Abstract
Oral lichen planus (OLP) is a chronic oral inflammatory disease of unknown etiology. According to reports, 1-2% of OLP patients develop oral squamous cell carcinoma (OSCC) in the long run. While World Health Organization (WHO) classifies OLP as “a potentially malignant disorder,” it is still a matter of debate which mechanisms drive OLP to such a condition. The current hypothesis connecting OLP and OSCC is that chronic inflammation results in crucial DNA damage which over time results in cancer development. Initial studies investigating the OLP and OSCC link were mainly retrospective clinical studies. Over the past years, several amount of information has accumulated, mainly from molecular studies on the OLP malignant potential. This article is a critical review of whether OLP has a malignant potential and, therefore, represents a model of preneoplastic inflammation.
1. Introduction
Oral lichen planus (OLP) is a chronic inflammatory oral condition of unknown aetiology characterized by T-cell-mediated chronic immune response and abnormal epithelial keratinization cycle [1]. The OLP lesions may coexist with cutaneous and genital lesions, or may be the only disease manifestations [2]. The epidemiology of OLP is not easy to calculate with reported incidence ranging between 1-2% of the general population. Recent meta-analysis calculated a 1.27% incidence in the general population [3]. The OLP lesions are consistently more persistent than the dermal lesions and have been reported to carry a risk of malignant transformation to oral squamous cell carcinoma (OSCC) of 1-2% (reported range of malignant transformation 0–12.5%) [4]. Clinically, OLP appears more commonly with the classic reticular form, which results from coalition of papules and may be asymptomatic or may cause mild discomfort. Erythema, erosions, and ulceration could also appear and these are the most painful OLP manifestations, while if the lesions become chronic they may become hyperplastic or atrophic [4]. The lesions of OLP tend to present symmetrically and bilaterally especially in the buccal mucosa [5]. Histological examination of OLP reveals, dense inflammatory infiltrate in the upper lamina propria, mainly consisting of T-cells, liquefaction degeneration of basal keratinocytes and basal membrane hyperkeratosis or atrophy of the keratin layer [6, 7]. The pathogenesis of OLP is very complex and involves possible antigen presentation by the oral keratinocytes that could be either of an exogenous or an endogenous origin [8–10]. This antigenic trigger is accompanied by a mixed inflammatory response comprising mainly T-cells, macrophages, and mast cells, as well as the associated cytokines and cytotoxic molecules [4, 8–10]. Officially, the World Health Organisation (WHO) classifies OLP as a “potentially malignant disorder” with unspecified malignant transformation risk and suggests that OLP patients should be under close monitoring [11]. The possible premalignant nature of OLP has been the subject of numerous studies and great controversies [4, 5]. Treatment of OLP is remarkably unsatisfying; topical steroids are the first treatment choice and systemic corticosteroids and immunosuppressants are the second line agents, but none of them can result in significant long-term disease control [12]. Severe erosive disease leaving mucosal atrophy and requiring systemic treatment is reported to carry the highest risk of malignant transformation [13]. There is no definite malignant transformation mechanism identified in OLP. The current hypothesis is that chronic stimulation from the inflammatory and stromal cells is providing the signals that are causing epithelial cells to derange their growth control and in cooperation with oxidative stress, from oxidative and nitrative products, it provokes DNA damage resulting in neoplastic changes [4, 14–17] (Figure 1). Recently, OLP has been proposed to be an ideal model of inflammation induced cancer [18].
Figure 1.
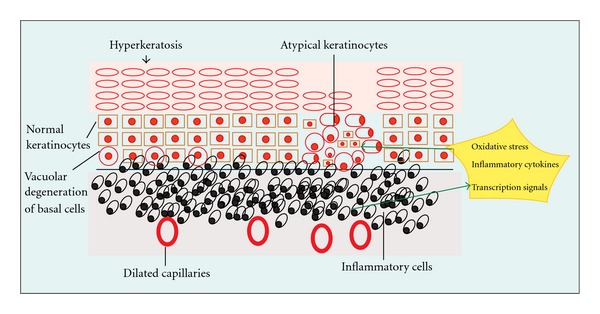
The current hypothesis on the development of dysplasia and cancer in OLP suggests that changes in epithelial cells (keratinocytes) is a result of detuning in cellular replication, DNA damage and disorganization of epithelial integrity, secondary to oxidative stress, cytokine, and transcription factor signals originating from the inflammatory infiltrate.
The advances in molecular information on this pathologic condition have shed new light on the complex pathogenesis of OSCC arising in OLP and this article is an attempt to review the currently available data.
2. Cell Cycle Control in Oral Lichen Planus
Apoptosis of basal keratinocytes, caused by the activity of cytotoxic T-cells, could be a possible explanation for one of the histopathologic hallmarks of OLP that is the vacuolar degeneration of basal membrane [8]. This is also supported by several molecular studies demonstrating the presence of apoptotic signals in OLP [10, 19, 20].
Nevertheless, if apoptosis was the main cellular event, then all cases of untreated OLP would end up with severe and extensive oral mucosa erosions [21]. However, this is not the case in the majority of OLP, as the most common clinical form of OLP is reticular lichen planus, while the erosive forms usually are limited in one or two oral sites [4, 5]. Therefore, a counterbalancing mechanism is expected as a response from the oral epithelium to maintain its integrity. In fact, several molecular studies indicated evidence of increased cellular turnover rate, in the form of increased cellular proliferation, in epithelial cells of oral lichen planus [21–24]. In addition, other authors have demonstrated mixed patterns of both apoptosis and increased cellular proliferation occurring simultaneously [25–27].
