Abstract
Porous scaffolds fabricated from biocompatible and biodegradable polymers play vital roles in tissue engineering and regenerative medicine. Among various scaffold matrix materials, poly(lactide-co-glycolide) (PLGA) is a very popular and an important biodegradable polyester owing to its tunable degradation rates, good mechanical properties and processibility, etc. This review highlights the progress on PLGA scaffolds. In the latest decade, some facile fabrication approaches at room temperature were put forward; more appropriate pore structures were designed and achieved; the mechanical properties were investigated both for dry and wet scaffolds; a long time biodegradation of the PLGA scaffold was observed and a three-stage model was established; even the effects of pore size and porosity on in vitro biodegradation were revealed; the PLGA scaffolds have also been implanted into animals, and some tissues have been regenerated in vivo after loading cells including stem cells.
Keywords: poly(lactide-co-glycolide) (PLGA), porous scaffolds, tissue engineering, biodegradation, mechanical properties
1. Introduction
Biodegradable materials are very important in medical applications such as tissue engineering, tissue induction, drug-controlled release and internal fixation [1–3]. A specific physical form of materials, porous scaffolds has been paid much attention in the latest decade owing to the emergence of modern regenerative medicine. This material form has a rich physics as well as broad potential applications. The present review will concern the progress of the pertinent interface between physics, biology and material sciences.
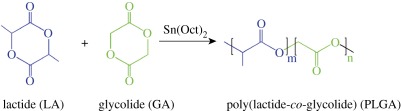
The development of tissue engineering, tissue induction and other types of regenerative medicine depend strongly upon the techniques of three-dimensional porous scaffolds composed of organic and inorganic substrates, very frequently, biocompatible and biodegradable polymers [4–7]. Acting as temporary substitutes for extracellular matrices (ECM) [8], the scaffolds provide an initial mechanical support and a three-dimensional niche for transplanted cells until the regenerated tissue can stabilize the initial structure. High porosities and interconnected pores are thus desired for scaffolds to facilitate cell seeding and adhesion, ECM secretion and eventual tissue regeneration [9]; on the other hand, appropriate mechanical properties and degradation rates of porous scaffolds are also very important [10,11]. Among a number of scaffold materials reported so far, poly(lactide-co-glycolide) (PLGA) is the most popular biodegradable polymer [3,4,9,12], owing to its prominent advantages such as adjustability of degradation rates, good mechanical properties especially toughness, and excellent processibility. PLGA was usually obtained via ring-opening copolymerization of lactide (LA) and glycolide (GA), as shown in scheme 1. LA could be formed by condensation of two l-lactic acids or d-lactic acids or a mixture of d- and l-configurations. d,l-lactide is used more frequently, not owing to the low cost (actually more expensive than l,l-lactide), but owing to the fact that the corresponding materials are more tough and the degradation rates are easily controlled for an amorphous polymer.
Scheme 1.
Schematic of synthesis of poly(lactide-co-glycolide) (PLGA).
The latest decade has witnessed much progress of the fundamental research of PLGA scaffolds as well as their medical applications. Owing to the wide applications of PLGA materials and the specific requirements of porous scaffolds in regenerative medicine, a focused review of PLGA porous scaffolds is very helpful for pertinent researchers and users in broad subjects, and might also be stimulating for research and design of other biodegradable materials. The present review will highlight the recent progress of PLGA scaffolds, especially about the corresponding fundamental research such as fabrication approaches, mechanical properties and degradation behaviours. The progress by Chinese researchers including the authors’ group will be emphasized since the manuscript is for a special issue of ‘Biomaterials Research in China’.
2. Approaches to fabricate porous scaffolds at non-high temperatures
Several strategies have been used to fabricate three-dimensional porous scaffolds in the past decades, such as porogen leaching [13], fibre bonding [14], phase separation [15], freeze drying [16], gas foaming [17], three-dimensional printing and fused deposition modelling [18], and electrospinning [19]. While each strategy has its advantage, porogen leaching has been taken by many groups owing to its great convenience in controling porosity and pore sizes. Nevertheless, how to combine the porogen leaching and moulding technique together still constitutes an important topic. Especially, the avoidance of high temperature should be taken into consideration in order to alleviate degradation in processing biodegradable polyesters. The Fudan group has improved the pertinent fabrication techniques at moderate or even room temperature.
2.1. Modified thermal compression moulding/particulate leaching method at moderate temperature
Compression moulding is widely used to shape plastic products with complicated geometry. Nevertheless, a simple combination of the compression moulding and porogen leaching led to PLGA porous scaffolds just with a simple shape. Potential clinical applications of tissue engineering require scaffolds resembling the anatomical shapes of the deficient tissues or organs. However in scaffold fabrication, the mould release of a sticky polymer material of a complicated shape became a challenging topic. The Fudan group developed a feasible and practical fabrication method called modified thermal compression moulding/particulate leaching approach (MTCM/PL) to simultaneously form an internal interconnected pore structure and an external complicated anatomical shape of the porous scaffolds [20]. In the MTCM/PL approach, a polymer–particulate mixture (PLGA and NaCl particles) was first prepared by the conventional solvent casting, and then compressively moulded in a specially designed flexible–rigid combined mould. The rigid part was made of metal, which made the mould pressure-loadable in moulding, and the flexible part was a silicone rubber, which enabled the mould release after moulding even a complicated scaffold such as an auricle scaffold. Our PLGA scaffolding could be carried out at a moderate temperature, which is above the glass transition temperature but below the flow temperature of PLGA. Finally, a highly interconnected porous scaffold with good mechanical properties was obtained after particulate leaching.
2.2. Room-temperature compression moulding/particulate leaching approach
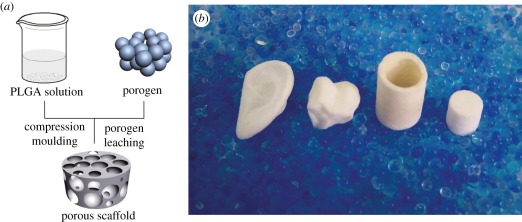
As convenience of scaffolding and alleviation of polyester degradation are concerned, the better choice of processing temperature is, of course, room temperature. It is also helpful not to have to use a high-pressure compressing machine in moulding. In order to avoid high temperature and high-pressure machinary, our group further put forward a ‘room-temperature’ compression moulding/particulate leaching approach (RTCM/PL) [21,22]. The fabrication process is schematically presented in figure 1a. A highly concentrated polymer solution instead of polymer melt was herein used, and the mixture of polymer–porogen was thus mouldable owing to assistance of the organic solvent of PLGA. In this strategy, two solvents must be used, one is an organic solvent to resolve polymer but not porogen, the other is water to resolve salt porogen particles but not the polyester.
Figure 1.
(a) Schematic of fabrication of PLGA scaffolds with the RTCM/PL approach; (b) photographs of typical PLGA porous scaffolds with complicated shapes (ear, joint, tube and cylinder). The image (b) is reproduced with permission from Jing et al. [22].
While this approach is very convenient to fabricate complex scaffolds at relatively low temperature and pressure, the as-shaped mixture of polymer-porogen must shrink during solvent evaporation after demoulding. Yet, the shrinkage was found tolerable under normal fabrications with high salt content, which is just what the preparation of highly porous scaffolds requires. Some representative porous scaffolds with anatomical shapes are shown in figure 1b.
2.3. Room-temperature injection moulding/particulate leaching approach
A processing technique more accurate than compression moulding is injection moulding in plastic engineering. However, polymer melt is used in injection moulding as usual, and the high temperature must lead to serious polyester degradation. So, again with the assistance of a solvent of PLGA, we developed another approach, which we called room-temperature injection moulding/particulate leaching (RTIM/PL). As is well known, tubular scaffolds with a small diameter and/or a thin wall are not easy to fabricate owing to difficulties to charge mould and control wall thickness. The feasibility of RTIM/PL was demonstrated by us to successfully fabricating highly porous scaffolds with tubular and other complicated shapes free of thermal degradation and a high-pressure machine [23]. It provides a technology with high repeatability and precision.
