Abstract
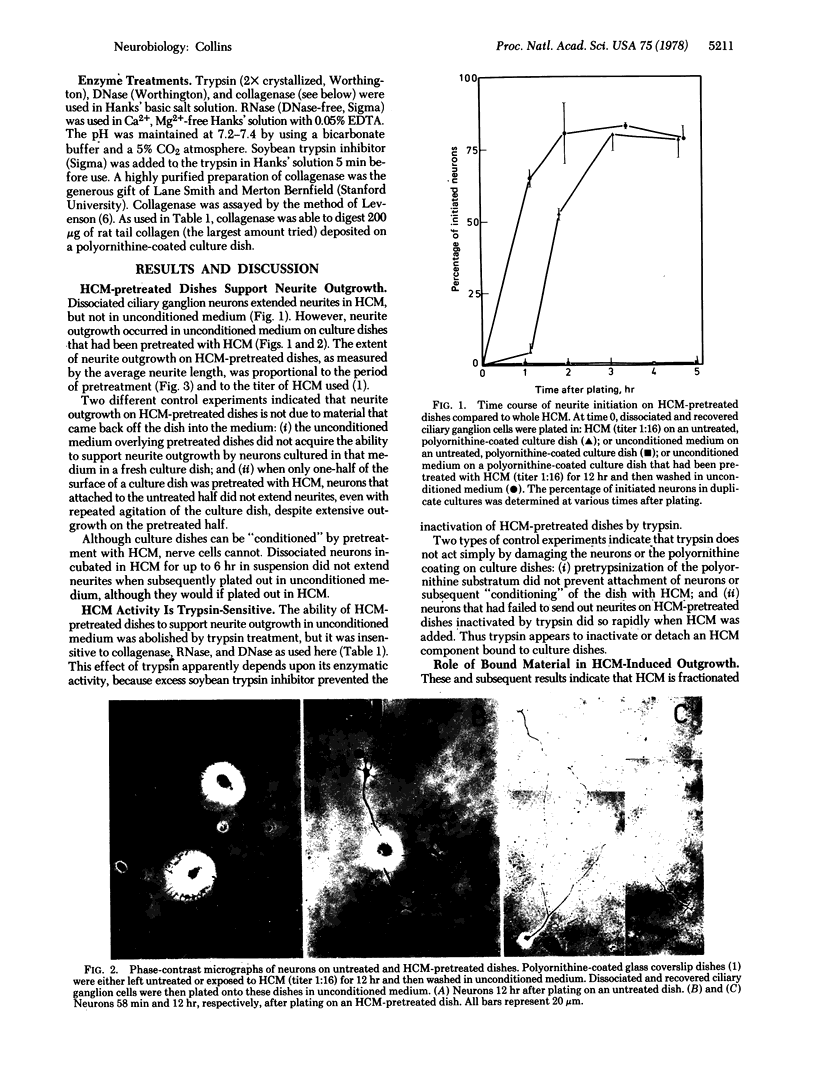
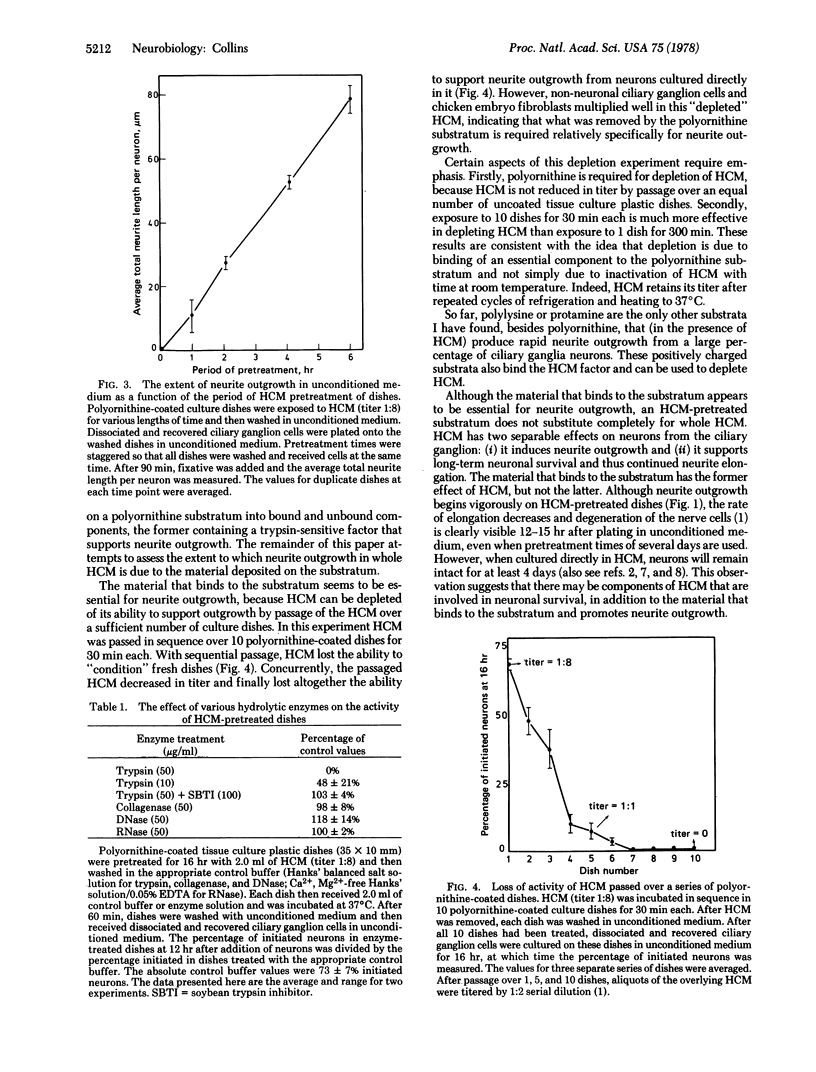
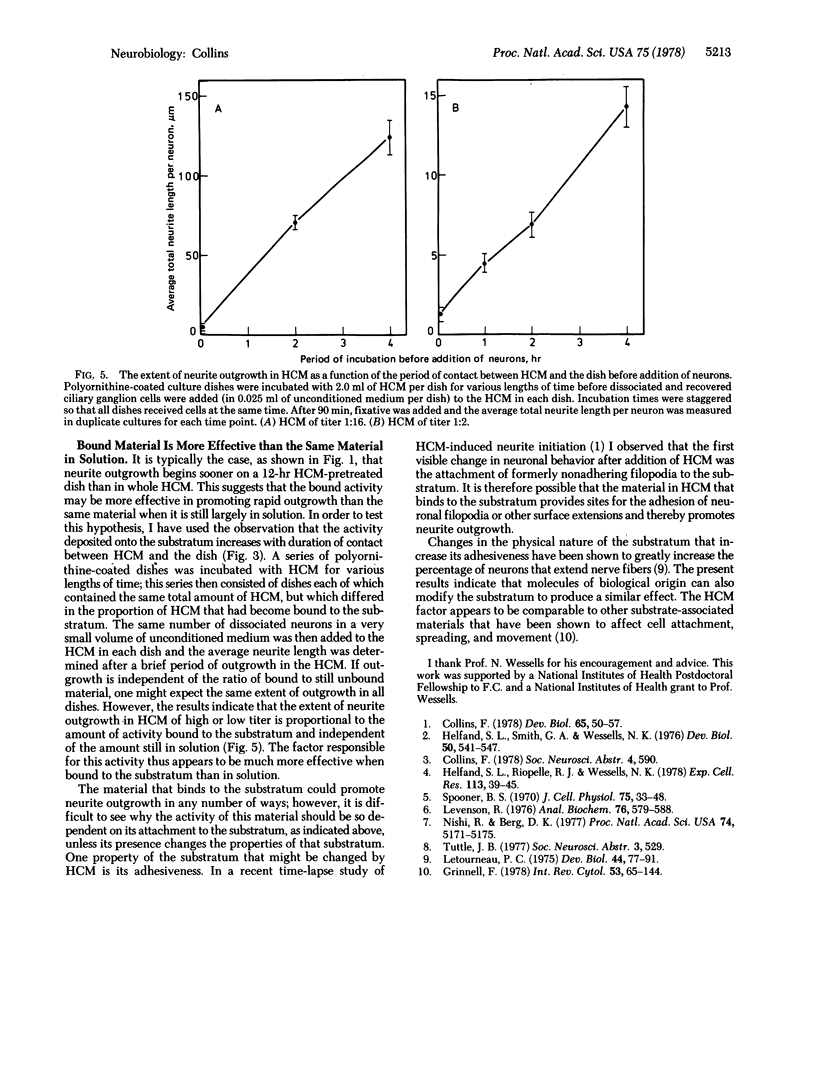
Heart-cell conditioned medium (HCM) induces rapid neurite outgrowth from isolated neurons in culture. The following evidence indicates that this action of HCM is due to a trypsin-sensitive factor which attaches to the polyornithinecoated culture substratum: (i) Pretreatment of the culture substratum with HCM allows rapid neurite outgrowth to occur even in unconditioned media. The active factor remains bound to the substratum during the period of neurite outgrowth. (ii) The substratum-bound activity is destroyed by trypsin treatment, but is insensitive to collagenase, RNase, and DNase. (iii) The factor that binds to the substratum is essential for neurite outgrowth, because HCM is no longer active when the material that binds to the polyornithine substratum has been removed by passage of the HCM over a series of culture dishes. However, this “depleted” HCM is still able to support the growth of nonneuronal cells. (iv) Most significantly, when neurons are cultured in whole HCM, the extent of neurite outgrowth is