Abstract
Biosystem–surface interactions play an important role in various biological events and determine the ultimate functionality of implanted devices. Endothelialization or mimicking of endothelium on the surface of cardiovascular materials is a promising way to solve the problems of material-induced thrombosis and restenosis. Meanwhile, a multifunctional surface design is needed as antithrombotic properties should be considered in the period when the implants are not yet completely endothelialized. In this article, we summarize some successful approaches used in our laboratory for constructing multifunctional endothelium-like surfaces on metallic cardiovascular biomaterials through chemical modification of the surface or by the introduction of specific biological molecules to induce self-endothelialization in vivo. Some directions on future research in these areas are also presented.
Keywords: endothelial cell, biomimetic modification, endothelialization, nitric oxide
1. Introduction
Metallic biomaterials, e.g. stainless steel, Ti alloys and Ni–Ti alloy, are commonly used in cardiovascular therapy. While such materials are favoured for their inertness and mechanical strength, negative clinic consequences often arise from limited biocompatibility [1–3]. Thrombosis and intimal hyperplasia are two main failure modes of cardiovascular devices. When a metallic biomaterial is implanted in vivo, the surface immediately contacts the surrounding biosystem, such as proteins, blood cells and the lining cells of the vessel wall, which will initiate cascaded reactions of thrombosis. Intimal hyperplasia is another issue caused by excessive growth of the tissues surrounding the device, which reduces the diameter of the vessels. For vascular stents, the proliferation of smooth muscle cells (SMCs) is thought to be the main reason of intimal hyperplasia during healing [4,5].
A healthy endothelium provides an anticoagulant and anti-proliferative surface through the expression of thrombomodulin, tissue factor pathway inhibitor, heparan sulphate proteoglycan, and through the release of prostacyclin and nitric oxide (NO). Among them, NO is of particular interest for endothelial cell (EC) function as NO produces multiple effects on the vessel wall. NO mediates a variety of physiological functions in vivo including regulation of blood vessel tone (vessel wall tension), vascular permeability and inhibition of leucocyte adhesion, platelet activation and SMC proliferation [6,7]. Inspired by the superb functionality of the endothelium, numerous studies in recent years have been focused on construction of the endothelium-mimicking surfaces on cardiovascular materials [8–12]. The strategies can be divided into two types—one is the mimicking function or composition of ECs by surface modification, and the other is aimed at the endothelialization of the material surface.
Although considerable progress has been made in the biomimetic modification of cardiovascular materials by both strategies, several challenges remain, especially for metallic cardiovascular materials. Firstly, long-term antithrombotic performance is of critical importance for implanted devices such as vascular stents, but it may be a great challenge [13]. Although surface heparinization has been widely studied since the 1960s, there are still no truly antithrombotic surfaces for long-term use today. The immobilized biomacromolecule will undergo rapid biological degradation in vivo, which leads to loss of antithrombotic activity in long-term usage. Secondly, covalent immobilization of organic or biological molecules on inorganic or metallic surface which is absent in reactive groups is also a challenge. Thirdly, multiple functions of antithrombogenicity, antiproliferation and rapid endothelialization are always simultaneously needed for cardiovascular devices. However, realization of rapid surface endothelialization and preservation of normal EC function are other challenges. In recent years, we have studied the long-term use of metallic cardiovascular biomaterials to surface modification, and some approaches are summarized below.
2. Mimicking function of endothelial cell by inducing NO release in vivo
Apart from other antithrombotic molecules, NO secreted by ECs has been shown to possess multiple vasoprotective activities [6,7]. In 1998, Murad and co-workers were awarded the Nobel Prize in Physiology for their discoveries regarding the important effects of NO on the cardiovascular system. Hence, NO is a more attractive agent to embody EC function for adapting into biomaterials.
NO-releasing polymers have been widely studied and proven to exhibit improved blood compatibility [14–16]. However, when such NO-releasing polymers were coated on the metallic stents, they cannot work effectively because of the rapid depletion of NO from a relatively thin coating of polymeric NO-donor reservoir [17]. For permanent devices fabricated with metal biomaterials, such as heart valve prosthesis and vascular stents, the long-term maintenance of haemocompatibility is required. In addition, NO generated from normal ECs has been estimated to be approximately 0.5–1 × 10–10 mol cm–2 min–1 [18,19], while the initial NO flux from NO-releasing polymers is always far greater than that of the normal EC owing to the initial burst [20]. Notably, some adverse effects are associated with the cytotoxicity of a high NO level caused by the formation of peroxynitrite (ONOO−), which will lead to tyrosine nitration, DNA damage and cell apoptosis [21,22]. Hence, NO release oriented at the physiological levels is mandatory.
A promising way of continuous NO release was developed by Oh & Meyerhoff [23]. Catalytic sites on the surface of polymer biomaterials are created, which continuously generate NO locally upon contact with blood from endogenous NO precursors and reducing agents already present in blood. For inorganic biomaterial of TiO2, a universal and convenient method of polydopamine was used in our study with full oxidation when compared with that of Lee et al. [24]. Simple immersion of substrates in a dilute aqueous solution of dopamine buffered to a pH typical of marine environments (2 mg ml−1 dopamine, 10 mM Tris, pH 8.5) with full oxidation, resulted in spontaneous deposition of a thin adherent polymer film [25]. It has been demonstrated that a compact and well-bonding polydopamine can be grafted on inorganic or metallic surfaces which can withstand powerful ultrasound treatment. The polydopamine coating worked out as an amazingly versatile platform for secondary reactions to immobilize bioactive molecules. Two kinds of low molecular weight (LMW) chemicals, cystamine and selenocystamine instead of biomacromolecules, were immobilized on the TiO2 surface as catalysts [25,26]. This approach reduced the inactivation by degradation, which typically occurs at grafting of a complete enzyme. S-nitrosothiols (RSNOs) present in the blood acts as the source of NO. Levels of RSNOs are constant at 7 µM [27] under normal physiological conditions which ensures steady and physiological levels of NO generation on the catalytically active surface.