Since in this approach, a ‘wet’ composite of particulate/polymer/solvent was used as the processing objective to inject into the mould, our scaffolding technique triggers the rheological research of these ‘complex fluids’. The salt particle suspension in a PLGA solution exhibited Newtonian behaviour at low shear frequency but non-Newtonian behaviour with a power-law relation at high frequency [24]. The viscosities of suspensions increased with the volume fraction of particles. We found that the viscosity increase with the fraction was sharper than that predicted by the Einstein equation, which implied significant particle interaction and possible particle reorganization under flow.
2.4. Control of pore shape and inter-pore connectivity by design of new porogens
Our motivation to control pore shape was triggered by the improvement of inter-pore connectivity. The inter-pore connectivity is important for cell loading in tissue engineering and cell in-growth in tissue induction. In general, the interconnectivity is enhanced with the increase of porosity. But how to enhance the interconnectivity of a scaffold under a given porosity? Preparation of an appropriate porogen affords a way.
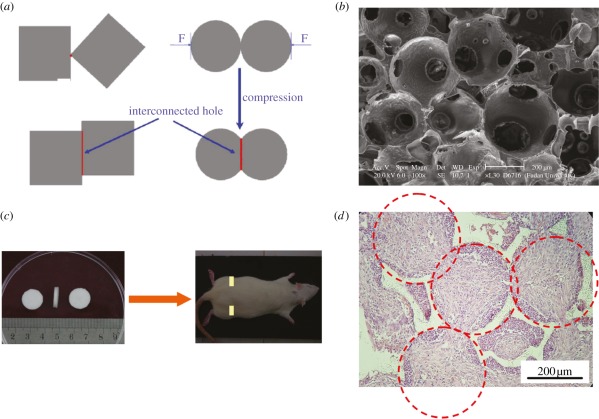
Several kinds of porogens have been used to fabricate porous scaffolds [9,13,25]. Inorganic salt porogens are widely applied owing to easy leaching by water, but limited by the crystal shape and eventually low interconnectivity of scaffolds. Spheric paraffin particles were tried as porogens by the Ma group [9]. They dropped poly(lactide) (PLA) or PLGA solution into the pre-stacked paraffin cluster, and the scaffolds after leaching paraffin exhibited well-controlled architecture. Our group combined this porogen technique with our room-temperature compression moulding approach to fabricate large polyester scaffolds efficiently [26]. Resultant scaffolds exhibited ordered macropores with good inter-pore connectivity as shown in figure 2. The porosity ranged from 77 to 97 per cent by varying porogen content. Cells adhered well on the pore walls. Gelatin particles were used as porogens by another Chinese group [28].
Figure 2.
(a) Schematic of interconnected tunnels of two kinds of porogens: cubic and hard salt, and spherical and soft paraffin under compression. Adapted from Zhang et al. [27]. (b) Scanning electron micrographs of a spherical-pore PLGA scaffold. Reproduced with permission from Zhang et al. [26]. (c) Global observations of PLGA scaffolds and SD rat after subcutaneous implanting of the scaffolds at the marked site. (d) Optical micrograph of haematoxylin-eosin-stained slice of PLGA scaffolds four weeks after subcutaneous implanting. The coloured region indicates fibroblasts migrating into the pores from the surrounding tissues, and the dashed circles reflect the positions of the initial porogens, which were removed after porogen leaching.
The good connectivity of the scaffold resulted from not only the well-ordered spheric porogens, but also the deformability of paraffin under compression [27], as indicated in figure 2a. The good interconnectivity was even indicated by those fibroblasts growing into the pores after implanting our PLGA scaffold subcutaneously into Sprague Dawley (SD) rats, as shown in figure 2c,d.
It is worthy of note that some other Chinese groups also developed the fabrication techniques of scaffolds of synthetic or natural polymers. For instance, a porous PLA scaffold of orientated microtubules was fabricated by Wang et al. through an improved thermal-induced phase separation technique [29]. The microtubule structure could be controlled by the temperature gradient in the formation process. A Tsinghua group [30] used a rapid prototyping technology to obtain three-dimensional structures composed of hepatocytes and gelatin hydrogel. These kind of techniques [6,30] employ an accurate three-dimensional micropositioning system with a pressure-controlled syringe, which can deposit cell/biomaterial with designed structures.
3. Mechanical properties of porous scaffolds
In regenerative medicine, a scaffold should keep its structural integrity in the early stage after implanting and provide an appropriate microstress stimulation to cells [8,11]. Many groups have reported the mechanical properties of porous scaffolds of different biomaterials fabricated by different approaches [9,15,17,18,31–35], including our group [20–22,26,27,36,37]. The mechanical properties of the porous scaffolds could be affected by several factors, and some factors were not well recognized or understood.
3.1. Effects of porosity on mechanical properties of porous scaffolds
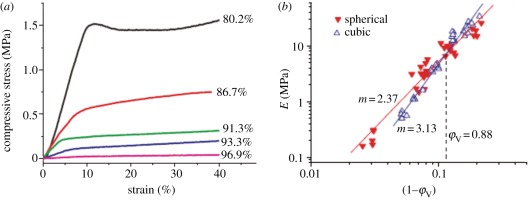
PLGA is basically an elastic-plastic material and yielding may happen in some compression tests, as demonstrated in figure 3a. The porous scaffolds exhibited striking yielding behaviours at low porosities, in which a stress peak appeared after an elastic deformation [27]. The yielding peak disappeared, however, at relative high porosities above approximately 80 per cent, and gave a way to a flexure point. The slope in the linear elastic region gives the compressive modulus E, and the flexure point or yielding point describes the compressive strength σy.
Figure 3.
(a) Some typical stress–strain curves of PLGA85/15 porous scaffolds with spherical macropores at indicated porosities. (b) Compressive modulus of PLGA85/15 foams as a function of relative density, 1−φV. The examined porosities are between 77 and 97%. Marked in the figure are slopes and cross points. Adapted from Zhang et al. [27].
Under a given molecular weight (MW) of polymers and content of porogens, the compressive modulus and strength of scaffolds fabricated with MTCM/PL were higher than those of the scaffolds fabricated with the RTCM/PL approach. Owing to solvent evaporation in RTCM/PL, more micropores in the walls of macropores act as material defects and contribute unfavourably to the mechanical properties [22]. Nevertheless, the mechanical properties of the scaffolds obtained via the RTCM/PL approach are still in the same order of magnitude as those prepared via the former approach, especially under high porosities. Because of the convenience of RTCM/PL, the following tests of our research were carried out for the scaffolds fabricated by this approach.
While a simple linear relationship between mechanical parameters and porosity φV has not been found, the PLGA scaffolds obeyed power-law relations between mechanical parameters and relative density (1−φV), expressed as:
| 3.1 |
and
| 3.2 |
Here, C1 and C2 are constants. The positive exponents m and n indicate reasonably a decrease of compressive modulus and strength with the increase of porosity. So, although a high porosity is beneficial for the nutrition exchange in tissue engineering, an overly high porosity such as over 95 per cent is not expected owing to poor mechanical properties. That is why the porosity φV of a PLGA scaffold is usually selected at around 90 per cent.
3.2. Effects of pore shape on mechanical properties
We found that our PLGA scaffolds of cubic pores and spheric pores exhibited both power-law relations of mechanical parameters versus (1−φV), as shown in figure 3b. Figure 3b also indicates different scaling exponents between two PLGA scaffolds. At low porosities, the modulus of a cubic-pore scaffold was higher than that of a sphere-pore scaffold, but a reverse trend was found at high porosities. Our fundamental research affords guidance of the corresponding scaffold design and parameter selection.
