Abstract
Background
The prevalence of chronic renal failure and End Stage Renal Disease (ESRD) has remained high worldwide and the epidemiology has changed significantly in the last decade in industrialised countries. While there have been significant improvements in these patient's outcomes in developed countries, their state and survival is still appalling in developing countries.
Objective
To determine the clinical pattern, presentation and management outcomes in our ESRD population over a 19-year period (1989–2007).
Methods
Seven hundred and sixty patients' records were reviewed. Data on major causes, clinical presentation, management and survival were retrieved and collated. Data was analysed using SPSS package version 16.
Results
Their ages ranged between 15–90 years (mean ± SD; 39.9±1.67years) with male preponderance (70.3%). Major presenting complaints were body swelling and uraemic symptoms in most studied patients. The predisposing conditions included chronic glomerulonephritis, hypertension, obstructive uropathy and diabetes mellitus. Renal replacement therapy offered included HD in 556(73.2%), Continous Ambulatory Peritoneal Dialysis (CAPD) in only 9(1.2%) patients and renal transplantation in only 7(0.9%). Only 38(6.8%) survived on HD for longer than three months while 7(77.8%) CAPD patients and all transplanted patients survived for between six months and four years (p<0.00001). Median duration of survival after diagnosis for all the patients was 2 weeks (range 0–50 months).
Conclusion
End stage renal disease is still prevalent with chronic glomerulonephritis and hypertension being the common causes. Prognosis is still grave hence subsidized renal replacement therapy and preventive nephrology should be targeted in such underserved populations.
Keywords: ESRD, dialysis, survival
Introduction
Chronic kidney disease (CKD) and end stage renal disease (ESRD) are highly prevalent in both industrialized and developing countries of the world and the epidemiology has changed remarkably in the last decade particularly in the developed countries with diabetic nephropathy now assuming epidemic proportions1–6. While most industrialised countries have developed efficient renal registries, its either none existent or at its infancy in most developing ones hence only hospital derived data are available for planning.
In addition, while major developments have occurred in the management of ESRD patients worldwide with significant improvements in outcomes, their clinical state and survival is still very poor in developing countries where GDP per capita is low and budgetary allocation on health is poor 1,2,4. The epidemiology of ESRD is strikingly different in sub Saharan Africa (SSA) compared with developed economies. While it predominantly affects the middle aged and elderly populations in developed countries, it picks out the young adults in their prime of life and the most economically productive years in SSA2,4,7–13. This may be attributable to the probable aetiology of CKD in the sub-region, and other factors that may contribute include poor access to care, poor knowledge of risk factors for CKD as well as deleterious socio-cultural practices. Diabetes Mellitus has emerged as the commonest cause of ESRD in developed countries followed by hypertension but it ranked a distant third amongst causes of ESRD in SSA with CGN and HTN being more prevalent. An earlier report on the causes of chronic renal failure (CRF) from our centre identified CGN and hypertension as the commonest causes while diabetic nephropathy (DN) was not reported at all among the causes of CRF9. Several other reports in Nigeria and other countries in SSA have established that HTN and CGN are the leading causes of ESRD, but the prevalence of diabetic nephropathy is rising and obstructive uropathy and toxic nephropathies also contribute significantly10–15.
The outcome of ESRD in SSA is very poor and this is principally as a result of poor awareness of kidney disease in the community, late presentation to the hospital, limited capacity of health workers in kidney disease prevention and limited accessibility as well as affordability of renal replacement therapy (RRT) 16–18. In fact in a recent publication by Grassman et al19, Africa contributed less than 5% of patients on RRT worldwide. As a consequence of these, preventive nephrology strategies would be most beneficial in the sub-region and everyone should participate including governments and nongovernmental organisations 2. However to be able to plan a useful CKD prevention program in any community, there would be a need for background statistics on possible magnitude of CKD, probable predisposing factors or causes and mode of presentation. In this short report we sought to determine epidemiology of ESRD, modes of presentation as well as determine the major causes of ESRD. This we believe is vital and useful in planning prevention program in our community which would be representative of most communities in SSA.