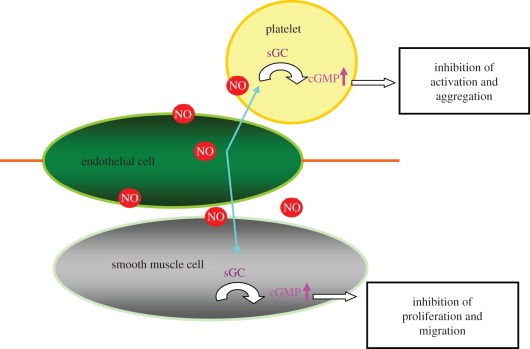
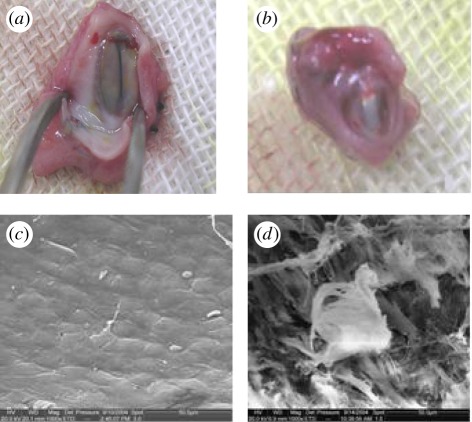
ECs continuously generate NO and the generated NO diffuses into platelets and the surrounding vascular SMCs and binds to soluble guanylate cyclase (sGC). Binding of NO to the haem-iron of sGC results in the formation of a pentacoordinate nitrosyl–haem complex, which breaks the bond to the axial histidine and activates the enzyme, leading to an increase in cyclic guanosine-3′,5′-monophosphate (cGMP). The NO/cGMP pathway regulates platelet activation and aggregation, as well as SMC proliferation and migration (figure 1) [28]. The initial evaluation of inducing NO release from vascular stents was performed in vitro and in vivo. NO generating catalytically active surfaces did show greatly reduced collagen-induced platelet activation with an elevated cGMP level, and this is beneficial for the application in vascular stents, as implantation of stents always causes endothelium injury and collagen exposure. The in vitro study also showed that the catalytic activity is preserved after incubation in PBS for three months. Selenocystamine-immobilized stents were implanted onto canine femoral arteries for two months and the intimal hyperplasia is significantly reduced (figure 2). These encouraging results suggest the potential application of this method in the modification of metallic stents. However, attention should be paid to the fact that when the surface is covered by protein, fibrin or cells owing to non-specific surface adsorption, the catalytic sites may be hidden. What is very interesting is that the surface of the selenocystamine-immobilized stent was covered with endothelial-like cells after two months of implantation (figure 3). The result indicates that NO release by this method shows no negative effects on endothelium healing. It is more beneficial than drug-eluting stents that significantly inhibit SMC proliferation simultaneously impeding endothelium healing.
Figure 1.
Schematic figure of NO–cGMP pathway in the inhibition of platelet activation and SMC proliferation.
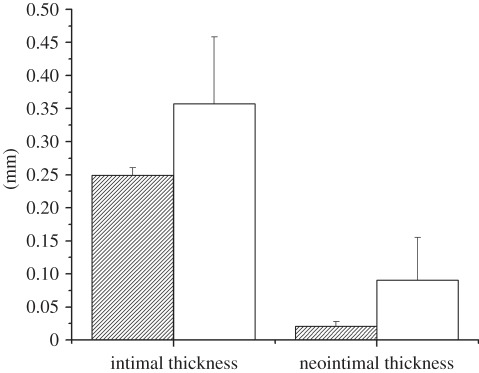
Figure 2.
Intimal and neointimal thickness analysis showing selenocystamine-immobilized stents (Se, striped bars) reduces the incidence of neointimal hyperplasia compared with that of stainless steel stents (SS, open bars).
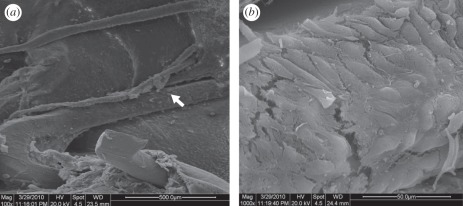
Figure 3.
Representative scanning electron photomicrographs showing endothelial cell-like cells covered the surface of the selenocystamine stents. (a) Lower magnification of 100×; (b) higher magnification of the region showing cobblestone-shaped cells on the surface of stent.
This approach is highly simplified, as NO formation is not regulated, whereas EC physiologically regulates NO generation upon mechanical or chemical disturbances. Nevertheless, the continuous NO generation for a comparably long term on the surface of metallic biomaterials may produce a healthy environment and support regeneration of the damaged implantation site. But it is not clear how long this LMW organic molecule-immobilized surface can be present in vivo, maybe endothelialization of the surface is the ultimate solution. Therefore, ‘repair instead of replace’ has become another topic for inducing endothelialization in vivo.
3. Inducing endothelialization in vivo
Ideally, when a metallic cardiovascular biomaterial is covered by a layer of healthy EC, problems such as thrombosis and intimal hyperplasia will not exist. But there are still two issues. Firstly, in the extracellular matrix (ECM), chemical cues that are present control all aspects of cell biology. ECM not only plays a mechanical role in supporting and maintaining tissue architecture but also acts as a dynamic meshwork in actively regulating critical cellular functions such as migration, survival, proliferation and differentiation [29,30]. Several in vitro studies have indicated that most of the metallic materials do not support cell growth owing to the lack of biorecognition and/or specific interaction [31,32]. When ECs are grown on bare material, they lose their normal phenotype and become more prothrombotic than they should be to prevent any thrombotic tendency of the device [32]. Secondly, endothelialization in vitro has two surgical procedures—one for obtaining the cells, and the other for implantation. This is inconvenient for the patient and it is not suitable for emergency cases at all. In addition, it requires a long cell culture time in vitro, which brings in concerns for potential phenotypic and genotypic changes, as well as bacterial contamination. An alternative approach to endothelialization in vitro would be to develop materials that better initiate a healing response in vivo.
