Abstract
Resources and predation are both known to be important in structuring communities; however the strength of one factor may be affected by the intensity of the other. This study used a fully crossed factorial experiment in laboratory microcosms to examine the ability of a predator, Corethrella appendiculata (Grabham), and basal resources (leaf litter) to differentially affect two competing species of mosquito prey. Increased resources resulted in shorter developmental time and increased survivorship, mass, and population performance for both prey species, except when predation levels were high. Increased levels of predation and resources reduced the negative competitive effects of Aedes albopictus (Skuse) on Ochlerotatus triseriatus (Say). At low levels of resources and predation, the superior competitor, A. albopictus had the higher survivorship, and at high levels of resources and predation, the inferior competitor’s survival was greater. Predators in high-resource treatments emerged larger than those in low resources, suggesting the occurrence of a bottom-up cascade or alternative feeding method. This study suggests that survival and coexistence of the two prey species may depend on the interaction of resources and predation, in that high levels of predation are important for the coexistence of both species.
Keywords: bottom-up, top-down, coexistence, treehole, mosquitoes
Recent work has attempted to disentangle the interacting effects of predation and resources on community structure in both terrestrial (Denno et al. 2002, 2003; Stadler 2004) and aquatic systems (Menge et al. 1997, Batzer 1998, Rosenfeld 2000, Pagano et al. 2003). Although many studies of interactions between predators and nutrient levels have been in large aquatic systems (McQueen et al. 1989, Brett and Goldman 1997), top-down and bottom-up effects may be even more apparent in relatively compact habitats such as water-holding tree holes and artificial containers (e.g., discarded automobile tires and cemetery vases) where space is limited and the resource base originates from allochthonous sources such as leaf litter (Maciá and Bradshaw 2000, Kitching 2001), animal detritus (Daugherty et al. 2000), and stemflow (Carpenter 1982). Several studies of both natural and artificial container communities have described the importance of predators (Bradshaw and Holzapfel 1983; Lounibos 1983, 1985; Chambers 1985; Fincke et al. 1997; Lounibos et al. 2001) or resource quantity (Fish and Carpenter 1982, Carpenter 1983, Leonard and Juliano 1995, Walker et al. 1997), whereas fewer have studied the combined effects of predation and resource levels on prey populations (Yanoviak 2001, Kneitel and Miller 2002). Interactions between these factors may be important for determining the ability of multiple prey species to coexist.
Top-down effects in container systems are somewhat variable, especially when predator populations are patchy (Lounibos et al. 1997). Temperate container systems typically contain only a few predator species (Kitching 2000). The most well studied of these predators, Toxorhynchites rutilus (Coquillett), exerts strong effects on prey assemblages (Bradshaw and Holzapfel 1983, Lounibos et al. 1993). However, T. rutilus is also highly cannibalistic, which may result in only a single predator per container when alternative prey is unavailable (Campos and Lounibos 2000). Larger numbers of a smaller predatory dipteran, Corethrella appendiculata (Grabham), are commonly found together in containers in Florida (Lounibos 1983), which may lead to intraspecific interactions among larvae of this species. However, unlike T. rutilus, cannibalism does not seem to occur commonly among fourth instars of C. appendiculata (M.W.G., unpublished data). Thus, increases in C. appendiculata density may have significant impacts on prey populations with limited intraspecific interference.
Bottom-up effects are assumed to be very strong in container habitats (Kitching 2001). When treeholes are first formed, or when containers are first colonized in the field, available resources may be scarce, but resources will increase as litter falls into these systems. Habitats will vary in quantity and quality of leaf litter depending on the season and the leaf species, which may ultimately affect its decay rate and microfauna (Dieng et al. 2002) and thus influence the nutrient levels available to mosquito larvae. Quantity of leaf litter is highly variable (Leonard and Juliano 1995), and dry weight has been found to range from 0 to 3.9 g in natural treeholes in Michigan (Walker and Merritt 1988). In the absence of further input, resource levels will be depleted over time, especially in the presence of detritivores such as mosquito larvae (Carpenter 1982). Resource levels in tropical container communities are important in determining species richness, especially early in the colonization process (Yanoviak 2001). Experiments manipulating resource quantity are useful and important to determine controls of community structure in these systems.
Aedes albopictus (Skuse) is an invasive mosquito species from Asia that has been broadly dispersed via used tires and has spread rapidly during the past two decades in the United States (O’Meara et al. 1995). Larvae of this species inhabit treeholes and artificial containers and feed by filtering or browsing upon microbes (Hawley 1988). In forested areas of the eastern United States, A. albopictus has invaded tree-holes and containers occupied by the native mosquito Ochlerotatus triseriatus (Say), whose larvae also acquire resources by browsing and filtering microbes (Jenkins and Carpenter 1946). Laboratory studies have shown that A. albopictus outcompetes O. triseriatus for limiting larval resources (Barrera 1996). Furthermore, these two species respond differently to the presence of predators. In the presence of T. rutilus, O. triseriatus reduces its activity to avoid predation, whereas A. albopictus does not (Kesavaraju and Juliano 2004). Thus, the combined effects of predation and resources should interact to affect coexistence among the prey species.