Even more, González et al. suggested that possibly epithelial cells in OLP respond to the inflammatory chronic attack by exhibiting a senescent phenotype instead of apoptosis. This hypothesis was based on the observed positive p21WAF1 expression in OLP, which is indicative of cell cycle arrest and possibly of senescence [28].
Cell cycle arrest helps in maintaining tissue integrity and facilitating DNA repair mechanisms, but at the same time entry into senescence could favor malignant transformation [29–31]. As the authors note too, positive p21WAF1 is only indicative of senescence, in contrast to the most established marker of senescence which is SA-beta gal staining [28]. Nevertheless, given that this staining method is not suitable for paraffin embedded tissues, [32] which is the most widely available material for OLP studies, data after applying this method are lacking.
In a similar hypothetical model, Poomsawat et al., considered their observations of increased p16INK4A and cdk4 expression in OLP as evidence of a precancerous OLP process [33]. In a contradictory study, Montebugnoli et al. did not find significant differences in p16INK4A expression in OLP and nonspecific oral inflammation and interpreted the p16INK4A expression only as a sign of inflammation [34].
3. The Role of p53 in OLP
Inactivation of p53 is a frequent phenomenon in OSCC. This is caused by mutations, presence of HPV virus and other molecular alteration occurring in the p53 pathway [35]. The studies investigating the expression of p53 in OLP have been recently reviewed by Ebrahimi et al. [36]. In their vast majority, they included immunohistochemistry-based reports and their results varied significantly, with reported expression percentages ranging from 0–100%. Nevertheless, most of them found significantly higher expression in OLP than in normal oral mucosa [36]. As p53 expression has been identified as a response to DNA damage, [37] the identification of p53 in OLP tissue is interpreted as an indication of precancerous potential by some researchers [24, 38]. In support to this concept, Chaiyarit et al. showed an i-NOS-dependent DNA damage and p53 elevated expression in OLP patients [39]. Another concept is that the high expression of p53 in OLP is a result of the higher cellular proliferation [22, 40]. To prove that p53 expression in OLP is not just a result of the inflammatory process, Safadi et al. [38] compared the immunohistochemical expression of p53 and of its downstream effector p21WAF1 between OLP and other inflammatory oral conditions and found significantly higher expression in OLP [38]. What is still unclear is the underlying mechanism that drives p53 expression in a significant percentage of OLP cases, but as p53 expression in OLP is comparable to that observed in dysplastic oral lesions, it is considered as a sign of malignant potential [36].
At this point, it is tempting to speculate that OLP as an inflammatory condition, along with the accompanying oxidative stress, probably induces a genotoxic stress. In addition, the high proliferation rates reported for the oral epithelium turnover in OLP may also create a replication stress. Such conditions should activate the DNA damage response (DDR) checkpoint [41, 42]. In turn, this pathway should elicit the p53-mediated antitumor barriers of apoptosis and senescence. Continuous activation of this checkpoint will eventually surpass the cell repair capacity predicting the emergence of genomic instability and finally selective p53 inactivation. Consecutively, this would result in the progression to malignancy. Nevertheless, this scenario requires experimental validation, despite the presence of experimental evidence compatible with it.
4. Chromosomal Instability in OLP
To verify the OLP malignant potential hypothesis, genetic alterations observed in epithelial cancers have also been studied in OLP. In 1997, Zhang et al. used microsatellite analysis to investigate loss of heterozygosity (LOH) at loci 3p, 9p, and 17p, which is frequently observed in oral cancers [43]. Despite they detected LOH, their results showed no different frequencies from the reactive irritation (benign inflammation). Nevertheless, while this result did not support OLP as a lesion at risk for malignant transformation, the authors could not exclude that OLP may undergo malignant transformation through other genetic pathways [43]. Following these results, the same authors performed the same loci analysis in dysplastic lesions in OLP patients and their results showed comparable rates of allelic loss with those observed in epithelial dysplasia even for cases of mild dysplasia [44]. From this finding they concluded that dysplasia observed in OLP cases is possibly an independent risk factor for malignant transformation and underlined that very diligent clinical and pathologic approach should be applied in the case of OLP biopsies [44]. Similar results and conclusions especially for LOH in chromosome 9 in OLP-associated dysplasia were reported by Kim et al. with the use of chromosomal in situ hybridization [45]. On the other hand, in a more recent study using laser capture microdissection and microsatellite analysis to identify LOH, the results were similar in benign lesions and OLP samples weakening the concept of malignant OLP potential, but these authors also emphasize on careful histopathologic examination of OLP samples [46]. Of note, all data available from LOH analyses are confined only to chromosomes 3, 9, and 17 [43–46]. To the best of our knowledge, genome-wide analyses in large cohorts of OLP are still missing.
Changes in DNA ploidy are also an indication of malignancy. DNA ploidy studies in OLP have demonstrated that some atrophic lesions may be found aneuploid, but the results are not indicative of a potentially malignant process [47–49]. Abnormal karyotypes and chromosomal alterations associated with p53 expression have also been detected in OLP, but the data are small to allow safe conclusions [50].