3.1. Construction of extracellular matrix on metallic biomaterials
ECM surrounding mature ECs is organized into two distinct compartments—the basement membrane and the interstitial matrix. The basement membrane consists of a network of molecules composed primarily of type IV collagen, laminin (Ln) and heparan sulphate proteoglycan (HSPG), whereas typical components of the interstitial matrix include fibrillar collagens and glycoproteins such as fibronectin (Fn) and vitronectin (Vn). An effective method of promoting integration and adhesion of the cells onto the metallic devices is to immobilize agents such as ECM proteins or other compositions directly onto the device surface, and then the presence of integrin-binding site will mediate EC adhesion and migration.
Numerous studies were carried out in the immobilization of a single adhesive molecule to mimick ECM, e.g. Fn, collagen or Ln [33–36]. But most of the adhesive molecules not only accelerate adherence of ECs, but platelet adhesion and proliferation of SMCs are also simultaneously enhanced [37,38]. As blood compatibility is the first consideration of cardiovascular devices, especially in the initial implantation, a simplified ECM mode of adhesive protein combined with an antithrombotic molecule was co-immobilized in our study. Immobilized proteins sometimes lose their activity on the surface by a conformational change. In physiological conditions, proteins always form supramolecular structures to keep activity or preserve conformation via ‘key and lock’ recognition or electrostatic interaction. Thus, construction of a supramolecular structure on metallic biomaterials by various methods was attempted in our study.
A technique of combining electrostatic interaction and covalent immobilization was developed to form heparin and fibronectin (Hep/Fn) films on aminosilanized Ti surfaces by Li et al. [39]. In this method, heparin and fibronectin were first blended to form a supramolecular complex by electrostatic interaction, and silane-based strategies were used for further covalent immobilization of the complex. Initial work shows that both the activity of heparin to bind with antithrombin-III and the activity of fibronectin on RGD exposure are preserved, and the Hep/Fn co-immobilized film keeps excellent bioactivity after immersion in PBS for 5 days. However, a longer time should be under consideration in future study to ensure synergistic effects of heparin on Fn preventing platelet adhesion and enhancing blood compatibility in the period when the implants are not yet completely covered by ECs. Collagen is one of the main components of ECM, and has been widely used in scaffold construction in tissue engineering [40,41], but it activates the extrinsic coagulation system when it is in contact blood. A combined immobilization of collagen and sulphated chitosan (SCS) as a supramolecular film was studied by Li et al. using a layer-by-layer (LBL) self-assembly technique based on electrostatic interaction [42]. LBL technique allows films with layer thickness and interlayer separation to be controlled on the nanometre scale, with controlled surface structure and charge, which in turn govern the biological responses. Collagens, adhesive proteins and proteoglycans—the three major constituents of ECM—usually form a three-dimensional nanoscale hydrogel in vivo. In our study, an ECM-like hydrogel coating of collagen and SCS was also obtained, which is beneficial for improved biocompatibility. A collagen and heparin LBL supramolecular film was also prepared by Chen et al. [43]. It shows that the modified surface reduces platelet adhesion under dynamic conditions and possesses the ability to maintain cell viability.
Although there are some disadvantages of over-simplification of ECM while mimicking real ECM by this method, it has two definite advantages: (i) as the composition is simple, it provides a clean background to interpret the results; (ii) it is much more convenient for fabrication and control the composition.
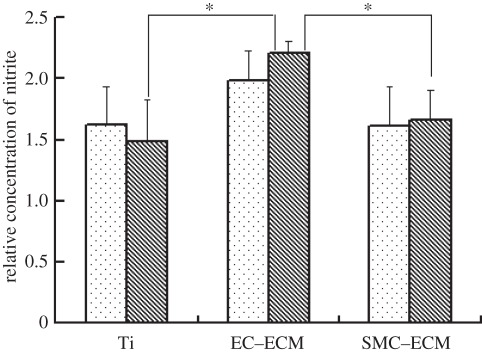
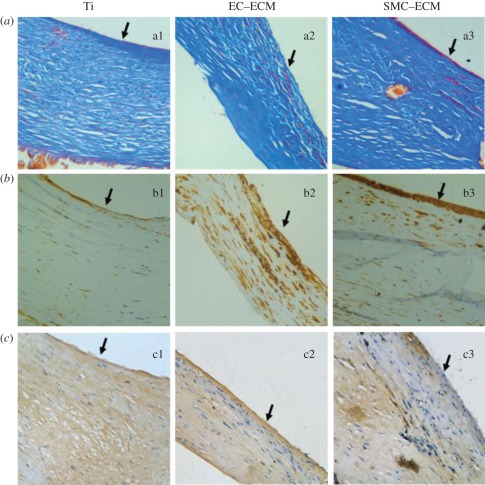
Decellularized ECMs with a more complex composition on metallic biomaterial were studied by Tu et al. [44]. In their study, titanium (Ti) discs were coated with ECM secreted by ECs (EC–ECM) or SMCs (SMC–ECM), so as to help ECs proliferate and migrate and to improve their endothelialization in vivo. It was found that a smooth surface of Ti does not support ECM adhesion, and the constructed ECM can be easily pulled off during incubation. A porous surface of Ti with enhanced hydrophilicity was obtained by NaOH treatment, which supported the initial cell adhesion and the anchorage of the ECM. It was found that ECM from EC (EC–ECM) was more bioactive and ECs growing on EC–ECM can secrete more NO than ECM secreted by SMCs (SMC–ECM; figure 4). ECM deposition not only stimulated EC proliferation, increased EC migration speed and re-cellularization speed, but also inhibited platelet adhesion. The exposure of ECM after removal of ECs is generally regarded as the reason for thrombi formation at an injured blood vessel in vivo, but in this study, both types of ECM coating resulted in significantly lower platelet adhesion than that on Ti discs. The reason is not completely clear now, but some factors, including the decellularized ECM, cannot release tissue factor, which induces activation of the clotting cascade and may play an important role. In vivo results show that ECM derived from ECs was superior to those derived from SMCs in accelerating endothelialization and inhibition of neointima hyperplasia (figure 5), which is in agreement with the in vitro study as more production of NO will inhibit SMC proliferation.
Figure 4.
Production of NO by ECs cultured on the control and ECM-modified Ti. *p < 0.001 compared with the control Ti. Dotted bars, 1 day; striped bars, 3 days.
Figure 5.