3.3. Effects of dry or wet state on mechanical properties of scaffolds
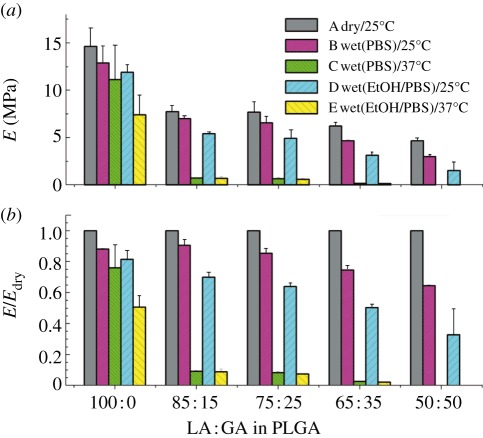
As the porous scaffolds are ultimately used in vivo, it is meaningful to evaluate them under a simulated physiological environment in vitro. While most of the data about mechanical properties of porous scaffolds were collected at the dry state in the literature, we examined the ‘wet-state’ mechanical properties under phosphate buffer saline (PBS) solution [37]. Surprisingly, even though PLGA is hydrophobic, the PLGA scaffolds were softened under the ‘wet’ environment when compared with the usual ‘dry/25°C’ condition, as shown in figure 4.
Figure 4.
Effects of environmental conditions on the compressive modulus E of PLGA porous scaffolds of different compositions with the indicated weight ratios of LA and GA. The measurements were carried out under the indicated conditions or pretreatment conditions marked by (A–E) in (a). For instance, in case C, the scaffolds were first wetted by PBS, and then measured at 37°C; in case D, the scaffolds were first treated by ethanol, then washed thoroughly by PBS, and the mechanical measurements of the wet scaffolds were carried out at 25°C. E/Edry in (b) is the ratio of the compressive modulus under a certain condition to the ‘initial’ one under the dry/25°C condition. Fraction of porogen: 90 wt%; porosity: 88%. Adapted from Wu et al. [37].
We further interpret those phenomena based on the glass transition temperature Tg. As PLGA has a Tg of 45–60°C not far away from the body temperature, the difference between Tg and the measurement temperature plays a critical role in the change of mechanical properties. Wetting of scaffolds results in plasticization of polymer and reduction of Tg, and consequently leads to the decrease of mechanical properties.
Ethanol treatment is a common sterilization approach of PLGA scaffolds. We found that if scaffolds were pre-wetted with ethanol ahead of being pre-wetted with PBS, the mechanical properties further decreased compared with those merely pre-wetted with PBS. Our findings and new insights are helpful for designing polyester porous scaffolds of desired mechanical properties for regenerative medicine.
3.4. Effects of copolymer composition of poly(lactide-co-glycolide) on mechanical properties
Besides MW, the composition of PLGA is also a key molecular parameter. For instance, PLGA85/15 means that the weight fractions of LA and GA are 85 and 15 per cent, respectively. Copolymer composition remarkably affects the mechanical properties of scaffolds. For amorphous PLGA, a higher GA content results in a decrease of Tg of PLGA and of the mechanical properties of the corresponding scaffolds. Figure 4 gives an excellent demonstration of the composition effect, and the mechanical properties of PLGA 50 : 50 scaffolds were even not sufficiently strong for mechanical tests in some cases.
3.5. Other factors to influence the mechanical properties of scaffolds
Some other factors such as degree of crystallization for crystalline polymers and detection temperature are also significant. We do not highlight the crystallization effect here, because PLGA is amorphous if just d, l-lactic acids are repeating units and the fraction of GA is not overly high. The temperature in medical applications is usually around 37°C, and it is less probable that one detects mechanical properties of tissue engineering scaffolds at very low or high temperatures. It is necessary to keep in mind many factors that influence the mechanical properties in selection of an appropriate scaffold.
4. Degradation of poly(lactide-co-glycolide) scaffolds
Degradation of porous scaffolds is necessary for a tissue engineering material, and it affects cell viability, cell growth and even host response in engineering a tissue [38]. The ideal in vivo degradation rate may be similar to that of tissue formation. PLGA degrades prevailingly via chemical hydrolysis of the hydrolytically unstable ester bonds into lactic acids and glycolic acids, which are non-toxic and can be removed from the body by normal metabolic pathways [39]. The degraded particles and fragments were selectively concentrated in the liver and kidney, following release of the degraded products into the bloodstream from the implantation site [40]. Our group confirmed that a solid film of polyester degraded much more rapidly than a porous scaffold [41]. This unexpected result is owing to the autocatalysis of the aliphatic polyester, that is, the degradation product accelerates the further degradation of the remaining polymer chains. So, although plenty of reports concern degradation of PLGA in various forms such as film and microsphere [42–44], the investigation of degradation of porous scaffolds is still valuable in its own right.
4.1. Three-stage kinetic model to describe in vitro degradation of amorphous poly(lactide-co-glycolide) scaffolds
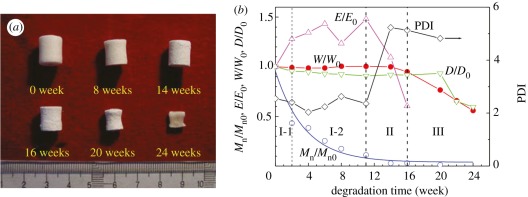
We observed the in vitro degradation of many PLGA porous scaffolds in PBS solution at 37°C in parallel with some results shown in figure 5a [21,36]. The MW of the remaining polymer decreased exponentially with time, indicating simultaneous degradation on the surface and in the interior of the materials. This is a typical characteristic of the bulk degradation mechanism of polyesters.
Figure 5.
(a) Photographs of porous PLGA85/15 scaffolds degraded in PBS solution at 37oC for indicated periods. (b) Three stages of in vitro degradation typically characterized by change of number average molecular weight Mn and polydisperse index (PDI) of remaining polymer, compressive modulus of the remaining scaffold E; the remaining weight W and diameter D of scaffolds. The subscript ‘0’ denotes the initial value. Adapted from Wu & Ding [21].
Besides the change in MW and its distribution of the polymer, we measured a group of other quantities such as weight, diameter and compressive modulus of scaffolds, as plotted in figure 5b [21]. Based on the comparison between the changes of those quantities, we divided the in vitro degradation process of porous scaffolds composed of amorphous PLGA into three stages: quasi-stable stage (stage I), decrease-of-strength stage (stage II), and loss-of-weight and disruption-of-scaffold stage (stage III). The first stage of degradation includes two sub-stages, I-1 and I-2: the I-1 stage was characterized by a probable decrease of the dimensions of the porous scaffolds and increase of mechanical properties, while the scaffold weight did not change significantly; at the I-2 stage, all quantities except MW did not change significantly. At stage II, we observed the decreased mechanical properties and broadened MW distribution with multiple peaks, yet still constant scaffold weight and dimensions. The phase that exhibited obvious weight loss, dimension decrease and eventual disruption of the scaffold was defined as stage III. Weight loss was accompanied by diffusion and dissolution of acidic degradation products, namely, oligomers, lactic acid and glycolic acid, into the PBS solution, thus resulting in decrease of medium pH.
4.2. Effects of porosity and pore size on scaffold degradation
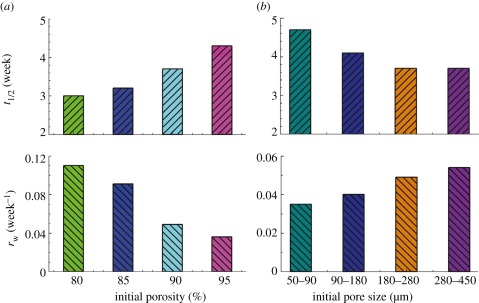
While many factors influence degradation of porous scaffolds, the effects of pore size and porosity were realized by only a few Western groups about one decade ago [45–47]. But their reports are controversial, and thus our group performed a very careful examination of these two effects. A series of scaffolds with porosities among 80–95% and pore sizes among 50–450 μm were fabricated, and the in vitro degradation profiles were observed in comparative tests. Significant effects were observed, as shown in figure 6 [36]. In all of the cases, the degradation processes obeyed the above-mentioned three-stage model. The half degradation time t1/2 in figure 6 refers to the degradation time at which the remaining polymers have average MW half of the initial value before biodegradation, and this value is readily obtained by linearly fitting the logarithmic MW versus t. We also define a term rw, which means the maximum rate of weight loss and could be calculated via scaffold weight as a function of degradation time. We unambiguously conclude that scaffolds with a higher porosity or a smaller pore size degraded more slowly than those with a lower porosity or a larger pore size.
