of puberty.
Cognitive Skills, Stress Sensitivity, and Symptoms of Psychopathology
Growth “spurts” are evident in the course of brain development and typically occur at critical periods in physical maturation (i.e., between ages 6-8, 10-12, and 14-16+ years; Kolb & Fantie, 2009). The cortical complexity achieved with this brain growth is associated with increasingly complex behavioral functions and is linked with higher level reasoning and information processing (Kolb & Fantie, 2009). Often, maturational changes in brain structure and function during adolescence have been attributed to puberty-related changes (Giedd et al., 2006); however, only a few studies have actually measured pubertal maturation (Pepera et al., 2009; Perrin et al., 2008). Research on developmental changes in cognitive functions during adolescence suggests that reasoning, logic, and self-regulation of emotions (i.e., executive skills) may be less related to pubertal maturation than age, as these skills continue developing long after puberty is over (Dahl, 2004; Nelson, Leibenluft, McClure, & Pine, 2005). In contrast, processes related to emotion, motivation, and arousal may be linked more strongly to puberty than to age, suggesting puberty-specific maturational changes (Dahl, 2004; Nelson et al., 2005). For example, research examining developmental changes in stress sensitivity suggests that sensitivity, as indexed by cortisol reactivity, increases across pubertal maturation, even after controlling for age (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009). Dahl (2004) and others (e.g., Ge & Natsuaki, 2009; Steinberg, 2008) have suggested a “disconnect” or mismatch between cognitive and emotional development may impart risk for emotional and behavioral problems. Further, Blair and Diamond (2008) posit that self-regulation of emotion and behavior problems reflects an emerging balance between emotional arousal and cognitive regulation. Integrating these perspectives with the early maturation hypothesis, if greater emotional sensitivity to challenging psychosocial experiences develops prior to the emergence of cognitive skills necessary to master strong emotional responses, an individual’s ability to navigate demanding transitions (e.g., pubertal maturation) may be compromised.
Considering the interconnectedness of cognition, stress sensitivity, and behavioral functioning, specific indices of cognition (i.e., executive functioning) and stress sensitivity (i.e., cortisol reactivity) may either provide benefits for navigating challenging demands of early adrenarche or exacerbate risk for subsequent emotional and behavioral problems. Further, because the potential for the “disconnect” between these systems is exaggerated in the case of early maturation (Ge & Natsuaki, 2009), girls with PA may be at heightened risk for symptoms of psychopathology. That is, girls with PA may have to rely on underdeveloped skills to manage the demands of early maturation that are more typically associated with later development (i.e., adolescence). On the other hand, girls who demonstrate cognitive skills that are more advanced may be better able to handle such demands and, in turn, be less likely to manifest problems.
Executive functioning
Executive functioning represents the coordination of higher level cognition and behavior (e.g., novel problem-solving, self-evaluation, behavioral modulation, perspective-taking; Kolb & Fantie, 2009) and may be particularly important to processing and navigating challenging transitions, such as early maturation. Research has demonstrated a consistent association between less developed executive functioning and social and emotional problems, particularly within populations where executive deficits are present (e.g., youth with Attention Deficit-Hyperactivity Disorder or ADHD; Barkley, 2002; Miller & Hinshaw, 2010; Wahlstedt, Thorell, & Bohim, 2008). Further, research has determined that executive problems provide unique predictive information concerning social problems in school age girls (Diamantopoulo, Rydell, Thorell, & Bohlin, 2007). A recent study exploring executive functioning in children treated for leukemia found that higher executive functioning was associated with more adaptive and active coping strategies, in turn reducing the likelihood of exhibiting internalizing and externalizing symptoms (Campbell et al., 2009). The importance of executive functioning as it relates to coping and adjustment may be particularly important for girls, for whom executive functioning in childhood is positively associated with social functioning and resiliency (e.g., Diamontapoulou et al., 2007). Thus, it is possible that the development of executive functioning may be particularly salient for girls with PA who are confronted earlier with psychosocial demands typically reserved for adolescence. Specifically, less developed executive functioning might result in symptoms of psychopathology, whereas more advanced executive functioning may be protective and contribute to adaptive adjustment.
Cortisol reactivity
Cortisol reactivity represents the body’s response to perceived and actual stress and its ability to regulate these responses. When individuals experience stress or novelty, the HPA axis responds with an increase in cortisol secretion (Cicchetti & Walker, 2001). Following this rise in circulating cortisol levels, the negative feedback loop of the HPA axis is activated, reducing the level of circulating cortisol back to homeostatic functioning (Cicchetti & Walker, 2001; Dickerson & Kemeny, 2004). Cortisol reactivity is a useful measure of stress sensitivity and emotional arousal in children and adolescents following stressful experiences (e.g., public speaking tasks, Kudielka, Hellhammer, & Kirschbaum, 2007; venipuncture, McCarthy et al., 2009). In turn, much attention has been given to the association between cortisol reactivity and the development of mood and behavior problems during childhood and adolescence.
Research on cortisol reactivity has found that greater cortisol reactivity (particularly hyper-reactivity) is associated with both internalizing and externalizing problems in children and adolescents (e.g., Granger, Weisz, & Kauneckis, 1994; Luby et al., 2003; Lopez-Duran, Olson, Hajal, Felt, & Vazquez, 2009; van West, Claes, Sulon, & Deboutte, 2008). Some studies have found blunted reactivity to be associated with aggressive behavior; however, these profiles are typically associated with more severe conduct problems or callous-unemotional personality characteristics (Shirtcliff et al., 2009). Given that clinically early maturation (i.e., PA) may contribute to the presence of non-normative stressors through increased parental and peer expectations, being more reactive may place these girls at greater risk for emotional and behavioral problems.
Despite a surge in cortisol research, findings on the association between cortisol reactivity and symptoms of psychopathology in youth are still somewhat inconsistent, particularly in community rather than clinical samples (Klimes-Dougan et al., 2001). These inconsistencies are likely due to the large heterogeneity in methodologies and age ranges of the samples (Klimes-Dougan et al., 2001; Shirtcliff & Essex, 2008). Additionally, some research suggests maturational changes in stress responsivity (i.e., greater cortisol reactivity) across pubertal development (Walker, Sabuwalla, & Huot, 2004); however, much of these findings have been based on associations with pubertal status (e.g., Gunnar et al., 2009; Stroud et al., 2009) with no evidence of changes as a result of adrenarche. Regardless, cortisol reactivity has emerged as an important marker of stress responsivity and a key component of risk for symptoms of psychopathology in youth. Hence, additional research is needed examining the association between cortisol reactivity and symptoms of psychopathology, especially within younger populations already at risk for symptoms of psychopathology (e.g., children with PA).