Micrographs of neointima growing on the surfaces of Ti and ECM-Ti exhibited by (a) Masson's Trichrome Stains and (b) immunohistochemical stains for α-SMA and (c) vWF; scale bar, 400×.
There are three mechanisms of EC ingrowth into a vascular graft in vivo. (i) The first is pannus ingrowth, i.e. the migration of ECs inward across the anastomosis from the native vessel. (ii) The second is transmural endothelialization. In this mode, EC is derived from the ingrowth of capillaries through porous grafts. (iii) The third is EC fallout deposition on the surface, which is based on capturing endothelial progenitor cells (EPCs) from circulating blood onto the luminal surface of the implants. All the above studies show that construction of ECM on Ti materials provides cues to direct cellular activity through integrin interactions that control an assortment of biological processes. Integrin-induced EC adhesion and growth mainly determine pannus ingrowth and transmural endothelialization. The degree of endothelialization there is limited because ECs are terminally differentiated cells with low ability for migration and proliferation (table 1).
Table 1.
Cues used in the study to induce or enhance the formation of an endothelial layer.
| cues | mechanism of enhancing endothelialization | effect of endothelialization |
|---|---|---|
| Fn, collagen or decellularized ECMs | integrin-induced endothelial cell adhesion | slow (for days) and limited endothelialization |
| CD34 antibody | capturing of EPCs (containing early EPCs) | rapid (for hours) endothelialization but the captured EPCs may differentiate into SMCs |
| peptide aptamer | capturing of late EPCs | rapid, pure but limited endothelialization due to small amount of late EPCs |
3.2. Endothelialization in vivo by capturing endothelial progenitor cells
As it is circulating EPCs, instead of mature ECs, that play the central role in endothelialization of longer grafts, we also focused on surface modification of cardiovascular devices using CD34 antibodies or aptamers for rapid EPC-induced endothelialization in vivo.
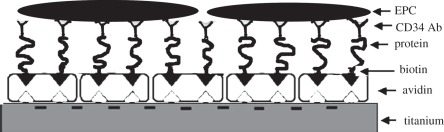
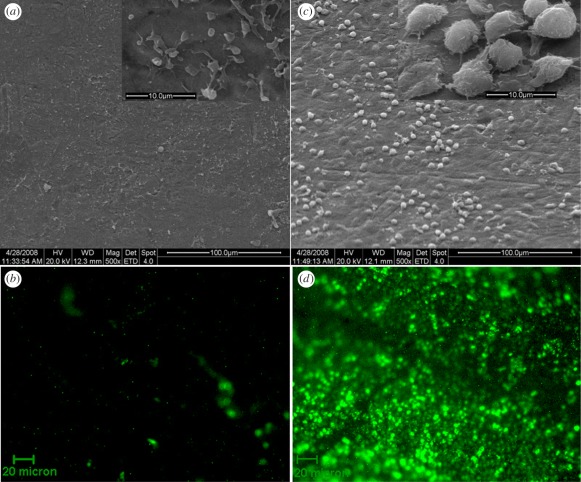
Circulating EPCs have been identified by the expression of cell surface antigen CD34; therefore, some studies attempted to accelerate the endothelialization of vascular stents by capturing circulating EPCs from the bloodstream onto a specially designed surface. The immobilization of CD34 antibodies on a material surface notably leads to capturing of EPCs from circulation. It has been reported that random immobilization generally leads to significant denaturation of the antibody [45]. Butler et al. [46] have reported that more than 90 per cent of monoclonal and 75 per cent of polyclonal anti-fluorescyl capture antibodies (CAbs) were denatured by passive adsorption in the immunochemistry of sandwich ELISAs. To improve the bioactivity of immobilized antibodies, anti-CD34 was immobilized onto titanium surfaces in upside orientation, such that the antigen-binding sites are oriented towards the medium in the study of Li et al. [47] (figure 6). The results suggest that the anti-CD34 antibody orderly immobilized surfaces could increase EPC attachment and capture, and induce rapid endothelialization of the luminal surface of the implant in vivo (figure 7). However, the specificity and selectivity of CD34 antibody to EPCs is controversial in clinical application [48,49]. It is well accepted that poly(ethylene glycol) (PEG) as spacer for surface immobilization of biomolecules is able to reduce non-specific interaction and potentiate specific bioactive interactions. Chen et al. [50] developed another method by covalently bonding PEG, and then bonding CD34 antibody on titanium surfaces. The final modified surface allows cell recognition which could promote EPC attachment and inhibit SMC attachment.
Figure 6.
Schematic figure of oriented immobilization of anti-CD34 Ab on Ti surface.
Figure 7.
Representative scanning electron photomicrographs showing EPCs captured in vivo at 2 h after implantation (a) and (b) untreated Ti (bare Ti); (c,d) anti-CD34-coated Ti; (a,c) SEM photograph (the inset is the high magnification). (b,d) FITC-fluorescent stain for EPCs/ECs marker of VEGFR-2 showing anti-CD34 antibody-coated surface was almost fully covered with EPCs/CEs marker positive cells.
EPCs are not sufficiently characterized by their CD34+ marker. Because of the heterogeneity of the CD34+ population and insufficient definition of further EPC surface antigens, it was reported that a population of CD34+ cells may also differentiate into SMCs [51,52]. So the success of rapid endothelialization by capturing EPCs depends on a better characterization and understanding of EPC biology. However, it is an exciting development towards endothelialization which mimics the repair potential of vascular wall regeneration.