Methods
Our hospital is a tertiary referral hospital based in south-western Nigeria and it served a population of above 15 million people in six contiguous states until the recent proliferation of renal care centres. We also have few referrals from eastern and northern states. Access to renal care is limited to major cities in Nigeria and renal replacement therapy is borne directly by the patients.
The case records of all patients with chronic renal failure (CRF) managed during the 19 year period (1989–2007) was retrieved. The data on major causes, clinical presentation, renal replacement therapy offered and survival were retrieved and collated. The CRF was defined as presence of persisting and progressive deterioration in renal function manifesting as recurrent features of uraemia, oedema and/or hypertension persisting for longer than three months and supported by renal ultrasonographic evidence of either reduced kidney size or cystic or hydronephrotic kidneys.
Diagnostic criteria used in categorizing the aetiologic factors were mainly clinical. They included the following:
Hypertensive Nephrosclerosis (HTN) was diagnosed in those patients that were 40 years or older with history of HT in the previous 5 years, features of hypertensive heart disease (HHD), grade 3 or 4 hypertensive retinopathy, minimal proteinuria 1+ to 2+.
Chronic Glomerulonephritis (CGN) was diagnosed in patients that were about 30 years (majority were younger than 25 years) with past history of acute or post infectious glomerulonephritis, nephrotic syndrome, recurrent body swelling, with moderate to massive proteinuria (>2+).
Diabetic nephropathy (DN) was diagnosed in those patients with diabetes mellitus (DM) diagnosed at least five years prior to presentation that also had proteinuria, hypertension, peripheral neuropathy, diabetic retinopathy and / or uraemic syndrome.
Patients with obstructive uropathy were usually referred by urologists and had either a benign prostatic hypertrophy (BPH) or prostatic cancer with recurrent episodes of bladder outlet obstruction as well as documented ultrasonographic evidence of advanced obstructive uropathy.
Adult polycystic kidney disease was diagnosed in patients that had uraemia, bilateral multicystic kidneys (with more than five cysts in each kidney) and family history.
Chronic tubulointerstitial nephritis (CTIN) was diagnosed in uraemic patients with significant analgesic consumption or exposure to metals, who had minimal oedema, and were either normotensive or had mild hypertension.
Patients that did not fit into any of these groups were classified as ESRD with undefined aetiology.
Standard haemodialysis (HD) prescription included dialysis duration of 12 hours per week subdivided into three 4-Hour sessions or two 6-hour sessions depending on affordability. For continuous ambulatory peritoneal dialysis (CAPD), the typical prescription was 3–4 daily 2L exchanges and an overnight dwell. On the current costing of various RRT modalities, a session of HD costs about 130 USD while a day cycle of CAPD costs 80 USD. The cost of renal transplantation along with three months of immunosuppressive therapy is about 20,000USD.
Statistical analysis
Data was analyzed using SPSS package version 16. Measures of central tendency (mean, median) and measures of variability (range, standard deviation) were used to describe quantitative/continuous variables. Similarly, frequencies, percentages and cross tabulations were used to summarize qualitative variables. Chi Square and Fishers exact tests were used as appropriate to compare different groups. Kaplan Meier Survival curve was used to assess survival.
Results
A total of 13,668 medical cases were admitted during the period while 1,208 (8.83%) patients had ESRD. Of note was the fact that ESRD admissions constituted only 6.1% of medical admissions in 1989 and had increased to 21.5% in 2006 and 18.9% in 2007.