Figure 6.
Dependence of half degradation time (t1/2) and maximum rate of weight loss (rw) upon (a) initial porosity and (b) pore size. Adapted from Wu & Ding [36].
We further gave a unified interpretation of the pore size effect and the porosity effect. The two effects are both attributed to a wall effect instead of a surface area effect. The scaffolds with lower porosities or larger pores possess thicker pore walls, which depress the diffusion of acidic degradation products and thus enhance the acid-catalysed hydrolysis. Our conclusion has been accepted by the field of biomaterials [48–51]. Very recently, the Albertsson group also reported similar phenomena for porous PLA scaffolds [52].
4.3. Other factors to influence scaffold degradation
4.3.1. Composition
Although porous scaffolds made from amorphous polyester-like PLGA are often regarded as hydrophobic biomaterials, they do exhibit a certain hydrophilicity to absorb water and thus degrade by cleavage of hydrolytically sensitive ester bonds. A higher content of less hydrophobic GA units in the copolymers facilitates the absorption and diffusion of water and thus the hydrolysis. For instance, the degradation rate increases in the order of PLGA85/15 < PLGA75/25 [21].
Not only the copolymer composition, but also the additives in scaffolds could affect the degradation behaviour. For example, the incorporation of tripolyphosphate (TPP) nanoparticles to PLGA scaffolds adjusted the acidic degradation of PLGA [53].
4.3.2. Degree of crystallization
While PLGA is amorphous if d,l-lactide instead of l-lactide is a monomer and the fraction of GA is not overly high, it is semi-crystalline under an appropriate GA content or in the case of l,l-lactide. The degree of crystallization must then influence the degradation behaviours significantly. Recently, Kofron et al. reported that the degradation behaviour of amorphous PLGA scaffolds was more suitable for bone tissue engineering because of more mineralized tissue formation at the matrix interior when compared with semi-crystalline PLGA scaffolds [51].
4.3.3. Temperature and pH
It is easy to understand that the material degradation strongly depends on temperature. By examining the half-life of MW of PLGA in the form of porous scaffolds under different temperatures [41], we confirmed that the biodegradation of porous scaffolds obeys the Arrhenius equation with an activation energy.
The pH effect is also not unexpected. PLGA degrades prevailingly via chemical hydrolysis, and a low pH or very high pH causes a significant effect to catalyse hydrolysis of an ester bond. So, temperature and pH should be strictly controlled for a convincing in vitro degradation test.
4.3.4. Mechanical loading
The degradation behaviours of porous scaffolds composed of PLGA and β-tricalcium phosphate (β-TCP) under mechanical loading were studied by Yuan & Fan's groups [50]. The cyclic loading under dynamic conditions accelerated the degradation of the PLGA component in the composite scaffolds with respect to that under static conditions. So, even the mechanical loading state should be taken into consideration in predicting a degradation behaviour.
4.3.5. In vivo microenvironment
For those enzyme-degradable materials [54], the degradation rate in vivo might be very different from that in vitro. Since the main degradation mechanism of polyester is hydrolysis, the difference of degradation rates in vitro and in vivo is not so significant. Nevertheless, the difference still cannot be neglected. By observation of PLGA75/25 scaffolds in cartilage defect of rabbits for 12 weeks, Gao et al. confirmed that in vivo degradation was faster than in vitro [55]. Such a phenomenon was also observed by our group in comparison of degradation of a thermogel of block copolymer composed of poly(ethylene glycol) (PEG) and PLGA [56] or PEG and poly(ε-caprolactone-co-lactide) [57] in vitro and in vivo. In most of the literature, the in vivo degradation was observed much less carefully than in vivo degradation. It is thus worthy of mentioning that the Song group patiently investigated the in vivo degradation of another biodegradable polymer poly(ε-caprolactone) in rats for as long as 2 years [58].
5. Surface modification of poly(lactide-co-glycolide) scaffolds or composites containing poly(lactide-co-glycolide)
Surface modification is very helpful for improvement of the performance of many biomaterials. The modification techniques could be classified into three strategies: morphological modifications (porosity and roughness), chemical modifications (composition and charge) and biological modifications, as summarized by Jiao & Cui [59]. While plenty of reports could be found about surface modification techniques of biomaterials in various forms, the most efficient technique for three-dimensional porous scaffolds developed in China is, in our opinion, the plasma treatment followed by collagen coating as put forward by the Wang group [60].
To mimic ECM of new tissues, many biocompatible materials have, besides collagen [60–62], been incorporated into the PLGA matrix, such as hydroxyapatite [63], TCP [64], TPP [53], chitosan [65], gelatin [66], alginate [67], peptide [68], elastin [69], galectin-1 [65], chondroitin sulphate [70], hyaluronic acid [70], and poly(β-hydroxybutyrate-co-β-hydroxyvalerate) [71].
6. Tissue repair and reconstruction based on poly(lactide-co-glycolide) scaffolds
Before engineering tissues, cells should evenly be loaded into porous scaffolds as usual. Distribution of mesenchymal stem cells (MSCs) in large porous polyester scaffolds was investigated by Chinese researchers to optimize the syringing depth during in vitro cell loading. It was found that an even distribution of cells was soon achieved if the initial cell suspension was seeded in the layer that was below the top surface but above the middle of scaffolds [72].
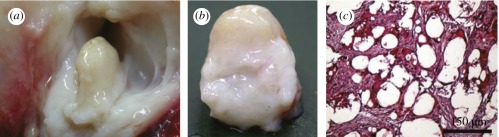
Our scaffolds were also tried in constructing tissues in large animals in cooperation with the Wang group [73]. The PLGA scaffolds loaded with autologous MSCs were implanted in a joint cavity of a sheep. Cartilage formation was observed after eight weeks, as shown in figure 7 [73]. So far, many cases of successful cartilage construction have been reported by several groups [55,61,67,70,74,75].
Figure 7.
(a) Photograph of MSC-PLGA constructs after eight weeks of implantation in a joint cavity of sheep. (b) Global observation of the constructed cartilage. (c) Optical micrograph of a tissue slice after Safranin-O staining, showing positive deposition of proteoglycan as an indicator of the cartilage tissue. Adapted from Chen et al. [73].
PLGA scaffolds were used to repair defects of other tissues such as bone [68,71,76–78], liver [79], nerve [66,80], skin [81] and blood vessel [69,82]. Drugs or proteins especially growth factors or genes to express growth factors were also loaded into porous scaffolds, which could significantly improve the regeneration of new tissues [12,55,75,83,84].
7. Summary and perspectives
In this paper, we have highlighted the fabrication approaches, mechanical properties, in vitro degradation and modification of PLGA scaffolds. These properties are very important for cell seeding and adhesion, ECM secretion and eventual tissue regeneration. The in vivo characterization of PLGA scaffolds and corresponding cell responses are still rather limited.
Since stem cells became the most important seeding cells in tissue engineering and tissue induction, specific attention could be focused upon the interaction between materials and stem cells. Besides chemical modification, surface topography and even material stiffness have been realized to influence basic behaviours of cells including stem cells [85–88]. Three-dimensional cell behaviour might be also different from on two-dimensional substrates. Besides macropores, the microscopic and nanoscopic cues are realized to play important roles, and thus a symphony of the hierarchical structures should be taken into consideration for a porous scaffold. The corresponding fundamental research of cell–biomaterial interactions is a central fundamental topic in biomaterials and regenerative medicine. Many techniques such as surface patterning have been employed to reveal the underlying science [87,89–94]. How to apply those principles into scaffolding, for instance, and how to control surface topography in the interior surfaces of a three-dimensional scaffold, remain as new challenging topics.