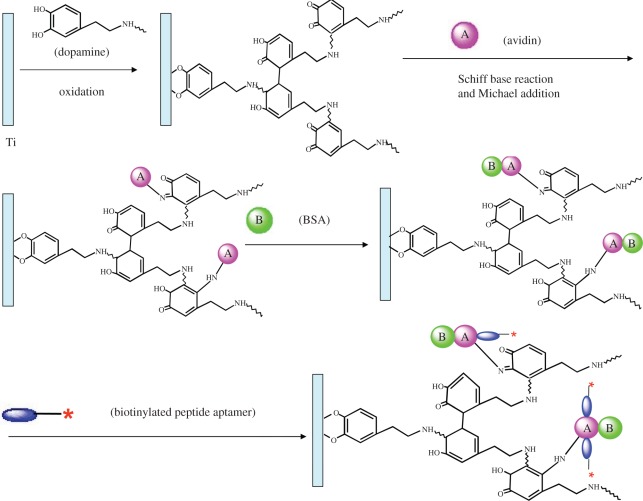
In contrast with EPCs or early EPCs, late EPCs will differentiate into ECs directly but never differentiate into SMCs. We have also pursued a new method for the design of rapid endothelialization of titanium surface using peptide aptamer evolved from late EPCs. Aptamers are a relatively new group of nucleic acid or peptide species that can bind with high affinity and specificity to a wide range of target molecules such as peptides, proteins, drugs, organic and inorganic molecules or even whole cells. Owing to degradation by nuclease, DNA aptamers will soon lose their activity in vivo. In this strategy, biotinylated peptide aptamer with a high affinity and specificity to late EPC was immobilized on the surface via the avidin–biotin system, and in addition, bovine serum albumin (BSA) was co-immobilized on the surface for reducing non-specific interaction and increasing specific bioactive interactions (figure 8). In vitro study shows that the surface leads to a remarkable enhancement in EPC adhesion and proliferation, as well as better haemocompatibility. The potential of such a multifunctional aptamer and BSA-co-immobilized surface for application in vascular stents is under study.
Figure 8.
A schematic figure showing biotinylated peptide aptamer conjugated to titanium. The surface of titanium was first deposited on layers of polydopamine and then reacted with avidin, which binds to BSA. The final reaction between avidin and biotinylated peptide aptamer linked the biotinylated peptide aptamer to the substrate.
4. Discussions and future directions
4.1. In vivo derivation of biomimetic surfaces and its influence on remodelled extracellular matrix
ECM or other components of cells are under a balance of generation and degradation to maintain normal functions in vivo. When an artificially constructed biomimetic surface is implanted in vivo, various proteases, lipases or amylases will still mediate the ultimate degradation of the immobilized protein, heparin or other active molecules. On the other hand, the immobilized biological molecule will initiate several biological reactions of protein adsorption, cell adhesion and tissue growth on the surface. However, the process of derivation of biomimetic surfaces in vivo is a ‘black box’ and we do not know how it influences tissue regeneration and remodelling; we do not know the change of interlayer between the substrate materials and the tissue when the immobilized biomolecule is cleaved by proteolysis; we even do not know if it is possible that there is only a single layer of EC without an underlayer of SMCs on the biomimetic surface. Detailed investigations should be carried out to look into the facts. For ECs or EPCs, the biomimetic surface provides chemical cues as stimuli for adhesion, proliferation or differentiation, which act as an artificial microenvironment. The artificial microenvironment will be eventually replaced by a remodelled microenvironment, namely of ECM. Simultaneously, the artificial microenvironment ultimately determines the remodelled ECM. The interaction between cells and ECM components finally determines the cellular behaviour on biomaterials. Appropriate ECM formation, turnover and remodelling on endothelialized surfaces are crucial for long-term function preservation of normal ECs. Then the main factors influencing the artificial microenvironment on ECM remodelling should be evaluated in the future.
4.2. The influence of metallic or inorganic substrate on endothelialization
In the study of Prasad & Krishnan, when ECs are grown on bare Ti material, they lose their normal phenotype and become prothrombotic in vitro [32]. In our previous study, two kinds of materials, Ti-O film-coated stainless steel (Ti-O) and stainless steel (SS), were implanted in canine femoral arteries for five months. The results show that the surface of Ti-O was covered by a thin layer of EC, while the surface of SS was covered with a thick fibrous texture which was prothrombotic (figure 9). Nevertheless, it is noteworthy that after oral anticoagulation with warfarin for the first 3 days, no further anticoagulating measures were adopted. In this case, endothelialization of Ti-O surface was mainly owing to migration of the surrounding EC from the vessel. There was no thrombosis and tissue proliferation on the surface of Ti-O indicating that normal EC phenotype or function was preserved. Thus, the substrates have different influences on endothelialization. Since the constructed biomimetic layer will be digested in vivo ultimately, the substrate may also influence the long-term function of the endothelialized surface, and yet we do not know to which extent. Although the mechanism of cell–material interactions on Ti-O is not known, a specific prohealing response should be initiated, e.g. a certain key protein or factor is preferentially adsorbed on the surface and then initiates the process. Future study should aim to look into the mechanisms and thus to direct the construction of more desirable surfaces to control the biological activation processes.
Figure 9.
(a,b) Digital images and (c,d) SEM images of (a,c) Ti-O and (b,d) SS at five months post implantation. It shows a thin and transparent tissue adherent on Ti-O (a), while SS is embedded in a thick fibrin tissue capsule (b). SEM image shows typical cobblestone-shaped endothelial cells on the surface of Ti-O (c) and fibrous tissue on the surface of SS (d).
4.3. Special challenge of endothelialization on heart valve prosthesis
Endothelialization of vascular stents is comparably easy. The bare metallic stent will be endothelialized one month after implantation although it may be over-healed owing to SMC proliferation. But when it comes to heart valve prosthesis, realization of surface endothelialization is quite difficult, especially for the valve leaflets. Since valve leaflets do not contact the surrounding tissue and keep on opening and closing, ECs cannot migrate to the surfaces. In addition, the surface areas of valve leaflets are large and there is high shear stress, and complete endothelialization is considered to be quite difficult. However, a ventricular assist device with textured surface design studied by Menconi et al. [53] was implanted for 324 days. In their study, the textured surface encouraged the formation of a tightly adherent, haemocompatible, viable pseudointima lining which never exceeded 150 μm in thickness, and remained free of pathological calcification. No clinical thromboembolic events or pump-related thromboembolism occurred. It provides us with a valuable reference and so we propose the hypothesis that maybe the surface of the heart valve prosthesis can be endothelialized. Surely, the amount of EPCs is too scarce to cover the heart valve prosthesis, and more stem cells other than EPCs should be mobilized to fulfil this dream. Moreover, natural processes of blood vessel healing should be referenced as a guide, to give the surface certain defined functional properties required to activate the prohealing response and appropriately direct cellular activity.