Based on empirical findings and the early maturation hypothesis, girls with PA who exhibit dysregulation of the HPA axis (i.e., hyper- or hypo-reactivity) are likely at greater risk for symptoms of psychopathology. In a recent study examining risk for psychopathology in a community sample of adolescent girls, Sontag, Graber, Brooks-Gunn, & Warren (2008) found no evidence that cortisol reactivity moderated the association between early maturation and internalizing and externalizing problems. Yet, in a study of 8 to 13 year old boys and girls, Susman and colleagues (2010) found that higher cortisol reactivity in later maturing boys was related to antisocial and rule-breaking behaviors; this effect did not emerge for girls. However, Susman and colleagues did not investigate an interactive effect of pubertal timing and stress reactivity on internalizing problems. Moreover, such research with clinically-defined early maturing samples (e.g., PA) has not yet been conducted. To date, only a few studies have examined the interactive effect between early maturation and cortisol reactivity on symptoms of psychopathology, and no studies have examined these interactive effects in girls with PA.
Goals and Hypotheses
The goal of the present study was to test the early maturation hypothesis (Brooks-Gunn et al., 1985; Petersen & Taylor, 1980) within a sample of PA girls and matched on-time girls. Aim 1 examined the interaction between premature adrenarche (PA vs. on-time) and executive functioning on symptoms of psychopathology. It was hypothesized that lower executive functioning would be associated with greater symptoms of psychopathology and that PA girls with lower executive functioning would demonstrate the greatest number of symptoms. Aim 2 examined the interaction between premature adrenarche (PA vs. on-time) and cortisol reactivity on symptoms of psychopathology. It was hypothesized that greater cortisol reactivity (stressor-related increases) would be associated with greater symptoms of psychopathology, and that PA girls with hyper-reactivity (i.e., increases in cortisol) would demonstrate the greatest symptoms.
Method
Participants
The study included 40 girls with premature adrenarche (PA) and 36 with on-time adrenarche. Due to the rare occurrence of PA in boys (10 to 1 ratio of girls to boys; Reiter & Kulin, 1972) and the significant risk for negative outcomes of early maturation for girls, only girls were recruited. Eligibility criteria for PA included: age 6 through 8 years old, benign PA documented by a pediatric endocrinologist, Tanner 1 breast (Marshall & Tanner, 1969), Tanner 2 or greater pubic hair, English speaking, IQ greater than or equal to 75 (Wechsler, 1991) and no acute or chronic disorders or medication regimens that could influence endocrine measures (e.g., steroids). Girls with on-time adrenarche (i.e., girls displaying physical development and adrenal hormone profiles expected for their chronological age) were recruited from the community and matched to the PA group on age (+ 6 months), ethnicity, gender, socioeconomic status (+ 10 points; Hollingshead, 1975), and body mass index (BMI + 20%). Eligibility criteria for on-time adrenarche girls were the same as the PA girls with the exception that they were Tanner 1 for both breast and pubic hair. Although judgment cannot be made about late timing of adrenarche, the term “on-time” commonly refers to children demonstrating physical maturation in line with their chronological age. Hence, consistent with clinical standards and for purposes of this study, these girls are considered “on-time”.
On average, the girls were 7.50 years old (SD = .85), had a socioeconomic status (SES) of 45.30 (range 17-66; (Hollingshead, 1975), and an estimated intellectual functioning of 107.16, SD = 17.24 (Weschler Intelligence Scale for Children—Third Edition; Wechsler, 1991). Approximately 65% of the girls were Caucasian, 25% African American, and 10% biracial or other ethnicities.
Procedure
Girls with PA were recruited from pediatric endocrine clinics in two large midwestern cities and from letters distributed to community clinicians in both cities. The study began as a single-site study but the investigator moved to a second site half-way through the study. On-time girls were recruited from both communities via advertisements and flyers. The study was approved by the Institutional Review Boards affiliated with each study site.
Of the 76 participants enrolled in this study, approximately half were recruited from each site (Site 1: n = 34; Site 2: n = 42) and approximately half of the participants from each site were PA (Site 1: 52.9%; Site 2: 52.4%). As indicated in prior publications (Dorn et al., 2008), the distribution of PA/on-time participants did not differ by site. Girls from Site 1 were slightly younger than their Site 2 counterparts, but no site differences emerged for grade in school, ethnicity, parent education, SES, Tanner stage, height, weight, or BMI.
Study visits were conducted in a General Clinical Research Center (GCRC) and informed consent and assent was obtained prior to study procedures for the parent and child, respectively (Dorn et al., 2008). The visit began between 11.30 and 13.00 hours to control for diurnal rhythms in cortisol. Girls fasted in the clinic for 120 minutes prior to blood sampling to eliminate potential postprandial increases in cortisol. A brief physical examination was conducted to determine eligibility. Anthropometric data and vital signs were collected. Girls were then separated from their caregivers while completing a neuropsychological battery by a trained administrator. Caregivers completed demographic information, birth history [e.g., birth weight and length used to determine low birth weight (LBW)], and measures of parental psychological functioning [as measured by the Brief Symptom Inventory (BSI; α = .94); Derogatis, 1993], which were used to identify potential control variables. Venipuncture as a stressor (Susman, Dorn, Inoff-Germain, Nottelmann, & Chrousos, 1997) was then performed with the caregiver usually in attendance. The study visit concluded with separate child and caregiver questionnaires assessing child psychosocial functioning.
Neuropsychological Tests and Measures of Psychopathology
Executive functioning
Executive functioning was assessed using the Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Talley, Kay, & Curtiss, 1993), a widely accepted measure of executive skills for children and adults that is sensitive to developmental and maturational changes. The WCST assesses abstract reasoning, logical analysis, and concept formation by assessing a child’s ability to sort 128 cards marked with a distinct form (i.e., shape), color, and number using examiner cues (i.e., “right” or “wrong”). Based upon the WCST factor structure (Greve, Ingram, & Bianchini, 1998) and a contemporaneous investigation of the WCST within this sample (Tissot et al., 2010), the following four WCST subscales were selected to represent aspects of executive functioning: Categories Completed, Perseverative Errors, Perseverative Responses, and Trials to Complete the First Category.
An executive functioning domain score was created using the previously described WCST subscales. The domain score was calculated by converting all subscale raw scores to z scores and summing subscale z scores to create an executive functioning domain z score. This approach is frequently utilized in neuropsychological research because of the sensitivity to differences in cognitive skills afforded by z scores (e.g., Whitaker, 2003). Additionally, consistent with other exploratory investigations of cognitive skills in case-matched designs (e.g., Ouimet, Stewart, Collins, Schindler, & Bielajew, 2009; Su, Chen, Kwan, Lin & Guo, 2007), subscale z scores were calculated based on the mean and standard deviation of the on-time group, as they represent a closely matched and typically developing (i.e., physiologically and cognitively) comparison group ensuring that any differences in cognitive performance are due to differences in the condition of premature adrenarche, and not other preexisting factors (Ouimet et al., 2009). Higher scores represented more advanced skills.
Parent report of psychopathology
Parent report of child symptoms was assessed using the Child Behavior Checklist (CBCL; Achenbach, 1991), which is designed to record emotional and behavior problems of children ages 4 to 16. Raw scores were converted to T-scores (M = 50, SD = 10) based on national norms to yield two broad-band scores, internalizing and externalizing problems. Reliability for both the internalizing and externalizing scores in this sample was α = .93.