5. Matrix Metalloproteinases (MMPs) and OLP
Sutinen et al. were among the first to investigate the expression of MMPs and their inhibitors TIMPs in clinical samples with OSCC, OLP, dysplasia, lymph node, metastases, and normal oral mucosa [51]. Though their findings showed significantly higher expression in OSCC in comparison to the other lesions, they first noted a weak MMP 1 and 2 expression in some OLP cases [51]. Subsequently, Zhou et al. reported increased expression of MMP 1–3 in the epithelial OLP cells and MMP-9 in the OLP inflammatory infiltrating cells, but not the TIMPs, and suggested a role of MMPs in the basement membrane disruption, which possibly enables intraepithelial inflammatory cell migration [52].
The role of MMPs in OLP was initially associated with apoptosis of epithelial cells and the level of inflammation [53]. Transforming growth factor beta (TGF-β) and the bone morphogenic protein-4 (BPM-4) were suggested as promoting signals for the upregulation of the MMPs [53, 54]. Chen et al., studied MMPs, TIMPs and TGF-b in OSCC that developed from previous OLP and found constant expression with levels comparable to those detected in atrophic OLP, which is the form of OLP reported to have the higher malignant potential [55]. They concluded that their findings are suggestive of the role MMPs have in the malignant transformation in OLP [55]. More recently, Tsai et al. detected elevated MMP-2 levels both in situ, and in peripheral blood of the same patients, interpreting their findings as indices of systemic inflammation, in OLP [56].
6. The Role of NF-KappaB and Associated Cytokines (IL-1α, IL-6, IL-8, TNF)
The transcription factor Nuclear Factor kappa betta (NF-kappaB) has been described as a major molecule associating chronic inflammation and cancer mainly by inhibiting apoptosis, promoting cellular proliferation and favoring metastatic phenotypes [57]. The expression of NF-kappaB has been reported higher in OLP than in cutaneous lichen planus (CLP), a fact that is considered consistent with the more persistent inflammation observed in OLP in comparison to CLP [58]. In support to the above, the levels of NF-kappaB associated cytokines (IL-1α, IL-6, IL-8, TNF) have been found increased in whole unstimulated saliva and other oral fluids of OLP patients [59–61] and also in OSCC patients [60]. These observations are suggestive for a role of NF-kappaB and of the associated cytokines in the inflammatory process of OLP and possibly also in the malignant transformation of OLP [59–61].
TNF is one of the most studied cytokines linking chronic inflammation and cancer by inducing neoplastic cellular phenotypes, and angiogenesis [62]. TNF involvement in the pathogenesis of OLP has been proposed for more than 15 years ago [63]. Since then, several studies demonstrated findings supporting the TNF involvement in OLP pathogenesis. These include TNF genetic polymorphisms with OLP susceptibility, [64–67] elevated serum and saliva TNF levels in patients with OLP, [68–71] and in situ detection of TNF in OLP epithelium [72, 73]. Its role is also supported by the favorable results of anti-TNF agents in patients with OLP [74, 75].
IL-6 expression in serum and saliva of OLP patients [76] is considered indicative of a Th2 cellular involvement in OLP, [77, 78] a fact that was underestimated initially in the pathogenesis of OLP [8]. Similarly, IL-6 has been associated with promoting colon cancer development in inflammatory bowel diseases [79, 80]. Furthermore, IL-6 and IL-8 expression is associated with the senescence phenotype and has been suggested that they promote senescence-related growth arrest [81].
7. Hepatitis C Virus (HCV) Infection and OLP Malignant Potential
HCV infection has been associated with OLP pathogenesis in certain ethnic populations, especially in the Mediterranean area [82]. HCV infection is a well-documented risk factor for hepatocellular carcinoma development [83]. Also, chronic HCV infection has been implicated with other malignancies like cholangiocarcinoma and lymphomas [84]. The pathogenetic mechanisms that connect OLP and HCV were based on the findings that circulating antibodies against the oral epithelium were identified in OLP patients with HCV infection, [85] and that OLP mediating cytokines are triggered by HCV infection [86]. A study in Japanese OLP patients identified HCV RNA in oral lesions and serum from OLP and OSCC patients and concluded that it may be involved in the pathogenesis of OSCC [87]. In contrast, in a study of oral epithelial dysplasia and HCV infection in British population, no such association was observed [88]. The association of HCV infection and OLP development in certain ethnic groups may be related to HLA subclasses presence, and though weak, some evidence exists to correlate these diseases suggesting further investigation [89]. Nevertheless, up to now no strong evidence exists so far to indicate a possible strong association of HCV infection with OLP progression to OSCC.
8. Similarities between Inflammatory Bowel Diseases (IBD) Associated Colorectal Carcinomas and OLP Associated OSCC
Inflammatory bowel diseases, ulcerative colitis (UC), and Crohn's disease (CD) are complicated with colorectal carcinomas in a percentage rate of 7–14% for UC in a 25 year time frame, and a 2.9% cumulative risk for CD in 10 years [90, 91].
Patients who develop colorectal carcinomas in IBD may present with multiple sites of cancer and areas of dysplasia in the same way that patients with OLP-associated OSCC may develop new primary tumors and dysplastic lesions in multiple oral sites [92–95]. T-cells and apoptotic mechanisms have an important role, both in OLP and IBD pathogenesis [96, 97].