Epidemiology and mode of presentation
Of the 1,208 ESRD admissions managed over the period, only 760 (62.9%) case records were reviewed as the others were either not found or contained insufficient information. The age range was 15–90 years (median; 36 years), and there was a male preponderance with 534 (70.3%) being males. Ninety percent of the patients were from western Nigeria while 7% and 3% were from the east and north respectively. Major presenting features were body swelling in 67.8%, uraemic symptoms (nausea, vomiting, hiccups or pruritus) in 60.4%, and dyspnoea on exertion or orthopnoea in 33%. Although 40.7% of the patients had headaches only 20.8% of them had associated blurring of vision. Twenty-two percent had no past history suggestive of renal disease. A high proportion of patients (72.4%) had stage 2 hypertension (JNC VII) with median (range) systolic, diastolic and mean arterial blood pressures of 160 mmHg (120 – 270 mmHg), 100 mmHg (50 – 209 mmHg) and 120 mmHg (83.3 – 169.3 mmHg) respectively, only 34.1% had clinical cardiomegaly (table 1). Seventy percent of those that had fundoscopic examination had either grade III or IV hypertensive retinopathy. Ascites and hepatomegaly were the commonest abdominal findings found in 55.3% and 39.2% respectively. Only 1.2 % of the patients had bone pains.
Table 1.
Baseline clinical and biochemical parameters in studied patients
| Parameters | Median | Range |
| Systolic BP (mmHg) | 160 | 120 – 270 |
| Diastolic BP (mmHg) | 100 | 50 – 209 |
| Mean arterial BP (mmHg) | 120 | 83.3 – 169.33 |
| Serum creatinine (µmol/L) | 1021.0 | 208.0 – 4728.0 |
| Serum urea (mmol/L) | 22.9 | 8.90 – 84.0 |
| Serum potassium (mmol/L) | 4.5 | 2.0 – 7.7 |
| Serum sodium (mmol/L) | 132.0 | 104.0 – 167.0 |
| Serum bicarbonate (mmol/L) | 19.0 | 12.0 – 30.0 |
| Serum Albumin (g/L) | 31.0 | 10.9 – 46 |
| Serum globulin (g/L) | 27 | 10.4 – 59 |
| PCV (%) | 23 | 8 – 50 |
Aetiology of ESRD
Aetiology of ESRD found in the patients based on our criteria outlined above included chronic glomerulonephritis (43.7%), hypertension (31.1%), obstructive uropathy (6.7%), diabetes mellitus (3.7%), tubulointerstitial nephritis (2.2%), polycystic kidney disease (0.7%) and in the remaining 12% of the patients; the cause could not be ascertained (table 2).
Table 2.
Distribution of studied ESRD patients according to aetiology
| Aetiology | Number of cases (n) |
Percentage (%) |
| Chronic Glomerulonephritis | 332 | 43.7 |
| Hypertensive Nephrosclerosis | 236 | 31.1 |
| Obstructive Uropathy | 51 | 6.7 |
| Diabetic Nephropathy | 28 | 3.7 |
| Chronic Tubulointerstitial Nephritis | 17 | 2.2 |
| ADPKD | 5 | 0.7 |
| Unknown | 91 | 12 |
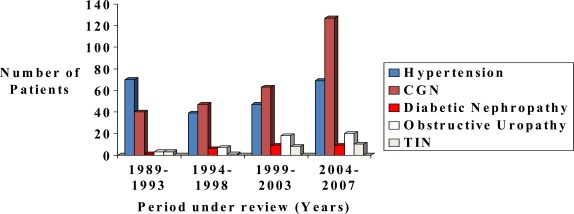
One hundred and sixty one (21.2%) had exposure to nephrotoxic agents out of which 53.4% were herbal remedies. Of note is the progressive increase in the proportions of patients with CGN and diabetic nephropathy over the study period while the proportion of patients with HTN did not change over the period (figure 1).
Figure 1.