Previous research has found that C. appendiculata may reduce competition among larvae of A. albopictus and O. triseriatus (Griswold and Lounibos 2005), most likely through differential predation, facilitating coexistence of these prey species. Because A. albopictus is the superior resource competitor (Barrera 1996) and the preferred prey of C. appendiculata (Griswold and Lounibos 2005), we predicted that predator density and resource density would interact, so that O. triseriatus will be more likely to coexist with A. albopictus at high resource levels and high predator densities. This hypothesis is based upon models that predict a trade-off between competitive ability and predation avoidance, such that good resource competitors will dominate at low resource levels in the absence of predation, but poor resource competitors, which are resistant to predation, will do better in the presence of predators (Holt et al. 1994, Leibold 1996, Chase 1999, Chase et al. 2002). Our approach used a factorial experiment to determine interactions between resource and predator densities and their effects on fitness of the two competing prey species.
Materials and Methods
Experimental Design
We conducted a laboratory experiment using plastic cups as treehole analogs to examine the roles of predators and basal resources on coexistence of two competing prey species. Water was collected from ≈40 discarded automobile tires adjacent to the University of Florida campus in Gainesville, FL, and sieved through a 180-μm filter to remove macroinvertebrates and their eggs. Water from multiple tires was pooled and mixed before being allocated to treatments. All of the species used in the study were found to occur in the tires. Although tire water is of lower nutritive value than treehole water (Livdahl and Willey 1991), the tires were close together and the species inhabiting them likely represented closely related individuals, whereas tree-holes were rare in the area. Sixty 400-ml plastic cups (11 cm in height by 8 cm in diameter) were used as containers for the predator and prey treatments. Live oak, Quercus virginiana Miller, leaves were collected from the ground during the early spring 2003 in Gainesville. Live oak is a common component of forested ecosystems in Florida, and leaf fall from this species into treeholes and artificial containers occurs in the spring. Leaves were washed and then dried at 65°C for 48 h, weighed in portions of 0.5 g, and then chopped into pieces ≈1 by 1 cm. Leaves (0.5, 1.0, or 2.0 g) were then added to each cup with 400 ml of tire water and soaked for 3 d to allow for microbial growth before the start of the experiment. Naturally occurring resource levels in treeholes average 0.015 g per larvae (Walker et al. 1991) and thus are similar to the intermediate level used here. Two days before the start of the experiment, field-collected C. appendiculata larvae that had molted to fourth instars in the past 24 h were given aquatic nematodes ad libitum. Fourth instars of C. appendiculata were used because this is the primary larval stage able to consume mosquito larvae (Lounibos 1985). During the initial spring generation in northern Florida, treeholes may contain third and fourth instars of C. appendiculata at the time of hatching of the summer generation of prey larvae (Bradshaw and Holzapfel 1984). Moreover, all instars of larvae of both species may be present year-round in subtropical southern Florida, justifying the stages used here. To standardize hunger, C. appendiculata were then starved 24 h before the start of the experiment. The cups were maintained during experiments at 25 ± 1°C with a photoperiod of 14:10 (L:D) h and ≈70% RH.
Four levels of C. appendiculata (zero, one, two, or four individuals) were fully crossed with three levels of oak leaves (0.5, 1.0, or 2.0 g, herein referred to as low, intermediate, and high) with treatments replicated five times by using a completely randomized design. C. appendiculata densities used were within the range found in southern Florida treeholes (Lounibos 1983). Each treatment received 50 first instars of A. albopictus and 50 first instars of O. triseriatus from locally derived F1 colonies of these species collected in southern Florida (Lounibos et al. 2001). Prey larvae <24 h old were added to each container and allowed to acclimate for 10 min before adding predators. The experiment was run until all prey larvae had died, were consumed, or had emerged. Emerged adults were frozen until all could be thawed together and then dried for 48 h at 65°C and weighed individually to the nearest 0.001 mg.