Another serious concern for the potential clinical applications of PLGA scaffolds and all other polyester medical materials is the adverse effect of the degradation products such as the aseptic inflammation in vivo. The underlying mechanism of the products to affect cell growth in vitro and in vivo is required to be revealed. In our opinion, the adverse effect of the degradation products of polyester seems to be exaggerated by some researchers, who have less knowledge of PLGA or intend to take PLGA as an imaginary less positive control for their own different materials in preparation of their grants and papers. As we know, serious inflammation of commercialized polyesters appears only in the cases of large amounts of implanting such as bone nail, etc. Even in the case of bone nail, serious inflammation happens just with a non-high probability, otherwise the polyester bone nail cannot be approved by the Food and Drug Administration of the USA, and the State Food and Drug Administration of China, and corresponding bureau in other countries, and be clinically applied in many hospitals every day. The adverse cell response is dependent on the amount of degradation products and the rate of the local fluid exchange. While the bone nail is a large solid implant, porous scaffolds contain much less polyester owing to the high porosity. The local fluid exchange is, also owing to high porosity of scaffolds, much accelerated. I would also like to stress here that a subcutaneous implanting usually results in a slower exchange rate than implanting into other sites such as joint cavities. So, one should be careful to take some negative results from a pre-experiment of subcutaneous implanting to simply predict the failure of a cartilage restoration. Very recently, our group measured the local pH of a block copolymer hydrogel composed of PEG and PLGA after subcutaneously injection. To our surprise, the local pH recovered to nearly neutral after 10 h although the initial pH as low as 4 [95]. So, even in the case of subcutaneous implanting, the body fluid exchange is faster than what we expected. In our opinion, the final conclusion might not be a simple deny or support, but an objective criterion based upon experimental tests or a series of criteria about which application case can or cannot be permitted. Hence, very careful and strong in vitro and in vivo tests are called for to collect sufficient data about the potential adverse effects.
Besides seeding cells and scaffold materials, successful tissue construction frequently requires the assistance of growth factors. The temporal and spatial releases of pertinent growth factors from porous scaffolds are also quite important, and corresponding material techniques need to be developed.
In summary, much progress for PLGA porous scaffolds, a specific physical form of a medical material, has been achieved in the latest decade along with the development of regenerative medicine. Future studies might be more focused upon the fundamental research of cell–material interactions, careful evaluation of the potential positive and adverse effects, the efficient and practical scaffolding and modification techniques based on those insights, and great efforts towards various clinical applications.
Acknowledgements
The authors are grateful for the financial support from the Chinese Ministry of Science and Technology (973 programmes no. 2009CB930000 and no. 2011CB606203), and NSF of China (grant no. 21034002).
References
- 1.Langer R., Vacanti J. P. 1993. Tissue engineering. Science 260, 920–926 10.1126/science.8493529 (doi:10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- 2.Yuan H. P., Kurashina K., de Bruijn J. D., Li Y. B., de Groot K., Zhang X. D. 1999. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 20, 1799–1806 10.1016/s0142-9612(99)00075-7 (doi:10.1016/s0142-9612(99)00075-7) [DOI] [PubMed] [Google Scholar]
- 3.Yu L., Ding J. 2008. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 37, 1473–1481 10.1039/b713009k (doi:10.1039/b713009k) [DOI] [PubMed] [Google Scholar]
- 4.Ishaug S. L., Crane G. M., Miller M. J., Yasko A. W., Yaszemski M. J., Mikos A. G. 1997. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 36, 17–28 10.1002/(SICI)1097-4636(199707)36:1%3C17::AID-JBM3%3E3.0.CO;2-O (doi:10.1002/(SICI)1097-4636(199707)36:1<17::AID-JBM3>3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 5.Du C., Cui F. Z., Zhu X. D., de Groot K. 1999. Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J. Biomed. Mater. Res. 44, 407–415 (doi:10.1002/(sici)1097-4636(19990315)44:4<407::aid-jbm6>3.0.co;2-t) [DOI] [PubMed] [Google Scholar]
- 6.Hutmacher D. W. 2000. Scaffolds in tissue engineering bone and cartilage. Biomaterials 21, 2529–2543 10.1016/s0142-9612(00)00121-6 (doi:10.1016/s0142-9612(00)00121-6) [DOI] [PubMed] [Google Scholar]
- 7.Zhou G., Liu W., Cui L., Wang X., Liu T., Cao Y. 2006. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 12, 3209–3221 10.1089/ten.2006.12.3209 (doi:10.1089/ten.2006.12.3209) [DOI] [PubMed] [Google Scholar]
- 8.Kim B. S., Mooney D. J. 1998. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 16, 224–230 10.1016/s0167-7799(98)01191-3 (doi:10.1016/s0167-7799(98)01191-3) [DOI] [PubMed] [Google Scholar]
- 9.Ma P. X., Choi J. W. 2001. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 7, 23–33 10.1089/107632701300003269 (doi:10.1089/107632701300003269) [DOI] [PubMed] [Google Scholar]
- 10.Holy C. E., Dang S. M., Davies J. E., Shoichet M. S. 1999. In vitro degradation of a novel poly(lactide-co-glycolide) 75/25 foam. Biomaterials 20, 1177–1185 10.1016/s0142-9612(98)00256-7 (doi:10.1016/s0142-9612(98)00256-7) [DOI] [PubMed] [Google Scholar]
- 11.Agrawal C. M., Ray R. B. 2001. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 55, 141–150 (doi:10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j) [DOI] [PubMed] [Google Scholar]
- 12.Shen H., Hu X. X., Bei J. Z., Wang S. G. 2008. The immobilization of basic fibroblast growth factor on plasma-treated poly(lactide-co-glycolide). Biomaterials 29, 2388–2399 10.1016/j.biomaterials.2008.02.008 (doi:10.1016/j.biomaterials.2008.02.008) [DOI] [PubMed] [Google Scholar]
- 13.Mikos A. G., Thorsen A. J., Czerwonka L. A., Bao Y., Langer R., Winslow D. N., Vacanti J. P. 1994. Preparation and characterization of poly(l-lactic acid) foams. Polymer 35, 1068–1077 10.1016/0032-3861(94)90953-9 (doi:10.1016/0032-3861(94)90953-9) [DOI] [Google Scholar]
- 14.Mikos A. G., Bao Y., Cima L. G., Ingber D. E., Vacanti J. P., Langer R. 1993. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J. Biomed. Mater. Res. 27, 183–189 10.1002/jbm.820270207 (doi:10.1002/jbm.820270207) [DOI] [PubMed] [Google Scholar]
- 15.Zhang R. Y., Ma P. X. 1999. Poly(α-hydroxyl acids) hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 44, 446–455 (doi:10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f) [DOI] [PubMed] [Google Scholar]