Acknowledgments
The financial support for this work by Key Basic Research Project (No. 2011CB606204), Natural Science Foundation of China (nos 30900295, 31170916, 31000426 and 30831160509) is greatly acknowledged.
References
- 1.Barnes K. 2008. Complications in patients with ventricular assist devices. Dimens. Crit. Care Nurs. 27, 233–241 10.1097/01.DCC.0000338867.24293.20 (doi:10.1097/01.DCC.0000338867.24293.20) [DOI] [PubMed] [Google Scholar]
- 2.Ruel M., Kulik A., Lam B. K., Rubens F. D., Hendry P. J., Masters R. G., Bédard P., Mesana T. G. 2005. Long-term outcomes of valve replacement with modern prostheses in young adults. Eur. J. Cardiothorac. Surg. Mar. 27, 425–433 10.1016/j.ejcts.2004.12.002 (doi:10.1016/j.ejcts.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Cheung Y. F., Sanatani S., Leung M. P., Human D. G., Chau A. K. T., Culham J. A. G. 2000. Early and intermediate-term complications of self-expanding stents limit its potential application in children with congenital heart disease. J. Am. Coll. Cardiol. 35, 1007–1015 10.1016/S0735-1097(99)00644-0 (doi:10.1016/S0735-1097(99)00644-0) [DOI] [PubMed] [Google Scholar]
- 4.Clark D. J., Lessio S., O'Donoghue M., Tsalamandris C., Schainfeld R., Rosenfield K. 2006. Mechanisms and predictors of carotid artery stent restenosis: a serial intravascular ultrasound study. J. Am. Coll. Cardiol. 47, 2390–2396 10.1016/j.jacc.2006.01.076 (doi:10.1016/j.jacc.2006.01.076) [DOI] [PubMed] [Google Scholar]
- 5.Chang C. C., Ong E. T. 2005. Coronary restenosis. Acta. Cardiol. Sin. 21, 177–189 [Google Scholar]
- 6.Santiago L., Pérez-Sala D., Moncada S. 1998. Nitric oxide: from discovery to the clinic. TiPS 19, 436–438 10.1016/S0165-6147(98)01265-6 (doi:10.1016/S0165-6147(98)01265-6) [DOI] [PubMed] [Google Scholar]
- 7.Naseem K. M. 2005. The role of nitric oxide in cardiovascular diseases. Mol. Aspects Med. 26, 33–65 10.1016/j.mam.2004.09.003 (doi:10.1016/j.mam.2004.09.003) [DOI] [PubMed] [Google Scholar]
- 8.Sperling C., Salchert K., Streller U., Werner C. 2004. Covalently immobilized thrombomodulin inhibits coagulation and complement activation of artificial surfaces in vitro. Biomaterials 25, 5101–5113 10.1016/j.biomaterials.2003.12.014 (doi:10.1016/j.biomaterials.2003.12.014) [DOI] [PubMed] [Google Scholar]
- 9.Wu B., Gerlitz B., Grinnell B. W., Meyerhoff M. E. 2007. Polymeric coatings that mimic the endothelium: combining nitric oxide release with surface-bound active thrombomodulin and heparin. Biomaterials 28, 4047–4055 10.1016/j.biomaterials.2007.06.002 (doi:10.1016/j.biomaterials.2007.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick C., Wadsworth R. M., Jones R. L., Kennedy S. 2007. Prostacyclin analogues: the next drug-eluting stent? Biochem. Soc. Trans. 35, 910–911 10.1042/BST0350910 (doi:10.1042/BST0350910) [DOI] [PubMed] [Google Scholar]
- 11.Xia Y., Boey F., Venkatraman S. S. 2010. Surface modification of poly(l-lactic acid) with biomolecules to promote endothelialization. Biointerphases 5, 32–40 10.1116/1.3467508 (doi:10.1116/1.3467508) [DOI] [PubMed] [Google Scholar]
- 12.Jun H. W., West J. 2004. Development of a YIGSR-peptide-modified polyurethaneurea to enhance endothelialization. J. Biomater. Sci. Polym. Edn. 15, 73–94 10.1163/156856204322752246 (doi:10.1163/156856204322752246) [DOI] [PubMed] [Google Scholar]
- 13.Ratner B. D. 2007. The catastrophe revisited: blood compatibility in the 21st century. Biomaterials 28, 5144–5147 10.1016/j.biomaterials.2007.07.035 (doi:10.1016/j.biomaterials.2007.07.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleser P. S., et al. 2004. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J. Vasc. Surg. 40, 803–811 10.1016/j.jvs.2004.07.007 (doi:10.1016/j.jvs.2004.07.007) [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Annich G. M., Miskulin J., Osterholzer K., Merz S. I., Bartlett R. H., Meyerhoff M. E. 2002. Nitric oxide releasing silicone rubbers with improved blood compatibility: preparation, characterization, and in vivo evaluation. Biomaterials 23, 1485–1494 10.1016/S0142-9612(01)00274-5 (doi:10.1016/S0142-9612(01)00274-5) [DOI] [PubMed] [Google Scholar]
- 16.Riccio D. A., Dobmeier K. P., Hetrick E. M., Privett B. J., Paul H. S., Schoenfisch M. H. 2009. Nitric oxide-releasing S-nitrosothiol-modified xerogels. Biomaterials 30, 4494–4502 10.1016/j.biomaterials.2009.05.006 (doi:10.1016/j.biomaterials.2009.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon J. H., Wu C. J., Homme J. 2002. Local delivery of nitric oxide from an eluting stent to inhibit neointimal thickening in a porcine coronary injury model. Yonsei. Med. J. 43, 242–251 [DOI] [PubMed] [Google Scholar]
- 18.Reynolds M. M., Frost M. C., Meyerhoff M. E. 2004. Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications. Free Radic. Biol. Med. 37, 926–936 10.1016/j.freeradbiomed.2004.06.019 (doi:10.1016/j.freeradbiomed.2004.06.019) [DOI] [PubMed] [Google Scholar]