Recently, the role of the neuronal axon guidance molecule netrin-1 and its receptors (DCC, UNC5H) have been discovered to play a pivotal role in progression of IBD to colon adenocarcinoma, [98, 99] and its expression is probably upregulated through NF-kappaB [100]. This molecule has not been investigated in OLP and could constitute a possible link between chronic OLP inflammation and cancer progression, a hypothesis that we currently investigate.
It is possible that both IBD and OLP, as chronic inflammatory conditions, provide the basis for the establishment of early preneoplastic lesions. These in turn, under the appropriate conditions, may further develop by progressing to malignant stages. The fact that, in contrast to IBD, OLP has less percentage of malignant potential is not against this model as IBD is common in younger adults while OLP is a disease mainly of post menopausal women. Thus, the time frame for malignant development is wider in IBD in contrast to OLP.
9. Future Prospects
It is clear that most of the studies so far have shown indicative results of a precancerous OLP nature. Studies that will include investigation of other unexplored pathways, like the DDR one, and in larger OLP cohorts, especially including all the spectrum of lesions up to full-blown cancer from the same patient, are a prerequisite. A series of evidence like the oxidative stress due to chronic inflammation, the potential replication stress as exemplified by the observed high cellular proliferation, the increased p53 expression, and the genomic instability in OLP are suggesting that DNA damage is taking place in OLP. The hypothesis that could fit in OLP carcinogenesis, based on the available data, is that p53 upregulation in response to continuous oxidative and replicative DNA damage, protects the OLP-affected cells from a malignant potential through activation of the cell cycle arrest, apoptosis and/or senescence. Nevertheless, sustained DDR activation will eventually overwhelm the cellular repair capacity. When this repair mechanism exceeds its potential, genomic instability will gradually accumulate, leading eventually to mutation fixation in critical genes. As a result the antitumor barriers will gradually diminish and dysplastic changes as well as further ones may occur.
There are still other important questions to consider: Which is/are the main signals that could promote toward cancer? What kind of treatment modalities should be applied? Could new treatment with new biologic agents (e.g., anti-TNF, anti-IL6 receptors) alter the cancer risk in OLP patients? The development of accurate OLP animal models, which currently lack, may also prove to be important tools in the molecular deciphering of both OLP and its progression to cancer. More research is required to acquire a full view of the precancerous nature of OLP and to be able to determine subclasses of OLP patients at increased risk of malignant transformation.
10. Conclusion
All the findings so far are indicative that the OLP is a preneoplastic inflammatory model. The fact that OLP lesions are found in an open cavity, such as the mouth, that is, accessible to regular monitoring and biopsy, is feasible without complications and high cost, render OLP an ideal disease to study the relationship between chronic inflammation and cancer. The focus should be on finding markers that delimit the patients at risk of OSCC progression.
Acknowledgments
A. Kotsinas is financially supported by the European Commission FP7 Projects INFLA-CARE (Contract no. 223151) and INsPiRE (Contract no. 284460; REGPOT).
References
- 1.Scully C, Carrozzo M. Oral mucosal disease: lichen planus. British Journal of Oral and Maxillofacial Surgery. 2008;46(1):15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]
- 2.Bidarra M, Buchanan JAG, Scully C, Moles DR, Porter SR. Oral lichen planus: a condition with more persistence and extra-oral involvement than suspected? Journal of Oral Pathology and Medicine. 2008;37(10):582–586. doi: 10.1111/j.1600-0714.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 3.Carrozzo M. How common is oral lichen planus? Evidence-Based Dentistry. 2008;9(4):112–113. doi: 10.1038/sj.ebd.6400614. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Diseases. 2008;14(3):229–243. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 5.Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2005;100(2):164–178. doi: 10.1016/j.tripleo.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 6.Van der Meij EH, Van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. Journal of Oral Pathology and Medicine. 2003;32(9):507–512. doi: 10.1034/j.1600-0714.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 7.Dorrego MV, Correnti M, Delgado R, Tapia FJ. Oral lichen planus: immunohistology of mucosal lesions. Journal of Oral Pathology and Medicine. 2002;31(7):410–414. doi: 10.1034/j.1600-0714.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Sugerman PB, Savage NW, Walsh LJ, et al. The pathogenesis of oral lichen planus. Critical Reviews in Oral Biology and Medicine. 2002;13(4):350–365. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 9.Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clinics in Dermatology. 2010;28(1):100–108. doi: 10.1016/j.clindermatol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2005;100(1):40–51. doi: 10.1016/j.tripleo.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 11.Warnakulasuriya S, Johnson NW, Van Der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. Journal of Oral Pathology and Medicine. 2007;36(10):575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 12.Keenan AV, Ferraiolo D. Insufficient evidence for effectiveness of any treatment for oral lichen planus. Evidence-Based Dentistry. 2011;12(3):85–86. doi: 10.1038/sj.ebd.6400814. [DOI] [PubMed] [Google Scholar]