- 16.Whang K., Thomas C. H., Healy K. E., Nuber G. 1995. A novel method to fabricate bioabsorbable scaffolds. Polymer 36, 837–842 10.1016/0032-3861(95)93115-3 (doi:10.1016/0032-3861(95)93115-3) [DOI] [Google Scholar]
- 17.Harris L. D., Kim B. S., Mooney D. J. 1998. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 42, 396–402 (doi:10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e) [DOI] [PubMed] [Google Scholar]
- 18.Zein I., Hutmacher D. W., Tan K. C., Teoh S. H. 2002. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23, 1169–1185 10.1016/s0142-9612(01)00232-0 (doi:10.1016/s0142-9612(01)00232-0) [DOI] [PubMed] [Google Scholar]
- 19.Zeng J., Chen X. S., Xu X. Y., Liang Q. Z., Bian X. C., Yang L. X., Jing X. B. 2003. Ultrafine fibers electrospun from biodegradable polymers. J. Appl. Polym. Sci. 89, 1085–1092 10.1002/app.12260 (doi:10.1002/app.12260) [DOI] [Google Scholar]
- 20.Wu L. B., Zhang H., Zhang J. C., Ding J. D. 2005. Fabrication of three-dimensional porous scaffolds of complicated shape for tissue engineering. I. Compression molding based on flexible-rigid combined mold. Tissue Eng. 11, 1105–1114 10.1089/ten.2005.11.1105 (doi:10.1089/ten.2005.11.1105) [DOI] [PubMed] [Google Scholar]
- 21.Wu L. B., Ding J. D. 2004. In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 25, 5821–5830 10.1016/j.biomaterials.2004.01.038 (doi:10.1016/j.biomaterials.2004.01.038) [DOI] [PubMed] [Google Scholar]
- 22.Jing D., Wu L., Ding J. 2006. Solvent-assisted room-temperature compression molding approach to fabricate porous scaffolds for tissue engineering. Macromol. Biosci. 6, 747–757 10.1002/mabi.200600079 (doi:10.1002/mabi.200600079) [DOI] [PubMed] [Google Scholar]
- 23.Wu L. B., Jing D. Y., Ding J. D. 2006. A ‘room-temperature’ injection molding/particulate leaching approach for fabrication of biodegradable three-dimensional porous scaffolds. Biomaterials 27, 185–191 10.1016/j.biomaterials.2005.05.105 (doi:10.1016/j.biomaterials.2005.05.105) [DOI] [PubMed] [Google Scholar]
- 24.Jing D., Ding J. 2007. Rheology of a polymer-based hybrid suspension composed of concentrated poly (d,l-lactide)-co-glycolide solution and inorganic salt particles. Macromol. Biosci. 7, 1290–1298 10.1002/mabi.200700113 (doi:10.1002/mabi.200700113) [DOI] [PubMed] [Google Scholar]
- 25.Chen G. P., Ushida T., Tateishi T. 2001. Preparation of poly(l-lactic acid) and poly(d,l-lactic-co-glycolic acid) foams by use of ice microparticulates. Biomaterials 22, 2563–2567 10.1016/s0142-9612(00)00447-6 (doi:10.1016/s0142-9612(00)00447-6) [DOI] [PubMed] [Google Scholar]
- 26.Zhang J. C., Zhang H., Wu L. B., Ding J. D. 2006. Fabrication of three dimensional polymeric scaffolds with spherical pores. J. Mater. Sci. 41, 1725–1731 10.1007/s10853-06-2873-7 (doi:10.1007/s10853-06-2873-7) [DOI] [Google Scholar]
- 27.Zhang J. C., Wu L. B., Jing D. Y., Ding J. D. 2005. A comparative study of porous scaffolds with cubic and spherical macropores. Polymer 46, 4979–4985 10.1016/j.polymer.20058.02.120 (doi:10.1016/j.polymer.20058.02.120) [DOI] [Google Scholar]
- 28.Gong Y., Zhou Q., Gao C., Shen J. 2007. In vitro and in vivo degradability and cytocompatibility of poly(L-lactic acid) scaffold fabricated by a gelatin particle leaching method. Acta Biomater. 3, 531–540 10.1016/j.actbio.2006.12.008 (doi:10.1016/j.actbio.2006.12.008) [DOI] [PubMed] [Google Scholar]
- 29.Yang F., Qu X., Cui W., Bei J., Yu F., Lu S., Wang S. 2006. Manufacturing and morphology structure of polylactide-type microtubules orientation-structured scaffolds. Biomaterials 27, 4923–4933 10.1016/j.biomaterials.2006.05.028 (doi:10.1016/j.biomaterials.2006.05.028) [DOI] [PubMed] [Google Scholar]
- 30.Wang X. H., et al. 2006. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 12, 83–90 10.1089/ten.2006.12.83 (doi:10.1089/ten.2006.12.83) [DOI] [PubMed] [Google Scholar]
- 31.Gomes M. E., Ribeiro A. S., Malafaya P. B., Reis R. L., Cunha A. M. 2001. A new approach based on injection moulding to produce biodegradable starch-based polymeric scaffolds: morphology, mechanical and degradation behaviour. Biomaterials 22, 883–889 10.1016/s0142-9612(00)00211-8 (doi:10.1016/s0142-9612(00)00211-8) [DOI] [PubMed] [Google Scholar]
- 32.Hutmacher D. W., Schantz T., Zein I., Ng K. W., Teoh S. H., Tan K. C. 2001. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 55, 203–216 (doi:10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7) [DOI] [PubMed] [Google Scholar]
- 33.Cai Q., Yang J. A., Bei J. Z., Wang S. G. 2002. A novel porous cells scaffold made of polylactide-dextran blend by combining phase-separation and particle-leaching techniques. Biomaterials 23, 4483–4492 10.1016/s0142-9612(02)00168-0 (doi:10.1016/s0142-9612(02)00168-0) [DOI] [PubMed] [Google Scholar]
- 34.Sander E. A., Alb A. M., Nauman E. A., Reed W. F., Dee K. C. 2004. Solvent effects on the microstructure and properties of 75/25 poly(d,l-lactide-co-glycolide) tissue scaffolds. J. Biomed. Mater. Res. Part A 70A, 506–513 10.1002/jbm.a.30109 (doi:10.1002/jbm.a.30109) [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Yuan X. Y., Cui Y. L., Ge Q. B., Yao K. D. 2004. Preparation and characterization of poly(l-lactide)/poly(ε-caprolactone) fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 91, 1676–1684 10.1002/app.13323 (doi:10.1002/app.13323) [DOI] [Google Scholar]
- 36.Wu L. B., Ding J. D. 2005. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 75A, 767–777 10.1002/jbm.a.30487 (doi:10.1002/jbm.a.30487) [DOI] [PubMed] [Google Scholar]
- 37.Wu L. B., Zhang J. C., Jing D. Y., Ding J. D. 2006. ‘Wet-state’ mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. Part A 76A, 264–271 10.1002/jbm.a.30544 (doi:10.1002/jbm.a.30544) [DOI] [PubMed] [Google Scholar]
- 38.Babensee J. E., Anderson J. M., McIntire L. V., Mikos A. G. 1998. Host response to tissue engineered devices. Adv. Drug Deliver. Rev. 33, 111–139 10.1016/s0169-409x(98)00023-4 (doi:10.1016/s0169-409x(98)00023-4) [DOI] [PubMed] [Google Scholar]
- 39.Gopferich A. 1996. Mechanisms of polymer degradation and erosion. Biomaterials 17, 103–114 10.1016/0142-9612(96)85755-3 (doi:10.1016/0142-9612(96)85755-3) [DOI] [PubMed] [Google Scholar]
- 40.Hua N., Sun J. 2008. Body distribution of poly(d,l-lactide-co-glycolide) copolymer degradation products in rats. J. Mater. Sci-Mater. M. 19, 3243–3248 10.1007/s10856-008-3460-z (doi:10.1007/s10856-008-3460-z) [DOI] [PubMed] [Google Scholar]
- 41.Jing D. Y., Zhang J. C., Wu L. B., Ding J. D. 2005. Degradation study of poly(lactic acid) porous scaffold at different temperatures. Polym. Mat. Sci. Eng. 21, 162–164 [In Chinese] [Google Scholar]
- 42.Li S. M., Garreau H., Vert M. 1990. Structure property relationships in the case of the degradation of massive poly(α-hydroxy acids) in aqueous-media 0.2. degradation of lactide-glycolide copolymers-PLA37.5GA25 and PLA75GA25. J. Mater. Sci-Mater. M. 1, 131–139 10.1007/bf00700872 (doi:10.1007/bf00700872) [DOI] [Google Scholar]