Data Analyses
Survivorship, median developmental time, and female dry mass were measured for each species in each replicate container. Survivorship was calculated by dividing the total number of emerging adults by the initial number of larvae present. The proportion of A. albopictus among total surviving prey was determined by dividing the number of A. albopictus surviving by the total number of prey surviving. Survivorship, developmental time, and dry mass of females for each replicate were used to calculate a composite index of performance based on r′ (Livdahl 1982, 1984; Livdahl and Sugihara 1984), which estimates the realized per capita rate of population change, λ′ = exp(r′), and is an analog of the finite rate of increase (Pianka 1988) estimated as follows:
where N0 is the initial number of females (assumed to be 50% of a cohort), Ax is the number of females eclosing on day x, wx is the mean dry mass of females eclosing on day x, and f(wx) is a function relating egg production to dry mass. D is the time from adult eclosion to reproduction, estimated as 12 d for O. triseriatus (Leonard and Juliano 1995) and 14 d for A. albopictus (Livdahl and Willey 1991). A regression relating adult dry mass to fecundity for A. albopictus was obtained from Lounibos et al. (2002):
and for O. triseriatus from Nannini and Juliano (1997):
When a replicate does not have any larvae surviving to adulthood, λ′ values are equal to zero and will ultimately result in greater variance for the treatment. Values of λ′ > 1 indicate that the cohort in question is increasing, values ≅1 indicate that the cohort is stable, and values <1 indicate that the cohort is decreasing (Juliano 1998).
Survivorship, female median developmental time, female dry mass, and proportion of A. albopictus surviving were each analyzed using a two-way analysis of variance (ANOVA) with resource and predator levels as independent variables by using PROC GLM in SAS (SAS Institute 1989) for each prey species separately. When significant main effects were found, all pairwise comparisons were made using the Tukey–Kramer method (SAS Institute 1989). Comparisons among treatments were run on least-square (LS) means, where one factor is fixed and the others vary (e.g., compare predator treatments with fixed resources). When significant interactions were found, pairwise comparisons were made between resources for a specific predator treatment (e.g., no predator: low resource versus high resource), and between treatments for a specific predator (e.g., low resource: no predator versus one predator), by using Bonferroni corrections (PDIFF makes Bonferroni corrections in PROC GLM) to control for experiment-wise error rate. Table-wide corrections were made for response variables for A. albopictus and O. triseriatus by using a sequential Bonferroni test (Rice 1989). This test corrects for multiple ANOVAs and did not change the overall results.
Assumptions of normality and homogeneity of variance were assessed visually through residual plots and through normality tests. Data that did not meet these assumptions were transformed. Survivorship (proportion prey surviving) was arcsine square-root transformed. Median developmental time for O. triseriatus was log (y + 1) transformed, and this variable for C. appendiculata was square root transformed. Female dry masses of O. triseriatus and median developmental times of A. albopictus were reciprocally transformed. Because λ′ values did not meet assumptions required for ANOVA and no typical transformation could remedy this, they were analyzed by both parametric and randomization ANOVAs (Manly 1991). Randomization ANOVAs are more robust than other nonparametric analyses, do not assume normality, and allow for testing interactions (Crowley 1992). Randomization ANOVAs were run in RT (Manly 1991, 1997) with 1000 randomizations. Because the parametric and randomization ANOVAs yielded the same conclusions, only analyses from the parametric ANOVAs are reported.
Results
Prey Performance
Survivorship
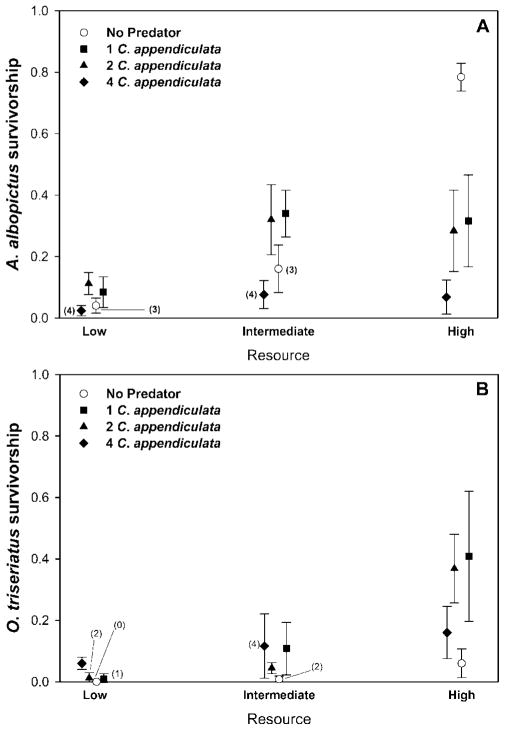
Predation, resource levels, and interactions between these factors influenced A. albopictus and O. triseriatus survivorship (Tables 1 and 2). Survivorship of A. albopictus at high levels of predation was significantly lower than treatments with one or two predators. However, when resources were high, survivorship of A. albopictus in the absence of predators was greater than for any of the predator treatments (Fig. 1A). At low and intermediate resource levels, survivorship was not significantly different among any of the predator treatments for O. triseriatus. O. triseriatus survivorship in treatments with one or two predators was significantly higher than in treatments with zero or four predators (Fig. 1B).
Table 1.