- 13.Barnard NA, Scully C, Eveson JW, Cunningham S, Porter SR. Oral cancer development in patients with oral lichen planus. Journal of Oral Pathology and Medicine. 1993;22(9):421–424. doi: 10.1111/j.1600-0714.1993.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 14.Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L, Bucci E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: is there any evidence? Oral Oncology. 2004;40(2):120–130. doi: 10.1016/j.oraloncology.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Battino M, Greabu M, Totan A, et al. Oxidative stress markers in oral lichen planus. BioFactors. 2008;33(4):301–310. doi: 10.1002/biof.5520330406. [DOI] [PubMed] [Google Scholar]
- 16.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biological Chemistry. 2006;387(4):365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 17.Ergun S, Troşala ŞC, Warnakulasuriya S, et al. Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. Journal of Oral Pathology and Medicine. 2011;40(4):286–293. doi: 10.1111/j.1600-0714.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Messadi DV, Wu H, Hu S. Oral lichen planus is a unique disease model for studying chronic inflammation and oral cancer. Medical Hypotheses. 2010;75(6):492–494. doi: 10.1016/j.mehy.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattila R, Syrjanen S. Caspase cascade pathways in apoptosis of oral lichen planus. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2010;110(5):618–623. doi: 10.1016/j.tripleo.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 20.Neppelberg E, Johannessen AC, Jonsson R. Apoptosis in oral lichen planus. European Journal of Oral Sciences. 2001;109(5):361–364. doi: 10.1034/j.1600-0722.2001.00081.x. [DOI] [PubMed] [Google Scholar]
- 21.Karatsaidis A, Hayashi K, Schreurs O, Helgeland K, Schenck K. Survival signalling in keratinocytes of erythematous oral lichen planus. Journal of Oral Pathology and Medicine. 2007;36(4):215–222. doi: 10.1111/j.1600-0714.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi Y, Nagao T, Maeda H, Kameyama Y, Warnakulasuriya KAAS. Epithelial cell proliferation in oral lichen planus. Cell Proliferation. 2002;35(1):103–109. doi: 10.1046/j.1365-2184.35.s1.11.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flatharta CÓ, Flint S, Toner M, Mabruk M. hTR RNA component as a marker of cellular proliferation in oral lichen planus. Asian Pacific Journal of Cancer Prevention. 2008;9(2):287–290. [PubMed] [Google Scholar]
- 24.Sousa FA, Paradella TC, Carvalho YR, Rosa LE. Immunohistochemical expression of PCNA, p53, bax and bcl-2 in oral lichen planus and epithelial dysplasia. Journal of Oral Science. 2009;51(1):117–121. doi: 10.2334/josnusd.51.117. [DOI] [PubMed] [Google Scholar]
- 25.Hirota M, Ito T, Okudela K, et al. Cell proliferation activity and the expression of cell cycle regulatory proteins in oral lichen planus. Journal of Oral Pathology and Medicine. 2002;31(4):204–212. doi: 10.1034/j.1600-0714.2002.310403.x. [DOI] [PubMed] [Google Scholar]
- 26.Tobón-Arroyave SI, Villegas-Acosta FA, Ruiz-Restrepo SM, Vieco-Durán B, Restrepo-Misas M, Londoño-López ML. Expression of caspase-3 and structural changes associated with apoptotic cell death of keratinocytes in oral lichen planus. Oral Diseases. 2004;10(3):173–178. doi: 10.1046/j.1601-0825.2003.00998.x. [DOI] [PubMed] [Google Scholar]
- 27.Mattila R, Alanen K, Syrjanen S. Immunohistochemical study on topoisomerase IIalpha, Ki-67 and cytokeratin-19 in oral lichen planus lesions. Archives of Dermatological Research. 2007;298(8):381–388. doi: 10.1007/s00403-006-0711-z. [DOI] [PubMed] [Google Scholar]
- 28.González-Moles MA, Bascones-Ilundain C, Gil Montoya JA, Ruiz-Avila I, Delgado-Rodríguez M, Bascones-Martínez A. Cell cycle regulating mechanisms in oral lichen planus: molecular bases in epithelium predisposed to malignant transformation. Archives of Oral Biology. 2006;51(12):1093–1103. doi: 10.1016/j.archoralbio.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Bascones-Ilundain C, Gonzalez-Moles MA, Esparza G, Gil-Montoya JA, Bascones-Martinez A. Significance of liquefaction degeneration in oral lichen planus: a study of its relationship with apoptosis and cell cycle arrest markers. Clinical and Experimental Dermatology. 2007;32(5):556–563. doi: 10.1111/j.1365-2230.2007.02457.x. [DOI] [PubMed] [Google Scholar]
- 30.Bascones C, Gonzalez-Moles MA, Esparza G, et al. Apoptosis and cell cycle arrest in oral lichen planus: hypothesis on their possible influence on its malignant transformation. Archives of Oral Biology. 2005;50(10):873–881. doi: 10.1016/j.archoralbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Moles MA, Gil-Montoya JA, Ruiz-Avila I, Esteban F, Bascones-Martinez A. Differences in the expression of p53 protein in oral lichen planus based on the use of monoclonal antibodies DO7 and pAb 240. Oral Oncology. 2008;44(5):496–503. doi: 10.1016/j.oraloncology.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poomsawat S, Buajeeb W, Khovidhunkit SOP, Punyasingh J. Overexpression of cdk4 and p16 in oral lichen planus supports the concept of premalignancy. Journal of Oral Pathology and Medicine. 2011;40(4):294–299. doi: 10.1111/j.1600-0714.2010.01001.x. [DOI] [PubMed] [Google Scholar]