- 43.Park T. G. 1995. Degradation of poly(lactic-co-glycolic acid) microspheres-effect of copolymer composition. Biomaterials 16, 1123–1130 10.1016/0142-9612(95)93575-x (doi:10.1016/0142-9612(95)93575-x) [DOI] [PubMed] [Google Scholar]
- 44.Lu L., Garcia C. A., Mikos A. G. 1999. In vitro degradation of thin poly(d,l-lactic-co-glycolic acid) films. J. Biomed. Mater. Res. 46, 236–244 (doi:10.1002/(sici)1097-4636(199908)46:2<236::aid-jbm13>3.0.co;2-f) [DOI] [PubMed] [Google Scholar]
- 45.Athanasiou K. A., Schmitz J. P., Agrawal C. M. 1998. The effects of porosity on in vitro degradation of polylactic acid polyglycolic acid implants used in repair of articular cartilage. Tissue Eng. 4, 53–63 10.1089/ten.1998.4.53 (doi:10.1089/ten.1998.4.53) [DOI] [Google Scholar]
- 46.Agrawal C. M., McKinney J. S., Lanctot D., Athanasiou K. A. 2000. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials 21, 2443–2452 10.1016/s0142-9612(00)00112-5 (doi:10.1016/s0142-9612(00)00112-5) [DOI] [PubMed] [Google Scholar]
- 47.Lu L., et al. 2000. In vitro and in vivo degradation of porous poly(d,l-lactic-co-glycolic acid) foams. Biomaterials 21, 1837–1845 10.1016/s0142-9612(00 (doi:10.1016/s0142-9612(00)00047-8) [DOI] [PubMed] [Google Scholar]
- 48.Williams D. F. 2008. On the mechanisms of biocompatibility. Biomaterials 29, 2941–2953 10.1016/j.biomaterials.2008.04.023 (doi:10.1016/j.biomaterials.2008.04.023) [DOI] [PubMed] [Google Scholar]
- 49.Yoshioka T., Kawazoe N., Tateishi T., Chen G. 2008. In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges. Biomaterials 29, 3438–3443 10.1016/j.biomaterials.2008.04.011 (doi:10.1016/j.biomaterials.2008.04.011) [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Zhao Y., Tang G., Li H., Yuan X., Fan Y. 2008. In vitro degradation of porous poly(l-lactide-co-glycolide)/β-tricalcium phosphate (PLGA/β-TCP) scaffolds under dynamic and static conditions. Polym. Degrad. Stabil. 93, 1838–1845 10.1016/j.polymdegradstab.2008.07.007 (doi:10.1016/j.polymdegradstab.2008.07.007) [DOI] [Google Scholar]
- 51.Kofron M. D., Griswold A., Kumbar S. G., Martin K., Wen X., Laurencin C. T. 2009. The implications of polymer selection in regenerative medicine: a comparison of amorphous and semi-crystalline polymer for tissue regeneration. Adv. Funct. Mater. 19, 1351–1359 10.1002/adfm.200801327 (doi:10.1002/adfm.200801327) [DOI] [Google Scholar]
- 52.Odelius K., Hoglund A., Kumar S., Hakkarainen M., Ghosh A. K., Bhatnagar N., Albertsson A.-C. 2011. Porosity and pore size regulate the degradation product profile of polylactide. Biomacromolecules 12, 1250–1258 10.1021/bm1015464 (doi:10.1021/bm1015464) [DOI] [PubMed] [Google Scholar]
- 53.Xie S., Zhu Q., Wang B., Gu H., Liu W., Cui L., Cen L., Cao Y. 2010. Incorporation of tripolyphosphate nanoparticles into fibrous poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 31, 5100–5109 10.1016/j.biomaterials.2010.03.004 (doi:10.1016/j.biomaterials.2010.03.004) [DOI] [PubMed] [Google Scholar]
- 54.Zhao Z., Yang L., Hu Y., He Y., Wei J., Li S. 2007. Enzymatic degradation of block copolymers obtained by sequential ring opening polymerization of l-lactide and ε-caprolactone. Polym. Degrad. Stabil. 92, 1769–1777 10.1016/j.polymdegradstab.2007.07.012 (doi:10.1016/j.polymdegradstab.2007.07.012) [DOI] [Google Scholar]
- 55.Wang W., Li B., Li Y., Jiang Y., Ouyang H., Gao C. 2010. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials 31, 5953–5965 10.1016/j.biomaterials.2010.04.029 (doi:10.1016/j.biomaterials.2010.04.029) [DOI] [PubMed] [Google Scholar]
- 56.Yu L., Zhang Z., Zhang H., Ding J. 2010. Biodegradability and biocompatibility of thermoreversible hydrogels formed from mixing a sol and a precipitate of block copolymers in water. Biomacromolecules 11, 2169–2178 10.1021/bm100549q (doi:10.1021/bm100549q) [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z., Ni J., Chen L., Yu L., Xu J., Ding J. 2011. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 32, 4725–4736 10.1016/j.biomaterials.2011.03.046 (doi:10.1016/j.biomaterials.2011.03.046) [DOI] [PubMed] [Google Scholar]
- 58.Sun H. F., Mei L., Song C. X., Cui X. M., Wang P. Y. 2006. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 27, 1735–1740 10.1016/j.biomaterials.2005.09.019 (doi:10.1016/j.biomaterials.2005.09.019) [DOI] [PubMed] [Google Scholar]
- 59.Jiao Y.-P., Cui F.-Z. 2007. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2, R24–R37 10.1088/1748-6041/2/4/r02 (doi:10.1088/1748-6041/2/4/r02) [DOI] [PubMed] [Google Scholar]
- 60.Yang J., Bei J. Z., Wang S. G. 2002. Enhanced cell affinity of poly (d,l-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 23, 2607–2614 [DOI] [PubMed] [Google Scholar]
- 61.Dai W., Kawazoe N., Lin X., Dong J., Chen G. 2010. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials 31, 2141–2152 10.1016/j.biomaterials.2009.11.070 (doi:10.1016/j.biomaterials.2009.11.070) [DOI] [PubMed] [Google Scholar]
- 62.Hao W., Pang L., Jiang M., Lv R., Xiong Z., Hu Y.-Y. 2010. Skeletal repair in rabbits using a novel biomimetic composite based on adipose-derived stem cells encapsulated in collagen I gel with PLGA-β-TCP scaffold. J. Orthopaed. Res. 28, 252–257 10.1002/jor.20969 (doi:10.1002/jor.20969) [DOI] [PubMed] [Google Scholar]
- 63.Cui Y., Liu Y., Cui Y., Jing X., Zhang P., Chen X. 2009. The nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with l-lactic acid oligomer for bone repair. Acta Biomater. 5, 2680–2692 10.1016/j.actbio.2009.03.024 (doi:10.1016/j.actbio.2009.03.024) [DOI] [PubMed] [Google Scholar]
- 64.Pang L., Hu Y., Yan Y., Liu L., Xiong Z., Wei Y., Bai J. 2007. Surface modification of PLGA/β-TCP scaffold for bone tissue engineering: hybridization with collagen and apatite. Surf. Coat. Tech. 201, 9549–9557 10.1016/j.surfcoat.2007.04.035 (doi:10.1016/j.surfcoat.2007.04.035) [DOI] [Google Scholar]
- 65.Chen S. J., Lin C. C., Tuan W. C., Tseng C. S., Huang R. N. 2010. Effect of recombinant galectin-1 on the growth of immortal rat chondrocyte on chitosan-coated PLGA scaffold. J. Biomed. Mater. Res. Part A 93A, 1482–1492 10.1002/jbm.a.32658 (doi:10.1002/jbm.a.32658) [DOI] [PubMed] [Google Scholar]
- 66.Li X. K., et al. 2007. Characteristics of PLGA-gelatin complex as potential artificial nerve scaffold. Colloid Surf. B 57, 198–203 10.1016/j.colsurfb.2007.02.010 (doi:10.1016/j.colsurfb.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 67.Bai H. Y., Chen G. A., Mao G. H., Song T. R., Wang Y. X. 2010. Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J. Biomed. Mater. Res. Part A 94A, 539–546 10.1002/jbm.a.32732 (doi:10.1002/jbm.a.32732) [DOI] [PubMed] [Google Scholar]
- 68.Ma S., Wang K., Dang X., Wang W., Zhang M., Wu D. 2008. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. J. Plast. Reconstr. Aes. 61, 1558–1560 10.1016/j.bjps.2008.03.040 (doi:10.1016/j.bjps.2008.03.040) [DOI] [PubMed] [Google Scholar]