Two-way ANOVA for λ′, survivorship to adulthood, female developmental time, and adult mass for A. albopictus
| Source |
λ′, Estimated finite rate of increase
|
Survivorship
|
Developmental time
|
Mass
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | P | F | P | F | P | F | P | |
| Predator | 3 | 1.22 | 0.312 | 29.98 | <0.001 | 6.51 | 0.001 | 5.50 | 0.003 |
| Resource | 2 | 7.99 | <0.001 | 58.82 | <0.001 | 23.93 | <0.001 | 11.58 | <0.001 |
| Predator * resource | 6 | 1.04 | 0.409 | 17.73 | <0.001 | 1.22 | 0.320 | 0.82 | 0.563 |
| Error df | 48a | ||||||||
Error degrees of freedom for developmental time was 36 and mass was 46.
Table 2.
Two-way ANOVA for λ′, survivorship to adulthood, female development time, and adult mass for O. triseriatus
| Source |
λ′, Estimated finite rate of increase
|
Survivorship
|
Developmental time
|
Mass
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | P | F | P | F | P | F | P | |
| Predator | 3 | 7.75 | <0.001 | 14.02 | <0.001 | 2.68 | 0.067 | 1.42 | 0.260 |
| Resource | 2 | 14.69 | <0.001 | 58.88 | <0.001 | 6.27 | 0.006 | 2.73 | 0.084 |
| Predator * resource | 6 | 1.49 | 0.203 | 5.72 | <0.001 | 0.50 | 0.770 | 1.39 | 0.262 |
| Error df | 48a | ||||||||
Error degrees of freedom for developmental time was 27 and mass was 33.
Fig. 1.
Mean ± SD survivorship (proportion of the original number of larvae surviving to adulthood) of A. albopictus (A) and O. triseriatus (B) at three levels of resources and four levels of predation. Numbers in parentheses denote the number of replicates producing adult mosquitoes. For data points without numbers, n = 5.
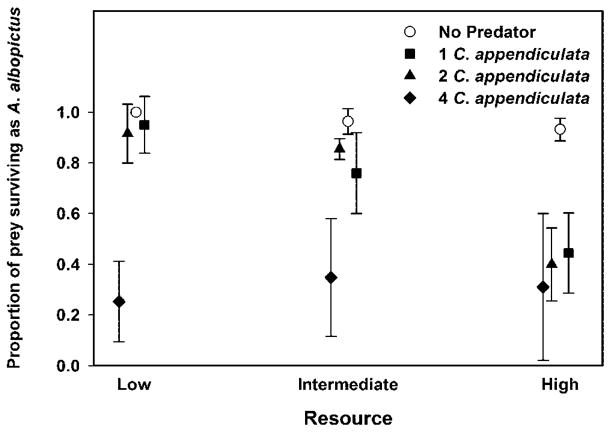
Predation (F3, 48 = 53.96; P < 0.0001), resource levels (F2, 48 = 26.51; P <0.0001), and their interaction (F3, 48 = 5.80; P = 0.0001) influenced the proportion of A. albopictus among total prey surviving. The interaction occurred because a smaller proportion of A. albopictus emerged with increasing resource levels at low and intermediate levels of predation. The proportion of A. albopictus surviving decreased with increasing resource levels in the presence of one or two predators (Fig. 2).
Fig. 2.
Mean ± SD proportion of A. albopictus surviving (number of A. albopictus surviving divided by the number of A. albopictus + O. triseriatus surviving) at three levels of resources and four levels of predation.
Developmental Time
Both resource levels and predation intensity influenced median developmental time of A. albopictus females (Table 1). Developmental time of A. albopictus was significantly greater in the low resource (21.67 ± 3.3 d) (mean ± SE) treatment compared with intermediate (11.34 ± 1.28 d) and high resource (9.35 ± 0.3 d) treatments. A. albopictus took longer to develop in the absence of predators (18.95 ± 3.8 d) compared with high levels of predation (10 ± 0.8 d).
Median developmental time of O. triseriatus adults was only affected by resource levels (Table 2). Because some containers did not produce any O. triseriatus adults, some comparisons were not conducted. O. triseriatus took significantly less time to reach adulthood in treatments with high levels (30.1 ± 3.4 d) of resource compared with intermediate resource levels (41.4 ± 2.7 d).
Adult Mass
Dry mass of A. albopictus females was significantly affected by predation intensity and resource levels (Table 1). Females emerging from treatments with four predators (423 ± 36 mg) were significantly larger than those emerging from treatments without predators (220 ± 36 mg), but not low (350 ± 37 mg) or intermediate (308 ± 31 mg) predator treatments. Females emerging from treatments with high resource levels (430 ± 26 mg) were significantly larger than those from treatments with low resource levels (225 ± 35 mg), but not intermediate resource levels (321 ± 30 mg). Mean adult female O. triseriatus mass was not significantly affected by predator or resource levels (Table 2).