Child report of psychopathology
Children completed questionnaire items and answer choices were read aloud by a research coordinator if the child indicated any difficulty completing the measure. Depressive symptoms were assessed using the Children’s Depression Inventory (CDI; Kovacs, 1992), a 27-item self-report measure of the severity of childhood depressive symptoms for individuals aged 7 to 17 years; this measure can also be administered to 6 year-olds for clinical and research purposes (Kovacs, 1992). The reliability coefficient of the CDI for the current sample was α = .87. Anxiety symptoms were measured using the State Trait Anxiety Inventory for Children (STAI-C; Spielberger, 1973), a 20-item self-report measure of transitory (i.e., state) and stable (i.e., trait) symptoms of anxiety in children under the age of 12 years. For the purposes of this research, only the trait anxiety subscale was used (α =.83). Hormonal Indices of Stress Reactivity and Premature Adrenarche
All hormones were assessed across three serum samples (0, 20, and 40 minutes) using an initial venipuncture and the use of an indwelling catheter for subsequent samples. Samples for adrenal hormones [dehydroepiandrosterone sulfate (DHEAS) and androstendione] were pooled for each participant. Samples were centrifuged and assayed, combining PA and on-time samples in the same batch. For data assurance purposes, assays for each hormone were run in a single research laboratory, in duplicate batches, and coefficients of variation were computed by each laboratory.
Cortisol reactivity
Cortisol reactivity was assessed using the venipuncture as a stressor (i.e., needle insertion and blood draw), which has been shown to reliably elicit responses in serum cortisol in child and adolescent samples (McCarthy et al., 2009; Susman et al., 1997). Individual samples were centrifuged and stored and -800 C and assayed by the GCRC Core laboratory at Site 2, combining PA and comparison samples in the same batch. Cortisol was assayed on the Nichols Advantage® Specialty System using chemiluminescence. Sensitivity was 0.8 μg/dl. The intra-assay CV ranged between 8.7% and 3.8% (average of 6.25%) at 2.3 μg/dl and 28.9 μg/dl. The interassay CV ranged between 17.4% and 8.7% at 2.3 μg/dl and 28.9 μg/dl.
Cortisol reactivity was calculated as the area under the curve (AUC) across the three samples accounting for Sample 1 (or ground), also known as AUC with respect to increase (AUCI; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Because AUCI accounts for ground, positive AUCI values represent relative increases in cortisol across the three samples, whereas negative AUCI values represent relative decreases.
Additionally, change-in-cortisol groups were created based on patterns of significant change in cortisol from Sample 1 to Sample 3 (increase, stable, or decrease) as a means of elaborating on potential effects of net change. The criterion for change was calculated as three times the average intra-assay coefficient of variation (18.75%) for the cortisol assay (Kao, Voina, Nichols, & Horton, 1975; Susman et al., 1997). Based on this criterion, “increasers” demonstrated an 18.75% or greater increase in cortisol level from Sample 1 to Sample 3, and “decreasers” an 18.75% or greater decrease in cortisol from Sample 1 to 3. All other participants were categorized as “stable.” In the current sample, 32.9% of girls were categorized as “increasers,” 38.6% as “stable,” and 28.6% as “decreasers.”
DHEAS
DHEAS was measured using a solid phase radioimmunoassay (RIA) (DSL, Inc., Webster, TX). Sensitivity for this assay was 1.7 μg/dL. Inter- and intra-assay coefficients of variation were 12% and 5% at 20 μg/dL.
Androstenedione
Androstenedione was assayed using a coated tube RIA (Coat-A-Count, DPC Los Angeles, CA). The minimal detectable level of this assay was 40 ng/dL and inter- and intra-assay coefficients of variation were 8% and 4%, respectively.
Statistical Analyses
All variables of interest were examined for normal distribution and outliers. All variables fell within accepted ranges of skewness and kurtosis (Tabachnick & Fidell, 2007); thus, data transformation was not necessary. Descriptive statistics and t-tests were used to explore group differences. Hierarchical linear regression analyses and univariate analyses of variance were used to test the hypotheses regarding main effects of executive functioning and cortisol reactivity (both AUCI and change in cortisol groups) on child symptoms of psychopathology, and the interaction between executive functioning or cortisol reactivity and early maturation (PA vs. on-time).
Covariates were examined based on reported associations between PA, LBW, parent symptoms of psychopathology, and child emotional and behavior problems (Dorn et al., 2008; Mericq, 2006; Moreno, Silverman, Saavedra, & Phares, 2008; Vostansis et al., 2006). Only those variables with a significant association with both premature adrenarche (PA vs. on-time) and child symptoms of psychopathology were retained as covariates in subsequent analyses.
The current research was designed to conduct multiple analyses (i.e., 12 regressions and post-hoc ANOVAs) without a multiplicity adjustment (e.g., Bonferroni procedure). This decision is based upon recommendations that multiplicity adjustments need not be applied to exploratory, low base rate biomedical research when specific a priori hypotheses are formulated (Bender & Lange, 1999; Perneger, 1998). Thus, this research was conducted to prioritize the limitation of the inflated Type II error that typically plagues small sample research by not statistically adjusting analyses of our a priori hypotheses. Although our results may provide groundwork for future confirmatory research with this under-researched population, without multiplicity adjustment the results of this research are considered to be exploratory.
Results
Descriptive and Covariate Analyses
Means and standard deviations for key variables are presented in Table 1. Girls with PA were taller and heavier than on-time girls and had significantly higher BMIs, which is congruent with the matching criteria (BMI + 20%) and the advanced skeletal growth associated with PA; no significant group differences emerged for other matching variables (i.e., age and SES). All girls (n = 76) were Tanner stage 1 for breast; for pubic hair, all on-time girls were Tanner stage 1 while 23 PA girls (57.5%) were Tanner stage 2 and 17 PA girls (42.5%) were Tanner stage 3. Additionally, PA girls demonstrated higher serum concentrations of DHEAS and androstenedione (Table 1). Finally, results indicated greater parent reported internalizing and externalizing problems for PA girls compared to on-time girls; however, no group differences for cognitive measures (i.e., IQ, executive functioning), cortisol reactivity, or child reported depressive and anxious symptoms were observed.
Table 1.
