Abstract
More than 300 million years ago, vertebrates emerged from the vast oceans to conquer gravity and the dry land. With this transition, new adaptations occurred that included ingenious changes in reproduction, waste secretion, and bone physiology. One new innovation, the egg shell, contained an ancestral protein (ovocleidin-116) that likely first appeared with the dinosaurs and was preserved through the theropod lineage in modern birds and reptiles. Ovocleidin-116 is an avian homolog of matrix extracellular phosphoglycoprotein (MEPE) and belongs to a group of proteins called short integrin-binding ligand-interacting glycoproteins (SIBLINGs). These proteins are all localized to a defined region on chromosome 5q in mice and chromosome 4q in humans. A unifying feature of SIBLING proteins is an acidic serine aspartate-rich MEPE-associated motif (ASARM). Recent research has shown that the ASARM motif and the released ASARM peptide have regulatory roles in mineralization (bone and teeth), phosphate regulation, vascularization, soft-tissue calcification, osteoclastogenesis, mechanotransduction, and fat energy metabolism. The MEPE ASARM motif and peptide are physiological substrates for PHEX, a zinc metalloendopeptidase. Defects in PHEX are responsible for X-linked hypophosphatemic rickets (HYP). There is evidence that PHEX interacts with another ASARM motif containing the SIBLING protein, dentin matrix protein-1 (DMP1). DMP1 mutations cause bone and renal defects that are identical with the defects caused by a loss of PHEX function. This results in autosomal recessive hypophosphatemic rickets (ARHR). In both HYP and ARHR, increased FGF23 expression plays a major role in the disease and in autosomal dominant hypophosphatemic rickets (ADHR), FGF23 half-life is increased by activating mutations. ASARM peptide administration in vitro and in vivo also induces increased FGF23 expression. FGF23 is a member of the fibroblast growth factor (FGF) family of cytokines, which surfaced 500 million years ago with the boney fish (i.e., teleosts) that do not contain SIBLING proteins. In terrestrial vertebrates, FGF23, like SIBLING proteins, is expressed in the osteocyte. The boney fish, however, are an-osteocytic, so a physiological bone-renal link with FGF23 and the SIBLINGs was cemented when life ventured from the oceans to the land during the Triassic period, approximately 300 million years ago. This link has been revealed by recent research that indicates a competitive displacement of a PHEX–DMP1 interaction by an ASARM peptide that leads to increased FGF23 expression. This review discusses the new discoveries that reveal a novel PHEX, DMP1, MEPE, ASARM peptide, and FGF23 bone-renal pathway. This pathway impacts not only bone formation, bone-renal mineralization, and renal phosphate homeostasis but also energy metabolism. The study of this new pathway is relevant for developing therapies for several diseases: bone–teeth mineral loss disorders, renal osteodystrophy, chronic kidney disease and bone mineralization disorders (CKD-MBD), end-stage renal diseases, ectopic arterial-calcification, cardiovascular disease renal calcification, diabetes, and obesity.
Keywords: MEPE, FGF23, DMP1, KLOTHO, Rickets, PHEX, rickets, osteomalacia, kidney disease
I. INTRODUCTION: A BRIEF HISTORICAL NARRATIVE
In 1856 the coffin of Princess Elizabeth (1635–1650), the second daughter of King Charles I, was discovered during the demolition of old St. Thomas’s Church at Newport, Isle of Wight, England. On detailed medical examination of her skeleton, it was clear that she suffered from a severe form of rickets. 1–3 The historical record suggests that her father King Charles I also suffered from this disease. Was the Princess’s illness inherited from her father, or was the disease dietary? The journey toward an answer to that question arguably began in 1650, the year that both the King and his daughter died. King Charles’s death was hastened after Oliver Cromwell injudiciously ordered the King’s execution by beheading. Of notable coincidence, that same year saw the first-ever documented medical monograph published in England by Glisson et al.4 This elegant monograph described rickets, the English disease that flourished in the nascent beginnings of the umbratic industrial revolution. In this early medical treatise, Glisson et al. described the clinical features of rickets “scientifically” but were not able to unravel the etiology of the disease. Nevertheless, Glisson’s early scientific studies lay the foundations for the discovery of key molecules and the causes of rickets (familial and dietary) 360 years later. These key molecules include 1,25 vitamin D3, PHEX, DMP1, FGF23, KLOTHO, PTH, and MEPE. Before this, researchers discovered the role of vitamin D (found in cod-liver oil) and its preconditional photoactivation by solar ultraviolet light.5,6
This review charts the exponential increase in our understanding of the roles of a new set of genes, proteins and hormones (FGF23, MEPE, DMP1, KLOTHO, PHEX, ASARM peptides) since the cloning of PHEX in 1995.7 An evidenced-based model (ASARM model) is also discussed. Many questions remain unanswered, but new molecular pathways have emerged that are highly complex. These pathways anastomose in ingeniously balanced ways that are designed to maintain a healthy skeleton, mineral homeostasis, and energy equilibrium.
II. PHEX, ITS PHYSIOLOGICAL SUBSTRATE (ASARM PEPTIDE), OSTEOMALACIA, AND HYPOPHOSPHATEMIC RICKETS
In 1995, the HYP consortium discovered a new gene PHEX (previously known as PEX, phosphate-regulating gene with homologies to endo-peptidases on the X chromosome) and identified its primary role in X-linked hypophosphatemic rickets (HYP also known as XLH).7–19 Following this event, matrix extracellular phosphoglycoprotein (MEPE) was cloned as a phosphaturic factor expressed from the tumor of a patient suffering from tumor-induced osteomalacia (TIO).19,20 Clinically, TIO shares overlapping pathophysiology with HYP. Loss of PHEX function causes defective mineralization, hypophosphatemia, and abnormal vitamin-D metabolism.21 PHEX is a zinc (Zn) metalloendopeptidase, and extensive studies have confirmed the protein binds with high specificity to MEPE, a bone-renal extracellular matrix-protein, and acidic serine aspartate-rich MEPE-associated (ASARM) peptide.21–24 MEPE has a motif (ASARM motif) located at the tip of the COOH terminus consisting of 23 residues enriched for aspartate, serine, and glutamate. The motif also occurs at the DMP1 COOH terminus (a related short integrin-binding ligand-interacting glycoprotein, i.e., SIBLING protein), but it is capped by an extra 33 residues.19,21,23 PHEX also binds to and hydrolyzes, with high affinity and specificity, phosphorylated and nonphosphorylated small ASARM peptides from MEPE and the related SIBLING protein osteopontin.21–27 The SIBLING motifs (ASARMs) and their potential role or roles were first described in the study in which MEPE was cloned from a tumor resected from a patient with tumor-induced osteomalacia (TIO).19 These ASARM peptides (~2.2 kDa) are essentially the released MEPE or SIBLING ASARM motif (DMP1, osteopontin, DSPP) and are the only known physiological substrates and/or ligands for PHEX.21,23,24,28,29 The ASARM peptide is otherwise resistant to proteolysis.11,19,21–24,29,30 This motif (ASARM), when released as a phosphorylated peptide (ASARM peptide), behaves like a biological bisphosphonate and inhibits mineralization and renal/intestinal phosphate uptake. 21,24,25,29,31–39 Indeed, like bisphosphonates and salivary–statherin,40–42 the ASARM peptide binds strongly to hydroxyapatite.21,23,25,32 Compelling evidence for the role of DMP1 (the closest relative to MEPE) in mineralization and phosphate regulation is the finding that DMP1-null mutations result in a phenotype identical with HYP. This newly characterized disease is called autosomal recessive hypophosphatemic rickets (ARHR).43,44 Null mutations in MEPE yield the following results: an opposite bone phenotype with an agedependent, increased, mineralization apposition rate (MAR); hastened mineralization in vitro; increased bone mass; increased trabecular volume; increased trabecular thickness/number; and increased cortical bone volume that is age dependent. 45 These findings are in agreement with several genome-wide studies in humans (males and females) that show strong correlations with volumetric bone mineral density (vBMD)46–49 and that confirm a major role for MEPE in osteocyte mechanotransductive response to load.50–55 Also, recent studies using MEPE transgenic mice show that MEPE and MEPE–PHEX interactions are a component of age- and diet-dependent pathways that regulate bone turnover, and suppress mineralization and renal calcification.26 This novel pathway also modulates bone-renal vascularization and bone turnover. In a separate study, ASARM peptides have been shown to be responsible for the in vitro mineralization defect in bone-marrow-stromal-cells (BMSCs) of HYP-mice.23 In these studies, a bioengineered, 4.2-kDa synthetic PHEX-peptide (SPR4) that specifically binds and neutralizes ASARM peptides was described. This peptide (SPR4) also corrects the mineralization defect in vitro and has marked effects on osteogenic and bone resorption markers.23,33 These discoveries (ASARM and SPR4 peptides) may help provide new strategies for treating select hypophosphatemic bone-mineralization disorders (HYP, ADHR, ARHR, and TIO) and for managing hyperphosphatemia in chronic kidney disease (CKD) and end-stage renal disease (ESRD).
Major progress has been made in understanding the molecular processes regulating bone formation remodeling and mineralization in disease and health.56,57 For example, the proteins of the PHEX, DMP1, FGF23, KLOTHO, and the MEPE/ASARM-peptide axis have emerged as novel and important regulators of phosphate homeostasis and bone-mineralization.57 More recent seminal and exciting discoveries have shifted the paradigm and have shown the skeleton to be a dynamic endocrine organ with major influence on regulating energy metabolism and fat mass.58 Accordingly, there is strong evidence that PHEX, DMP1, FGF23, and ASARM peptides are also involved in orchestrating cross talk between bone and energy metabolism.26,34,59
III. SIBLING PROTEINS, MEPE, DMP1 AND THE ASARM/DENTONIN MOTIFS
Human MEPE was first cloned in 2000 from a tumor resected from a patient with TIO.19 Later that year, rat MEPE was cloned;60 this was followed by the cloning of murine MEPE in 2001.61 The human-MEPE study reported by Rowe et al. in 2000 also mapped the MEPE gene to chromosome 4q and first described the close similarity of MEPE to a group of bone dentin non-collagenous matrix proteins (NCPs) that all clustered on chromosome 5q in mice and on 4q in humans (Figure 1).19 These proteins include DMP1, dentin sialo-phosphoprotein (DSPP), osteopontin (SPP1), bone sialoprotein (BSP), enamelin, and statherin. A key COOH terminal motif was discovered and named ASARM.19 Of note, DSPP is cleaved into three proteins, an N-terminal dentin sialoprotein (DSP),62,63 a COOH-terminal portion dentin phosphoprotein (DPP),64–66 and dentin glycoprotein (Dgp).67 The COOH-terminal DPP portion of this protein (DSPP) contains an RGD integrin binding sequence and a long “repeat” of the SIBLING ASARM motif (Figure 2B).19 Cleavage and release of the DPP (ASARM containing) portion of DSPP is catalyzed by a group of astacin Zn metallopeptidases that include BMP1 (tolloid), MEP1A, and MEP1B.68 These proteases are closely related to PHEX, the enzyme defective in HYP,7 a disease with increased levels of ASARM peptides.21,24,25,28,29,32–34 Specifically, BMP1, MEP1A, and MEP1B belong to the M12 family and the astacin subfamily of Zn metallopeptidases, and PHEX belongs to the M13 family and the MA clan of Zn metallopeptidases that also include the following (1) endothelin converting enzyme-1 (ECE-1α, ECE-Iβ, and ECE-II), (2) ECE-like enzyme/distress-induced neuronal endopeptidase (ECEL1/DINE), (3) soluble endopeptidase/NEP-like enzyme-1/neprilysin 2 (SEP/NL1/NEP2), (4) membrane metallo-endopeptidase-like 2 (MMEL2), and (5) Kell blood group protein antigen (KELL).21,69–72 Other shared genetic and structural features of the SIBLING proteins include the following: (1) a small non-translational first exon; (2) a start codon in the second exon; (3) the last exon, which contains a large coding segment (the number of exons varies among the different genes); (4) common exon–intron features; (5) an integrin-binding tripeptide Arg-Gly-Asp (RGD) motif that mediates cell attachment/signaling via interaction with cell surface integrins; (6) conserved phosphorylation and N-glycosylation sites; and (7) a strong signal peptide for extracellular release (Figures 3 and 4).19,21,73–75 A remarkable association to an ancestral mineralization gene statherin that also contains an ASARM motif and maps to the same region of chromosome 5 was confirmed in subsequent papers.21,31 Statherin, a small, 63-residue salivary protein, maintains the mineral solution dynamics of enamel through its ability to inhibit spontaneous precipitation and crystal growth from supersaturated solutions of calcium phosphate minerals.59–61 The role of statherins in preserving the calcium-phosphate-supersaturated state of saliva is crucial for recalcification and stabilization of tooth enamel and for the inhibition of mineral accretion on tooth surfaces. In addition, statherin has been proposed to function in the transport of calcium and phosphate during secretion in the salivary glands.59 As with the MEPE ASARM peptide, a single cathepsin-B site is present in statherin that would potentially release the highly charged and phosphorylated aspartate-serine rich statherin ASARM peptide. In recognition of these similarities, Fisher et al. coined the name SIBLING proteins (short integrin-binding ligand-interacting glycoproteins) as a family name for this group of unique proteins.74
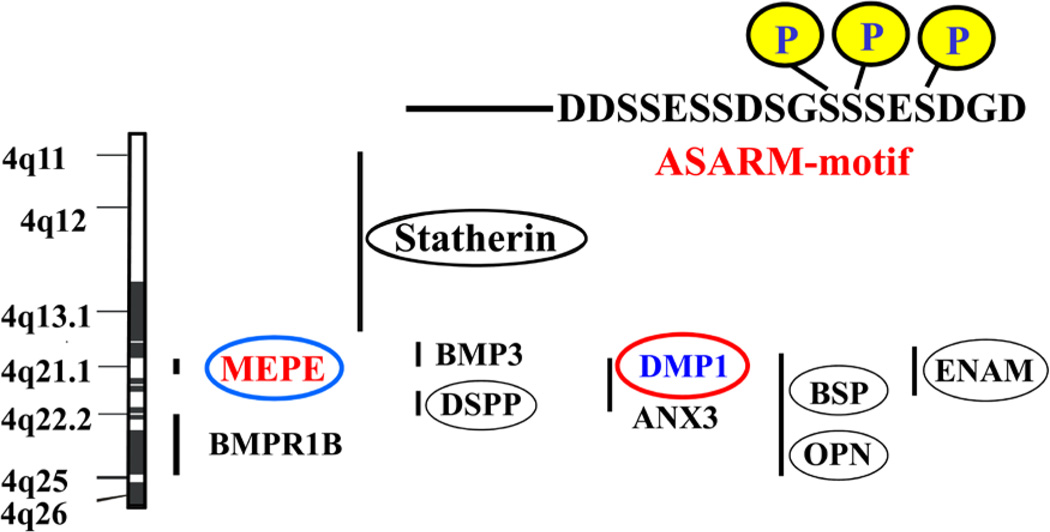
FIGURE 1.