- 19.Vaughn M. W., Kuo L., Liao J. C. 1998. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am. J. Physiol. 274, H2163–H2176 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds M. M., Hrabie J. A., Oh B. K. 2006. Nitric oxide releasing polyurethanes with covalently linked diazeniumdiolated secondary amines. Biomacromolecules 7, 987–994 10.1021/bm060028o (doi:10.1021/bm060028o) [DOI] [PubMed] [Google Scholar]
- 21.Pryor W. A., Squadrito G. L. 1995. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 268, L699–L722 [DOI] [PubMed] [Google Scholar]
- 22.Amirmansour C., Vallance P., Bogle R. G. 1999. Tyrosine nitration in blood vessels occurs with increasing nitric oxide concentration. Br. J. Pharmacol. 127, 788–794 10.1038/sj.bjp.0702590 (doi:10.1038/sj.bjp.0702590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh B. K., Meyerhoff M. E. 2004. Catalytic generation of nitric oxide from nitrite at the interface of polymeric films doped with lipophilic Cu(II)-complex: a potential route to the preparation of thromboresistant coatings. Biomaterials 25, 283–293 10.1016/S0142-9612(03)00530-1 (doi:10.1016/S0142-9612(03)00530-1) [DOI] [PubMed] [Google Scholar]
- 24.Lee H., Dellatore S. M., Miller W. M., Messersmith P. B. 2007. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 10.1126/science.1147241 (doi:10.1126/science.1147241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng Y. J., Song Q., Zhou Y. J., Zhang L. P., Wang J., Chen J. Y., Leng Y. X., Li S. Y., Huang N. 2011. Immobilization of selenocystamine on TiO2 surfaces for in situ catalytic generation of nitric oxide and potential application in intravascular stents. Biomaterials 32, 1253–1263 10.1016/j.biomaterials.2010.10.039 (doi:10.1016/j.biomaterials.2010.10.039) [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y. J., Weng Y. J., Zhang L. P., Jing F. J., Huang N., Chen J. Y. 2011. Cystamine immobilization on TiO2 film surfaces and the influence on inhibition of collagen-induced platelet activation. Appl. Surf. Sci. 258, 1776–1783 10.1016/j.apsusc.2011.10.043 (doi:10.1016/j.apsusc.2011.10.043) [DOI] [Google Scholar]
- 27.Kelm M. 1999. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta 1411, 273–289 10.1016/S0005-2728(99)00020-1 (doi:10.1016/S0005-2728(99)00020-1) [DOI] [PubMed] [Google Scholar]
- 28.Burstyn J. N., Yu A. E., Dierks E. A., Hawkins B. K., Dawson J. H. 1995. Studies of the heme coordination and ligand binding properties of soluble guanylyl cyclase (sGC): characterization of Fe(II)sGC and Fe(II)sGC(CO) by electronic absorption and magnetic circular dichroism spectroscopies and failure of CO to activate the enzyme. Biochemistry 34, 5896–5903 10.1021/bi00017a019 (doi:10.1021/bi00017a019) [DOI] [PubMed] [Google Scholar]
- 29.Attiah D. G., Kopher R. A., Desai T. A. 2003. Characterization of PC12 cell proliferation and differentiation-stimulated by ECM adhesion proteins and neurotrophic factors. J. Mater. Sci. Mater. Med. 14, 1005–1009 10.1023/A:1026363018805 (doi:10.1023/A:1026363018805) [DOI] [PubMed] [Google Scholar]
- 30.Ferreira K. S., Almeida S. R., Ribeiro C. H., Mariano M., Lopes J. D. 2003. Modulation of proliferation, differentiation and cytokine secretion of murine B-1b cells by proteins of the extracellular matrix. Immunol. Lett. 86, 15–21 10.1016/S0165-2478(02)00266-3 (doi:10.1016/S0165-2478(02)00266-3) [DOI] [PubMed] [Google Scholar]
- 31.Gupta A., Majumdar P., Amit J., Rajesh A., Singh S. B., Chakraborty M. 2006. Cell viability and growth on metallic surfaces: in vitro studies. Trends Biomater. Artif. Organs 20, 84–89 [Google Scholar]
- 32.Prasad C. K., Krishnan L. K. 2008. Regulation of endothelial cell phenotype by biomimetic matrix coated on biomaterials for cardiovascular tissue engineering. Acta Biomater. 4, 182–191 10.1016/j.actbio.2007.05.012 (doi:10.1016/j.actbio.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 33.Volcker N., Klee D., Hocker H., Langefeld S. 2001. Functionalization of silicone rubber for the covalent immobilization of fibronectin. J. Mater. Sci.: Mater. Med. 12, 111–119 10.1023/A:1008938525489 (doi:10.1023/A:1008938525489) [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Wang W., Feng Q., Cui F., Xu Y. 2006. A novel method to immobilize collagen on polypropylene film as substrate for hepatocyte culture. Mater. Sci. Eng.: C 26, 657–663 10.1016/j.msec.2005.08.039 (doi:10.1016/j.msec.2005.08.039) [DOI] [Google Scholar]
- 35.Ge S. N., Chen J. Y., Leng Y. X., Huang N. 2007. Laminin immobilized on titanium oxide films for enhanced human umbilical vein endothelial cell adhesion and growth. Key Eng. Mater. 342–343, 305–308 10.4028/www.scientific.net/KEM.342-343.305 (doi:10.4028/www.scientific.net/KEM.342-343.305) [DOI] [Google Scholar]
- 36.Zhang Y., Chai C., Jiang X. S., Teoh S. H., Leong K. W. 2007. Fibronectin immobilized by covalent conjugation or physical adsorption shows different bioactivity on aminated-PET. Mater. Sci. Eng.: C 27, 213–219 10.1016/j.msec.2006.03.013 (doi:10.1016/j.msec.2006.03.013) [DOI] [Google Scholar]