Physical Development, Demographics, and Key Variables for Girls with Premature Adrenarche (PA) and On-Time Adrenarche
| PA (n = 40) | On-Time (n = 36) | T-test | Effect Size | |
|---|---|---|---|---|
| M (SD) | M (SD) | t | Cohen’s d | |
| Age | 7.65 (.90) | 7.53 (.77) | −1.53 | .14 |
| Height (cm) | 128.53 (6.77) | 123.72 (7.03) | −3.03** | .67 |
| Weight (kg) | 31.58 (8.99) | 26.48 (6.01) | −2.94** | .67 |
| BMI | 18.86 (4.08) | 17.14 (2.68) | −2.19* | .50 |
| DHEAS (μg/dL) | 30.58 (37.92) | 77.95 (61.16) | −3.81 | .93 |
| Androstenedione (ng/dL) | 42.82 (32.89) | 81.88 (36.83) | −4.60 | 1.12 |
| Socioeconomic Status (SES) |
43.73 (13.10) | 47.06 (11.07) | 1.19 | .27 |
| IQ (WISC-III) | 106.05 (16.50) | 108.44 (18.22) | .59 | .14 |
| Executive Functioning Z score Domaina |
−.13 (4.18) | −.02 (3.15) | .13 | .03 |
| Cortisol Reactivity (AUCI) |
61.24 (113.30) | 47.69 (128.77) | −.47 | .11 |
| CBCL Internalizingb | 50.70 (10.45) | 46.06 (8.99) | −2.01* | .48 |
| CBCL Externalizingb | 50.28 (9.02) | 45.00 (8.67) | −2.53** | .60 |
| CDI Depressionb | 49.60 (12.69) | 45.81 (6.29) | −1.54 | .38 |
| STAI-C Trait Anxietyb | 48.05 (11.88) | 46.50 (12.75) | −.55 | .13 |
Note. Abstracted from the Wisconsin Card Sort Test (see methods section);
values represent standardized T-scores;
p < .05;
p < .01;
WISC-III = Wechsler Intelligence Scale for Children- III; CBCL = Child Behavior Checklist; CDI = Children’s Depression Inventory; STAI-C = State Trait Anxiety Inventory for Children
Exploration of potential covariates revealed that PA girls were slightly more likely to have had LBW (χ2 (1) = 2.62, p = .11), but LBW was not related to any measure of child psychopathology. Similarly, although parent symptoms of psychopathology were significantly correlated with parent report of child internalizing (r = .48) and externalizing (r = .26) symptoms, parent symptoms of psychopathology were unrelated to PA status (PA vs. on-time). Hence, because LBW and parent symptoms of psychopathology did not meet our inclusion criteria for covariates, no covariates were included in subsequent analyses.
Aim 1: Interaction between Premature Adrenarche and Executive Functioning
Multiple regression analyses were used to examine the interaction between premature adrenarche (PA vs. on-time) and executive functioning on child psychopathology (parent- and child-reports); centered interaction terms were computed These analyses were run separately for each outcome (CBCL internalizing, CBCL externalizing, CDI depression, and STAI-C trait anxiety).
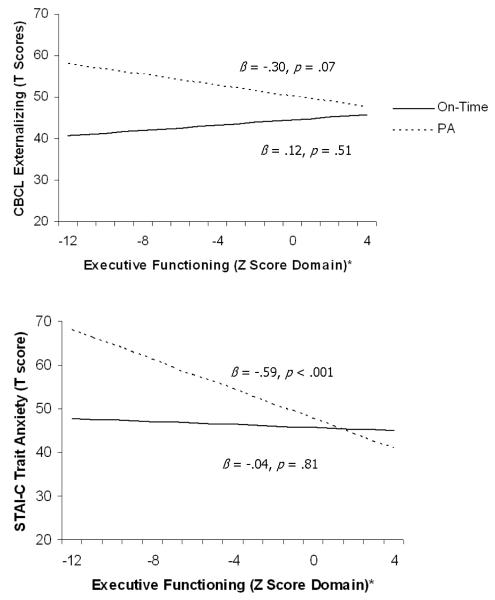
Analyses indicated two main effects for executive functioning, such that higher levels of executive functioning were associated with fewer child-reported depressive symptoms (β = -.23, p < .10) and anxious symptoms (β = -.30, p < .01). In addition, results suggested interaction effects between early maturation and executive functioning when examining parent-reported child externalizing symptoms (β = -.20, p < .10; Δ R2 = .03) and child-reported anxious symptoms (β = -.23, p < .05; Δ R2 = .05). Post hoc examination of these interaction effects indicated that girls with PA and lower levels of executive functioning were more likely to report higher levels of externalizing and anxiety symptoms. This same effect did not emerge for on-time girls, such that executive functioning was unrelated to externalizing and anxious symptoms. No main effects or interaction effects emerged for parent-reported child internalizing problems. See Table 2 and Figure 1 for illustrations of the significant interaction effects.
Table 2.
Interaction Between Premature Adrenarche and Executive Functioning (or Cortisol Reactivity) on Parent and Child Report of Symptoms of Psychopathology
| CBCL Internalizing | CBCL Externalizing | CDI Depression | STAI-C Trait Anxiety | |||||
|---|---|---|---|---|---|---|---|---|
| β | t | β | t | β | t | β | t | |
| Aim 1:Executive functioning as moderator |
||||||||
| PA Status | .22 | 1.90† | .31 | 2.74** | .19 | 1.68† | .09 | .80 |
| Executive Functioning | −.10 | −.81 | −.08 | −.66 | −.23 | −1.90† | −.30 | −2.63** |
| PA Status × Executive | −.14 | −1.17 | −.20 | −1.63† | −.13 | −1.07 | −.23 | −2.01* |
| Aim 2: Cortisol reactivity as moderator |
||||||||
| PA Status | .30 | 2.18* | .39 | 2.96** | .13 | .98 | .10 | .73 |
| AUCI | −.06 | −.51 | .08 | .68 | −.21 | −1.77† | −.33 | −2.79** |
| PA Status × AUCI | .08 | .61 | .16 | 1.23 | −.12 | −.92 | −.02 | −.17 |
Note. p < .10;
p < .05;
p < .01;
PA Status = Premature adrenarche (PA) vs. on-time; CBCL = Child Behavior Checklist; CDI = Children’s Depression Inventory; STAI-C = State Trait Anxiety Inventory for Children; AUCI = Area Under the Curve from increase (Pruessner et al., 2003)
Figure 1.
Interaction between executive functioning and premature adrenarche (PA vs. on-time) on symptoms of psychopathology
Note. Standardized beta coefficients are presented; * Abstracted from the Wisconsin Card Sort Test (see methods section); PA = premature adrenarche;
CDI = Childhood Depression Inventory; STAI-C = State Trait Anxiety Inventory for Children; CBCL = Child Behavior Checklist
Aim 2: Interaction between Premature Adrenarche and Cortisol Reactivity
The interaction between premature adrenarche (PA vs. on-time) and cortisol reactivity was examined via a combination of multiple regression analyses and two-way ANOVAs. Specifically, the interaction between PA and cortisol reactivity was examined for both the AUCI and change-in-cortisol groups. Analyses including AUCI utilized multiple regression analyses (with centered interaction terms), whereas analyses including change-in-cortisol groups (i.e., increasers, stable, and decreasers) utilized two-way ANOVAs. For both sets of analyses, models were run separately for each outcome (CBCL internalizing, CBCL externalizing, CDI depression, and STAI-C trait anxiety).