Scheme showing the chromosomal locations of MEPE and SIBLING proteins, (Rowe et al 2000)19. All the SIBLINGs including MEPE map to the long arm of chromosome 4 (4q12) between markers D4S1534 and D4S3381 in humans and chromosome 5 in mice. Codes are translated as follows: (1) BMP3, bone morphogenetic protein 3; (2) DSPP, dentin sialo phospho protein; (3) OPN, osteopontin; (4) DMP-1, dentin matrix protein 1; (5) ANX, annexin; (6) BSP, integrin binding sialo protein/bone sialoprotein II; (7) BMPR1B, bone morphogenetic receptor protein 1B; 8, MEPE, matrix extracellular phosphoglycoprotein; (9) statherin, salivary statherin; (10) ENAM, enamelin. Human ASARM sequence is shown above the scheme with conserved casein kinase serine-phosphorylation sites. See also Figure 6 and 7 for illustrations of the casein kinase ASARM-conservation across species for DMP1 and MEPE.
FIGURE 2.
Scheme illustrating the sequence alignments and positions of the ASARM-peptide in MEPE, DMP1, DSPP, osteopontin (OPN), and statherin.19 The sequence similarity analysis was carried out using “sim” and Lalnview mathematical and software tools.190–192 In each computation the gap open penalty was set to 12, and the gap extension penalty was 4. Comparison matrix was set to BLOSUM62 with similarity a configured score of 70%. The highlighted and colored blocks shown on each protein scheme represent sequence percentage homologies that are color indexed on the similarity scale at the top of the figure. Notably, in MEPE versus DSPP (B) there are several repeated ASARM homologous blocks that extend across the COOH terminal dentin phosphoprotein (DPP) region of DSPP. This region also contains a single integrin binding RGD motif. The MEPE and DMP1 ASARM motif sequence (DDSSESSDSGSSSESDGD) is shown in Figures 6 and 7. For other SIBLING ASARM sequences, see Rowe et al. 200019 and other recent publications.25,32,39
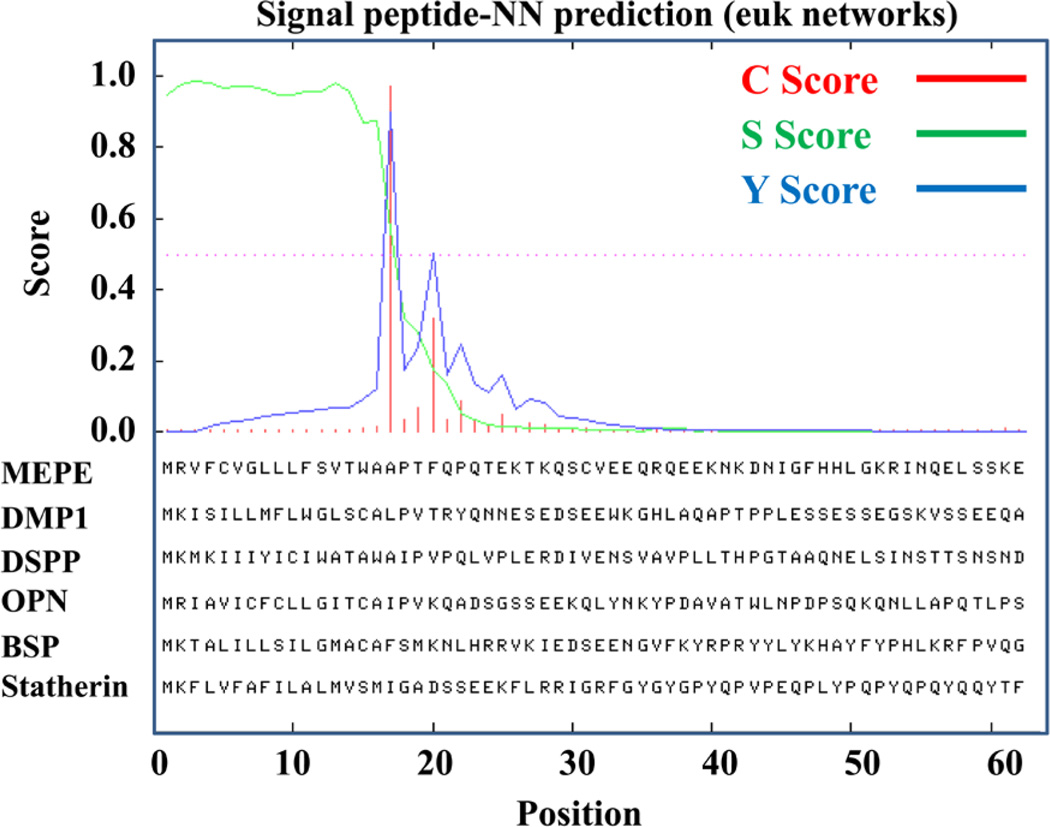
FIGURE 3.
Signal peptide analysis prediction using signal peptide-NN software confirming that all SIBLING proteins have strong signal-peptide motifs and are therefore secreted extracellular matrix proteins. Analysis was conducted using the SignalP 4.0 software193 and server at http://www.cbs.dtu.dk/services/SignalP/. Three scores (C, S, and Y) are provided as shown in the scheme. The red line shows the C-score or “cleavage site” score. The C-score is calculated for each sequence position. In all Siblings (MEPE, DMP1, DSPP, OPN, BSP, and statherin) a highly significant cleavage score (>0.8) was reported for the exact same sequence position. Y-max is a derivative of the C-score combined with the S-score resulting in a better cleavage site prediction than the raw C-score alone (blue peak). This is due to the fact that multiple high-peaking C-scores can be found in one sequence, where only one is the true cleavage site. The cleavage site is assigned from the Y-score where the slope of the S-score is steep and a significant C-score is found. The S-mean is the average of the S-score, ranging from the N-terminal amino acid to the amino acid assigned with the highest Y-max score, thus the S-mean score is calculated for the length of the predicted signal peptide. The calculated S-mean score for all SIBLINGs indicates strongly that all the SIBLINGs are secretory proteins.
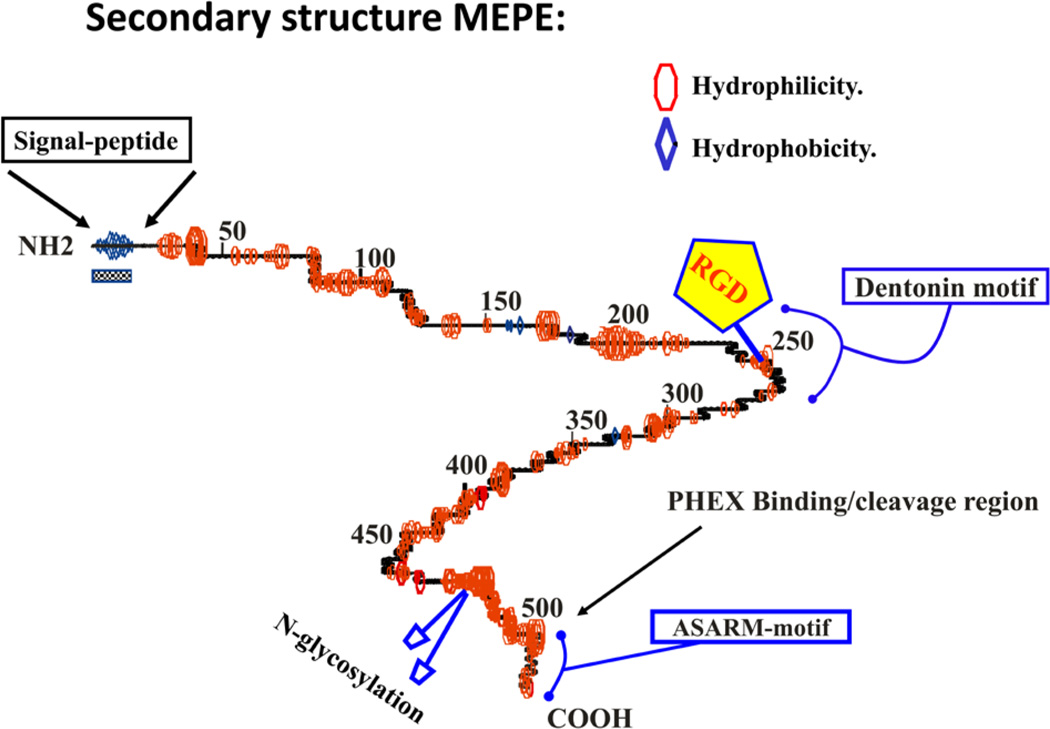
FIGURE 4.
Secondary structure prediction for MEPE as calculated using GCG peptide structure software19,194 (see also Accelrys computer platform software details at http://accelrys.com/products/). The primary amino acidbackbone is shown as a central line with curves indicating regions of predicted turn. Regions of hydrophilicity and hydrophobicity are represented as ellipsoids (red) and diamonds (blue) respectively. The RGD motif is highlighted with a pentagon. The N-glycosylation sites are represented as blue ellipsoids on stalks (C-terminus) and an alpha helix is indicated by undulating regions on the primary backbone. The signal peptide is indicated by a checkered box and coincides with a hydrophobic region at the N-terminus. The COOH-terminal ASARM motif is highlighted and the PHEX binding region is indicated.
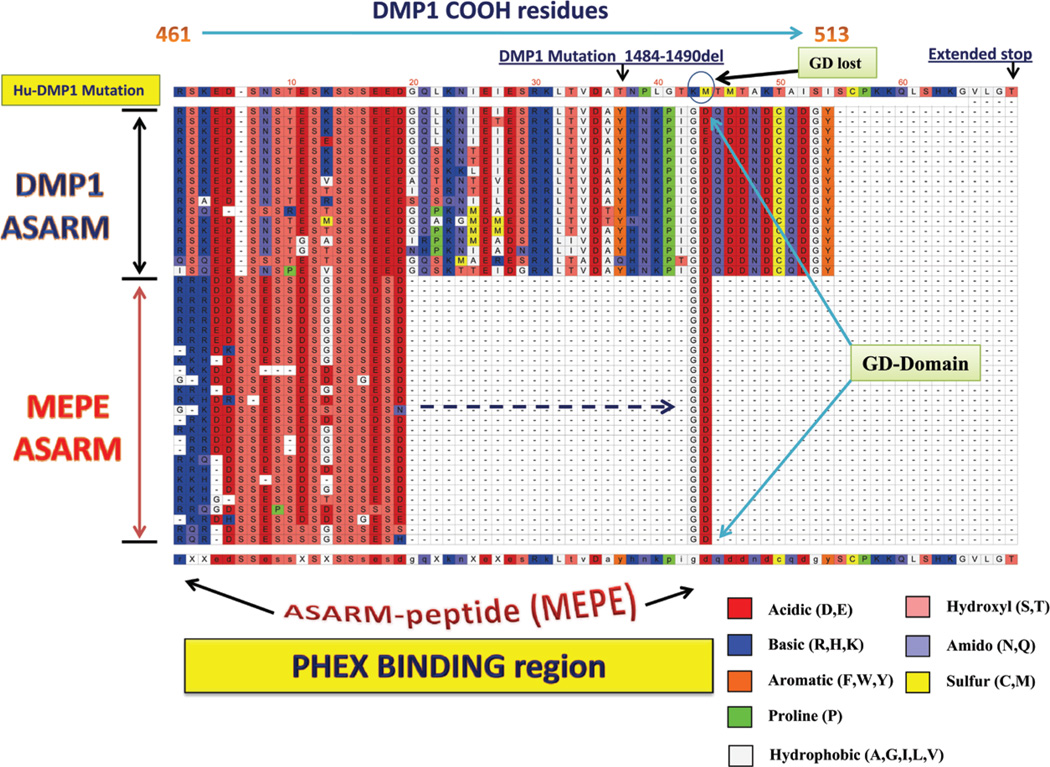
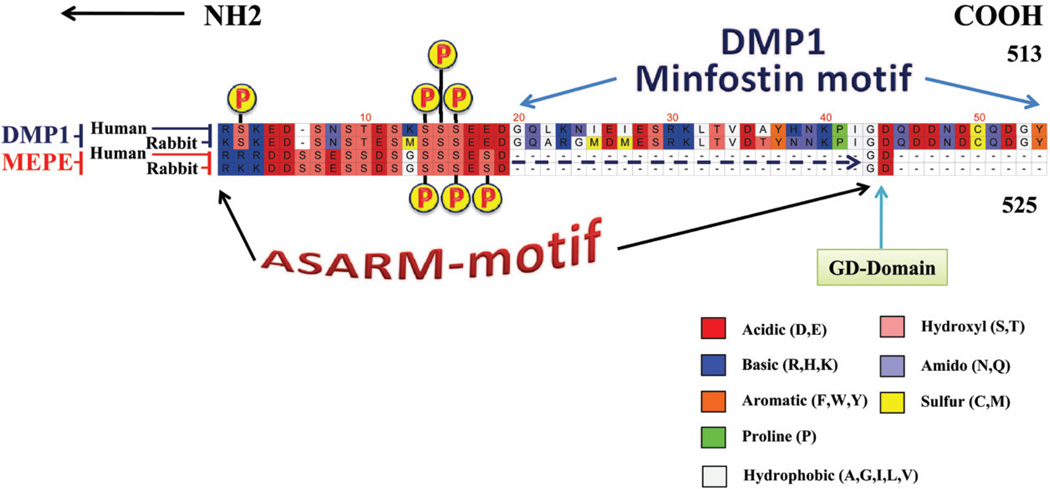
Two key motifs of MEPE, the ASARM motif and an Arg-Gly-Asp (RGD) integrin binding motif (also known as dentonin or AC100), are highly conserved across species.19,75,76 The dentonin motif contains two cell-matrix adhesion sequences (RGD and SGDG), and the PFSSGDGQ is almost immutable (Figure 5). Dentonin or AC100 has potent anabolic activity on osteoblast and odontoblasts pulp precursor cells in vitro, and these effects require the induction of COX-2.77–84. The ASARM motif is not only conserved in MEPE but also in other SIBLINGs; Figure 6 illustrates an alignment both the MEPE and DMP1 ASARM regions for different species. Figure 2 shows the position of the ASARM motif across the human SIBLING proteins as calculated using Lalnview software and alignment algorithms. 190–192 Of note, several conserved serines within the ASARM motif are substrates for casein kinase. As shown in Figure 6, a block of serines is invariant not only in MEPE but also in the DMP1 ASARM motif. This is further illustrated in Figure 7, which shows a comparison of DMP1 and MEPE ASARM motifs from two representative species (rabbit and human). The casein kinase serine residues that are substrates for phosphorylation are indicated in the figure. These phosphoserines are of key importance not only for interactions with hydroxyapatite but also for binding and hydrolysis with PHEX in both DMP1 and MEPE.19,23–25,32,39,85–87 Free ASARM peptide is a potent mineralization inhibitor in bone and teeth and suppresses renal calcification (in vivo and in vitro).23–26,31–33,39,86,88,89 The Gly-Asp (GD) domains (Figures 6 and 7) are “invariant” for DMP1 and MEPE across all species and have key roles in the kinetics of PHEX binding and hydrolysis. The ASARM motif in DMP1 is capped by 35 residues and is also highly conserved in DMP1 across species. This region is labeled as the minfostin motif because a frame-shift mutation that alters this region (i.e., DMP1 minfostinmotif) results in ARHR.43,44,90 The DMP1 minfostin region is thus required to foster or promote mineralization.23,34Also, this frame-shift mutation results in the loss of the GD residues that are conserved in both MEPE and DMP1 and that play a key role in the binding kinetics and hydrolysis of PHEX (Figures 6 and 7).23,24
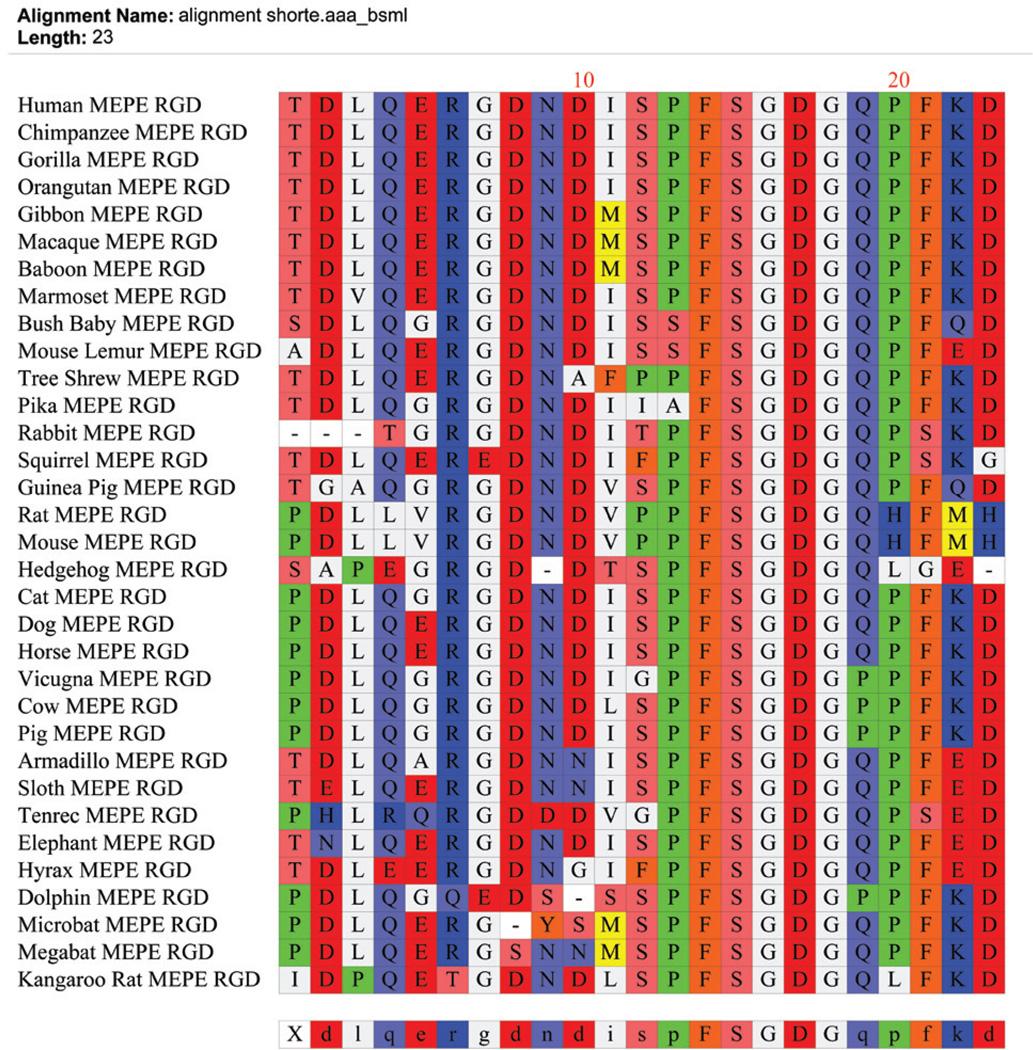
FIGURE 5.