- 34.Montebugnoli L, Venturi M, Gissi DB, et al. Immunohistochemical expression of p16(INK4A) protein in oral lichen planus. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2011;112(2):222–227. doi: 10.1016/j.tripleo.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Gasco M, Crook T. The p53 network in head and neck cancer. Oral Oncology. 2003;39(3):222–231. doi: 10.1016/s1368-8375(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 36.Ebrahimi M, Nylander K, van der Waal I. Oral lichen planus and the p53 family: what do we know? Journal of Oral Pathology and Medicine. 2011;40(4):281–285. doi: 10.1111/j.1600-0714.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- 37.Meek DW. The p53 response to DNA damage. DNA Repair. 2004;3(8-9):1049–1056. doi: 10.1016/j.dnarep.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Safadi RA, Jaber SZA, Hammad HM, Hamasha AAH. Oral lichen planus shows higher expressions of tumor suppressor gene products of p53 and p21 compared to oral mucositis. An immunohistochemical study. Archives of Oral Biology. 2010;55(6):454–461. doi: 10.1016/j.archoralbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Chaiyarit P, Ma N, Hiraku Y, et al. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Science. 2005;96(9):553–559. doi: 10.1111/j.1349-7006.2005.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JJ, Kuo MY, Cheng SJ, et al. Higher expressions of p53 and proliferating cell nuclear antigen (PCNA) in atrophic oral lichen planus and patients with areca quid chewing. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2005;99(4):471–478. doi: 10.1016/j.tripleo.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Bartkova J, Rezaei N, Liontos M, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444(7119):633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 42.Gorgoulis VG, Vassiliou LVF, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434(7035):907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Michelsen C, Cheng X, Zeng T, Priddy R, Rosin MP. Molecular analysis of oral lichen planus: a premalignant lesion? American Journal of Pathology. 1997;151(2):323–327. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Cheng X, Li YH, et al. High frequency of allelic loss in dysplastic lichenoid lesions. Laboratory Investigation. 2000;80(2):233–237. doi: 10.1038/labinvest.3780026. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Yook JI, Lee EH, et al. Evaluation of premalignant potential in oral lichen planus using interphase cytogenetics. Journal of Oral Pathology and Medicine. 2001;30(2):65–72. doi: 10.1034/j.1600-0714.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- 46.Accurso BT, Warner BM, Knobloch TJ, et al. Allelic imbalance in oral lichen planus and assessment of its classification as a premalignant condition. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2011;112(3):359–366. doi: 10.1016/j.tripleo.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Femiano F, Scully C. DNA cytometry of oral leukoplakia and oral lichen planus. Medicina Oral Patologia Oral y Cirugia Bucal. 2005;10(supplement 1):E9–E14. [PubMed] [Google Scholar]
- 48.Hosni ES, Yurgel LS, Silva VDD. DNA ploidy in oral lichen planus, determined by image cytometry. Journal of Oral Pathology and Medicine. 2010;39(3):206–211. doi: 10.1111/j.1600-0714.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 49.Rode M, Flezar MS, Kogoj-Rode M, et al. Image cytometric evaluation of nuclear texture features and DNA content of the reticular form of oral lichen planus. Analytical & Quantitative Cytology & Histology. 2006;28(5):262–268. [PubMed] [Google Scholar]
- 50.Montebugnoli L, Farnedi A, Marchetti C, Magrini E, Pession A, Foschini MP. High proliferative activity and chromosomal instability in oral lichen planus. International Journal of Oral and Maxillofacial Surgery. 2006;35(12):1140–1144. doi: 10.1016/j.ijom.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Sutinen M, Kainulainen T, Hurskainen T, et al. Expression of matrix metalloproteinases (MMP-1 and -2) and their inhibitors (TIMP-1, -2 and -3) in oral lichen planus, dysplasia, squamous cell carcinoma and lymph node metastasis. British Journal of Cancer. 1998;77(12):2239–2245. doi: 10.1038/bjc.1998.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou XJ, Sugerman PB, Savage NW, Walsh LJ. Matrix metalloproteinases and their inhibitors in oral lichen planus. Journal of Cutaneous Pathology. 2001;28(2):72–82. doi: 10.1034/j.1600-0560.2001.280203.x. [DOI] [PubMed] [Google Scholar]
- 53.Mazzarella N, Femiano F, Gombos F, De Rosa A, Giuliano M. Matrix metalloproteinase gene expression in oral lichen planus: erosive vs. reticular forms. Journal of the European Academy of Dermatology and Venereology. 2006;20(8):953–957. doi: 10.1111/j.1468-3083.2006.01693.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim SG, Chae CH, Cho BO, et al. Apoptosis of oral epithelial cells in oral lichen planus caused by upregulation of BMP-4. Journal of Oral Pathology and Medicine. 2006;35(1):37–45. doi: 10.1111/j.1600-0714.2005.00373.x. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Zhang W, Geng N, Tian K, Windsor LJ. MMPs, TIMP-2, AND TGF-β1 in the cancerization of oral lichen planus. Head and Neck. 2008;30(9):1237–1245. doi: 10.1002/hed.20869. [DOI] [PubMed] [Google Scholar]