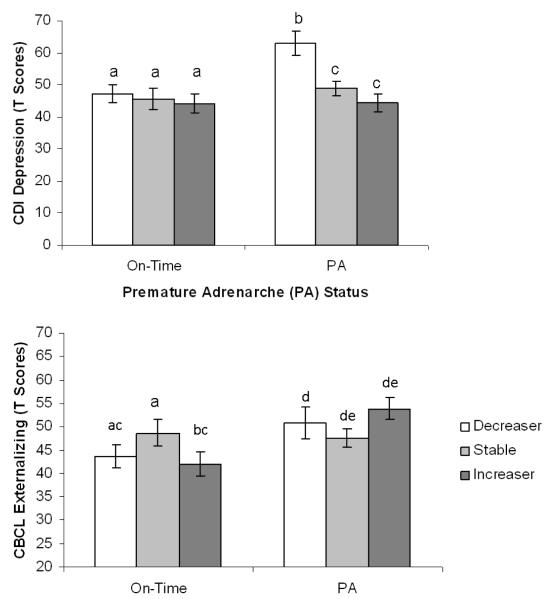
Results indicated a main effect of cortisol reactivity (AUCI) on child-reported depressive symptoms (β = -.21, p = .08) and anxious symptoms (β = -.33, p = .01), such that lower AUCI or decreases in cortisol were associated with greater depressive and anxious symptoms. No interaction effects between early maturation and AUCI emerged (see Table 2). However, when cortisol reactivity was reclassified as change-in-cortisol groups (i.e., increasers, stable, decreasers) and examined, significant interaction effects emerged for parent-reported externalizing symptoms [F(2,64) = 3.52, p = .05; η2 = .09] and child-reported depressive symptoms [F(2,64) = 3.19, p = .04; η2 = .10]. Specifically, girls with PA who demonstrated cortisol increases (i.e., increasers) had higher externalizing symptoms than girls with PA in the stable cortisol group. Interestingly, on-time girls with stable cortisol levels also presented with more elevated levels of externalizing problems, particularly when compared to on-time girls in the increaser group. With respect to child-reported depressive symptoms, girls with PA who demonstrated cortisol decreases had greater depressive symptoms than other PA girls. Although not directly tested, results suggested that PA girls in the decreaser group demonstrated the greatest number of depressive symptoms. See Figure 2 for illustrations of significant interaction effects.
Figure 2.
Estimated marginal means of externalizing problems and depressive symptoms by premature adrenarche status (PA vs. On-time) and cortisol reactivity
Note. Post hoc comparisons were conducted separately by PA status group; different subscript letters indicate significant differences at p < .05 with a Bonferroni correction, means with the same subscript did not differ significantly;
CBCL = Child Behavior Checklist; CDI = Children’s Depression Inventory
Contrary to expectation, as indicated by the analyses for AUCI and change-in-cortisol groups, girls who were decreasers rather than increasers were more likely to report depressive and anxious symptoms. Additional post hoc exploration of cortisol reactivity found that decreasers were actually more likely to exhibit higher Sample 1 concentrations of cortisol [F(2,67) 1.77, p = .18, η2 = .05] compared to the stable or increaser groups, suggesting that they may have been stressed in anticipation of the blood draw rather than in response to the blood draw itself.
Additional Analyses on Adrenal Hormones
Although results indicated significant and trend interaction effects between PA status (PA vs. on-time) and executive functioning and cortisol reactivity, the possibility exists that some of these effects were due to hormonal differences between the two groups of girls rather than as a result of early maturation from an experiential standpoint. As indicated, premature adrenarche is characterized by clinically elevated levels of the adrenal hormones DHEAS and androstenedione (Table 1). Due to sample size constraints, these hormones were not incorporated into Aim1 and Aim 2 analyses. However, in order to explore whether the PA effect was driven by hormonal effects (i.e., DHEAS and androstenedione) additional analyses examined whether the interactions that emerged for PA status also emerged for DHEAS and androstenedione. Specifically, analyses examined (1) the interactions between adrenal hormones (i.e., DHEAS and androstenedione) and executive functioning on externalizing problems and trait anxiety symptoms, and (2) the interactions between adrenal hormones and cortisol reactivity groups (i.e., increasers, stable, decreasers) for externalizing problems and depressive symptoms. Interestingly, no significant main effects of DHEAS or androstenedione on symptoms of psychopathology were observed. Additionally, only one trend interaction between DHEAS and executive functioning on parent-report of externalizing problems (β = -.23, p < .07; ΔR2 = .05) emerged. Exploration of this interaction revealed a similar pattern to what was observed for PA status (Figure 1), such that girls with higher levels of DHEAS and lower levels of executive functioning skills demonstrated the greatest number of externalizing problems. No significant interaction effects were observed between adrenal hormones and cortisol reactivity groups.
Discussion
The goal of the present study was to evaluate the early maturation hypothesis (Brooks-Gunn et al., 1985; Petersen & Taylor, 1980) by examining the interaction between early maturation, executive functioning, and stress reactivity on symptoms of psychopathology within a sample of girls with and without PA. As expected, girls with PA who demonstrated lower levels of executive functioning had higher levels of externalizing and anxious symptoms. The differing association between executive functioning and symptoms of psychopathology by maturation group was true in spite of the fact that on average PA girls and on-time girls had similar IQ and executive functioning scores. Concerning cortisol reactivity, findings supported our hypothesis that girls with PA who demonstrated cortisol increases had higher externalizing symptoms. However, contrary to expectation, girls with PA who demonstrated cortisol decreases had higher depressive symptoms.
Executive Functioning as a Risk Factor
Results of this research provide critical information concerning the influence of executive functioning on the behavior of girls with PA. Specifically, this study determined that for PA girls only, higher levels of executive functioning were protective against symptoms of psychopathology, such that PA girls with higher executive functioning scores were no different in their level of externalizing and anxious symptoms compared to on-time healthy girls. This is a significant contribution to the pubertal timing literature as well as to our understanding of a notably under-researched population. Although research has shown that early maturing girls are at greater risk for developing emotional and behavioral problems (e.g., see Mendle, Turkheimer, & Emery, 2007 for a review), the contribution of specific cognitive skills to pubertal timing has not been previously investigated. Executive functioning undergoes critical development around the time of puberty, affording an individual increased cognitive control, enhancing planning, aiding inhibition and possibly promoting social cognition (Blakemore & Choudhury, 2006). Moreover, integration of the cognitive and affective development occurring during puberty is essential for effective navigation of the complex psychosocial demands of puberty (Dahl, 2004). This research demonstrated that a relatively high level of executive functioning represents an advantage in effectively managing the emotional states and navigating the psychosocial demands of early maturation. While not specifically assessed by this study, the converse is also plausible, such that a lower level of executive functioning may be a risk for psychopathology as it represents lesser ability to manage these affective and psychosocial demands.
An alternate or parallel explanation of these findings is that, because higher executive functioning was protective in PA girls only, changes in the endocrine system during adrenarche may be playing a more complex role in the connection between executive functioning and externalizing and anxious symptoms. That is, hormones may be affecting structural and organizational brain development during adrenarche, as has been shown during puberty (Pepera et al., 2009; Paus, 2005; Sisk & Zehr, 2005). This may be especially important since the PA girls are only experiencing hormone changes of adrenarche and not gonadarche. It is the later phase of puberty (i.e., gonadarche) that the literature assumes to be relevant to cognitive changes. Interestingly, however, we are unaware of any studies that have identified functional brain changes associated with pubertal maturation that have actually measured pubertal onset or stage. Instead, many studies rely on chronological age as an assumed marker of puberty. Our findings support the importance of assessing pubertal stage and specifically exploring the relevance of adrenarche (both early and on-time), an understudied component of puberty, in relation to cognitive functioning and psychopathology.