The MEPE RGD integrin binding region (dentonin or AC100) is highly conserved across species as illustrated in the clustalW alignment. There is complete conservation of a sequence (FSGDG) N-terminal to the RGD region and the consensus sequence RGDNDISPFSGDGQ is highly conserved. Species sequences for MEPE were searched for and downloaded from the “Ensembl” project resource at http://www.ensembl.org. The dentonin/AC100 peptide is a strong stimulator of bone/teeth formation in vitro and in vivo.77,78,80–83,195–197 This contrasts with the ASARM peptide, an inhibitor of mineralization in vitro and in vivo (minhibin).23–26, 29,31–33,39,86,88,89
FIGURE 6.
DMP1 ASARM region (COOH residues 464 to 478) shows strong homology to MEPE ASARM peptide (across species) and the free ASARM peptide likely competes for PHEX binding.34 Flanking this region is a sequence also conserved in DMP1 (minfostin motif) and mutations here result in ARHR.43,90 Since MEPE and osteopontin ASARM peptides bind specifically to PHEX,23–25,27,32,34 we would propose the DMP1 ASARM motif also interacts with PHEX protein. Thus, free ASARM peptides likely compete with PHEX for DMP1 binding as described in Figure 9. The ASARM region (DMP1 and MEPE) consists of serine residues interspersed with acidic aspartate (D) and glutamic acid (E) residues. The serine residues are phosphorylated and the SXSSSE(S/D) conserved sequence of DMP1 and ASARM contains identical consensus casein-kinase II phosphorylation sites (see also Figure 7). Of relevance, a highly conserved GD sequence occurs in both MEPE-ASARM peptide and DMP1-ASARM motif as highlighted in the alignment. The GD sequence resides in the DMP1 minfostin region and likely plays an important role in PHEX binding. A DMP1 frame-shift mutation in this minfostin region that alters the COOH-terminal sequence at position 498 following residues LTVDA (with loss of the GD domain and an increase of 13 residues) results in ARHR.11,12 The species alignments (top to bottom) for DMP1 are human, chimpanzee, gibbon, macaque, cat, elephant, tarsier, cow, dolphin, tenrec, kangaroo, rabbit, squirrel, mouse, rat, hedgehog, and vicuna. The species alignments for MEPE (top to bottom) are human, chimpanzee, orangutan, gorilla, gibbon, macaque, rhesus monkey, marmoset, cow, tree shrew, sloth, alpaca, rock hyrax, tarsier, elephant, rabbit, squirrel, ground squirrel, cat, dog, dolphin, pig, microbat, hedgehog, kangaroo, rat and mouse. The above species sequences for MEPE and DMP1 were searched for and downloaded from the “Ensembl” project resource at http://www.ensembl.org.
FIGURE 7.
DMP1 ASARM region (COOH residues 464 to 478) shows strong homology to MEPE ASARM peptide (across species) and the free ASARM-peptide likely competes for PHEX binding.34 The ASARM region in both DMP1 and MEPE also contain casein kinase II serine phosphorylation sites as depicted in the scheme. These phosphoserine sites are highly conserved between DMP1 and MEPE and across species. The clustal W alignment compares DMP1 and MEPE and two representative species (human and rabbit).
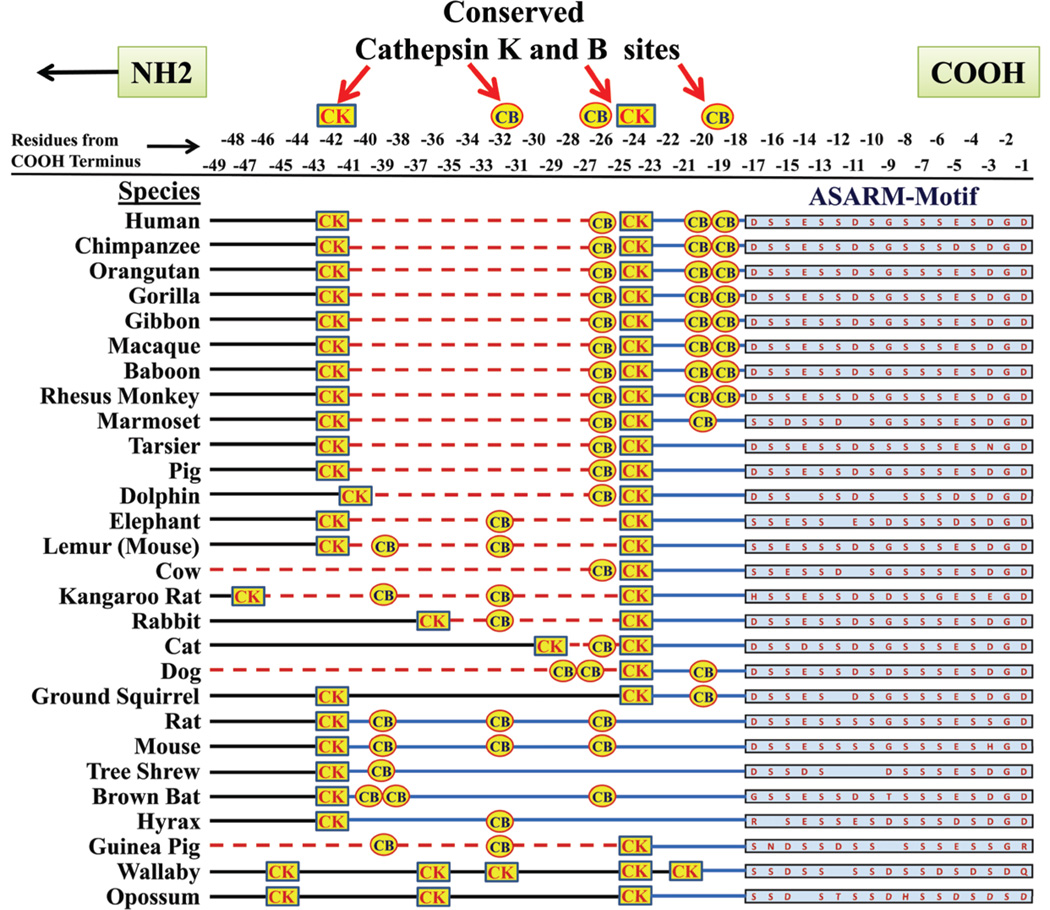
Another feature of the ASARM region in MEPE is the high degree of conservation of cathepsin K and cathepsin B protease sites that are N-terminal to and juxtaposed to the ASARM motif (Figure 8). Release of the ASARM motif by cysteine proteases as a protease-resistant ASARM peptide likely has a major role in mineralization as well as energy metabolism.23–25,27,30,32–34 The biological relevance of these cleavage sites is discussed in more detail in the following sections.
FIGURE 8.
Cleavage sites for cysteine proteases (cathepsin K and B) that are N-terminal and in close proximity to the MEPE ASARM motif are highly conserved as shown in the alignment. The ASARM motif and peptide sequence is resistant to proteases (except for PHEX). The ASARM peptide is the only known physiological substrate for PHEX. Species sequences for MEPE were searched for and downloaded from the “Ensembl” project resource at http://www.ensembl.org.
IV. ASARM PEPTIDES AND RENAL CALCIFICATION
Because MEPE ASARM peptides are mineralization inhibitors,22–24,26,31,32,86 their presence in urine26,34,91 may help suppress renal calcification. Indeed, transgenic mice overexpressing MEPE (MEPE tgn) are resistant to diet-induced renal calcification.26 In these mice, the urinary calcium phosphate (CaPO4) product correlates positively with urinary ASARM peptides. This is accompanied by a suppressed dietary renal calcification. Also, in normal mice, urinary ASARM peptides are significantly higher in mice fed high-phosphate diets.34 Intriguingly, null-mutant, Na-dependent, phosphate co-transporter (NPT2a−/−) mice92–94 are hypophosphatemic with increased 1,25 vitamin D3 and massive renal stones.93 Secondary ablation of the 1-α-hydroxylase gene resulting in a doublenull mutant (NPT2a−/−/1-α−/−) corrects the renalstone defect.95 Because MEPE expression (protein and mRNA) is suppressed by 1,25 vitamin D3,26,31,34,61 the increased size and frequency of renal stones in the NPT2a−/− mice may well have been precipitated by an increased urinary CaPO4 product and exacerbated by low urinary MEPE ASARM peptides. Further studies are required to confirm this.
MEPE also has a significant effect on neovascularization and likely mediates this by interacting with αvβ3 integrin through the MEPE RGD miotif.26 Specifically, renal-bone angiogenesis and vascular endothelial growth factor (VEGF) secretion is increased in transgenic mice overexpressing MEPE (MEPE trg).26 Coincident with increased vascularization, urinary aldosterone excretion is increased with hypokalemia and normal urinary output. Aldosterone exerts powerful effects on blood vessels, independent of blood-pressure rise mediated via regulation of the salt and water balance.96 Specifically, aldosterone increases neovascularization through an angiotensin II (AngII)-dependent pathway with commensurate increase in VEGF expression.97 Also, aldosterone inhibits vascular reactivity and tone as calculated by intravital video-microscopic measurement of vessel diameter.98 Of further relevance, activation of the renin–angiotensin–aldosterone system (RAAS) in mice markedly influences osteoclastogenesis also independent of hypertension.99 Indeed, AngII, a component of the RAAS system, increases aldosterone expression that stimulates receptor activator of nuclear factor κB ligand (RANKL) expression in osteoblasts.100 The changes in MEPE tgn renal vascularization may also combine with the factors discussed previously, contributing to the reduced susceptibility to renal calcification. Of relevance, recent studies have confirmed that in chronic kidney disease and mineralization bone disorders (CKD-MBD) there is cross talk between the RAAS and the vitamin-D, FGF23 klotho pathway. 101
V. THE ASARM MODEL, BONE-RENAL MINERALIZATION, AND PHOSPHATE HOMEOSTASIS
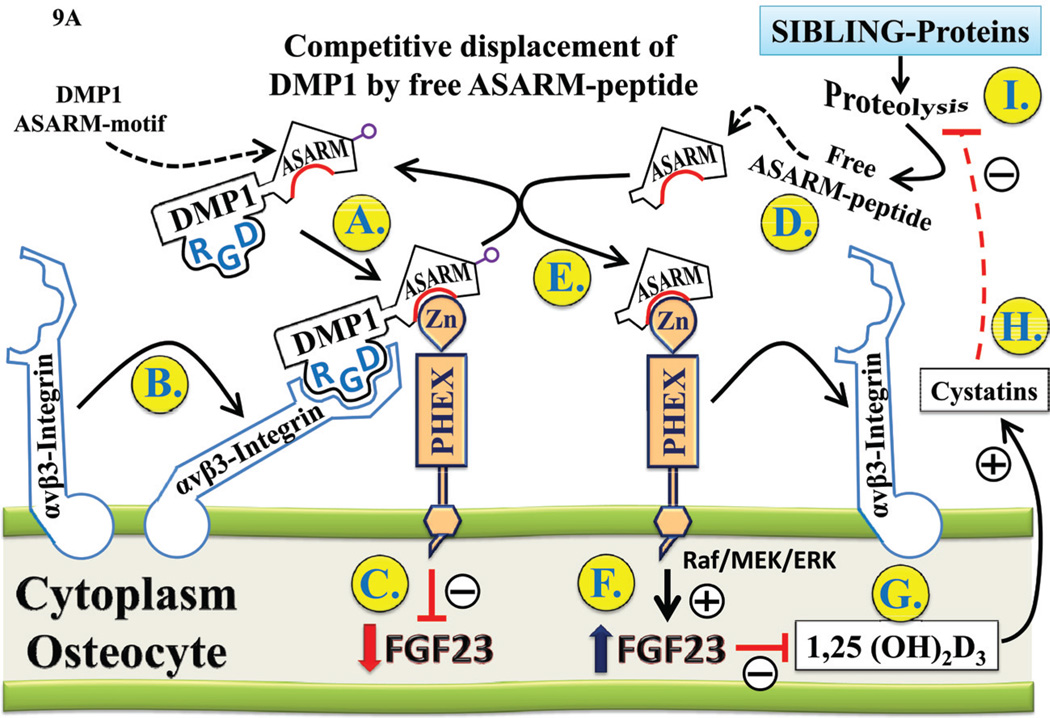
It is clear that PHEX regulates (directly or indirectly) FGF23 expression and or stability because the loss of PHEX activity leads to increased FGF23 expression.57 Loss of DMP1 (a SIBLING protein) has the same effect as loss of PHEX function; notably, increased FGF23 expression and ARHR.43,44 Both HYP (PHEX defect)7 and ARHR (DMP1 defect)43,44 have increased ASARM peptides in circulation, bone, and teeth where they inhibit mineralization and have component roles in the hypophosphatemia.23,24,28,29,33,34,102 Thus, inactivation of PHEX or DMP1 could “logically” either inactivate or activate a mineralization inhibitor and phosphate regulator.”In this regard, the experimental evidence suggests that a PHEX–DMP1 interaction is responsible for locally orchestrating mineralization and phosphate homeostasis. Figure 9A–C provides schemes illustrating component parts of the ASARM-model that incorporate this fact. The following text is labeled in alphabetic sequence and contains a detailed and evidence-based description for each of the corresponding labels in the diagrams:
FIGURE 9.