Proportion of patients with different Aetiologies
Baseline serum chemistry in studied patients revealed median (range) serum creatinine, urea, potassium, sodium and bicarbonate of 1021 µmol/L (208 4728 µmol/L), 22.5 mmol/L (8.9 – 84 mmol/L), 4.5 mmol/L (2.0 – 7.7mmol/L), 132 mmol/L (104 –167mmol/L) and 19 mmol/L (12.0 – 30mmol/L) respectively (table 1). A high percentage of patients (50.8%) had serum creatinine above 1000 µmol/L at presentation while in 32.8% it varied between 500µmol/L and 1000 µmol/L and in the last 16.4% it was less than 500 µmol/L. Malnutrition was prevalent as 75.7% had hypoalbuminaemia (median for serum albumin; 31.0g/L (range: 10.9 – 46 g/ L)). Majority of the patients (88.9%) had anaemia with median packed cell volume (PCV) of 24.0% (range: 8.0–50 %). Twenty four percent of the patients had PCV of <20%, 64.9% had PCV between 20% and <33%, while in the remaining 11.1% it was greater than 33%. On serological screening, 95 (12.5%) of the patients were Hepatitis B surface Antigen (HBsAg) positive while only 15 (1.97%) and 12 (1.58%) were seropositive to Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV) respectively. During the period reviewed, these patients were managed conservatively as there was no dedicated machine for them. However, we now have dedicated machines for them but with strict barrier nursing.
Management of ESRD and outcome
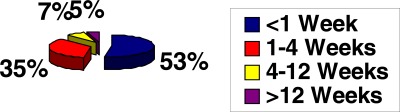
Renal replacement therapy offered included haemodialysis (HD) in 556 (73.2%), CAPD in only nine(1.2%) patients and renal transplantation in only seven(0.9%). The remaining 188 (25%) patients were managed conservatively as they could not afford the various RRT options or were either HBsAg positive or seropositive to Hepatitis C Virus (HCV) or Human Immunodeficiency Virus (HIV) as our centre lacked dedicated machines for these categories of patients at that time. Payment for RRT in our setting is borne by patient's out of pocket expenses, except for the extremely few number of patients assisted by corporate organisations or non governmental agencies. There is as yet no health insurance coverage for all modalities of RRT. Cost consideration was a major limiting factor for sustainability of RRT, out of the patients managed with HD only 38 (5.0%) could sustain the treatment for more than 12 weeks. These included 30 (3.9%) self sponsored patients and additional 8 (1.1%) patients supported by corporate or governmental agencies (figure 2).
Figure 2.
Affordability of HD amongst studied patients
Only these 38 patients survived on HD for longer than three months while seven CAPD patients and all transplanted patients survived for between six months and three years (P<0.00001) (Table 3).
Table 3.
Distribution of patients on various modalities of RRT and survival
| Modalities of RRT | Number of deaths less than 3 months after diagnosis |
Number of survivors more than 3 months after diagnosis |
P-value |
| Haemodialysis | 518 | 38 | p < 0.0001 (Fisher's exact test) |
| CAPD | 2 | 7 | |
| Renal Transplant | 0 | 7 | |
| Total | 520 | 52 | 572 |
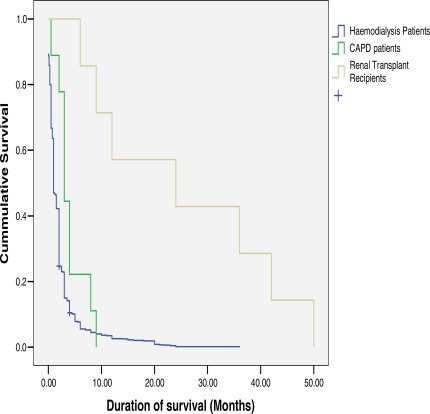
Majority (90%) of undialysed patients died within the first two weeks of presentation. Cause of death in most cases was advanced uraemia (70%) and congestive cardiac failure (25%) but was unexplained in 5% of cases especially those that discharged against medical advice and opted for care outside the hospital setting. Median duration of survival after diagnosis for all the patients was two weeks with range was 0 – 50 months. Kaplan Meier Survival Curve (figure 3) revealed that about 87% of our ESRD patients die in the first month of treatment and survival was significantly better in CAPD patients and transplant recipients.