- 56.Tsai LL, Yang SF, Tsai CH, Chou MY, Chang YC. Concomitant upregulation of matrix metalloproteinase-2 in lesions and circulating plasma of oral lichen planus. Journal of Dental Sciences. 2009;4(1):7–12. [Google Scholar]
- 57.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harbor Perspectives in Biology. 2009;1(5):p. a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santoro A, Majorana A, Bardellini E, Festa S, Sapelli P, Facchetti F. NF-κB expression in oral and cutaneous lichen planus. Journal of Pathology. 2003;201(3):466–472. doi: 10.1002/path.1423. [DOI] [PubMed] [Google Scholar]
- 59.Rhodus NL, Cheng B, Myers S, Bowles W, Ho V, Ondrey F. A comparison of the pro-inflammatory, NF-κB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clinical Immunology. 2005;114(3):278–283. doi: 10.1016/j.clim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Rhodus NL, Cheng B, Myers S, Miller L, Ho V, Ondrey F. The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Molecular Carcinogenesis. 2005;44(2):77–82. doi: 10.1002/mc.20113. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Lin M, Zhang S, et al. NF-κB-dependent cytokines in saliva and serum from patients with oral lichen planus: a study in an ethnic Chinese population. Cytokine. 2008;41(2):144–149. doi: 10.1016/j.cyto.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Frontiers in Bioscience. 2008;13(13):5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 63.Sugermann PB, Savage NW, Seymour GJ, Walsh LJ. Is there a role for tumor necrosis factor-alpha (TNF-α) in oral lichen planus? Journal of Oral Pathology and Medicine. 1996;25(5):219–224. doi: 10.1111/j.1600-0714.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 64.Carrozzo M, De Capei MU, Dametto E, et al. Tumor necrosis factor-α and interferon-γ polymorphisms contribute to susceptibility to oral lichen planus. Journal of Investigative Dermatology. 2004;122(1):87–94. doi: 10.1046/j.0022-202X.2003.22108.x. [DOI] [PubMed] [Google Scholar]
- 65.Bai J, Jiang L, Lin M, Zeng X, Wang Z, Chen Q. Association of polymorphisms in the tumor necrosis factor-α and interleukin-10 genes with oral lichen planus: a study in a Chinese cohort with Han ethnicity. Journal of Interferon and Cytokine Research. 2009;29(7):381–388. doi: 10.1089/jir.2008.0089. [DOI] [PubMed] [Google Scholar]
- 66.Kimkong I, Hirankarn N, Nakkuntod J, Kitkumthorn N. Tumour necrosis factor-alpha gene polymorphisms and susceptibility to oral lichen planus. Oral Diseases. 2011;17(2):206–209. doi: 10.1111/j.1601-0825.2010.01722.x. [DOI] [PubMed] [Google Scholar]
- 67.Xavier GM, Sá ARD, Guimarães ALS, Silva TAD, Gomez RS. Investigation of functional gene polymorphisms interleukin-1β, interleukin-6, interleukin-10 and tumor necrosis factor in individuals with oral lichen planus. Journal of Oral Pathology and Medicine. 2007;36(8):476–481. doi: 10.1111/j.1600-0714.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 68.Sklavounou-Andrikopoulou A, Chrysomali E, Iakovou M, Garinis GA, Karameris A. Elevated serum levels of the apoptosis related molecules TNF-α, Fas/Apo-1 and Bcl-2 in oral lichen planus. Journal of Oral Pathology and Medicine. 2004;33(7):386–390. doi: 10.1111/j.1600-0714.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 69.Pezelj-Ribaric S, Prso IB, Abram M, Glazar I, Brumini G, Simunovic-Soskic M. Salivary levels of tumor necrosis factor-α in oral lichen planus. Mediators of Inflammation. 2004;13(2):131–133. doi: 10.1080/09629350410001688530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhodus NL, Cheng B, Bowles W, Myers S, Miller L, Ondrey F. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Diseases. 2006;12(2):112–116. doi: 10.1111/j.1601-0825.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 71.Ghallab NA, El-Wakeel N, Shaker OG. Levels of salivary IFN-gamma, TNF-alfa, and TNF receptor-2 as prognostic markers in (Erosive) oral lichen planus. Mediators of Inflammation. 2010;2010:7 pages. doi: 10.1155/2010/847632. Article ID 847632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thongprasom K, Dhanuthai K, Sarideechaigul W, Chaiyarit P, Chaimusig M. Expression of TNF-α in oral lichen planus treated with fluocinolone acetonide 0.1% Journal of Oral Pathology and Medicine. 2006;35(3):161–166. doi: 10.1111/j.1600-0714.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 73.Sklavounou A, Chrysomali E, Scorilas A, Karameris A. TNF-α expression and apoptosis-regulating proteins in oral lichen planus: a comparative immunohistochemical evaluation. Journal of Oral Pathology and Medicine. 2000;29(8):370–375. doi: 10.1034/j.1600-0714.2000.290802.x. [DOI] [PubMed] [Google Scholar]
- 74.Chao TJ. Adalimumab in the management of cutaneous and oral Lichen planus. Cutis. 2009;84(6):325–328. [PubMed] [Google Scholar]
- 75.Yarom N. Etanercept for the management of oral lichen planus. American Journal of Clinical Dermatology. 2007;8(2):p. 121. doi: 10.2165/00128071-200708020-00010. [DOI] [PubMed] [Google Scholar]