A: ASARM displacement of the PHEX-DMP1-integrin complex regulates FGF23 expression (see text for detailed discussion of the scheme). The highlighted and encircled letters in the diagram link to the discussion in subsection-V1 titled “ASARM displacement of PHEX-DMP1-integrin complex regulates FGF23”.
B: ASARM-peptide regulation of bone mineral and renal phosphate regulation through FGF23 and 1,25 Vit D3 (see text for detailed discussion of the scheme). The highlighted and encircled letters in the diagram link to the discussion in subsection titled: “V(2) ASARM & bone-renal PO4 regulation through FGF23 & 1,25 VitD3”.
C: The ASARM pathway and the processing of BMP1 and DMP1 by SPC2 convertase and its co-activator 7B2 (see text for detailed discussion of the scheme). The highlighted and encircled letters in the diagram link to the discussion in subsection titled: “V(3) The ASARM pathway: processing of BMP1 & DMP1 by SPC2 & 7B2”.
A. ASARM displacement of PHEX-DMP1-integrin complex regulates FGF23
As illustrated in Figure 9A, PHEX, DMP1, and cell-surface αvβ3 integrin, when bound, are proposed to “co-activate” a pathway that leads to suppression of FGF23 expression and/or decreased FGF23 stability.21,23,34,103 Also, competitive displacement of DMP1 by ASARM-peptides modulates the PHEX–DMP1-mediated FGF23 expression, and this may have an important role in energy metabolism and vascularization and may contribute to the cross talk between bone and energy metabolism.34 In overview, the experimental data supports the following global hypotheses: (1) PHEX binding to DMP1 via the DMP1–ASARM motif leads to decreased active FGF23. (2) ASARM peptide competitive displacement of DMP1–PHEX interaction increases FGF23 activity. (3) ASARM peptide–PHEX interactions further modulate fat mass and bone-renal vascularization. (4) ASARM peptides influence glucose and insulin metabolism (phosphate and vitamin D-dependent). The bold letters (A to I) in Figure 9A sequentially highlight the key points of the pathway:
(A) PHEX (a Zn metalloendopeptidase) is proposed to interact with DMP1 by binding to the DMP1–ASARM motif adjacent to and N-terminal to the DMP1 ‘minfostin’ motif.34 The DMP1 min-fostin-motif23 refers to a region that when mutated results in Autosomal Recessive Hypophosphatemic Rickets (ARHR)43,44,90 and is thus required to foster or promote mineralization (Figure 6 and 7).23, 34 Note that a DMP1 frame-shift mutation (Figure 6) results in the loss of the GD residues that are conserved in both MEPE and DMP1 and have key roles in the binding kinetics and hydrolysis of PHEX to the ASARM region.23,24 Recent experiments further support the notion that FGF23 is regulated through a PHEX–DMP1 common pathway involving FGF-receptor (FGFR) signaling.103 This was done by comparing phenotypes of compound- and single-mutant DMP1 and PHEX mice.
(B) DMP1 also contains an RGD motif that interacts with αvβ3 integrin and stimulates phosphorylation of focal adhesion kinase (FAK), leading to downstream activation of the MAPK pathway.104
(C) The PHEX–DMP1–integrin cell-surface complex may be involved in suppressing FGF23 expression and possibly increases FGF23 protein degradation through 7B2 coactivation of SPC2 proprotein convertase as proposed by Drezner et al. (discussed further in section VC: Figure 9C).105,106 Thus, in HYP and ARHR, mutations in PHEX and DMP1 respectively result in hypophosphatemia through increased FGF23 expression and stability. There is precedent for this result because PHEX binds with high affinity and specificity to ASARM peptides (MEPE- and osteopontin-derived) and MEPE protein.23–25,32,33 PHEX also cleaves ASARM peptides, the only known physiological substrate for PHEX,23–25,27,32 and SIBLINGs (like DMP1) activate PHEX-related Zn matrix metalloproteinases (MMPs) by direct binding interactions that also involve cell-surface integrins.104,107–111 DMP1, for example, binds and activates a PHEX-related Zn matrix metalloproteinase 9 (MMP9)108,110 and signals through cell-surface interactions with αvβ3 integrin in human mesenchymal cells and osteoblast-like cells.104 Also, as discussed earlier, DSPP (a SIBLING protein) is cleaved into three proteins: DSP,62,63 DPP,64–66 Dgp.67 Like DMP1, the DPP protein fragment of DSPP contains a SIBLING RGD motif and a COOH-terminal ASARM motif. In DPP, the ASARM motif is repeated multiple times (Figure 2). Of relevance to this motif sequence and alignment similarity, recent experiments have confirmed that DPP (like DMP1) binds to cell-surface integrins via the RGD-motif and activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells and dental cells.112 The cell-surface binding generates intracellular signals that are channeled along cytoskeletal filaments and activates the non-receptor tyrosine kinase FAK, which plays a key role in signaling at sites of cellular adhesion.112 This is the same signaling pathway activated by DMP1 cellsurface integrin binding in human mesenchymal cells and osteoblast-like cells.104 Because DMP1 and DSPP both contain RGD and ASARM motifs and PHEX is expressed in the cell lines investigated in these studies,23,113–115 this result also supports a cell-surface PHEX–DMP1–integrin axis for signal transduction through a focal adhesion kinase (FAK) and downstream MAPK pathways, namely ERK and JNK.104,112 Moreover, the in vitro addition of recombinant DMP1 to UMR-106 cells causes a dose-dependent decrease in FGF23 expression (mRNA and protein),116 and FGF23 upregulates Dmp1 expression in MLO-Y4 cells (a well-established murine osteocyte cell line) in the presence of KLOTHO.
Notably, Narayanan et al. attribute “dual” functionality to DMP1. Specifically, they propose that DMP1 is both a “nuclear” transcriptional coactivator and acts as an extracellular matrix orchestrator of mineralization.117 However, as indicated previously, recent research suggests that DMP1 and DPP both signal on the cell surface through integrin interactions. 104,112 Also, a more recent report that used DMP-null mice (DMP1-KO) found no rescue of DMP1-KO mice by the targeted re-expression of artificially localized nuclear DMP1 (nlsDMP1) in osteoblast/osteocytes.118 Yuan et al., in agreement with previous studies,104,112 concluded that DMP1 is not a “nuclear co-transcription factor” but an extracellular matrix protein.118 Clearly, DMP1 may have dual functions (nuclear and extracellular), and the extracellular signaling appears well established (consistent with the ASARM model).
(D) Specific cleavage of MEPE and/or other bone SIBLING proteins (DSPP, OPN, DMP1, etc.) generates free protease-resistant ASARM peptides. 23,28,29,33,34,102 In HYP, MEPE and several bone proteases, including cathepsins, ECEL1/DINE, and NEP, are markedly increased,23,29,119–121 resulting in excess ASARM peptide production and inhibition of mineralization.23,28,29,32,102
(E) Increased ASARM peptides are proposed to competitively displace the DMP1–PHEX complex in “normal mice” by forming a high-affinity/high-specificity PHEX–ASARM complex23,24,27 that is slowly hydrolyzed (low Kcat/Km) by PHEX.25,27,32 Loss of PHEX and DMP1 in HYP and ARHR, respectively, results in “ASARM independent” constitutive overexpression and increased stability of FGF23.
(F) The competitive displacement of DMP1 by ASARM peptide(s) in normal mice results in increased FGF23 expression. This is supported by in vivo and in vitro murine experiments using ASARM peptides with bolus, osmotic-pump infusion, perfusion, renal/intestinal micropuncture, ex vivo cell-culture experiments with transgenic FGF23 green-fluorescence-protein (GFP) promoter reporters, and transgenic mice models.22,23,31,34–37 Additional compelling support for this hypothesis comes from in vitro observations showing that MEPE/PHEX mRNA and protein ratios are excellent indicators of mineralization progression.122 Specifically, the MEPE/PHEX ratio is low when osteoblasts are actively differentiating to the mineralization stage and high when the mineralization stage is reached. At the late mineralization stage the osteocyte is thus presumed to release ASARM peptide to maintain a hypomineralized space around the osteocytic lacuna via a MEPE–ASARM–peptide– BMP2 pathway.122 Also, ASARM peptides, MEPE and DMP1 are key players in regulating the osteocyte-mediated mechanotransductive response to load, bone formation, and osteoclastogenesis.23,26,50–53,55,77,122–126
(G) Increased FGF23 results in decreased serum 1,25(OH)2D3 by the well-documented alteration of renal 1-α-hydroxylase and 24-hydroxylase expression and activities.127
(H) 1,25(OH)2D3 regulates several proteases and protease inhibitors in different cell types, including bone.128–131 Cystatins, for example, are strong inhibitors of the cathepsin protease family (e.g., cathepsins B, D, and K) and 1,25(OH)2D3 is a potent stimulator of cystatin expression.128 Also, cystatin C stimulates differentiation of mouse osteoblastic cells, bone formation, and mineralization in vitro and ex vivo, consistent with a suppression of cathepsin-mediated release of ASARM peptide.131 As depicted schematically in Figure 8, the cathepsin B and K regions N-terminal to and adjacent to the ASARM motif are highly conserved. 21,23,24,29,31 Recent phylogenetic analyses of MEPE have confirmed that this region is also under positive selection.75,76
(I) Thus, FGF23 suppression of 1,25(OH)2D3 in diseases with increased FGF23 levels may be partly responsible for the markedly increased levels of osteoblastic proteases in HYP and ADHR.23,26,29,119,120,132 In turn (as shown in part D of the figure), the increased osteoblastic-protease activity is likely responsible for the increased proteolytic release of protease-resistant ASARM peptides from SIBLING proteins, including MEPE and DMP1.21,23,24,28,29,33,34,102 Notably, classic experiments show that treatment of HYP and ARHR with phosphate supplements does not correct the endosteal mineralization defect but does partially correct the growth defect.133–135 Co-supplementation with 1,25(OH)2D3 is required to impact the mineralization defect, but this treatment is still not completely satisfactory because it results in increased FGF23 production (vicious cycle).135 The partial correction of the mineralization defect by 1,25(OH)2D3 supplementation is thus consistent with the ASARM model. This is because the 1,25(OH)2D3 dietary supplements may help reduce free ASARM peptide production by inhibiting extracellular matrix proteases (cathepsins, for example) and suppressing MEPE expression. In addition, in vivo administration of cathepsin protease inhibitors pepstatin and CAO74 partially corrects the mineralization defect in HYP mice.29 This sequence of events leads to a coordinated feedback loop involving 1,25(OH)2D3, PHEX, DMP1, FGF23, and ASARM peptides as illustrated in further detail in Figure 9B.
B. ASARM & bone-renal PO4 regulation through FGF23 & 1,25 VitD3
The physiological loop described in Figure 9A also impacts bone mineralization and renal phosphate regulation as illustrated in Figure 9B.
(A–B) Specifically, accumulation of ASARM-peptides inhibits mineralization and coordinately inhibits Na+-dependent phosphate uptake in the kidney as shown in vitro and in vivo.24,26,31,34–37,136 The pathophysiological ASARM pathway inhibits renal phosphate uptake independent of FGF23 and exacerbates the FGF23-induced hypophosphate-mia found in familial HYP, ARHR and ADHR.21,34 ASARM peptides are acidic, phosphorylated and highly charged, with low isolectric points (pI). They are extraordinarily resistant to a wide range of proteases and have physicochemical similarities to bisphosphonates, phosphonoformic acid (PFA), and phosphonoacetic acid (PAA). They also share biological properties in vivo and in vitro with bisphosphonates, PFA, and PAA in that they all inhibit mineralization and interfere with renal phosphate handling and vitamin D metabolism. 23,26,31,34–37,137–149 Thus in this abnormal context, ASARM peptides likely have direct but component roles in the hypophosphatemic pathology2.3,24,26,28,29,31–38 Specifically, in HYP and ARHR, FGF23 is the chief hypophosphatemic stimulus, and ASARM peptides exacerbate the renal phosphate leak by virtue of their overabundance and intrinsic hypophosphatemic and physicochemical properties.24,31,34–38 In further support of the secondary ASARM inhibition of phosphate uptake in the renal proximal tubules of the kidney, in 2004 Baum et al. observed phosphatonin washout in HYP mice renal proximal tubules.150 In these elegant experiments, evidence for a secondary posttranscriptional defect in HYP mice that complemented the down-regulation of Na-dependent phosphate cotransporters (NPT2a) in mRNA expression was provided. Baum et al. concluded that a secondary posttranscriptional mechanism regulates renal phosphate uptake, but they were unable to define the factor(s) involved. Specifically, Baum et al. observed a temporal normalization of the phosphatetransport defect in HYP proximal tubule cells perfused in vitro (independent of protein synthesis) that was consistent with phosphatonin washout. Similar findings were reported independently by researchers using immortalized HYP renal proximal cell lines.151,152 Thus, ASARM peptides may also inhibit phosphate transport by directly binding to the phosphate transporter as demonstrated with PFA and etidronate138,139,143–146,149,153,154 and in turn exacerbating the effects of FGF23 on NPT2 transcription.34
(C–E) As shown, free ASARM peptides competitively displace the DMP1–PHEX–integrin complex.
(F–I) This results in up-regulation of FGF23 expression, down-regulation of 1,25(OH)2D3, and inhibition of renal phosphate uptake or hypophosphatemia. 22–24,31,34,61
(J) The decrease in 1,25(OH)2D3 also increases expression of a 100-kDa transcription factor (100 kDa-TRP) that is required for PHEX expression.155,156
(K) This results in increased PHEX expression that fulfills a classic feedback loop involving FGF23, DMP1, PHEX and 1,25(OH)2D3. Specifically, increased PHEX favors increased hydrolysis of ASARM peptides and increased PHEX–DMP1 binding. This leads to reduced FGF23 expression and increased 1,25(OH)2D3. Increased 1,25(OH)2D3 results in reduced PHEX expression and decreased PHEX–DMP1 binding that leads to increased FGF23 and suppression of 1,25(OH)2D3 synthesis. The reduced 1,25(OH)2D3 results in increased protease and MEPE expression, and thus increased ASARM peptide production, and this feeds back to reduce FGF23 expression by modulating DMP1 processing and FGF23 stability and possibly by directly suppressing FGF23 expression via 1,25(OH)2D3, as illustrated in Figure 9C.