- 37.Beumer S., Heijnen-Snyder G. J., IJsseldijk M. J., de Groot P. G., Sixma J. J. 2000. Fibronectin in an extracellular matrix of cultured endothelial cells supports platelet adhesion via its ninth type III repeat: a comparison with platelet adhesion to isolated fibronectin. Arterioscler. Thromb. Vasc. Biol. 20, 16–25 10.1161/01.ATV.20.4.e16 (doi:10.1161/01.ATV.20.4.e16) [DOI] [PubMed] [Google Scholar]
- 38.Hirst S. J., Twort C. H. C., Lee T. H. 2000. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am. J. Respir. Cell. Mol. Biol. 23, 335–344 [DOI] [PubMed] [Google Scholar]
- 39.Li G. C., Yang P., Qin W., Maitz M. F., Zhou S., Huang N. 2011. The effect of coimmobilizing heparin and fibronectin on titanium on hemocompatibility and endothelialization. Biomaterials 32, 4691–4703 10.1016/j.biomaterials.2011.03.025 (doi:10.1016/j.biomaterials.2011.03.025) [DOI] [PubMed] [Google Scholar]
- 40.Glowacki J., Mizuno S. 2008. Collagen scaffolds for tissue engineering. Biopolymers 89, 338–344 10.1002/bip.20871 (doi:10.1002/bip.20871) [DOI] [PubMed] [Google Scholar]
- 41.Parenteau-Bareil R., Gauvin R., Berthod F. 2010. Collagen-based biomaterials for tissue engineering applications. Materials 3, 1863–1887 10.3390/ma3031863 (doi:10.3390/ma3031863) [DOI] [Google Scholar]
- 42.Li Q. L., Huang N., Chen J. L., Wan G. J., Zhao A. S., Chen J. Y., Wang J., Yang P., Leng Y. X. 2008. Anticoagulant surface modification of titanium via layer-by-layer assembly of collagen and sulfated chitosan multilayers. J. Biomed. Mater. Res. 89A, 575–584 10.1002/jbm.a.31999 (doi:10.1002/jbm.a.31999) [DOI] [PubMed] [Google Scholar]
- 43.Chen J. L., Chen C., Chen Z. Y., Chen J. Y., Li Q. L., Huang N. 2010. Collagen/heparin coating on titanium surface improves the biocompatibility of titanium applied as a blood-contacting biomaterial. J. Biomed. Mater. Res. A 95, 341–349 10.1002/jbm.a.32847 (doi:10.1002/jbm.a.32847) [DOI] [PubMed] [Google Scholar]
- 44.Tu Q. F., Zhao Y. C., Xue X. Q., Wang J., Huang N. 2010. Improved endothelialization of titanium vascular implants by extracellular matrix secreted from endothelial cells. Tissue Eng.: Part A 16, 3635–3645 10.1089/ten.tea.2010.0088 (doi:10.1089/ten.tea.2010.0088) [DOI] [PubMed] [Google Scholar]
- 45.Butler J. E., Ni L., Nessler R., Joshi K. S., Suter M., Rosenberg B., Chang J., Brown W. R., Cantarero L. A. 1992. The physical and functional behavior of capture antibodies adsorbed on polystyrene. J. Immunol. Methods 150, 77–90 10.1016/0022-1759(92)90066-3 (doi:10.1016/0022-1759(92)90066-3) [DOI] [PubMed] [Google Scholar]
- 46.Butler J. E., Ni L., Brown W. R., Joshi K. S., Chang J., Rosenberg B., Voss E. W. 1993. The immunochemistry of sandwich ELISAs–VI. Greater than 90% of monoclonal and 75% of polyclonal anti-fluorescyl capture antibodies (CAbs) are denatured by passive adsorption. Mol. Immunol. 30, 1165–1175 10.1016/0161-5890(93)90135-X (doi:10.1016/0161-5890(93)90135-X) [DOI] [PubMed] [Google Scholar]
- 47.Li Q. L., Huang N., Chen C., Chen J. L., Xiong K. Q., Chen J. Y., You T. X., Jin J., Liang X. 2010. Oriented immobilization of anti-CD34 antibody on titanium surface for self-endothelialization induction. J. Biomed. Mater. Res. A 94A, 1283–1293 10.1002/jbm.a.32812 (doi:10.1002/jbm.a.32812) [DOI] [PubMed] [Google Scholar]
- 48.Beijk M. A., et al. 2010. Genous endothelial progenitor cell capturing stent versus the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur. Heart. J. 31, 1055–1064 10.1002/jbm.a.32812 (doi:10.1002/jbm.a.32812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garg S., Duckers H. J., Serruys P. W. 2010. Endothelial progenitor cell capture stents: will this technology find its niche in contemporary practice? Eur. Heart. J. 31, 1032–1055 10.1093/eurheartj/ehp591 (doi:10.1093/eurheartj/ehp591) [DOI] [PubMed] [Google Scholar]
- 50.Chen J. L., Cao J. J., Wang J., Chen Z. Y., Zhao Y. C., Li Q. l., Xiong K. Q., Huang N. 2012. Investigation on hemocompatibility and endothelialization of titanium vascular implants modified by PEG and CD34 antibody. J. Colloid Interface Sci. 368, 636–647 10.1016/j.jcis.2011.11.039 (doi:10.1016/j.jcis.2011.11.039) [DOI] [PubMed] [Google Scholar]
- 51.Shimizu K., Sugiyama S., Aikawa M., Fukumoto Y., Rabkin E., Libby P., Mitchell R. N. 2001. Host bone-marrow cells are a source of donor intimal smooth muscle-like cells in murine aortic transplant arteriopathy. Nat. Med. 7, 738–741 10.1038/89121 (doi:10.1038/89121) [DOI] [PubMed] [Google Scholar]
- 52.Simper D., Stalboerger P. G., Panetta C. J., Wang S., Caplice N. M. 2002. Smooth muscle progenitor cells in human blood. Circulation 106, 1199–1204 10.1161/01.CIR.0000031525.61826.A8 (doi:10.1161/01.CIR.0000031525.61826.A8) [DOI] [PubMed] [Google Scholar]
- 53.Menconi M. J., Pockwinse S., Owen T. A., Dasse K. A., Stein G. S., Lian J. B. 1995. Properties of blood-contacting surfaces of clinically implanted cardiac assist devices: gene expression, matrix composition, and ultrastructural characterization of cellular linings. J. Cell. Biochem. 57, 557–573 10.1002/jcb.240570320 (doi:10.1002/jcb.240570320) [DOI] [PubMed] [Google Scholar]