Population Performance (λ′)
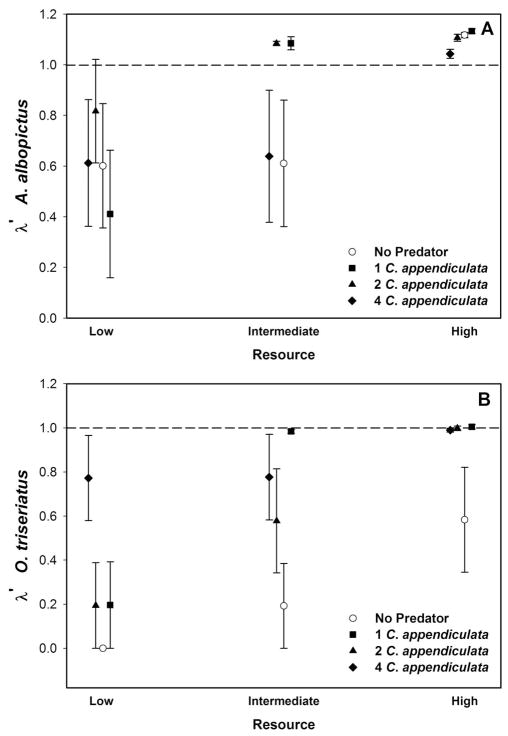
λ′ values for A. albopictus were only affected by changing resource levels (Table 1). λ′ values for treatments with high resources were significantly higher than low resources (Fig. 3A). Predator and resource levels affected λ′ values for O. triseriatus (Table 2). λ′ values for treatments with one or four predators were significantly greater than those for treatments without predators. LS mean λ′ values for treatments with low resource levels were significantly lower than means from intermediate and high resource level (Fig. 3B).
Fig. 3.
Mean ± SE estimates of population performance (λ′, an analog of the finite rate of increase for the cohort) for A. albopictus (A) and O. triseriatus (B) at three levels of resources and four levels of predation. The line at λ′ = 1 is where population growth is estimated to be 0.
Predator Performance
All treatments had predators that emerged as adults, resulting in a sample size of five for each treatment. Survivorship of C. appendiculata was not significantly different among any of the treatments. There were significant effects of predator level (F2, 34 = 5.24; P = 0.020), but not resource or the interaction on developmental time. C. appendiculata took significantly longer to develop in treatments with four predators (16.9 ± 0.9 d) than in those with one predator (13.7 ± 0.8). Dry mass of C. appendiculata adults was significantly affected by resource levels (F2, 34 = 9.64; P < 0.001), but not by predator levels or the interaction of these factors. Overall, predators with high resources (156 ± 4 mg) emerged significantly larger than those in low (134 ± 5 mg) and intermediate (133 ± 5 mg) resource levels.
Discussion
In this study, top-down and bottom-up effects dictated prey fitness and coexistence. In general, increased resources resulted in greater survivorship (Fig. 1) and larger and faster developing prey. Predation was important in mediating coexistence between prey species by selective consumption of the superior competitor. Bottom-up and top-down effects are important in other aquatic systems, interacting in some cases (Pagano et al. 2003), but acting independently in others (Forrester et al. 1999, Yanoviak 2001, Nystrom et al. 2003). Regardless of whether these effects interact, predation and resource quantity both regulate community structure, and the balance and importance of each may vary by environment (Leibold 1989).
As expected (Kitching 2001), bottom-up effects were strong in this experiment (Fig. 1). As shown previously (Leonard and Juliano 1995, Daugherty et al. 2000), increased resource levels generally resulted in increased prey growth rates, survivorship, and mass and resulted in increased values for λ′ for both prey species. In the absence of predators, O. triseriatus may be driven to extinction via competition; thus, predation is very important for offsetting the negative effects of resource competition. Numerous studies have shown that competition with A. albopictus produces strong negative effects on O. triseriatus (Livdahl and Willey 1991, Barrera 1996); however, A. albopictus and O. triseriatus seem to coexist in Florida treeholes (Lounibos et al. 2001). The low numbers of O. triseriatus surviving at high resource levels in the absence of predators suggest other mechanisms may affect coexistence in nature.
Increased resource levels also were beneficial to the predators, consistent with the finding of Yanoviak (2001) that leaf litter benefited odonate predators in tropical treeholes by increasing their growth rates. At high nutrient levels, C. appendiculata adults emerged larger than those at lower nutrient levels. Because fecundity typically increases with mass (Armbruster and Hutchinson 2002), higher nutrient levels may generate more predators and thus more intense predation throughout the community. Increased resources available to the prey could have increased the amount of biomass available to C. appendiculata. However, increased prey growth rate at high resource levels also may allow prey to escape predation by C. appendiculata, which can only consume prey smaller than themselves (Lounibos 1983). In addition, C. appendiculata could have been directly consuming microfauna (Grabham 1906) produced as a result of increasing leaf litter. When alternative prey is not available, C. appendiculata will browse on leaves (M.W.G., unpublished data). As a result, C. appendiculata may act as an intraguild predator and compete with its prey for resources. Menge (1992) hypothesized that linkage of top-down and bottom-up effects should be manifested as stronger predation with increased nutrients, because increased nutrients support more prey and thus more predators. Although predators emerged larger with increased resources, field experiments over more than one generation are needed to determine long-term effects. Further studies should investigate the natural diet of this predator species and its role in intraguild predation among mosquito larvae.