C. The ASARM pathway: processing of BMP1 & DMP1 by SPC2 & 7B2
The ASARM pathway is also regulated by the processing of DMP1 by BMP1, and BMP1 is in turn processed by 7B2-activated SPC2 proproteinconvertase.157,158 FGF23 is also proposed to be processed by 7B2-activated SPC2.105,106
(A–D) As discussed previously, competitive displacement of the DMP1–PHEX–Integrin complex results in increased expression of FGF23.
(E) Recent exciting developments indicate that the PHEX–DMP1 axis may also coordinately regulate expression of 7B2.105,106 (F) 7B2 protein is a coactivator of SPC2 proprotein convertase, and in HYP mice there is constitutively decreased 7B2 expression, presumably due to a loss of PHEX. Cell-surface PHEX is required for normal 7B2 expression, and this is likely coactivated by the PHEX–DMP1–integrin complex. In HYP, absence of cell-surface PHEX results in constitutive overexpression of FGF23 because the PHEX–DMP1–integrin complex is required to suppress FGF23 and activate 7B2. Thus, in HYP and ADHR, loss or suppression of 7B2 leads to increased stability of FGF23 (reduced 7B2 and thus SPC2 proproteinconvertase activation).105,106 Therefore, in HYP mice there is increased FGF23 expression and stability and consequential bone-renal pathology. In normal mice, the 7B2–PHEX–DMP1–FGF23 axis coordinates a feedback loop to exquisitely regulate bone-renal metabolism.
Specifically, in normal mice the PHEX– DMP1–integrin complex results in increased expression of 7B2 and decreased expression of FGF23.
(F–J) Protein 7B2 coactivates SPC2 that in turn cleaves and inactivates FGF23. SPC2 activation also cleaves a protease “pro-BMP1” (also called tolloid, a member of the astacin Zn metal-loendopeptidase family) that in turn cleaves pro-DMP1 into an active form (57 kDa) and an inactive form (37 kDa).157,158 Of relevance, experiments by Feng et al. show conclusively that the 57-kDa BMP1 cleaved form of DMP1 is indeed the active form. Specifically, transgenic overexpression and reintroduction of 57-kDa DMP1 in DMP1-null mice almost completely correct the bone-renal phenotype.125
(K) The activated 57-kDa DMP1 then binds to PHEX and integrin, further suppressing FGF23 expression. This is coordinated in turn by an increase in 1,25(OH)2D3 consequential to the reduced FGF23. A recent finding shows that the regulatory loop is closed by strong BMP2 that stimulates MEPE expression.122 Thus, the competitive displacement of DMP1 by ASARM peptides provides an additional “bone” fine-tuning of FGF23 and bone-renal phosphate mineralization.
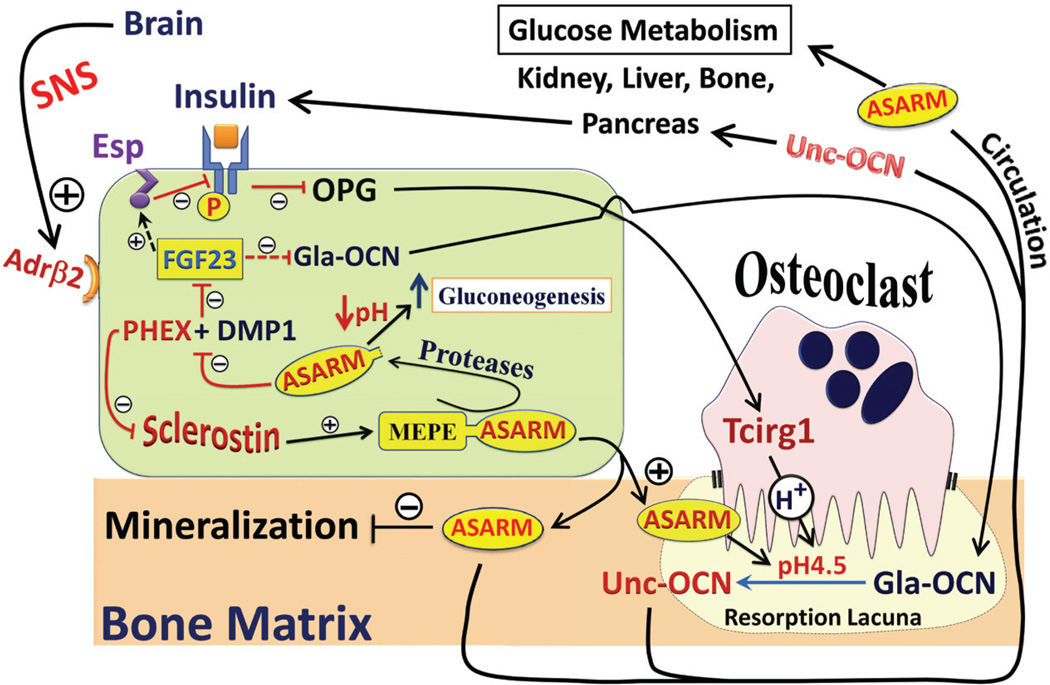
VI. PHEX, DMP1, ASARM REGULATES FGF23 AND MODULATES ENERGY METABOLISM
Recent seminal discoveries have revealed an integrated relationship between bone and energy metabolism.58,159 Leptin, an adipocyte hormone-regulating appetite, influences osteoblast function and bone formation peripherally and centrally.159,160 In turn, osteocalcin, a bone hormone produced by osteoblasts, influences energy metabolism.159–161_ Recent studies show that serotonin has a major role in regulating bone remodeling through contrasting pathways.159,160 These pathways originate from the duodenum (via LRP5), the brain (via Leptin), and bone (via LRP5).159 Notably, the duodenal serotonin pathway is currently controversial.162–164 Osteoblasts and osteocytes have key roles in glucose homeostasis, and they mediate insulin signaling in pancreatic β cells, hepatocytes, and adipocytes.165,166 The surface area of the lacuno-canalicular Haversian complex is vast, containing large numbers of osteocytes. Relevant to this, osteocytic expression of FGF23, DMP1, and MEPE is high (mRNA and protein).26,51,53,122,167 Thus, this new microcosmic endocrine system undoubtedly plays a major role in disease and health. Several studies confirm overexpression or infusion of ASARM peptides or MEPE results in major changes in fat mass, energy metabolism, vascularization, and soft-tissue metastatic calcification (renal).26,34,168 Also, these changes are PHEX-, phosphate-, and vitamin D-dependent. These findings are consistent with several independent studies showing that HYP mice are hypoinsulinemic and hyperglycemic with increased gluconeogenesis in bone, liver, and kidney.151,152,169–176 The reverse is the case for FGF23-null mice (hypoglycemic).177 Klotho (an FGF23 coactivator), and or vitamin D likely have direct or indirect roles.177–181 Indeed, earlier seminal studies by Avioli et al. showed that the HYP osteoblast has a markedly higher rate of gluconeogenesis.174 These studies not only confirmed that osteoblasts are capable of glucose production but also clearly showed that the HYP osteoblast has decreased intracellular pH. The acidic intracellular milieu was proposed to be the chief reason for the increased gluconeogenesis, but the cause of the decreased pH was unknown. Our studies show that increased acidic ASARM peptide production (pH 3 in solution) is chiefly responsible for the altered pH and increased gluconeogenesis.26 Also, acidic ASARM-peptides impact osteocalcin expression26,34,168 and may activate osteocalcin by acidic γ-de-carboxylation (Figure 10). Osteocalcin is a key osteoblast-derived protein that links bone and energy metabolism by regulating insulin secretion, insulin sensitivity, and energy expenditure (Figure 10).58 The changes in gluconeogenesis in HYP, vitamin D-receptor-null mice (VDR−/−) and FGF23-null mice occur in bone,172–174 liver,175 and kidney.151,152,169–172,176 The kidney is of nearly equal importance to the liver in glucose homeostasis, supplying >40% of glucose output.182,183 Of relevance, specific expression of MEPE and acidic ASARM peptides occurs in the proximal convoluted tubules, and acidosis also increases renal and bone gluconeogenesis.26,28,34,91,174,183 Extraosseous infusion, bolus, or micropunture administration of MEPE or MEPE-derived ASARM peptides in vivo and in vitro induces hypophosphatemia and inhibits intestinal and renal, Na-dependent, phosphate cotransport. 31,34–38 Also, hypophosphatemic rodents or humans develop hyperglycemia with reduced insulin secretion and sensitivity.184–186 Moreover, our data confirms that this accompanies an increase in circulating ASARM peptides in mice.26,34 Consistent with this, increased glucose-6-phosphatase activity (a gluconeogenic enzyme) occurs in rats fed with PI-deficient diets.187,188 These PI-diet-restricted animals are also hypophosphatemic, hypoinsulinemic, and hyperglycemic (35% increase).187,188 Furthermore, clinical hypophosphatemia is associated with impaired glucose tolerance and insulin resistance.185,186 Also, in type 2 diabetes patients subjected to a 4-h euglycemic–hyperinsulinemic clamp show major increases in FGF23 that correlate positively with insulin infusion.189
FIGURE 10.
Competitive displacement of DMP1 by ASARM peptide modulates PHEX–DMP1-mediated FGF23 expression (see central osteoblast in green). Specifically, DMP1 and PHEX interact and signal a down regulation of FGF23 as discussed in the text. ASARM disrupts this binding (PHEX + DMP1), resulting in an up-regulation of FGF23 signaling as discussed in Figure 9. This in turn leads to major changes in bone mineralization, bone turnover, vitamin D metabolism with hypophosphatemia. Recent evidence indicates that this also impacts fat energy metabolism pathways as depicted in the scheme and discussed in the text. Profound changes in fat mass, weight, glucose metabolism, insulin sensitivity, leptin levels, serotonin levels, sympathetic tone, aldosterone levels, vascularization, and sympathetic tone, occur in mice with defects in PHEX, DMP1, FGF23, MEPE, and ASARM expression. 26,34,171,174–178,180,185,187,198–201 Index: SNS, sympathetic nervous system; Adrβ2, Osteoblast β2 adrenergic receptors (responsive to epinephrine/norepinephrine); Gla-OCN, γ-carboxylated osteocalcin (inactive form); Unc-OCN, γ-de-carboxylated osteocalcin (active form); Esp, Gene for OST-PTP (tyrosine phosphatase) that phosphorylates and inactivates the insulin receptor. This directly/indirectly results in reduced active osteocalcin (reduced γ-decarboxylation); Tcirg1, Osteoclast proton (H+) pump. Increases resorption lacuna acidity (acidic ASARM-peptides likely contribute) and thereby increases active osteocalcin (Unc-OCN) by acidic γ-decarboxylation; ASARM, Acidic Serine Aspartate Rich MEPE associated peptide or motif.
Mice overexpressing MEPE, ASARM peptides, or infused ASARM peptides using osmotic pumps provide further support for a SIBLING, FGF23, PHEX energy metabolism link.16,26,34 Intriguingly, as well as changes in mineralization, bone formation and phosphate regulation, these mice develop increased fat mass, leptin, adiponectin, osteocalcin, and sympathetic tone. The mice are also hyperglycemic, with changes in circulating insulin, serotonin, VEGF, and urinary aldosterone. These changes are more profound in mutant-MEPE and ASARM-overexpressing transgenic mice (MEPE-trg and ASARM-trg) or ASARM-infused mice fed a low-phosphate and vitamin D3-deficient diet. Figure 10 highlights the areas impacted by changes in DMP1, PHEX, ASARM, and FGF23. Notably, the key hormones from adipocytes, osteoblasts, kidney (adrenal glands), and liver are altered as indicated in the scheme. We previously reported an increase in urinary aldosterone and VEGF for our MEPE-transgenic mice and showed that this accompanied increased vascularization in bone and kidney.26 Notably, aldosterone suppresses vascular glucose-6-phosphatedehydrogenase activity (glycolysis), and this leads to reduced vascular reactivity.98 Also, through the renin-aldosterone-angiotensin pathway (RAAS), aldosterone increases osteoclastogenesis by increasing RANKL production.99,100 Consistent with this, we have shown that aldosterone is increased in MEPE-tgn mice with increased osteoclast progenitor cells.26 Of relevance, recent studies confirm that in chronic kidney disease there is cross talk between the RAAS and the vitamin-D–FGF23– KLOTHO pathway.101
VII. CONCLUSION
The skeleton and in particular the osteocytes of the bone lacuno-canalicular Haversian complex are endocrine systems exquisitely attuned to communicating with other organs (e.g., gut, brain, kidney, liver, and pancreas). A new group of key osteocyte-expressed proteins (FGF23, DMP1, PHEX, MEPE, and ASARM peptides) link to form a new hormonal internuncial pathway that integrates bone turnover, bone-renal mineralization, bone-teeth mechanical loading, renal mineral homeostasis, mineralization, and vascularization. The SIBLING proteins DMP1 and MEPE play a coordinate role in this pathway and share an ASARM motif. This motif likely appeared 300 million years ago in ancestral bone–egg proteins coincident with the vertebrate conquest of the terrestrial environment. Nature parsimoniously used the properties of this peptide and motif to both transduce and suppress FGF23 signaling. The FGF23 signaling is primarily orchestrated by competitive displacement of a DMP1–ASARM motif and PHEX interaction by free ASARM peptide. Therapies for several diseases may result from the study of this new pathway. These diseases include bone-teeth mineral loss disorders, renal osteodystrophy, renal transplantation, chronic and end-stage renal diseases, ectopic arterial calcification, renal calcification, diabetes, and obesity.
ACKNOWLEDGMENTS
The author acknowledges with gratitude the financial support and awards from the National Institute of Arthritis and Musculoskeletal and Skin Diseases National Institutes of Health (NIH, NIAMS: Grant No. 5R01AR051598).