- 76.Gu GM, Martin MD, Darveau RP, et al. Oral and serum IL-6 levels in oral lichen planus patients. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2004;98(6):673–678. doi: 10.1016/j.tripleo.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Rhodus NL, Cheng B, Ondrey F. Th1/Th2 cytokine ratio in tissue transudates from patients with oral lichen planus. Mediators of Inflammation. 2007;2007 doi: 10.1155/2007/19854. Article ID 19854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalogerakou F, Albanidou-Farmaki E, Markopoulos AK, Antoniades DZ. Detection of T cells secreting type 1 and type 2 cytokines in the peripheral blood of patients with oral lichen planus. Hippokratia. 2008;12(4):230–235. [PMC free article] [PubMed] [Google Scholar]
- 79.Matsumoto S, Hara T, Mitsuyama K, et al. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. Journal of Immunology. 2010;184(3):1543–1551. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 80.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. Journal of Leukocyte Biology. 2006;80(2):227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 81.Bhaumik D, Scott GK, Schokrpur S, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1(4):402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carrozzo M. Oral diseases associated with hepatitis C virus infection. Part 2: lichen planus and other diseases. Oral Diseases. 2008;14(3):217–228. doi: 10.1111/j.1601-0825.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 83.Kumar A. MicroRNA in HCV infection and liver cancer. Biochimica et Biophysica Acta. 2011;1809(11-12):694–699. doi: 10.1016/j.bbagrm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 84.Vlaar APJ, Rietdijk ST, Zeerleder SS, Boerman T, Beuers U. Malignancies associated with chronic hepatitis C: case report and review of the literature. Netherlands Journal of Medicine. 2011;69(5):211–215. [PubMed] [Google Scholar]
- 85.Lodi G, Olsen I, Piattelli A, D’Amico E, Artese L, Porter SR. Antibodies to epithelial components in oral lichen planus (OLP) associated with hepatitis C virus (HCV) infection. Journal of Oral Pathology and Medicine. 1997;26(1):36–39. doi: 10.1111/j.1600-0714.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 86.Femiano F, Scully C. Functions of the cytokines in relation oral lichen planus-hepatitis C. Medicina Oral Patologia Oral y Cirugia Bucal. 2005;10:E40–E44. [PubMed] [Google Scholar]
- 87.Nagao Y, Sata M, Noguchi S, et al. Detection of hepatitis C virus RNA in oral lichen planus and oral cancer tissues. Journal of Oral Pathology and Medicine. 2000;29(6):259–266. doi: 10.1034/j.1600-0714.2000.290604.x. [DOI] [PubMed] [Google Scholar]
- 88.Jaber MA, Porter SR, Bain L, Scully C. Lack of association between hepatitis C virus and oral epithelial dysplasia in British patients. International Journal of Oral and Maxillofacial Surgery. 2003;32(2):181–183. doi: 10.1054/ijom.2002.0258. [DOI] [PubMed] [Google Scholar]
- 89.Alves MGO, Almeida JD, Cabral LAG. Association between hepatitis C virus and oral lichen planus. Hepatitis Monthly. 2011;11(2):132–133. [PMC free article] [PubMed] [Google Scholar]
- 90.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Alimentary Pharmacology and Therapeutics. 2006;23(8):1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91(4):854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 92.Choi PM, Zelig MP. Similarity of colorectal cancer of Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35(7):950–954. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fedele S, Lo Russo L, Mignogna C, Staibano S, Porter SR, Mignogna MD. Macroscopic classification of superficial neoplastic lesions of the oral mucosa: a preliminary study. European Journal of Surgical Oncology. 2008;34(1):100–106. doi: 10.1016/j.ejso.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 94.Mignogna MD, Lo Russo L, Fedele S, Ruoppo E, Califano L, Lo Muzio L. Clinical behaviour of malignant transforming oral lichen planus. European Journal of Surgical Oncology. 2002;28(8):838–843. doi: 10.1053/ejso.2002.1302. [DOI] [PubMed] [Google Scholar]
- 95.Sigel JE, Petras RE, Lashner BA, Fazio VW, Goldblum JR. Intestinal adenocarcinoma in Crohn’s disease: a report of 30 cases with a focus on coexisting dysplasia. American Journal of Surgical Pathology. 1999;23(6):651–655. doi: 10.1097/00000478-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 96.Veltkamp C, Anstaett M, Wahl K, et al. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut. 2011;60(10):1345–1353. doi: 10.1136/gut.2010.217117. [DOI] [PubMed] [Google Scholar]
- 97.Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Current Opinion in Gastroenterology. 2008;24(6):733–741. doi: 10.1097/mog.0b013e328311f26e. [DOI] [PubMed] [Google Scholar]
- 98.Aherne CM, Collins CB, Masterson JC, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61(5):733–741. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paradisi A, Maisse C, Coissieux MM, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(40):17146–17151. doi: 10.1073/pnas.0901767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paradisi A, Maisse C, Bernet A, et al. NF-κB regulates netrin-1 expression and affects the conditional tumor suppressive activity of the netrin-1 receptors. Gastroenterology. 2008;135(4):1248–1257. doi: 10.1053/j.gastro.2008.06.080. [DOI] [PubMed] [Google Scholar]