Cortisol Reactivity as a Risk Factor
Cortisol reactivity findings provided important biopsychosocial insight into the associations between early maturation, psychosocial demands of puberty, and psychopathology in girls. Results revealed that whereas cortisol increases (i.e., increasers) corresponded to higher externalizing symptoms in PA girls compared to PA girls with stable patterns, stable cortisol reactivity represented the greatest risk for externalizing symptoms in on-time girls. This finding may suggest a fundamental difference between the behavioral endocrinology of PA and on-time girls of this age. These insights are even more important because little research examining cortisol reactivity and psychopathology in youth has utilized pre-adolescent samples or explored the influence of pubertal maturation as measured by physiological markers.
Concerning the association of cortisol reactivity and psychopathology in on-time girls, the finding that stable cortisol reactivity was associated with higher externalizing symptoms is consistent with extensive research demonstrating a link between blunted cortisol reactivity or low cortisol levels and antisocial behavior in children and adolescents (see van Goozen, Fairchild, Snoek, & Harold, 2007 for a review). In contrast, girls with PA who were increasers had higher externalizing symptoms. Interestingly, the finding for PA girls is consistent with some findings obtained with healthy adolescent samples (e.g., Susman et al., 1997). These group differences in the association between cortisol reactivity and externalizing symptoms may suggest that changes during adrenarche influence stress reactivity and its subsequent association with psychopathology. However, it is important to note that within the child and adolescent literature, consistent links between cortisol reactivity and symptoms of psychopathology have not been found across all studies, especially in community rather than clinical samples (Klimes-Dougan et al., 2001). Additional research examining the influence of adrenarche on the association between cortisol reactivity and symptoms of psychopathology is needed.
With respect to cortisol reactivity and internalizing symptoms, results indicated that girls with PA who demonstrated a decrease in cortisol had higher depressive symptoms. Although this finding is contrary to several studies that have demonstrated a link between hyper-reactivity or elevated levels of cortisol and internalizing problems in youth samples (e.g., Granger et al., 1994; Luby et al., 2003; van West et al., 2008), findings may also reflect developmental differences in the stress reactivity systems from childhood to adolescence as supported by others (Gunnar et al., 2009; Stroud et al., 2009). It is possible that the findings in this study reflect a pre-to-post pubertal shift from hypo- to hyper-reactivity as a risk factor for internalizing symptoms that occurs with gonadarche rather than adrenarche (Hankin, Badanes, Abela, & Watamura, 2010). Further examination of cortisol reactivity groups demonstrated that PA decreasers exhibited higher cortisol concentrations for Sample 1. Based on this finding and other studies on cortisol and internalizing symptoms in youth (Lopez-Duran et al., 2009; Shirtcliff, 2008), it is also possible that elevated levels at Sample 1 were reflective of higher basal concentrations of cortisol. However, because the decreaser group demonstrated significant decreases in cortisol concentrations from Sample 2 to Sample 3, it is equally plausible that elevated levels of cortisol at Sample 1 were reflective of being stressed in anticipation of the blood draw. That is, the stressor was imposed when the HPA axis was already activated and, therefore, in the context of negative feedback regulation of the axis cortisol levels declined (Gunnar et al., 2009). Thus, our decreaser and increaser groups may both reflect HPA axis hyper-reactivity, with decreasers representing girls with an earlier (i.e., anticipatory) hyper-activation and increasers representing hyper-activation at venipuncture administration. However, this distinction between anticipatory arousal and then decline versus arousal in direct response to a stressor (i.e., venipuncture) is an important one, since decreasers demonstrated higher levels of depressive symptoms whereas increasers demonstrated higher levels of externalizing problems.
Adrenal Hormones as Risk Factors
Although findings suggested that the mismatch of school age cognitive and emotion regulation skills with more advanced physical development may place PA girls at greater risk for psychopathology, the exploration of premature adrenarche as a context of risk is challenging as PA is essentially defined by concentrations of adrenal androgens; thus “group” and hormone concentration overlap. As follow-up to the examination of PA group effects, separate analyses addressed the potential influence of elevated adrenal hormones on symptoms of psychopathology. Findings suggested that higher levels of DHEAS may partly explain risk for externalizing problems in PA girls. However, most of the effects of the current study were not accounted for by hormonal influences, suggesting the importance of considering the psychosocial context of PA and social and emotional challenges PA elicits for school age girls.
Integration of Findings
Taken together, findings support the early maturation hypothesis in that school age girls whose cognitive skills demonstrate a maturational mismatch with their more advanced physical development may be at greatest risk for symptoms of psychopathology. The finding that girls with PA with higher levels of executive functioning were no more at risk for symptoms of psychopathology than their on-time counterparts further supports the notion that more developed executive skills may be protective in managing affective states and navigating the premature psychosocial demands precipitated by PA. The interactive role of executive functioning crucially informs our understanding of the potential psychosocial outcomes of PA, which is typically considered to be a “benign” condition (Dorn & Rotenstein, 2004). Specifically, these results suggest that for girls with higher levels of executive functioning for their age who are better able to navigate the premature demands of early maturation, PA may, in fact, be benign. Yet, PA represents a potentially more challenging condition for those girls whose executive functioning is less well developed. Moreover, this research demonstrates that intra-individual psychobiological mechanisms may convey additional risk for psychopathology related to PA. Specific reactivity profiles (i.e., increaser, decreaser) were associated with more symptoms of psychopathology. This finding is consistent with both the early maturation hypothesis, and more broadly the diathesis-stress perspective (e.g., Susman, 2006), which posits that major transitions or stressful events (e.g., early maturation) interact with prior vulnerabilities to psychopathology (i.e., lower executive skills and hyper cortisol reactivity) and increase an individual’s susceptibility to psychopathology.
In light of the contribution of these findings to the literature, it is important to note that effects differed somewhat depending on the source of reporting (i.e., parent-versus child-report). Specifically, results demonstrated significant effects for parent report of externalizing problems, but only child report for internalizing problems. Exploratory correlation analyses indicated that parent report of internalizing symptoms was not significantly related to child report of depressive symptoms (r = .14, p = .24) or anxiety symptoms (r = .10, p = .39). Moreover, the correlations between parent-report of externalizing symptoms and child-report of depressive symptoms (r = .22, p = .06) and anxiety symptoms (r = .16, p = .17) were not significant. This cross-informant disagreement is consistent with extant investigations (e.g., Luby, Belden, Sullivan & Spitznagel, 2007) and underscores the issue of low correlation between parent- and child-reports of symptoms on well-validated measures of psychopathology (e.g., Rothen et al., 2009). Although parent- and child-report of psychopathology may disagree, research generally supports the notion that this disagreement does not suggest invalidity of either report. Instead, parent-child inconsistencies reflect different dimensions of symptomatology, particularly related to internalizing symptoms (e.g., Cole, Hoffman, Tram & Maxwell, 2000; Luby et al., 2007). Thus, the current study provides valuable information about the relationships of interest across domains of psychopathology as observed by both parent- and child-reports of symptoms of psychopathology.