ABBREVIATIONS
- 1, 25(OH)2D3
1, 25-dihydroxycholecalciferol
- ADHR
autosomal dominant hypophosphatemic rickets
- ARHR
autosomal recessive hypophosphatemic rickets
- ASARM
acidic serine aspartate-rich MEPE associated motif
- BMSC
bone marrow stromal cells
- BSP
bone sialoproptein
- CKD-MBD
chronic kidney disease and mineralization bone disorders
- Dgp
dentin glyco protein
- DMP1
dentin matrix protein 1
- DPP
dentin phosphoprotein
- DSP
dentin sialo protein
- DSPP
dentinsialophosphoprotein
- ESRD
end-stage renal disease
- FEP
fractional excretion of phosphate
- FGF-23
fibroblast growth factor 23
- HYP
X-linked hypophosphatemic rickets
- MEPE
matrix extracellular phosphoglycoprotein
- NPT2a, -b, or -c
sodium dependent phosphate co-transporters
- OCN
osteocalcin
- OPG
osteoprotegerin
- OPN
osteopontin
- PHEX
phosphate-regulating gene with homologies to endopeptidases on the X chromosome
- PTH
parathyroid hormone 1–34
- RAAS
renin-angiotensin-aldosterone system
- RANKL
receptor activator for nuclear factor κB ligand
- SIBLING
short integrin-binding ligand-interacting glycoproteins
- TIO
tumor-induced osteomalacia
- VEGF
vascular endothelial growth factor
- WT
wild-type mice
REFERENCES
- 1.Burland C. An historic case of rickets: being an account of the medical examination of the remains of Princess Elizabeth, daughter of King Charles I. who died at Carrisbrooke Castle, September 8, 1650. Practitioner. 1918;100:391–395. [Google Scholar]
- 2.TEC J. Princess Elizabeth, second daughter of King Charles 1, and rickets. Pediatrics. 1979;64(2):241. [Google Scholar]
- 3.O’Riordan JL. Rickets in the 17th century. J Bone Miner Res. 2006;21(10):1506–1510. doi: 10.1359/jbmr.060703. [DOI] [PubMed] [Google Scholar]
- 4.Glisson F, Bate G, Regemorter A. A treatise of the rickets: being a disease common to children. London: Peter Cole, Cornhill Royal Exchange (Bedford Royal Infirmary Medical Library); p. 1651. [Google Scholar]
- 5.Rafter GW. Elmer McCollum and the disappearance of rickets. Perspect Biol Med. 1987;30(4):527–534. doi: 10.1353/pbm.1987.0005. [DOI] [PubMed] [Google Scholar]
- 6.Palm T. The geographical distribution and aetiology of rickets. Practitioner. 1890;45(270–279):321–342. [Google Scholar]
- 7.HYP-consortium. Francis F, Hennig S, Korn b, Reinhardt R, de Jong D, Poustka A, Lehrach H, Rowe PSN, Goulding JN, Summerfield T, Mountford RC, Read AP, Popowska E, Pronicka E, Davies KE, O’Riordan JLH, Econs MJ, Nesbitt T, Drezner MK, Oudet C, Pannetier S, Hanauer A, Strom TM, Meindl A, Lorenz B, Cagnoli M, Mohnike KL, Murken J, Meitinger T. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11(2):130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 8.Econs MJ, Francis F, Rowe PSN, Speer MC, O’Riordan JLH, Lehrach H, Becker PA. Dinucleotide repeat polymorphism at the DXS1683 locus. Hum Mol Genet. 1994;3(4):680. doi: 10.1093/hmg/3.4.680. [DOI] [PubMed] [Google Scholar]
- 9.Francis F, Strom TM, Hennig S, Boeddrich A, Lorenz B, Brandau O, Mohnike KL, Cagnoli M, Steffens C, Klages S, Borzym K, Pohl T, Oudet C, Econs MJ, Rowe PSN, Reinhardt R, Meitinger T, Lehrach H. Genomic organisation of the human PEX gene mutated in X-linked dominant hypophosphataemic rickets. Gen Res. 1997;7(6):573–585. doi: 10.1101/gr.7.6.573. [DOI] [PubMed] [Google Scholar]
- 10.Rowe PSN. Molecular biology of hypophosphataemic rickets and oncogenic osteomalacia. Hum Genet. 1994;94(5):457–467. doi: 10.1007/BF00211008. [DOI] [PubMed] [Google Scholar]
- 11.Rowe PSN. The role of the PHEX gene (PEX), in families with X-linked hypophosphataemic rickets. Curr Opin Nephrol Hyperten. 1998;7(4):367–376. doi: 10.1097/00041552-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rowe PSN. Finding mutations in disease genes. In: Econs MJ, editor. Genetics of osteoporosis and metabolic bone disease. 1 ed. Totowa, NJ: Humana Press; 2000. pp. 431–446. [Google Scholar]
- 13.Rowe PSN, Francis F, Goulding J. Rapid isolation of DNA sequences flanking microsatellite repeats. Nucleic Acids Res. 1994;22(23):5135–5136. doi: 10.1093/nar/22.23.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe PSN, Goulding J, Read A, Lehrach H, Francis F, Hanauer A, Oudet C, Biancalana V, Kooh SW, Davies KE, Oriordan JLH. Refining the genetic-map for the region flanking the X-linked hypophosphatemic rickets locus (Xp22.1-22.2) Hum Genet. 1994;93(3):291–294. doi: 10.1007/BF00212025. [DOI] [PubMed] [Google Scholar]
- 15.Rowe PSN, Goulding J, Read AP, Mountford RC, Hanauer A, Oudet C, Whyte MP, Meier-Ewert S, Lehrach H, Davies KE, O’Riordan JLH. New markers for linkage analysis of hypophosphataemic rickets. Hum Genet. 1993;91:571–575. doi: 10.1007/BF00205082. [DOI] [PubMed] [Google Scholar]
- 16.Rowe PSN, Goulding JN, Francis F, Oudet C, Econs MJ, Hanauer A, Lehrach H, Read AP, Mountford RC, Summerfield T, Weissenbach J, Fraser W, Drezner MK, Davies KE, Oriordan JLH. The gene for X-linked hypophosphataemic rickets maps to a 200–300 kb region in Xp22.1, and is located on a single YAC containing a putative vitamin D response element (VDRE) Hum Genet. 1996;97(3):345–352. doi: 10.1007/BF02185769. [DOI] [PubMed] [Google Scholar]
- 17.Rowe PSN, Oudet C, Francis F, Sinding C, Pannetier S, Econs MJ, Strom TM, Meitinger T, Garabedian M, David A, Macher M-A, Questiaux E, Popowska E, Pronicka E, Read AP, Mokrzycki A, Glorieux FH, Drezner MK, Hanauer A, Lehrach H, Goulding J, O’Riordan JLH. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP) Hum Mol Genet. 1997;6:539–549. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]
- 18.Rowe PSN, Read AP, Mountford RC, Benham F, Kruse TA, Camarino G, Davies KE, O’Riordan JLH. Three DNA markers for hypophosphataemic rickets. Hum Genet. 1992;89:539–542. doi: 10.1007/BF00219180. [DOI] [PubMed] [Google Scholar]
- 19.Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67(1):54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 20.Rowe PSN, Ong A, Cockerill F, Goulding J, Hewison M. Candidate 56 and 58 kDa protein(s) responsible for mediating the renal defects in oncogenic hypophosphataemic osteomalacia. Bone. 1996;18(2):159–169. doi: 10.1016/8756-3282(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 21.Rowe PSN. The wrickkened-pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15(5):264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD. Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192(1):261–267. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (min-hibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149(4):1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe PSN, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE. Surface Plasmon Resonance (SPR) confirms MEPE binds to PHEX via the MEPE-ASARM-motif: A model for impaired mineralization in X-linked rickets (HYP) Bone. 2005;36(1):33–46. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addison W, Masica D, Gray J, McKee MD. phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res. 2009;25(4):695–705. doi: 10.1359/jbmr.090832. [DOI] [PubMed] [Google Scholar]
- 26.David V, Martin A, Hedge AM, Rowe PS. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology. 2009;150(9):4012–4023. doi: 10.1210/en.2009-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos M, Couture C, Hirata IY, Juliano MA, Loisel TP, Crine P, Juliano L, Boileau G, Carmona AK. Human recombinant endopeptidase PHEX has a strict S1’ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem J. 2003;373(1):271–279. doi: 10.1042/BJ20030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bresler D, Bruder J, Mohnike KL, Fraser D, Rowe PSN. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–R9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe PS, Matsumoto N, Jo OD, Shih RN, Oconnor J, Roudier MP, Bain S, Liu S, Harrison J, Yanagawa N. Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone. 2006;39(4):773–786. doi: 10.1016/j.bone.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo R, Rowe PS, Liu S, Simpson LG, Xiao ZS, Quarles LD. Inhibition of MEPE cleavage by PHEX. Biochem Biophys Res Commun. 2002;297(1):38–45. doi: 10.1016/s0006-291x(02)02125-3. [DOI] [PubMed] [Google Scholar]
- 31.Rowe PSN, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34(2):303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Addison W, Nakano Y, Loisel T, Crine P, McKee M. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite—an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23(10):1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 33.Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM. Sclerostin is a locally acting regulator of late-osteoblast/pre-osteocyte differentiation and regulates mineralization through a MEPE-ASARM dependent mechanism. J Bone Miner Res. 2011;27(7):1425–1436. doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.David V, Martin AC, Hedge AM, Drezner MK, Rowe PS. ASARM peptides: PHEX-dependent and independent regulation of serum phosphate. Am J Physiol Renal Physiol. 2011;300(3):F783–F791. doi: 10.1152/ajprenal.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobbie H, Unwin RJ, Faria NJ, Shirley DG. Matrix extracellular phosphoglycoprotein causes phosphaturia in rats by inhibiting tubular phosphate reabsorption. Nephrol Dial Transplant. 2008;23(2):730–733. doi: 10.1093/ndt/gfm535. [DOI] [PubMed] [Google Scholar]
- 36.Marks J, Churchill LJ, Edward SD, Unwin R. The phosphatonin matrix extracellular phosphoglycoprotein (MEPE) inhibits renal intestinal phosphate transport in vivo. J Am Soc Nephrol. 2008;19(12):2313–2320. doi: 10.1681/ASN.2008030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirley DG, Faria NJ, Unwin RJ, Dobbie H. Direct micropuncture evidence that matrix extracellular phosphoglycoprotein inhibits proximal tubular phosphate reabsorption. Nephrol Dial Transplant. 2010;25(10):3191–3195. doi: 10.1093/ndt/gfq263. [DOI] [PubMed] [Google Scholar]
- 38.Friedlander G. Welcome to MEPE in the renal proximal tubule. Nephrol Dial Transplant. 2010;25(10):3135–3136. doi: 10.1093/ndt/gfq516. [DOI] [PubMed] [Google Scholar]
- 39.Hoyer JR, Asplin JR, Otvos L. Phosphorylated osteopontin peptides suppress crystallization by inhibiting the growth of calcium oxalate crystals. Kidney Int. 2001;60(1):77–82. doi: 10.1046/j.1523-1755.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 40.Francis MD, Russell RG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science. 1969;165(899):1264–1266. doi: 10.1126/science.165.3899.1264. [DOI] [PubMed] [Google Scholar]
- 41.Goobes R, Goobes G, Shaw WJ, Drobny GP, Campbell CT, Stayton PS. Thermodynamic roles of basic amino acids in statherin recognition of hydroxyapatite. Biochemistry. 2007;46(16):4725–4733. doi: 10.1021/bi602345a. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger DH, Hay DI. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977;252(5):1689–1695. [PubMed] [Google Scholar]
- 43.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38(11):1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278(3):1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 46.Zmuda JM, Yerges-Armstrong LM, Moffett SP, Klei L, Kammerer CM, Roeder K, Cauley JA, Kuipers A, Ensrud KE, Nestlerode CS, Hoffman AR, Lewis CE, Lang TF, Barrett-Connor E, Ferrell RE, Orwoll ES. Genetic analysis of vertebral trabecular bone density and cross-sectional area in older men. Osteoporosis international. 2011;22(4):1079–1090. doi: 10.1007/s00198-010-1296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jemtland R, Holden M, Reppe S, Olstad OK, Reinholt FP, Gautvik VT, Refvem H, Frigessi A, Houston B, Gautvik KM. Molecular disease map of bone characterizing the postmenopausal osteoporosis phenotype. J Bone Miner Res. 2011;26(8):1793–1801. doi: 10.1002/jbmr.396. [DOI] [PubMed] [Google Scholar]
- 48.Styrkarsdottir U, Halldorsson BV, Gudbjartsson DF, Tang NL, Koh JM, Xiao SM, Kwok TC, Kim GS, Chan JC, Cherny S, Lee SH, Kwok A, Ho S, Gretarsdottir S, Kostic JP, Palsson ST, Sigurdsson G, Sham PC, Kim BJ, Kung AW, Kim SY, Woo J, Leung PC, Kong A, Thorsteinsdottir U, Stefansson K. European bone mineral density loci are also associated with BMD in East-Asian populations. PLoS One. 2010;5(10):e13217. doi: 10.1371/journal.pone.0013217. PMCID: 2951352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcified Tissue Int. 2010;87(5):461–468. doi: 10.1007/s00223-010-9407-7. PMCID: 2964475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gluhak-Heinrich J, Pavlin D, Yang W, MacDougall M, Harris SE. MEPE expression in osteocytes during orthodontic tooth movement. Archiv Oral Biol. 2007;52(7):684–690. doi: 10.1016/j.archoralbio.2006.12.010. PMCID: 1868431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashibara T, Hiraga T, Sugita A, Wang L, Hata K, Ooshima T, Yoneda T. Regulation of osteoclast differentiation and function by phosphate: potential role of osteoclasts in the skeletal abnormalities in hypophosphatemic conditions. J Bone Miner Res. 2007;22(11):1743–1751. doi: 10.1359/jbmr.070709. [DOI] [PubMed] [Google Scholar]
- 53.Harris SE, Gluhak-Heinrich J, Harris MA, Yang W, Bonewald LF, Riha D, Rowe PS, Robling AG, Turner CH, Feng JQ, McKee MD, Nicollela D. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 2007;7(4):313–315. [PMC free article] [PubMed] [Google Scholar]
- 54.Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I, Miyashita H, Yamada T, Matsukawa N, Matsumoto M, Morimoto S, Ogihara T, Ochi T, Yoshikawa H. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab. 2004;22(3):176–184. doi: 10.1007/s00774-003-0468-9. [DOI] [PubMed] [Google Scholar]
- 55.Amir LR, Jovanovic A, Perdijk FB, Toyosawa S, Everts V, Bronckers AL. Immunolocalization of SIBLING and RUNX2 proteins during vertical distraction osteogenesis in the human mandible. J Histochem Cytochem. 2007;11:11. doi: 10.1369/jhc.6A7162.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab. 2009;20(5):230–236. doi: 10.1016/j.tem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Kiela PR, Ghishan FK. Recent advances in the renal-skeletal-gut axis that controls phosphate homeostasis. Lab Invest. 2009;89(1):7–14. doi: 10.1038/labinvest.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310(1–2):21–29. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David V, Martin A, Hedge AM, Rowe PS. PHEX & MEPE ASARM-motif regulate a novel bone-renal and fat-mass pathway (Abstract) J Bone Miner Res. 2009;24(Suppl 1) Abstract available from: http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=d79d078c-e7b7-48de-ab35-83b08139efdd. [Google Scholar]
- 60.Petersen DN, Tkalcevic GT, Mansolf AL, Rivera-Gonzalez R, Brown TA. Identification of osteoblast/osteocyte factor 45 (OF45), a bone-specific cDNA encoding an RGD-containing protein that is highly expressed in osteoblasts and osteocytes. J Biol Chem. 2000;275(46):36172–36180. doi: 10.1074/jbc.M003622200. [DOI] [PubMed] [Google Scholar]
- 61.Argiro L, Desbarats M, Glorieux FH, Ecarot B. MEPE, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74(3):342–351. doi: 10.1006/geno.2001.6553. [DOI] [PubMed] [Google Scholar]