Figure 3.
Kaplan Meier survival curves of patients according to renal replacement therapy options
Discussion
Chronic kidney disease (CKD) has assumed epidemic proportion worldwide hence its being regarded as a major public health challenge. While data on incidence and prevalence of CKD and ESRD are available in developed countries because of reliable and functional renal registries, they are either unavailable or unreliable in developing countries 1,2. Thus, the exact prevalence of CKD or ESRD in developing countries is unknown hence reliance on hospital derived data 8–12. In this study, which reviewed available data from 760 ESRD admissions (62.9%) of 1,208 ESRD patients seen over the 19 year period, ESRD admissions constituted 8.83% of total medical admissions. Of particular interest was the progressive increase in the percentage of ESRD admissions over the study period. Even though this is hospital data, it further support the increasing prevalence of CKD reported in community studies.
In agreement with previous studies in SSA, our patients were young. This is in sharp contrast with reports from developed countries where CKD and ESRD affects the middle aged and elderly populations5,6,20,21. The male preponderance observed further supports findings from previous studies which may reflect the background prevalence of the predisposing illnesses and risk factors in males3,13,14. Socio-cultural and economic factors which preferentially favour males in our communities may also contribute.
Even though the categorizations of the causes of CKD were mainly clinical without renal biopsies or autopsy reports, chronic glomerulonephritis and hypertension were the common aetiological factors in our setting. The occurrence of diabetic nephropathy is however increasing. This agrees with other reports from the sub-region and may reflect the high prevalence of chronic parasitic, bacterial, and viral infections particularly with the scourge of HIV / AIDS4,7–17. In addition hypertensive nephrosclerosis remains a disease of blacks whose prevalence is not only high but is also increasing 9–14.
Majority of ESRD patients in our setting, present late to the hospital. This was exemplified by the huge proportion of our reviewed cases that had features of uraemia even at first presentation. While this is similar to previous reports from SSA, it calls for urgent steps aimed at early detection and subsequent treatment10,12,17,22. Anaemia and malnutrition were also found to be common in our patients and may negatively impact on quality of life, cardiovascular disease and survival 23,24. The high prevalence of positivity to HBsAg reflect the high prevalence in the community and indicate that these categories of patients must also be offered different dialysis modalities. This has prompted the setting up of an isolation unit with dedicated machines for them but with strict barrier nursing. Despite the relatively low seropositivity to HCV and HIV observed, the patients should also be offered dialysis modalities and possibly transplantation if conditions are favourable.
On the use of renal replacement therapies, majority of our ESRD patients had haemodialysis mainly because it was and still is the most readily available treatment modality 2. It is however sad to note that only 6.8% could afford the treatment for longer than three months principally because the cost (about 130 USD per session) is borne directly by the patients. As mentioned earlier, government subsidy is almost non-existent and the newly introduced National Health Insurance Scheme (NHIS) does not cover advanced care for renal disease including various modalities of RRT. This is a major reason for the very high mortality rate for ESRD in the subregion7,10,12,16. Only 1.2% had continuous ambulatory peritoneal dialysis (CAPD) (a day cycle of CAPD costs about 80 USD), and the major limitations to its continuous use included non-availability of consumables and very high infection rate 18,25. Similarly, renal transplantation whose cost along with three months of immunosuppressive therapy is about 20,000 USD, is still at its developmental stage in Nigeria as less than 1% of our patients could afford the procedure. In our series only 0.9% of our ESRD patients received renal allograft. The major limitations included funding, shortage of donor organs and lack of deceased organ donation as well as lack of appropriate legislation26.