proportional to the amount of substratum-bound activity and not to the amount in solution, indicating that the substratum-bound form of the factor is more active. Previous observations [Collins, F. (1978) Dev. Biol. 65, 50-57] suggest that HCM promotes neurite outgrowth by increasing the adhesion between nerve cell surface extensions and the polyornithine-coated culture substratum. It is possible, therefore, that the factor in HCM that binds to the substratum possesses sites to which nerve cell surface components adhere.
Keywords: nerve cell culture, substrate-associated material, parasympathetic neurons
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins F. Axon initiation by ciliary neurons in culture. Dev Biol. 1978 Jul;65(1):50–57. doi: 10.1016/0012-1606(78)90178-1. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Cellular adhesiveness and extracellular substrata. Int Rev Cytol. 1978;53:65–144. doi: 10.1016/s0074-7696(08)62241-x. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Riopelle R. J., Wessells N. K. Non-equivalence of conditioned medium and nerve growth factor for sympathetic, parasympathetic, and sensory neurons. Exp Cell Res. 1978 Apr;113(1):39–45. doi: 10.1016/0014-4827(78)90085-x. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Smith G. A., Wessells N. K. Survival and development in culture of dissociated parasympathetic neurons from ciliary ganglia. Dev Biol. 1976 Jun;50(2):541–547. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Possible roles for cell-to-substratum adhesion in neuronal morphogenesis. Dev Biol. 1975 May;44(1):77–91. doi: 10.1016/0012-1606(75)90378-4. [DOI] [PubMed] [Google Scholar]
- Levenson R. A collagen film microassay for tissue collagenase. Anal Biochem. 1976 Dec;76(2):579–588. doi: 10.1016/0003-2697(76)90352-3. [DOI] [PubMed] [Google Scholar]
- Nishi R., Berg D. K. Dissociated ciliary ganglion neurons in vitro: survival and synapse formation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5171–5175. doi: 10.1073/pnas.74.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B. S. The expression of differentiation by chick embryo thyroid in cell culture. I. Functional and fine structural stability in mass and clonal culture. J Cell Physiol. 1970 Feb;75(1):33–47. doi: 10.1002/jcp.1040750105. [DOI] [PubMed] [Google Scholar]