- 62.Butler WT, Bhown M, Brunn JC, D’Souza RN, Farach-Carson MC, Happonen RP, Schrohenloher RE, Seyer JM, Somerman MJ, Foster RA, Tomana M, Van Dijk S. Isolation, characterization and immunolocalization of a 53-kDal dentin sialoprotein (DSP) Matrix. 1992;12(5):343–351. doi: 10.1016/s0934-8832(11)80030-2. [DOI] [PubMed] [Google Scholar]
- 63.Ritchie HH, Hou H, Veis A, Butler WT. Cloning and sequence determination of rat dentin sialoprotein, a novel dentin protein. J Biol Chem. 1994;269(5):3698–3702. [PubMed] [Google Scholar]
- 64.Linde A, Bhown M, Butler WT. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980;255(12):5931–5942. [PubMed] [Google Scholar]
- 65.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 66.Ritchie HH, Wang LH. Sequence determination of an extremely acidic rat dentin phosphoprotein. J Biol Chem. 1996;271(36):21695–21698. doi: 10.1074/jbc.271.36.21695. [DOI] [PubMed] [Google Scholar]
- 67.Yamakoshi Y, Hu JC, Fukae M, Zhang H, Simmer JP. Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J Biol Chem. 2005;280(17):17472–17479. doi: 10.1074/jbc.M413220200. [DOI] [PubMed] [Google Scholar]
- 68.Tsuchiya S, Simmer JP, Hu JC, Richardson AS, Yamakoshi F, Yamakoshi Y. Astacin proteases cleave dentin sialophosphoprotein (DSPP) to generate dentin phosphoprotein (Dpp) J Bone Min Res. 2011;26(1):220–228. doi: 10.1002/jbmr.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bianchetti L, Oudet C, Poch O. M13 endopeptidases: new conserved motifs correlated with structure, and simultaneous phylogenetic occurrence of PHEX and the bony fish. Proteins. 2002;47(4):481–488. doi: 10.1002/prot.10075. [DOI] [PubMed] [Google Scholar]
- 70.Rawlings ND, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 1999;27:325–331. doi: 10.1093/nar/27.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turner AJ. Exploring the structure and function of zinc metallopeptidases: old enzymes and new discoveries. Biochem Soc Trans. 2003;31(Pt 3):723–727. doi: 10.1042/bst0310723. [DOI] [PubMed] [Google Scholar]
- 72.Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23(3):261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 73.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(Suppl 1):33–40. [PubMed] [Google Scholar]
- 74.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280(2):460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 75.Machado JP, Johnson WE, O’Brien SJ, Vasconcelos V, Antunes A. Adaptive evolution of the Matrix Extracellular Phosphoglycoprotein in mammals. BMC Evol Biol. 2011;11(1):342. doi: 10.1186/1471-2148-11-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bardet C, Delgado S, Sire JY. MEPE evolution in mammals reveals regions and residues of prime functional importance. Cell Mol Life Sci. 2010;67(2):305. doi: 10.1007/s00018-009-0185-1. 2–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang HG, Kawashima N, Iwata T, Xu J, Takahashi S, Sugiyama T, Suda H. MEPE activated by furin promotes pulpal cell adhesion. J Dent Res. 2011;90(4):529–534. doi: 10.1177/0022034510391057. [DOI] [PubMed] [Google Scholar]
- 78.Sprowson AP, McCaskie AW, Birch MA. ASARM-truncated MEPE and AC-100 enhance osteogenesis by promoting osteoprogenitor adhesion. J Orthop Res. 2008;26(9):1256–1262. doi: 10.1002/jor.20606. [DOI] [PubMed] [Google Scholar]
- 79.Gaucher C, Boukpessi T, Septier D, Jehan F, Rowe PS, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin noncollagenous matrix proteins in familial hypophosphatemic rickets. Cells Tissues Organs. 2009;189(1–4):219–223. doi: 10.1159/000151382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagel DE, Khosla S, Sanyal A, Rosen DM, Kumagai Y, Riggs BL. A fragment of the hypophosphatemic factor, MEPE, requires inducible cyclooxygenase-2 to exert potent anabolic effects on normal human marrow osteoblast precursors. J Cell Biochem. 2004;93(6):1107–1114. doi: 10.1002/jcb.20249. [DOI] [PubMed] [Google Scholar]
- 81.Goldberg M, Lacerda-Pinheiro S, Jegat N, Six N, Septier D, Priam F, Bonnefoix M, Tompkins K, Chardin H, Denbesten P, Veis A, Poliard A. The impact of bioactive molecules to stimulate tooth repair and regeneration as part of restorative dentistry. Dent Clin North Am. 2006;50(2):277–298. doi: 10.1016/j.cden.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 82.Liu H, Li W, Gao C, Kumagai Y, Blacher RW, DenBesten PK. Dentonin, a fragment of MEPE, enhanced dental pulp stem cell proliferation. J Dent Res. 2004;83(6):496–499. doi: 10.1177/154405910408300612. [DOI] [PubMed] [Google Scholar]
- 83.Six N, Septier D, Chaussain-Miller C, Blacher R, Den-Besten P, Goldberg M. Dentonin, a MEPE fragment, initiates pulp-healing response to injury. J Dent Res. 2007;86(8):780–785. doi: 10.1177/154405910708600818. [DOI] [PubMed] [Google Scholar]
- 84.Chen S, Chen L, Jahangiri A, Chen B, Wu Y, Chuang HH, Qin C, MacDougall M. Expression and processing of small integrin-binding ligand N-linked glycoproteins in mouse odontoblastic cells. Arch Oral Biol. 2008;53(9):879–889. doi: 10.1016/j.archoralbio.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boskey AL. Osteopontin and related phosphorylated sialoproteins: effects on mineralization. Ann N Y Acad Sci. 1995;760:249–256. doi: 10.1111/j.1749-6632.1995.tb44635.x. [DOI] [PubMed] [Google Scholar]
- 86.Boskey AL, Chiang P, Fermanis A, Brown J, Taleb H, David V, Rowe PS. MEPE’s diverse effects on mineralization. Calcif Tissue Int. 2010;86(1):42–46. doi: 10.1007/s00223-009-9313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deshpande AS, Fang PA, Zhang X, Jayaraman T, Sfeir C, Beniash E. Primary structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules. 2011;12(8):2933–2945. doi: 10.1021/bm2005214. PMCID: 3171794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boukpessi T, Gaucher C, Leger T, Salmon B, Le Faouder J, Willig C, Rowe PS, Garabedian M, Meilhac O, Chaussain C. Abnormal presence of the matrix extracellular phosphoglycoprotein-derived acidic serine- and aspartate-rich motif peptide in human hypophosphatemic dentin. Am J Pathol. 2010;177(2):803–812. doi: 10.2353/ajpath.2010.091231. PMCID: 2913338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int. 2006;79(5):294–300. doi: 10.1007/s00223-006-0182-4. [DOI] [PubMed] [Google Scholar]
- 90.Jiang B, Cao Z, Lu Y, Janik CB, Lauziere S, Xie Y, Poliard A, Qin C, Ward LM, Feng JQ. DMP1 C-terminal mutant mice recapture the human ARHR tooth phenotype phenotype. J Bone Miner Res. 2010;25(10):2155–2164. doi: 10.1002/jbmr.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68(1):155–166. doi: 10.1111/j.1523-1755.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 92.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chau H, El-Maadawy S, McKee MD, Tenenhouse HS. Renal calcification in mice homozygous for the disrupted type IIa Na/Pi cotransporter gene Npt2. J Bone Miner Res. 2003;18(4):644–657. doi: 10.1359/jbmr.2003.18.4.644. [DOI] [PubMed] [Google Scholar]
- 94.Gupta A, Tenenhouse HS, Hoag HM, Wang D, Khadeer MA, Namba N, Feng X, Hruska KA. Identification of the type II Na(+)-Pi cotransporter (Npt2) in the osteoclast and the skeletal phenotype of Npt2−/− mice. Bone. 2001;29(5):467–476. doi: 10.1016/s8756-3282(01)00601-9. [DOI] [PubMed] [Google Scholar]
- 95.Tenenhouse HS, Gauthier C, Chau H, St-Arnaud R. 1{alpha}-Hydroxylase gene ablation and Pi supplementation inhibit renal calcification in mice homozygous for the disrupted Na/Pi cotransporter gene Npt2a. Am J Physiol Renal Physiol. 2003;286(4):F675–F681. doi: 10.1152/ajprenal.00362.2003. [DOI] [PubMed] [Google Scholar]
- 96.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47(3):312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 97.Michel F, Ambroisine ML, Duriez M, Delcayre C, Levy BI, Silvestre JS. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation. 2004;109(16):1933–1937. doi: 10.1161/01.CIR.0000127112.36796.9B. [DOI] [PubMed] [Google Scholar]
- 98.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13(2):189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asaba Y, Ito M, Fumoto T, Watanabe K, Fukuhara R, Takeshita S, Nimura Y, Ishida J, Fukamizu A, Ikeda K. Activation of renin-angiotensin system induces osteoporosis independently of hypertension. J Bone Miner Res. 2009;24(3):241–250. doi: 10.1359/jbmr.081006. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, Tomita T, Yoshikawa H, Ogihara T, Morishita R. Angiotensin II accelerates osteoporosis by activating osteoclasts. Faseb J. 2008;22(7):2465–2475. doi: 10.1096/fj.07-098954. [DOI] [PubMed] [Google Scholar]
- 101.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603–1609. doi: 10.1681/ASN.2010121251. PMCID: 3171931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, Xie Y, Drezner MK. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118(2):722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. Faseb J. 2011;25(8):2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu H, Teng PN, Jayaraman T, Onishi S, Li J, Bannon L, Huang H, Close J, Sfeir C. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J Biol Chem. 2011;286(34):29462–29469. doi: 10.1074/jbc.M110.194746. PMCID: 3190986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan B, Bowman S, Blank R, Lindberg I, Drezner MK. Mechanism of hexa-D-arginine curative effects on the HYP phenotype (abstract) J Bone Miner Res. 2011;26(Suppl 1):SA0032. doi: 10.1002/jbmr.1738. Abstract available from: http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007509-52&terms=. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan B, Meudt J, Blank R, Feng J, Drezner MK. Hexa-D-arginine reversal of osteoblast 7B2 dysregulation in Hyp-mice normalizes the HYP biochemical phenotype (abstract) J Bone Miner Res. 2010;25(Suppl 1) (Presentation No: #1015). Abstract available from: http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=c5c1b1c6-9d98-4ec8-bafa-833ec5088097. [Google Scholar]
- 107.Jain A, Fisher LW, Fedarko NS. Bone sialoprotein binding to matrix metalloproteinase-2 alters enzyme inhibition kinetics. Biochemistry. 2008;47(22):5986–5995. doi: 10.1021/bi800133n. PMCID: 2484124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin-binding ligand N-linked glycoproteins (SIB-LINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18(6):734–736. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 109.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7(12):4060–4066. [PubMed] [Google Scholar]
- 110.Karadag A, Fedarko NS, Fisher LW. Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res. 2005;65(24):11545–11552. doi: 10.1158/0008-5472.CAN-05-2861. PMCID: 1350722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Bone sialoprotein, matrix metalloproteinase 2, and alpha(v) beta3 integrin in osteotropic cancer cell invasion. J Natl Cancer Inst. 2004;96(12):956–965. doi: 10.1093/jnci/djh169. [DOI] [PubMed] [Google Scholar]
- 112.Eapen AS, Ramachandran A, George A. DPP activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells. J Biol Chem. 2011 doi: 10.1074/jbc.M111.290080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bleicher F, Couble ML, Buchaille R, Farges JC, Magloire H. New genes involved in odontoblast differentiation. Adv Dent Res. 2001;15:30–33. doi: 10.1177/08959374010150010701. [DOI] [PubMed] [Google Scholar]
- 114.Nakashima M, Iohara K, Ishikawa M, Ito M, Tomokiyo A, Tanaka T, Akamine A. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Hum Gene Ther. 2004;15(11):1045–1053. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- 115.Alos N, Ecarot B. Downregulation of osteoblast PHEX expression by PTH. Bone. 2005;37(4):589–598. doi: 10.1016/j.bone.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 116.Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150(11):4835–4845. doi: 10.1210/en.2009-0472. [DOI] [PubMed] [Google Scholar]
- 117.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278(19):17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 118.Yuan B, Lin S, Cao Z, Liu Y, Lu Y, Drezner MK, Feng J. DMP1 does not function as a co-transcriptional factor (abstract) J Bone Miner Res. 2011;26(Suppl 1):MO0112. Abstract available from: ( http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007014-127&terms=). [Google Scholar]
- 119.Matsumoto N, Jo OD, Shih RN, Brochmann EJ, Murray SS, Hong V, Yanagawa J, Yanagawa N. Increased cathepsin D release by Hyp mouse osteoblast cells. Am J Physiol Endocrinol Metab. 2005;289(1):E123–E132. doi: 10.1152/ajpendo.00562.2004. [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto N, Jo OD, Shih RN, Yanagawa N. Altered cathepsin D metabolism in PHEX antisense human osteoblast cells. Biochem Biophys Res Commun. 2005;332(1):248–253. doi: 10.1016/j.bbrc.2005.04.116. [DOI] [PubMed] [Google Scholar]
- 121.Shih NR, Jo OD, Yanagawa N. Effects of PHEX anti-sense in human osteoblast cells. J Am Soc Nephrol. 2002;13(2):394–399. doi: 10.1681/ASN.V132394. [DOI] [PubMed] [Google Scholar]
- 122.Cho YD, Yoon WJ, Woo KM, Baek JH, Lee G, Cho JY, Ryoo HM. Molecular regulation of matrix extracellular phosphoglycoprotein expression by bone morphogenetic protein-2. J Biol Chem. 2009;284(37):25230. doi: 10.1074/jbc.M109.008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26(11):2634–2646. doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tu X, Rhee Y, Condon K, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2011;50(1):209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, McKee MD, Drezner MK, Bonewald LF, Feng JQ. The biological function of DMP-1 in osteocyte maturation is mediated by its 57–kDa c-terminal fragment. J Bone Miner Res. 2011;26(2):331–340. doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, Mac-Dougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res. 2003;18(5):807–817. doi: 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- 127.Prie D, Torres PU, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;4:1–8. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 128.Alvarez-Diaz S, Larriba MJ, Lopez-Otin C, Munoz A. Vitamin D: proteases, protease inhibitors and cancer. Cell Cycle. 2010;9(1):32–37. doi: 10.4161/cc.9.1.10266. [DOI] [PubMed] [Google Scholar]
- 129.Bao BY, Yeh SD, Lee YF. 1α,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis. 2006;27(1):32–42. doi: 10.1093/carcin/bgi170. [DOI] [PubMed] [Google Scholar]
- 130.Brage M, Abrahamson M, Lindstrom V, Grubb A, Lerner UH. Different cysteine proteinases involved in bone resorption and osteoclast formation. Calcified Tissue Int. 2005;76(6):439–447. doi: 10.1007/s00223-004-0043-y. [DOI] [PubMed] [Google Scholar]
- 131.Danjo A, Yamaza T, Kido MA, Shimohira D, Tsukuba T, Kagiya T, Yamashita Y, Nishijima K, Masuko S, Goto M, Tanaka T. Cystatin C stimulates the differentiation of mouse osteoblastic cells and bone formation. Biochem Biophys Res Commun. 2007;360(1):199–204. doi: 10.1016/j.bbrc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 132.Dubois SG, Ruchon AF, Delalandre A, Boileau G, Lajeunesse D. Role of abnormal neutral endopeptidase-like activities in Hyp mouse bone cells in renal phosphate transport. Am J Physiol Cell Physiol. 2002;283(5):C1414–C1421. doi: 10.1152/ajpcell.00135.2002. [DOI] [PubMed] [Google Scholar]