Predation typically results in decreased developmental time of prey, either by release from competition (Fauth 1990) or by preferential consumption of slowly developing prey (Travis et al. 1985, Wilbur 1987). Also, predators may indirectly affect prey foraging rates, thereby slowing prey development (Eklov 2000). Although C. appendiculata did not significantly affect developmental time for O. triseriatus in the current study, the developmental time of A. albopictus was decreased (Tables 1 and 2). At high predator densities, A. albopictus survival decreased to 5–10% of the initial prey densities (Fig. 1A). Developmental time did not differ among treatments at intermediate or high resources, although competition should have been reduced. The predators may have been competing with prey for resources and switched to alternative prey, such as microorganisms, when mosquito prey is not available. Aggression among C. appendiculata has been observed and could have reduced predation in the presence of multiple predators.
These results diverge from models predicting that good resource competitors who are vulnerable to predation will dominate at low resource levels, but poor resource competitors who are resistant to predation will replace the other species as resource levels increase (Holt et al. 1994, Leibold 1996). O. triseriatus was dominant at high resources and levels of predation, but A. albopictus was not driven extinct (Fig. 2). Therefore, our results are consistent with those of Proulx and Mazumder (1998), who stated that in herbivore– grazer systems at high levels of productivity, predators would allow persistence of the less vulnerable species, but not extinction of the more vulnerable species, due to its higher growth rate. In the current study, the more vulnerable species, A. albopictus, took less time to reach adulthood than the less vulnerable species, O. triseriatus. Survivorship for the two prey species was similar at high resource levels with low and intermediate levels of predation, indicating potential for coexistence (Fig. 1). However, λ′ values suggest that at high resource levels, O. triseriatus will be stable in the presence of the predators and A. albopictus populations will increase. Although predation strongly enhances the survival of O. triseriatus, our results indicate that competition with A. albopictus, via resources or interference will strongly influence persistence of O. triseriatus.
Although bottom-up and top-down effects had distinct consequences for consumers, these effects were interdependent. At low-to-intermediate resource levels, predation effects were minimal, suggesting the prey were resource limited and chose foraging over predator avoidance. In ephemeral habitats, such as treeholes and artificial containers, the aquatic inhabitants must develop quickly to escape desiccation. However, at high resource levels, top-down effects limited A. albopictus survivorship and enhanced O. triseriatus survivorship. The λ′ values for A. albopictus suggest a reaction to bottom-up and top-down effects, so that ideal conditions exist at intermediate levels of predation and resources. However, O. triseriatus requires greater resources and the addition of a predator to persist. A. albopictus has higher feeding and growth rates, which may allow consumption of more resources than O. triseriatus (Ho et al. 1992, Barrera 1996). Furthermore, at the temperatures used in this study, A. albopictus requires less degree-days to develop (Teng and Apperson 2000). Thus, the ability of C. appendiculata to cull early stages of larvae allows persistence of both prey species by reducing the overall number of potential competitors and selectively consuming the superior competitor.
Recent work suggests confounding effects in communities, depending on both detrital and autotrophic pathways (Moore et al. 2004). Even in such a simple community, there were a number of complex interactions occurring that may have confounded the results, emphasizing the importance of behavioral studies over the life of the predator and prey. Further studies also should focus on long-term, large-scale studies in both temperate and tropical container systems to examine more complex food webs composed of both vertebrate and invertebrate predators (Fincke 1999).
Acknowledgments
We thank R. Escher, J. Butler, and P. Shirk for providing prey larvae, insectary space, and equipment use, respectively. Previous drafts of the manuscript were improved by comments from B. Alto, J. Rey, S. Yanoviak, and D. Yee. This research was supported in part by National Institutes of Health Grant R01 (AI)-44793. This is University of Florida Experiment Station Journal Series No. R-10724.