The prognosis for ESRD in SSA is still very poor as very few of our patients survived for longer than three months. Unfortunately, this is in agreement with previous studies 4,7,10,12,16,17,27. The risk of death is inexorably high in our subset of ESRD patients with a very high percentage dying within a few weeks of diagnosis. This situation is different in states with relatively freer and more equitable access to the various renal replacement therapy options either through health insurance or other state sponsored payment modalities 19,28–30. These high mortality figures should stimulate subsidy for and standardization of renal care in Nigeria and other developing countries. It should also herald commencement of preventive nephrology programs which could be primordial, primary, secondary or tertiary in all developing countries. The objectives of the prevention programs would be prevention or early detection of CKD, retardation of progression of CKD as well as appropriate planning for the initiation of various renal replacement therapy options 31. This would in the long term lead to a reduction in the number of patients that would require RRT, consequently leading to better survival and improved health related quality of life as well as a reduction in healthcare expenditure of the state. As much as possible, all prevention programs should include a treatment arm to be able to achieve these goals 31.
Conclusion
Advanced CKD presenting as ESRD is still prevalent with chronic glomerulonephritis and hypertension being the common causes and the prevalence of diabetic nephropathy is increasing. Presentation is late and prognosis and survival are still unacceptably poor. We therefore recommend that provision of renal replacement therapy in SSA should be supported and subsidized by governments, non-governmental agencies and health insurance companies where such exists. Renal transplantation should be encouraged in those that can support the treatment and above all preventive nephrology should be targeted in all underserved economies.
References
- 1.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–999. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 2.Arogundade FA, Barsoum RS. CKD prevention in sub-Saharan Africa: A call for Government, Non-governmental and community support. Am J Kid Dis. 2008;51(3):515–523. doi: 10.1053/j.ajkd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Dubose TD., Jr American society on Nephrology presidential Address 2006: Chronic kidney disease as a public health threat- New strategy for a growing problem. J Am Soc Nephrol. 2007;18:1038–1045. doi: 10.1681/ASN.2006121347. [DOI] [PubMed] [Google Scholar]
- 4.Naicker S. End-stage renal disease in sub-Saharan Africa. Ethn Dis. 2009;19(1 Suppl 1):S113–S115. [PubMed] [Google Scholar]
- 5.United States Renal Data System, author. Annual data report: Incidence and prevalence of ESRD. Am J Kid Dis. 2003;42(suppl5):s37–s173. [Google Scholar]
- 6.Atkins RC. Global perspective of renal failure. Kid Int. 2005;67:S14–S18. [Google Scholar]
- 7.Mabayoje MO, Bamgboye EL, Odutola TA, Mabadeje AFB. Chronic renal failure at the Lagos university teaching hospital: A 10 year review. Transplant Proc. 1992;24:1851–1852. [PubMed] [Google Scholar]
- 8.Oyediran AB, Akinkugbe OO. Chronic renal failure in Nigeria. Trop Geog Med. 1970;22:41–45. [PubMed] [Google Scholar]
- 9.Akinsola W, Odesanmi WO, Ogunniyi JO, Ladipo GOA. Diseases causing chronic renal failure in Nigerians- a prospective study of 100 cases. Afr J Med Sci. 1989;18:131–137. [PubMed] [Google Scholar]
- 10.Matekole M, Affram K, Lee SJ, Howie AJ, Michael J, Adu D. Hypertension and end-stage renal failure in tropical Africa. J Hum Hypertens. 1993;7:443–446. [PubMed] [Google Scholar]
- 11.Abboud OL, Osman EM, Musa AR. The aetiology of chronic renal failure in adult Sudanese patients. Ann Trop Med Parasito. 1989;83:411–414. doi: 10.1080/00034983.1989.11812365. [DOI] [PubMed] [Google Scholar]