Limitations
Although the findings of this research are compelling, conclusions based upon this research are limited by several methodological issues. Beyond those limitations already outlined, the cross-sectional and exploratory nature of the study design limits the generalizability of these findings. Future investigations are needed to replicate the findings of this study, as well as utilize longitudinal design to better understand the temporal associations of early maturational timing, executive functioning, cortisol reactivity, and symptoms of psychopathology. Additionally, this study focused exclusively on girls. Although there is clinical rationale (i.e., very low prevalence of PA in boys) and theoretical rationale (i.e., early maturation is often most problematic for girls), limiting the study to girls precludes the investigation of potentially important gender differences in the processes investigated in this study. Future studies are needed to assess these relationships within samples of both girls and boys with PA, and within a sample of healthy children and adolescents with normative deviations in maturation (e.g., early versus on-time pubertal timing). Future investigations may also benefit by measuring cortisol reactivity with a more standard stress paradigm [e.g., the Trier Social Stress Test for Children (TSST-C); Kudielka et al., 2007]. Although the venipuncture has been used successfully as a stressor in other studies, it does have its limitations. Use of the TSST-C would also allow examination of both arms of the stress axis (e.g., the HPA axis and the autonomic nervous system via salivary alpha amylase). Finally, due to the small sample size, exploration of more complex interactions was limited. For instance, findings from this study for executive functioning and cortisol suggest that girls who demonstrate lower executive skills and reactive cortisol patterns (i.e., increaser or decreaser) may be at greatest risk for mood and behavior problems. Future studies would benefit from investigating the dual influence of these factors on maturational timing and psychopathology.
Conclusions
The current research achieved the primary aim of examining the moderating roles of executive functioning and cortisol reactivity on the association between PA and symptoms of psychopathology and providing support for the early maturation hypothesis. Importantly, these effects were shown to emerge in a sample of school age girls, suggesting that these effects are salient not only during early adolescence, but also in childhood. Understanding that a girl’s response to complex psychosocial demands of early maturation is significantly affected by her cognitive functioning as well as by her intrinsic physiological reactivity may be clinically relevant. Specifically, this information aids in the identification of individuals who may be at highest risk for maladjustment as a result of PA (i.e., lower executive functioning, cortisol hyper-reactivity). Further, it provides prescriptive information concerning the type of preventive and intervention efforts from which girls with PA may be more likely to benefit (i.e., externalizing vs. internalizing). These findings may be used to inform screening procedures and the design and implementation of short-term academic, social, and behavior interventions for promoting successful management of the pubertal transition and limiting emotional and behavioral problems for girls with PA as well as those on the broader spectrum of early maturation. In sum, the findings of this research critically extend our understanding of physiological and developmental processes that potentially exert their influence prior to gonadarche; and thus, may affect a girl’s adjustment to early maturation.
Acknowledgements
This study was supported in part by the NIMH R01 MH59892 to Dr. Dorn; by the USPHS GCRC grant M01 RR 08084 from the National Center for Research Resources, NIH, to Cincinnati Children’s Hospital Research Foundation, and grant M01 RR00084 to Children’s Hospital of Pittsburgh General Clinical Research Center. This project was also supported by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS), through a National Research Service Award Training Grant (T32HP10027-12; PI: Kristen Copeland, M.D.). The information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by the BHPR, HRSA, DHHS or the U.S. Government.
References
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. [Google Scholar]
- Barkley RA. ADHD: Long-term course, adult outcome, and comorbid disorders. In: Jensen PS, Cooper JR, editors. Attention-deficit/hyperactivity disorder: State of the science and best practices. Civic Research Institute; Kingston, NJ: 2002. pp. 4-1–4-12. [Google Scholar]
- Bender R, Lange S. Multiple test procedures other than Bonferroni’s deserve wider use. British Medical Journal. 1999;318:600. doi: 10.1136/bmj.318.7183.600a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychcopathology. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Petersen AC, Eichorn D. The study of maturational timing effects in adolescence. Journal of Youth and Adolescence. 1985;14:149–161. doi: 10.1007/BF02090316. [DOI] [PubMed] [Google Scholar]
- Campbell LK, Scaduto M, Van Slyke D, Niarhos F, Whitlock JA, Compas BE. Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. Journal of Pediatric Psychology. 2009;34:317–327. doi: 10.1093/jpepsy/jsn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Lynam D, Moffitt TE, Silva PA. Unraveling girls’ delinquency: Biological, dispositional, and contextual contributions to adolescent misbehavior. Developmental Psychology. 1993;29:19–30. [Google Scholar]
- Cicchetti D, Walker EF. Stress and development: Biological and psychological consequences. Development and Psychopathology. 2001;13:413–418. [PubMed] [Google Scholar]
- Cole DA, Hoffman K, Tram JM, Maxwell SE. Structural differences in parent and child reports of children’s symptoms of depression and anxiety. Psychological Assessment. 2000;12:174–85. doi: 10.1037//1040-3590.12.2.174. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- de Zegher F, Ibáñez L. Early origins of polycystic ovary syndrome: Hypotheses may change without notice. Journal of Clinical Endocrinology and Metabolism. 2009;94:3682–3685. doi: 10.1210/jc.2009-1608. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Administration, Scoring, and Procedures Manual. 4th Ed National Computer Systems; Minneapolis, MN: 1993. BSI Brief Symptom Inventory. [Google Scholar]
- Diamantopoulou S, Rydell AM, Thorell LB, Bohlin G. Impact of executive functioning and symptoms of attention deficit hyperactivity disorder on children’s peer relations and school performance. Developmental Neuropsychology. 2007;32:521–542. doi: 10.1080/87565640701360981. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorn LD. Psychological and social problems in children with premature adrenarche and precocious puberty. In: Pescovitz OH, Walvoord EC, editors. When Puberty is Precocious: Scientific and Clinical Aspects. Humana Press; Totowa, NJ: 2007. pp. 309–327. [Google Scholar]
- Dorn LD, Hitt SF, Rotenstein D. Biopsychological and cognitive differences in children with premature vs. on-time adrenarche. Archives of Pediatric & Adolescent Medicine. 1999;153:137–146. doi: 10.1001/archpedi.153.2.137. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Rose SR, Rotenstein D, Susman EJ, Huang B, Loucks TL, et al. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. Journal of Pediatric Endocrinology and Metabolism. 2008;21:439–448. doi: 10.1515/jpem.2008.21.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Rotenstein D. Early puberty in girls: The case of premature adrenarche. Women’s Health Issues. 2004;14:177–183. doi: 10.1016/j.whi.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ehrhardt AA, Meyer-Bahlburg HFL. Idiopathic precocious puberty in girls: Long-term effects on adolescent behavior. Acta Endocrinologica. 1986;112:247–253. doi: 10.1530/acta.0.112s247. [DOI] [PubMed] [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL, Murry VM. Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Developmental Psychology. 2002;38:42–54. doi: 10.1037//0012-1649.38.1.42. [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN. In search of explanations for early pubertal timing effects on developmental psychopathology. Current Directions in Psychological Science. 2009;18:327–331. [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Greve KW, Ingram F, Bianchini KJ. Latent Structure of the Wisconsin Card Sorting Test in a Clinical Sample. Archives of Clinical Neuropsychology. 1998;13:597–609. [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in HPA activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abuela J, Watamura SE. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin card sorting test manual: Revised and expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University Press; New Have, CT: 1975. [Google Scholar]