- 133.Harrell RM, Lyles KW, Harrelson JM, Friedman NE, Drezner MK. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia. Induction and maintenance with phosphorus and calcitriol. J Clin Invest. 1985;75:1858–1868. doi: 10.1172/JCI111900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marie PJ, Travers R, Glorieux FH. Healing of bone lesions with 1,25-dihydroxyvitamin D3 in the young X-linked hypophosphataemic male mouse. Endocrinology. 1982;111:904–911. doi: 10.1210/endo-111-3-904. [DOI] [PubMed] [Google Scholar]
- 135.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95(4):1846–1850. doi: 10.1210/jc.2009-1671. PMCID: 2853995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoffman WH, Jain A, Chen H, Fedarko NS. Matrix extracellular phosphoglycoprotein (MEPE) correlates with serum phosphorus prior to and during octreotide treatment and following excisional surgery in hypophosphatemic linear sebaceous nevus syndrome. Am J Med Genet A. 2008;146A(16):2164–2168. doi: 10.1002/ajmg.a.32395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bonjour JP, Trechsel U, Fleisch H, Schenk R, DeLuca HF, Baxter LA. Action of 1,25-dihydroxyvitamin D3 and a diphosphonate on calcium metabolism in rats. Am J Physiol. 1975;229(2):402–408. doi: 10.1152/ajplegacy.1975.229.2.402. [DOI] [PubMed] [Google Scholar]
- 138.Lawson-Matthew PJ, Guilland-Cumming DF, Yates AJ, Russell RG, Kanis JA. Contrasting effects of intravenous and oral etidronate on vitamin D metabolism in man. Clin Sci (Lond) 1988;74(1):101–106. doi: 10.1042/cs0740101. [DOI] [PubMed] [Google Scholar]
- 139.Loghman-Adham M, Dousa TP. Dual action of phosphonoformic acid on Na(+)-phosphate cotransport in opossum kidney cells. Am J Physiol. 1992;263(2 Pt 2):F301–F310. doi: 10.1152/ajprenal.1992.263.2.F301. [DOI] [PubMed] [Google Scholar]
- 140.Loghman-Adham M, Szczepanska-Konkel M, Dousa TP. Phosphate transport in brush border membranes from uremic rats. Response to phosphonoformic acid. J Am Soc Nephrol. 1992;3(6):1253–1259. doi: 10.1681/ASN.V361253. [DOI] [PubMed] [Google Scholar]
- 141.Loghman-Adham M, Szczepanska-Konkel M, Yusufi AN, Van Scoy M, Dousa TP. Inhibition of Na+-Pi cotransporter in small gut brush border by phosphonocarboxylic acids. Am J Physiol. 1987;252(2 Pt 1):G244–G249. doi: 10.1152/ajpgi.1987.252.2.G244. [DOI] [PubMed] [Google Scholar]
- 142.McCloskey EV, Yates AJ, Beneton MN, Galloway J, Harris S, Kanis JA. Comparative effects of intravenous diphosphonates on calcium and skeletal metabolism in man. Bone. 1987;8(Suppl 1):S35–S41. [PubMed] [Google Scholar]
- 143.McCloskey EV, Yates AJ, Gray RE, Hamdy NA, Galloway J, Kanis JA. Diphosphonates and phosphate homeostasis in man. Clin Sci (Lond) 1988;74(6):607–612. doi: 10.1042/cs0740607. [DOI] [PubMed] [Google Scholar]
- 144.Muhlbauer RC, Bonjour JP, Fleisch H. Tubular handling of phosphate along the nephron of thyroparathyroidectomized rats injected with ethane-1-hydroxy-1,1-diphosphonate. Clin Sci (Lond) 1981;60(2):171–177. doi: 10.1042/cs0600171. [DOI] [PubMed] [Google Scholar]
- 145.Walton RJ, Russell RG, Smith R. Changes in the renal and extrarenal handling of phosphate induced by disodium etidronate (EHDP) in man. Clin Sci Mol Med. 1975;49(1):45–56. doi: 10.1042/cs0490045. [DOI] [PubMed] [Google Scholar]
- 146.Stoll R, Fleisch H, Bonjour JP. Effect of diphosphonate treatment on phosphate transport by renal brush border vesicles. Am J Physiol. 1980;239(1):F13–F16. doi: 10.1152/ajprenal.1980.239.1.F13. [DOI] [PubMed] [Google Scholar]
- 147.Szczepanska-Konkel M, Yusufi ANK, VanScoy M, Webster SK, Dousa TP. Phosphonocarboxylic acids as specific inhibitors of Na+- dependent transport of phosphate across renal brush border membrane. J Biol Chem. 1986;261:6375–6383. [PubMed] [Google Scholar]
- 148.Trechsel U, Schenk R, Bonjour JP, Russell RG, Fleisch H. Relation between bone mineralization, Ca absorption, and plasma Ca in phosphonate-treated rats. Am J Physiol. 1977;232(3):E298–E305. doi: 10.1152/ajpendo.1977.232.3.E298. [DOI] [PubMed] [Google Scholar]
- 149.VanScoy M, Loghman-Adham M, Onsgard M, Szczepanska-Konkel M, Homma S, Knox FG, Dousa TP. Mechanism of phosphaturia elicited by administration of phosphonoformate in vivo. Am J Physiol. 1988;255(5 Pt 2):F984–F994. doi: 10.1152/ajprenal.1988.255.5.F984. [DOI] [PubMed] [Google Scholar]
- 150.Baum M, Moe OW, Zhang J, Dwarakanath V, Quigley R. Phosphatonin washout in Hyp mice proximal tubules: evidence for posttranscriptional regulation. Am J Physiol Renal Physiol. 2005;288(2):F363–F370. doi: 10.1152/ajprenal.00217.2004. Epub 2004 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nesbitt T, Byun JK, Drezner MK. Normal phosphate transport in cells from the S2 and S3 segments of Hyp-mouse proximal renal tubules. Endocrinology. 1996;137:943–948. doi: 10.1210/endo.137.3.8603607. [DOI] [PubMed] [Google Scholar]
- 152.Nesbitt T, Econs MJ, Byun JK, Martel J, Tenenhouse HS, Drezner MK. Phosphate transport in immortalized cell cultures from the renal proximal tubule of normal and Hyp mice: evidence that the HYP gene locus product is an extrarenal factor. J Bone Miner Res. 1995;10:1327–1333. doi: 10.1002/jbmr.5650100909. [DOI] [PubMed] [Google Scholar]
- 153.Szczepanska-Konkel M, Yusufi AN, Dousa TP. Interactions of [14C]phosphonoformic acid with renal cortical brush-border membranes. Relationship to the Na+-phosphate co-transporter. J Biol Chem. 1987;262(17):8000–8010. [PubMed] [Google Scholar]
- 154.Ullrich KJ, Rumrich G, Burke TR, Shirazi-Beechey SP, Lang H. Interaction of Alkyl/Arylphosphonates, phosphonocarboxylates and diphosphonates with different anion transport systems in the proximal renal tubule. J Pharmacol Exp Ther. 1997;283(3):1223–1229. [PubMed] [Google Scholar]
- 155.Ecarot B, Desbarats M. 1,25-(OH)(2)D-3 down-regulates expression of Phex, a marker of the mature osteoblast. Endocrinology. 1999;140(3):1192–1199. doi: 10.1210/endo.140.3.6593. [DOI] [PubMed] [Google Scholar]
- 156.Hines ER, Kolek OI, Jones MD, Serey SH, Sirjani NB, Kiela PR, Jurutka PW, Haussler MR, Collins JF, Ghishan FK. 1,25-dihydroxyvitamin D3 down-regulation of PHEX gene expression is mediated by apparent repression of a 110 kDa transfactor that binds to a polyadenine element in the promoter. J Biol Chem. 2004;279(45):46406–46414. doi: 10.1074/jbc.M404278200. [DOI] [PubMed] [Google Scholar]
- 157.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279(2):980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 158.von Marschall Z, Fisher LW. Dentin matrix protein-1 isoforms promote differential cell attachment and migration. J Biol Chem. 2008;283(47):32730–32740. doi: 10.1074/jbc.M804283200. PMCID: 2583300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. doi: 10.1016/j.cell.2008.09.059. PMCID: 2614332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yadav VK, Oury F, Tanaka K, Thomas T, Wang Y, Cremers S, Hen R, Krust A, Chambon P, Karsenty G. Leptin-dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208(1):41–52. doi: 10.1084/jem.20101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19(5):161–166. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 162.Goltzman D. LRP5, serotonin and bone: complexity, contradictions and conundrums. J Bone Miner Res. 2011;26(9):2002–2011. doi: 10.1002/jbmr.462. [DOI] [PubMed] [Google Scholar]
- 163.Cui Y, Niziolek PJ, Macdonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nature Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. PMCID: 3113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Price S. Bone: evidence for local effects of LRP5 on bone mass. Nat Rev Rheumatol. 2011;7(7):373. doi: 10.1038/nrrheum.2011.84. [DOI] [PubMed] [Google Scholar]
- 165.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2010 doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 166.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66(2):236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ubaidus S, Li M, Sultana S, de Freitas PH, Oda K, Maeda T, Takagi R, Amizuka N. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 2009;58(6):381–392. doi: 10.1093/jmicro/dfp032. [DOI] [PubMed] [Google Scholar]
- 168.Rowe PS, Hedge AM, David V, Zelenchuk L. PHEX and Fat Energy Metabolism across a Bone-Renal Axis (Abstract) J Bone Miner Res. 2011;26(Suppl 1) Abstract available from: http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007466-89&terms=. [Google Scholar]
- 169.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119(1):84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 170.Cade C, Norman AW. Rapid normalization/stimulation by 1,25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology. 1987;120(4):1490–1497. doi: 10.1210/endo-120-4-1490. [DOI] [PubMed] [Google Scholar]
- 171.Capparelli AW, Roh D, Dhiman JK, Jo OD, Yanagawa N. Altered proximal tubule glucose metabolism in X-linked hypophosphatemic mice. Endocrinology. 1992;130(1):328–334. doi: 10.1210/endo.130.1.1309337. [DOI] [PubMed] [Google Scholar]
- 172.Hruska KA, Rifas L, Cheng SL, Gupta A, Halstead L, Avioli L. X-linked hypophosphatemic rickets and the murine Hyp homologue. Am J Physiol. 1995;268:F357–F362. doi: 10.1152/ajprenal.1995.268.3.F357. [DOI] [PubMed] [Google Scholar]
- 173.Rifas L, Cheng S, Halstead LR, Gupta A, Hruska KA, Avioli LV. Skeletal casein kinase activity defect in the HYP mouse. Calcif Tissue Int. 1997;61:256–259. doi: 10.1007/s002239900331. [DOI] [PubMed] [Google Scholar]
- 174.Rifas L, Gupta A, Hruska KA, Avioli LV. Altered osteoblast gluconeogenesis in X-linked hypophosphatemic mice is associated with a depressed intracellular pH. Calcif Tissue Int. 1995;57(1):60–63. doi: 10.1007/BF00298998. [DOI] [PubMed] [Google Scholar]
- 175.Xie W, Mechin MC, Dubois SG, Lajeunesse D, van de Werve G. Up-regulation of liver glucose-6-phosphatase in x-linked hypophosphatemic mice. Horm Metab Res. 2002;34(6):288–292. doi: 10.1055/s-2002-33256. [DOI] [PubMed] [Google Scholar]
- 176.Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17(3):509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- 177.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26(2):75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 178.Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, Kuro-o M, Nabeshima Y, Nagai R. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49(9):1118–1123. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 179.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 180.Lorenzi O, Veyrat-Durebex C, Wollheim CB, Villemin P, Rohner-Jeanrenaud F, Zanchi A, U MV. Evidence against a direct role of klotho in insulin resistance. Pflugers Arch. 2010;459(3):465–473. doi: 10.1007/s00424-009-0735-2. [DOI] [PubMed] [Google Scholar]
- 181.Drueke TB. Klotho, FGF23, and FGF receptors in chronic kidney disease: a yin-yang situation? Kidney Int. 2010;78(11):1057–1060. doi: 10.1038/ki.2010.339. [DOI] [PubMed] [Google Scholar]
- 182.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009;53(5):875–883. doi: 10.1053/j.ajkd.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 183.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24(2):382–391. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 184.Kurokawa K, Rasmussen H. Ionic control of renal gluconeogenesis. IV. Effect of extracellular phosphate concentration. Biochim Biophys Acta. 1973;313(1):59–71. doi: 10.1016/0304-4165(73)90188-8. [DOI] [PubMed] [Google Scholar]
- 185.Paula FJ, Plens AE, Foss MC. Effects of hypophosphatemia on glucose tolerance and insulin secretion. Horm Metab Res. 1998;30(5):281–284. doi: 10.1055/s-2007-978884. [DOI] [PubMed] [Google Scholar]
- 186.DeFronzo RA, Lang R. Hypophosphatemia and glucose intolerance: evidence for tissue insensitivity to insulin. N Engl J Med. 1980;303(22):1259–1263. doi: 10.1056/NEJM198011273032203. [DOI] [PubMed] [Google Scholar]
- 187.Xie W, Li Y, Mechin MC, Van De Werve G. Up-regulation of liver glucose-6-phosphatase in rats fed with a P(i)-deficient diet. Biochem J. 1999;343(Pt 2):393–396. doi: 10.1042/bj3430393. PMCID: 1220566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xie W, Tran TL, Finegood DT, van de Werve G. Dietary P(i) deprivation in rats affects liver cAMP, glycogen, key steps of gluconeogenesis and glucose production. Biochem J. 2000;352(Pt 1):227–232. PMCID: 1221451. [PMC free article] [PubMed] [Google Scholar]
- 189.Winther K, Nybo M, Vind B, Pedersen SM, Hojlund K, Rasmussen LM. Acute hyperinsulinemia is followed by increased serum concentrations of fibroblast growth factor 23 in type 2 diabetes patients. Scand J Clin Lab Invest. 2011 doi: 10.3109/00365513.2011.640407. [DOI] [PubMed] [Google Scholar]
- 190.Duret L, Gasteiger E, Perriere G. LALNVIEW: a graphical viewer for pairwise sequence alignments. Comput Appl Biosci. 1996;12:507–510. doi: 10.1093/bioinformatics/12.6.507. [DOI] [PubMed] [Google Scholar]
- 191.Huang X, Miller W, Schwartz S, Hardison RC. Parallelization of a local similarity algorithm. Comput Appl Biosci. 1992;8:155–165. doi: 10.1093/bioinformatics/8.2.155. [DOI] [PubMed] [Google Scholar]
- 192.Huang XQ, Hardison RC, Miller W. A space-efficient algorithm for local similarities. Comput Appl Biosci. 1990;6:373–381. doi: 10.1093/bioinformatics/6.4.373. [DOI] [PubMed] [Google Scholar]
- 193.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 194.Rice P. Programme Manual for the EGCG package. Cambridge, CB10 1RQ, England: Hinxton Hall; 1995. [Google Scholar]
- 195.Goldberg M, Six N, Chaussain C, DenBesten P, Veis A, Poliard A. Dentin extracellular matrix molecules implanted into exposed pulps generate reparative dentin: a novel strategy in regenerative dentistry. J Dent Res. 2009;88(5):396–399. doi: 10.1177/0022034509337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hayashibara T, Hiraga T, Yi B, Nomizu M, Kumagai Y, Nishimura R, Yoneda T. A synthetic peptide fragment of human MEPE stimulates new bone formation in vitro and in vivo. J Bone Miner Res. 2004;19(3):455–462. doi: 10.1359/JBMR.0301263. [DOI] [PubMed] [Google Scholar]
- 197.Vordemvenne T, Paletta JR, Hartensuer R, Pap T, Raschke MJ, Ochman S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet Disord. 2011;12(1):263. doi: 10.1186/1471-2474-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105(13):5266–5270. doi: 10.1073/pnas.0711119105. PMCID: 2278202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. PMCID: 2910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95(11):4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 201.Zhou XJ, Fadda GZ, Perna AF, Massry SG. Phosphate depletion impairs insulin secretion by pancreatic islets. Kidney Int. 1991;39(1):120–128. doi: 10.1038/ki.1991.15. [DOI] [PubMed] [Google Scholar]