References Cited
- Armbruster P, Hutchinson RA. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) J Med Entomol. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- Batzer DP. Trophic interactions among detritus, benthic midges, and predatory fish in a freshwater marsh. Ecology. 1998;79:1688–1698. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitos in southeastern North America. Oecologia (Berl) 1983;57:239–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Seasonal development of tree-hole mosquitoes (Diptera, Culicidae) and chaoborids in relation to weather and predation. J Med Entomol. 1984;21:366–378. [Google Scholar]
- Brett MT, Goldman CR. Consumer versus resource control in freshwater pelagic food webs. Science (Wash DC) 1997;275:384–386. doi: 10.1126/science.275.5298.384. [DOI] [PubMed] [Google Scholar]
- Campos RE, Lounibos LP. Natural prey and digestion times of Toxorhynchites rutilus (Diptera: Culicidae) in southern Florida. Ann Entomol Soc Am. 2000;93:1280–1287. doi: 10.1093/jmedent/37.3.385. [DOI] [PubMed] [Google Scholar]
- Carpenter SR. Stemflow chemistry - effects on population-dynamics of detritivorous mosquitos in tree-hole ecosystems. Oecologia (Berl) 1982;53:1– 6. doi: 10.1007/BF00377128. [DOI] [PubMed] [Google Scholar]
- Carpenter SR. Resource limitation of larval tree-hole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Chambers RC. Competition and predation among larvae of three species of treehole-breeding mosquitoes. In: Lounibos LP, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach, FL: 1985. pp. 25–53. [Google Scholar]
- Chase JM. To grow or to reproduce? The role of life-history plasticity in food web dynamics. Am Nat. 1999;154:571–586. doi: 10.1086/303261. [DOI] [PubMed] [Google Scholar]
- Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ. The interaction between predation and competition: a review and synthesis. Ecol Lett. 2002;5:302–315. [Google Scholar]
- Crowley PH. Resampling methods for computation intensive data analysis in ecology and evolution. Annu Rev Ecol Syst. 1992;23:405– 447. [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denno RF, Gratton C, Peterson MA, Langellotto GA, Finke DL, Huberty AF. Bottom-up forces mediate natural-enemy impact in a phytophagous insect community. Ecology. 2002;83:1443–1458. [Google Scholar]
- Denno RF, Gratton C, Dobel H, Finke DL. Predation risk affects relative strength of top-down and bottom-up impacts on insect herbivores. Ecology. 2003;84:1032–1044. [Google Scholar]
- Dieng H, Mwandawiro C, Boots M, Morales R, Satho T, Tuno N, Tsuda Y, Takagi M. Leaf litter decay process and the growth performance of Aedes albopictus larvae (Diptera: Culicidae) J Vector Ecol. 2002;27:31–38. [PubMed] [Google Scholar]
- Eklov P. Chemical cues from multiple predator-prey interactions induce changes in behavior and growth of anuran larvae. Oecologia (Berl) 2000;123:192–199. doi: 10.1007/s004420051005. [DOI] [PubMed] [Google Scholar]
- Fauth JE. Interactive effects of predators and early larval dynamics of the treefrog Hyla chrysoscelis. Ecology. 1990;71:1609–1616. [Google Scholar]
- Fincke OM. Organization of predator assemblages in Neotropical tree holes: effects of abiotic factors and priority. Ecol Entomol. 1999;24:13–23. [Google Scholar]
- Fincke OM, Yanoviak SP, Hanschu RD. Predation by odonates depresses mosquito abundance in water-filled tree holes in Panama. Oecologia (Berl) 1997;112:244–253. doi: 10.1007/s004420050307. [DOI] [PubMed] [Google Scholar]
- Fish D, Carpenter SR. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology. 1982;63:283–288. [Google Scholar]
- Forrester GE, Dudley TL, Grimm NB. Trophic interactions in open systems: effects of predators and nutrients on stream food chains. Limnol Oceanogr. 1999;44:1187–1197. [Google Scholar]
- Grabham M. A new Corethrella from Jamaica. Entomol News. 1906;17:343–345. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation allow invasive and native mosquito larvae to coexist in Florida? Ecol. Entomol. 2005;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4(suppl):1–39. [PubMed] [Google Scholar]
- Ho BC, Khoo H, Chew LM, Wong KP, Ewert A. Food ingestion and digestive enzymes in larval Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 1992;29:960–964. doi: 10.1093/jmedent/29.6.960. [DOI] [PubMed] [Google Scholar]
- Holt RD, Grover J, Tilman D. Simple rules for interspecific dominance in systems with exploitative and apparent competition. Am Nat. 1994;144:741–771. [Google Scholar]
- Jenkins DW, Carpenter SJ. Ecology of the treehole breeding mosquitoes of nearctic North America. Ecol Monogr. 1946;16:33– 47. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching RL. Food webs and container habitats: the natural history and ecology of phytotelmata. Cambridge University Press; Cambridge, NY: 2000. [Google Scholar]
- Kitching RL. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Annu Rev Entomol. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Kneitel JM, Miller TE. Resource and top-predator regulation in the pitcher plant (Sarracenia purpurea) inquiline community. Ecology. 2002;83:680– 688. [Google Scholar]
- Leibold MA. Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am Nat. 1989;134:922–949. [Google Scholar]