- Ibáñez L, Díaz R, López-Bermejo A, Marcos MV. Clinical spectrum of premature pubarche: Links to metabolic syndrome and ovarian hyperandrogenism. Reviews in Endocrine & Metabolic Disorders. 2009;10:63–76. doi: 10.1007/s11154-008-9096-y. [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche—Normal variant or forerunner of adult disease. Endocrine Reviews. 2000;21:671–696. doi: 10.1210/edrv.21.6.0416. [DOI] [PubMed] [Google Scholar]
- Kao M, Voina S, Nichols A, Horton R. Parallel radioimmunoassay for plasma cortisol and 11-deoxycortisol. Clinical Chemistry. 1975;21:1644–1647. [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kolb B, Fantie BD. Development of the child’s brain and behavior. In: Reynolds CR, Fletcher-Janzen E, editors. Handbook of clinical child neuropsychology. 3rd ed Springer; New York: 2009. pp. 19–46. [Google Scholar]
- Kovacs M. Children’s depression inventory manual. Multi-Health Systems, Inc.; North Tonawanda, NY: 1992. [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test - revisited. In: Harmon-Jones E, Winkleman P, editors. Social neuroscience: Integrating biological and psychological explanations. Guilford Press; New York: 2007. pp. 56–83. [Google Scholar]
- Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. Journal of Abnormal Child Psychology. 2009;37:169–182. doi: 10.1007/s10802-008-9263-3. [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden A, Sullivan J, Spitznagel E. Preschoolers’ contribution to their diagnosis of depression and anxiety: Uses and limitations of young child self-report of symptoms. Child Psychiatry & Human Development. 2007;38:321–338. doi: 10.1007/s10578-007-0063-8. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger AK, Mrakotsky C, Brown KM, Hessler MJ, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, Hanrahan K, Kleiber C, Zimmerman MB, Lutgendorf S, Tsalikian E. Normative salivary cortisol values and responsivity in children. Applied Nursing Research. 2009;22:54–62. doi: 10.1016/j.apnr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, Emery RE. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review. 2007;27:151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericq V. Low birth weight and endocrine dysfunction in postnatal life. Pediatric Endocrinology Reviews. 2006;4:3–14. [PubMed] [Google Scholar]
- Miller M, Hinshaw SP. Does childhood executive function predict adolescent functional outcomes in girls with ADHD. Journal of Abnormal Child Psychology. 2010;38:315–326. doi: 10.1007/s10802-009-9369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Silverman WK, Saavedra LM, Phares V. Fathers’ ratings in the assessment of their child’s anxiety symptoms: A comparison to mothers’ ratings and their associations with paternal symptomatology. Journal of Family Psychology. 2008;22:915–919. doi: 10.1037/a0014097. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:I163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Ouimet LA, Stewart A, Collins B, Schindler D, Bielajew C. Measuring neuropsychological change following breast cancer treatment: An analysis of statistical models. Journal of Clinical and Experimental Neuropsychology. 2009;31:73–89. doi: 10.1080/13803390801992725. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Science. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pepera JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. British Medical Journal. 1998;316:1236–38. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Hervé P, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. The Journal of Neuroscience. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Taylor B. The biological approach to adolescence: Biological and psychosocial adaptation. In: Adelson J, editor. Handbook of the psychology of adolescence. John Wiley & Sons; New York: 1980. pp. 115–155. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Reiter EO, Kulin H. Sexual maturation in the female: Normal development and precocious puberty. Pediatric Clinics of North America. 1972;19:581–603. doi: 10.1016/s0031-3955(16)32740-7. [DOI] [PubMed] [Google Scholar]
- Reiter EO, Saenger P. Premature adrenarche. The Endocrinologist. 1997;7:85–88. [Google Scholar]
- Rothen S, Vandeleur CL, Lustenberger Y, Jeanpretre N, Ayer E, Gamma F, Halfon O, Fornerod D, Ferrero F, Preisig M. Parent-child agreement and prevalence estimates of diagnoses in childhood: Direct interview versus family history method. International Journal of Methods in Psychiatric Research. 2009;18:96–109. doi: 10.1002/mpr.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf A, Gostisha A, Merz JL, Zahn-Waxler C. Neurobiology of Empathy and Callousness: Implications for the Development of Antisocial Behavior. Behavioral Sciences and the Law. 2009;27:1–35. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and development. Journal of Clinical Endocrinology and Metabolism. 1980;51:548–556. doi: 10.1210/jcem-51-3-548. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Children. Consulting Psychologists Press; Manual. Palo Alto, CA: 1973. [Google Scholar]
- Sontag LM, Graber JA, Brooks-Gunn J, Warren MP. Coping with social stress: Implications for psychopathology in young adolescent girls. Journal of Abnormal Child Psychology. 2008;36:1159–1174. doi: 10.1007/s10802-008-9239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud L, Foster E, Handwerger K, Papandonatos GD, Granger D, Kivlinghan KT, et al. Stress response and the adolescent transition: Performance versus peer rejection stress. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Chen HM, Kwan AL, Lin YH, Guod NW. Neuropsychological impairment after hemorrhagic stroke in basal ganglia. Archives of Clinical Neuropsychology. 2007;22:465–474. doi: 10.1016/j.acn.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, et al. Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35:557–569. doi: 10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD. Puberty: Its role in development. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. 3rd ed Vol. 1. John Wiley & Sons; Hoboken, NJ: 2009. pp. 116–151. [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behavior, behavior problems, and emotionality in young adolescents: A longitudinal perspective. Journal of Research on Adolescence. 1997;7:81–105. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed Allyn and Bacon; Boston: 2007. [Google Scholar]
- Tissot A, Dorn LD, Rotenstein D, Rose SR, Sontag LM, Jillard CL, et al. Neuropsychological functioning in girls with premature adrenarche: An investigation of hormonal influences. 2010. Manuscript under review. [DOI] [PMC free article] [PubMed]
- van Goozen S, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van West D, Claes S, Sulon J, Deboutte D. Hypothalamic-pituitary-adrenal reactivity in prepubertal children with social phobia. Journal of Affective Disorders. 2008;111:281–290. doi: 10.1016/j.jad.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Vostanis P, Graves A, Meltzer H, Goodman R, Jenkins R, Brugha T. Relationship between parental psychopathology, parenting strategies and child mental health. Social Psychiatry and Psychiatric Epidemiology. 2006;41:509–514. doi: 10.1007/s00127-006-0061-3. [DOI] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huout R. Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology. 2004;16:807–824. doi: 10.1017/s0954579404040027. [DOI] [PubMed] [Google Scholar]
- Weschler D. Weschler Intelligence Scale for Children. 3rd ed Psychological Corp.; San Antonio: 1991. [Google Scholar]
- Wahlstedt C, Thorell LB, Bohlin G. ADHD symptoms and executive function impairment: Early predictors of later behavioral problems. Developmental Neuropsychology. 2008;33:160–78. doi: 10.1080/87565640701884253. [DOI] [PubMed] [Google Scholar]
- Whittaker D. The use of z scores in assessing neuropsychological change after cardiac operations. The Annals of Thoracic Surgery. 2003;75:1066. doi: 10.1016/s0003-4975(02)04270-4. [DOI] [PubMed] [Google Scholar]