- 69.Han J., Lazarovici P., Pomerantz C., Chen X., Wei Y., Lelkes P. I. 2011. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 12, 399–408 10.1021/bm101149r (doi:10.1021/bm101149r) [DOI] [PubMed] [Google Scholar]
- 70.Fan H., Tao H., Wu Y., Hu Y., Yan Y., Luo Z. 2010. TGF-β 3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J. Biomed. Mater. Res. Part A 95A, 982–992 10.1002/jbm.a.32899 (doi:10.1002/jbm.a.32899) [DOI] [PubMed] [Google Scholar]
- 71.Huang W., Shi X., Ren L., Du C., Wang Y. 2010. PHBV microspheres - PLGA matrix composite scaffold for bone tissue engineering. Biomaterials 31, 4278–4285 10.1016/j.biomaterials.2010.01.059 (doi:10.1016/j.biomaterials.2010.01.059) [DOI] [PubMed] [Google Scholar]
- 72.Wang Z., Zhang Z., Zhang J. C., She Z. J., Ding J. D. 2009. Distribution of bone marrow stem cells in large porous polyester scaffolds. Chinese Sci. Bull. 54, 2968–2975 10.1007/s11434-009-0181-8 (doi:10.1007/s11434-009-0181-8) [DOI] [Google Scholar]
- 73.Chen J. W., et al. 2005. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 319, 429–438 10.1007/s00441-004-1025-0 (doi:10.1007/s00441-004-1025-0) [DOI] [PubMed] [Google Scholar]
- 74.Fan H., Hu Y., Zhang C., Li X., Lv R., Qin L., Zhu R. 2006. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 27, 4573–4580 10.1016/j.biomaterials.2006.04.013 (doi:10.1016/j.biomaterials.2006.04.013) [DOI] [PubMed] [Google Scholar]
- 75.Wang W., et al. 2010. The restoration of full-thickness cartilage defects with BMSCs and TGF-β 1 loaded PLGA/fibrin gel constructs. Biomaterials 31, 8964–8973 10.1016/j.biomaterials.2010.08.018 (doi:10.1016/j.biomaterials.2010.08.018) [DOI] [PubMed] [Google Scholar]
- 76.Ren T. B., Ren J., Jia X. Z., Pan K. F. 2005. The bone formation in vitro and mandibular defect repair using PLGA porous scaffolds. J. Biomed. Mater. Res. Part A 74A, 562–569 10.1002/jbm.a.30324 (doi:10.1002/jbm.a.30324) [DOI] [PubMed] [Google Scholar]
- 77.Yu D., Li Q., Mu X., Chang T., Xiong Z. 2008. Bone regeneration of critical calvarial defect in goat model by PLGA/TCP/rhBMP-2 scaffolds prepared by low-temperature rapid-prototyping technology. Int. J. Oral Max. Surg. 37, 929–934 10.1016/j.ijom.2008.07.012 (doi:10.1016/j.ijom.2008.07.012) [DOI] [PubMed] [Google Scholar]
- 78.Ge Z., Tian X., Heng B. C., Fan V., Yeo J. F., Cao T. 2009. Histological evaluation of osteogenesis of 3D-printed poly-lactic-co-glycolic acid (PLGA) scaffolds in a rabbit model. Biomed. Mater. 4, 021001. 10.1088/1748-6041/4/2/021001 (doi:10.1088/1748-6041/4/2/021001) [DOI] [PubMed] [Google Scholar]
- 79.Li J., et al. 2010. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell-derived hepatocytes. Stem Cells Dev. 19, 1427–1436 10.1089/scd.2009.0415 (doi:10.1089/scd.2009.0415) [DOI] [PubMed] [Google Scholar]
- 80.Xiong Y., et al. 2009. Synaptic transmission of neural stem cells seeded in 3-dimensional PLGA scaffolds. Biomaterials 30, 3711–3722 10.1016/j.biomaterials.2009.03.046 (doi:10.1016/j.biomaterials.2009.03.046) [DOI] [PubMed] [Google Scholar]
- 81.Yang J., Shi G. X., Bei J. Z., Wang S. G., Cao Y. L., Shang Q. X., Yang G. G., Wang W. J. 2002. Fabrication and surface modification of macroporous poly(l-lactic acid) and poly(l-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 62, 438–446 10.1002/jbm.10318 (doi:10.1002/jbm.10318) [DOI] [PubMed] [Google Scholar]
- 82.Hu X., Shen H., Yang F., Bei J., Wang S. 2008. Preparation and cell affinity of microtubular orientation-structured PLGA(70/30) blood vessel scaffold. Biomaterials 29, 3128–3136 10.1016/j.biomaterials.2008.04.010 (doi:10.1016/j.biomaterials.2008.04.010) [DOI] [PubMed] [Google Scholar]
- 83.Wang X. L., et al. 2009. In vitro release of osteoinductive molecule Icaritin from porous PLGA/TCP/Icaritin scaffolds for repairing steroid-associated osteonecrosis lesion. Bone 45, S105. 10.1016/j.bone.2009.04.176 (doi:10.1016/j.bone.2009.04.176) [DOI] [Google Scholar]
- 84.Yang Y., Tang G., Zhang H., Zhao Y., Yuan X., Wang M., Yuan X. 2011. Controllable dual-release of dexamethasone and bovine serum albumin from PLGA/β-tricalcium phosphate composite scaffolds. J. Biomed. Mater. Res. Part B Applied Biomat. 96B, 139–151 10.1002/jbm.b.31752 (doi:10.1002/jbm.b.31752) [DOI] [PubMed] [Google Scholar]
- 85.Discher D. E., Janmey P., Wang Y. L. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 10.1126/science.1116995 (doi:10.1126/science.1116995) [DOI] [PubMed] [Google Scholar]
- 86.Wan Y. Q., Wang Y., Liu Z. M., Qu X., Han B. X., Bei J. Z., Wang S. G. 2005. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(l-lactide). Biomaterials 26, 4453–4459 10.1016/j.biomaterials.2004.11.016 (doi:10.1016/j.biomaterials.2004.11.016) [DOI] [PubMed] [Google Scholar]
- 87.Fu J. P., Wang Y. K., Yang M. T., Desai R. A., Yu X. A., Liu Z. J., Chen C. S. 2010. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 7, 733–736 10.1038/nmeth.1487 (doi:10.1038/nmeth.1487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan Z., Yan C., Peng R., Zhao Y., He Y., Ding J. 2012. Control of cell nucleus shapes via micropillar patterns. Biomaterials 33, 1730–1735 10.1016/j.biomaterials.2011.11.023 (doi:10.1016/j.biomaterials.2011.11.023) [DOI] [PubMed] [Google Scholar]
- 89.Huang J., Grater S. V., Corbellinl F., Rinck S., Bock E., Kemkemer R., Kessler H., Ding J., Spatz J. P. 2009. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 9, 1111–1116 10.1021/nl803548b (doi:10.1021/nl803548b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun J., Tang J., Ding J. 2009. Cell orientation on a stripe-micropatterned surface. Chin. Sci. Bull. 54, 3154–3159 10.1007/s11434-009-0240-1 (doi:10.1007/s11434-009-0240-1) [DOI] [Google Scholar]
- 91.Huang J., Ding J. 2010. Nanostructured interfaces with RGD arrays to control cell-matrix interaction. Soft Matter 6, 3395–3401 10.1039/b927168f (doi:10.1039/b927168f) [DOI] [Google Scholar]
- 92.Tang J., Peng R., Ding J. D. 2010. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials 31, 2470–2476 10.1016/j.biomaterials.2009.12.006 (doi:10.1016/j.biomaterials.2009.12.006) [DOI] [PubMed] [Google Scholar]
- 93.Peng R., Yao X., Ding J. 2011. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials 32, 8045–8057 10.1016/j.biomaterials.2011.07.035 (doi:10.1016/j.biomaterials.2011.07.035) [DOI] [PubMed] [Google Scholar]
- 94.Yan C., Sun J., Ding J. 2011. Critical areas of cell adhesion on micropatterned surfaces. Biomaterials 32, 3931–3938 10.1016/j.biomaterials.2011.01.078 (doi:10.1016/j.biomaterials.2011.01.078) [DOI] [PubMed] [Google Scholar]
- 95.Chang G., Ci T., Yu L., Ding J. 2011. Enhancement of the fraction of the active form of an antitumor drug topotecan via an injectable hydrogel. J. Control Release 156, 21–27 10.1016/j.jconrel.2011.07.008 (doi:10.1016/j.jconrel.2011.07.008) [DOI] [PubMed] [Google Scholar]