- Leibold MA. A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat. 1996;147:784– 812. [Google Scholar]
- Leonard PM, Juliano SA. Effect of leaf-litter and density on fitness and population performance of the treehole mosquito Aedes triseriatus. Ecol Entomol. 1995;20:125–136. [Google Scholar]
- Livdahl TP. Competition within and between hatching cohorts of a treehole mosquito. Ecology. 1982;63:1751– 1760. [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. J Anim Ecol. 1984;53:573–580. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion - competition between Aedes albopictus and native Aedes triseriatus. Science (Wash DC) 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of treeholes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Plexus; Medford, NJ: 1983. p. 293. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach, FL: 1985. pp. 65–77. [Google Scholar]
- Lounibos LP, Escher RL, Nishimura N, Juliano SA. Long-term dynamics of a predator used for biological control and decoupling from mosquito prey in a subtropical treehole ecosystem. Oecologia (Berl) 1997;111:189–200. doi: 10.1007/s004420050225. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito - influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions. 2001;3:151–166. [Google Scholar]
- Lounibos LP, Suarez S, Menendez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- Maciá A, Bradshaw WE. Seasonal availability of resources and habitat degradation for the western tree-hole mosquito, Aedes sierrensis. Oecologia (Berl) 2000;125:55– 65. doi: 10.1007/PL00008891. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization and Monte Carlo methods in biology. Chapman & Hall; London, United Kingdom: 1991. [Google Scholar]
- Manly BFJ. RT: a program for randomizing testing, version 2.1. West Incorporated; Cheyenne, WY: 1997. [Google Scholar]
- Menge BA. Community regulation: under what conditions are bottom-up factors important on rocky shores? Ecology. 1992;73:755–765. [Google Scholar]
- Menge BA, Daley BA, Wheeler PA, Dahlhoff E, Sanford E, Strub PT. Benthic-pelagic links and rocky intertidal communities: bottom-up effects on top-down control? Proc Nat Acad Sci USA. 1997;94:14530– 14535. doi: 10.1073/pnas.94.26.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DJ, Johannes MRS, Post JR, Stewart TJ, Lean DRS. Bottom-up and top-down impacts on fresh-water pelagic community structure. Ecol Monogr. 1989;59:289–309. [Google Scholar]
- Moore JC, Berlow EL, Coleman DC, de Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall Diana H. Detritus, trophic dynamics and biodiversity. Ecology Letters. 2004;7:584– 600. [Google Scholar]
- Nannini MA, Juliano SA. Effects of developmental asynchrony between Aedes triseriatus (Diptera: Culicidae) and its predator Toxorhynchites rutilus (Diptera: Culicidae) J Med Entomol. 1997;34:457– 460. doi: 10.1093/jmedent/34.4.457. [DOI] [PubMed] [Google Scholar]
- Nystrom P, McIntosh AR, Winterbourn MJ. Top-down and bottom-up processes in grassland and forested streams. Oecologia (Berl) 2003;136:596– 608. doi: 10.1007/s00442-003-1297-1. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Aedes aegypti (Diptera, Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Pagano M, Koffi MA, Cecchi P, Corbin D, Champalbert G, Saint-Jean L. An experimental study of the effects of nutrient supply and Chaoborus predation on zooplankton communities of a shallow tropical reservoir (Lake Brobo, Cote d’Ivoire) Freshw Biol. 2003;48:1379–1395. [Google Scholar]
- Pianka ER. Evolutionary ecology. 4. Harper & Row; New York: 1988. [Google Scholar]
- Proulx M, Mazumder A. Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient-rich ecosystems. Ecology. 1998;79:2581–2592. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J. Effects of fish predation in erosional and depositional habitats in a temperate stream. Can J Fish Aquat Sci. 2000;57:1369–1379. [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. SAS Institute; Cary, NC: 1989. [Google Scholar]
- Stadler B. Wedged between bottom-up and top-down processes: aphids on tansy. Ecol Entomol. 2004;29:106– 116. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: Effect of density, food, and competition on response to temperature. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Travis J, Keen WH, Juilianna J. The role of relative body size in a predator-prey relationship between dragonfly naiads and larval anurans. Oikos. 1985;45:59– 65. [Google Scholar]
- Walker ED, Kaufman MG, Ayres MP, Riedel MH, Merritt RW. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera: Culicidae) Can J Zool. 1997;75:706–718. [Google Scholar]
- Walker ED, Merritt RW. The significance of leaf detritus to mosquito (Diptera: Culicidae) productivity from treeholes. Environ Entomol. 1988;17:199–206. [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- Wilbur HM. Regulation of structure in complex-systems–experimental temporary pond communities. Ecology. 1987;68:1437–1452. [Google Scholar]
- Yanoviak SP. Predation, resource availability, and community structure in Neotropical water-filled tree holes. Oecologia (Berl) 2001;126:125–133. doi: 10.1007/s004420000493. [DOI] [PubMed] [Google Scholar]