- 12.Diouf B, Ka EF, Niang A, Diouf ML, Mbenue M, Diop TM. Etiologies of chronic renal insufficiency in adult internal medicine services in Dakar. Dakar Med. 2000;45:62–65. [PubMed] [Google Scholar]
- 13.Du-Toit E, Pascoe M, MacGregor K, Thompson PD, editors. South Africa Dialysis and Transplantation Registry Report. Cape Town, South Africa: 1994. Combined report on maintenance dialysis and Transplantation in the Republic of South Africa. [Google Scholar]
- 14.Akinsola A, Adelekun TA, Arogundade FA, Sanusi AA. Magnitude of the problem of CRF in Nigerians. Afr J Nephrol. 2004;8:24–26. [Google Scholar]
- 15.Bamgboye E. End-stage renal disease in sub Saharan Africa. Ethn Dis. 2006;16(suppl2):S2–S5. [PubMed] [Google Scholar]
- 16.Arije A, Kadiri S, Akinkugbe OO. The viability of haemodialysis as a treatment option for renal failure in developing countries. Afr J Med Sci. 2000;29:311–314. [PubMed] [Google Scholar]
- 17.Alebiosu CO, Ayodele OO, Abbas A, Olutoyin AI. Chronic renal failure at the Olabisi Onabanjo University teaching hospital, Sagamu, Nigeria. Afr Health Sci. 2006;6(3):132–138. doi: 10.5555/afhs.2006.6.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinsola A, Adelekun TA, Arogundade FA. CAPD practice in OAUTHC, Ile-Ife, Nigeria; A preliminary experience. Dial Transplant. 2000;29:774–782. [Google Scholar]
- 19.Grassman A, Gioberge S, Moeller S, Brown G. End-stage renal disease: global demographics in 2005 and observed trends. Artif Organs. 2006;30(12):895–897. doi: 10.1111/j.1525-1594.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 20.Feest TG, Mistry CD, Grimes DS, Mallick NP. Incidence of advanced chronic renal failure and the need for end-stage renal replacement treatment. BMJ. 1990;301:897–900. doi: 10.1136/bmj.301.6757.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mc Geown Prevalence of advanced renal failure in Northern Ireland. BMJ. 1990;301:900–903. doi: 10.1136/bmj.301.6757.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLigeyo SO, Kayima JK. Evolution of Nephrology in East Africa in the last seventy years-Studies and Practice. East Afr Med J. 1993;70:362–368. [PubMed] [Google Scholar]
- 23.Maj Z, Ebben J, Xia H, et al. Haematocrit level and associated mortality in haemodialysis patient. J Am Soc Nephrol. 1999;6:610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 24.Forley RN, Parfrey PS, Morgan J, et al. Effect of haemoglobin levels in haemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2002;58:1325–1335. doi: 10.1046/j.1523-1755.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 25.Arogundade FA, Olatunde LO, Ishola AA, jr, Bappa A, Sanusi AA, Akinsola A. PD (peritoneal dialysis) peritonitis: Still a major limiting factor in peritoneal dialysis management today. Afr J Nephrol. 2004;8:52–56. [Google Scholar]
- 26.Badmus TA, Arogundade FA, Sanusi AA, et al. Kidney transplantation in a developing economy: Challenges and initial report of three cases in a Nigerian Teaching Hospital. Cent Afr J Med. 2005;51:102–106. [PubMed] [Google Scholar]
- 27.Bamgboye EL. Haemodialysis as management problem in developing countries with Nigeria as a surrogate. Kidney Int. 2003;(Suppl 83):S93–S95. doi: 10.1046/j.1523-1755.63.s83.19.x. [DOI] [PubMed] [Google Scholar]
- 28.United States Renal Data System, author. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 29.UK Renal Registry Report. 2009. [23rd March 2010]. Accessed at http://www.renalreg.com/Reports/2009.html.
- 30.Forth Report of the Singapore Renal Registry Report. [23rd March 2010]. Accessed at http://www.nrdo.gov.sg/uploadedFiles/NRDO/Publications/SRR%20Report%202001%202002.pdf.
- 31.Persy VP, Remuzzi G, Perico N, et al. Prevention and Transplantation in Chronic Kidney Disease: What Is Achievable in Emerging Countries?. Meeting Report: Bamako Meeting December 46, 2008. Nephron Clin Pract. 2010;115(2):c122–c132. doi: 10.1159/000312875. [DOI] [PubMed] [Google Scholar]