Abstract
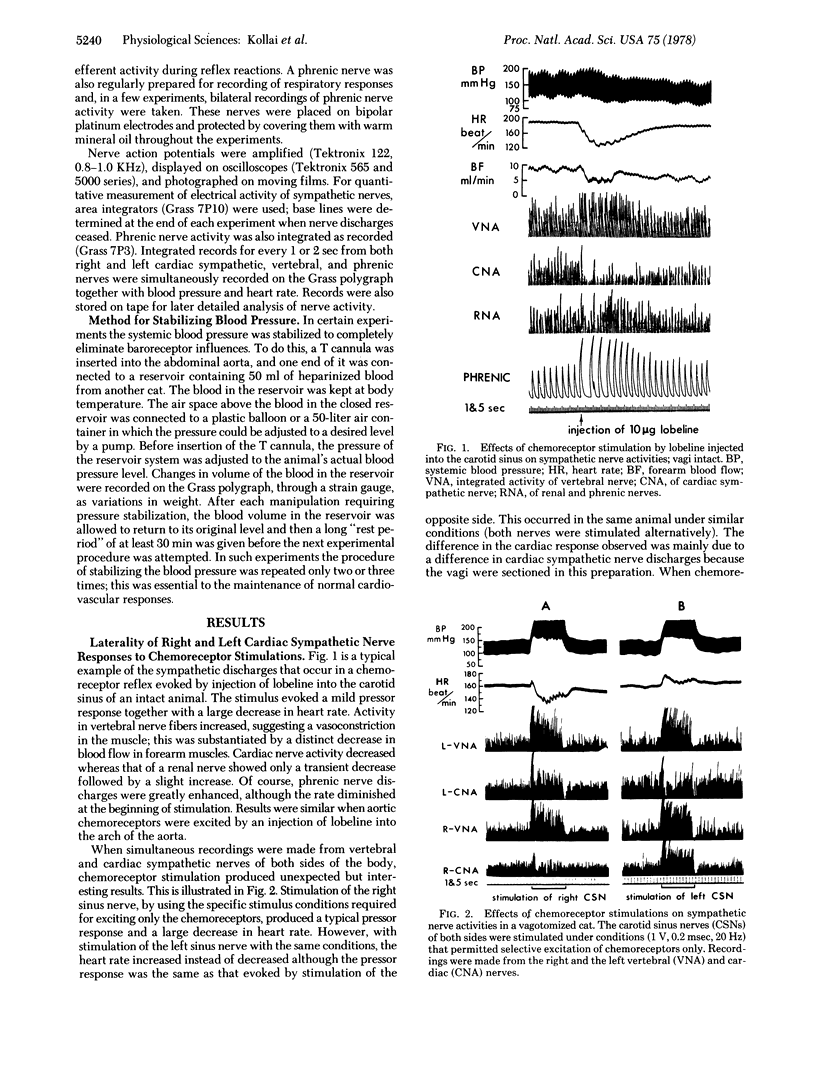
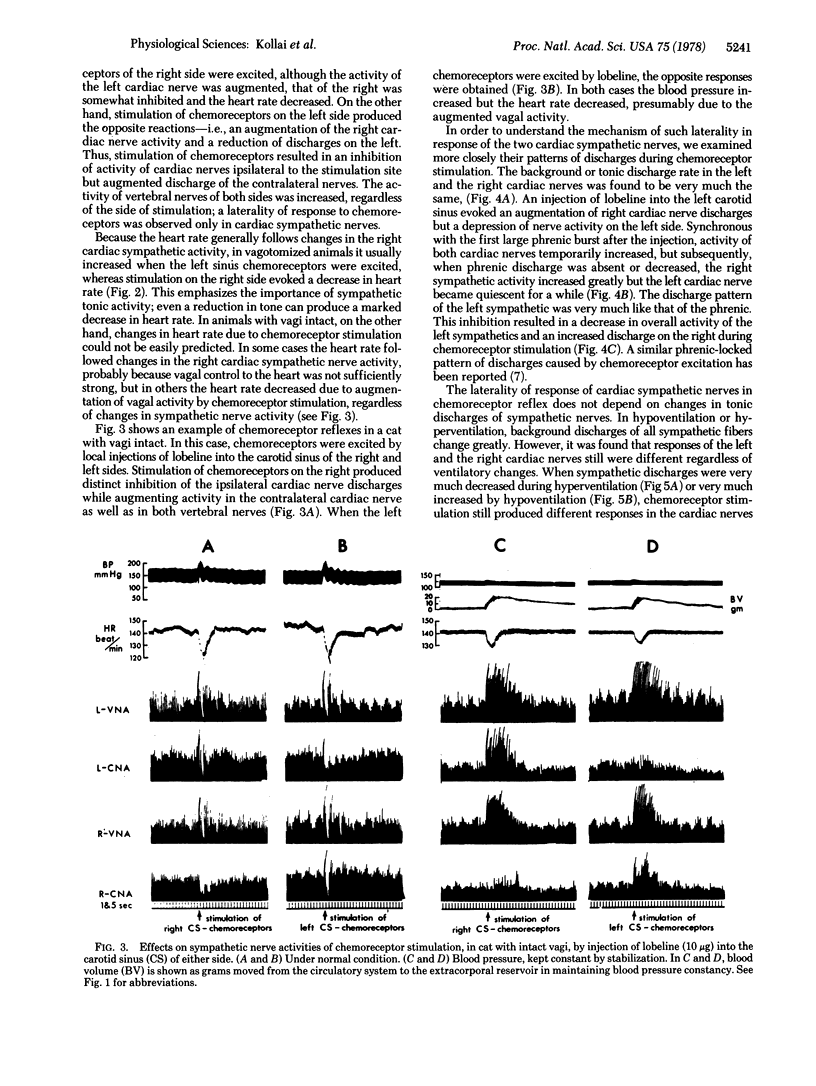
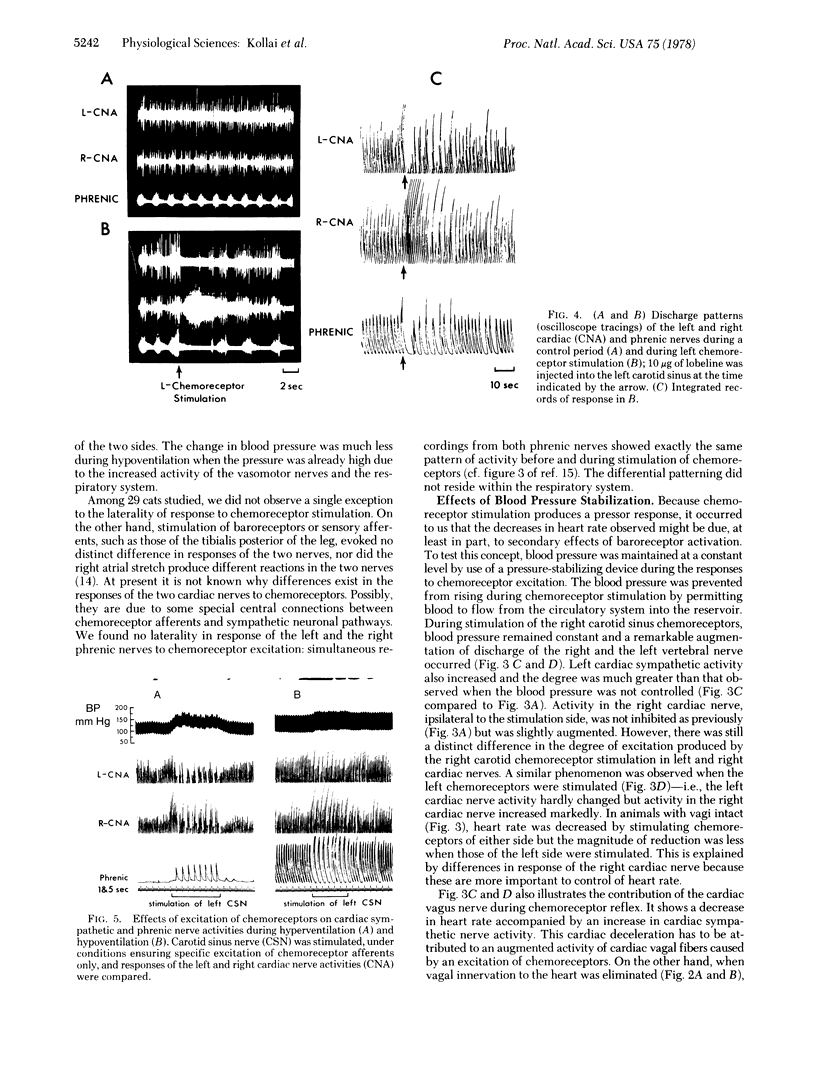
In a study of autonomic reflexes it was found that some produce a generalized, bilaterally uniform response whereas others have an asymmetric or laterality of action. Recordings from vertebral nerve fibers (mainly vasoconstrictors to forelimb muscles), right and left cardiac sympathetics, and renal nerves show that baroreceptors evoke a bilaterally uniform inhibition but chemoreceptors of the carotid sinus and aortic arch initiate a differential discharge. In the chemoreceptor reflex the vagi are activated and bradycardia generally occurs. Vertebral and renal sympathetic fibers increase their activity bilaterally commensurate with the increase in arterial pressure. Sympathetic discharges to the heart, however, are not uniform; they show ipsilateral inhibition and a strong contralateral increase in activity. Stabilization of blood pressure or inactivation of baroreceptors abolishes the ipsilateral inhibition. In isolation, therefore, the chemoreceptor-induced cardiac sympathetic discharge is just quantitatively stronger contralaterally. In the absence of vagi, heart rate changes differ depending on which chemoreceptors are stimulated, because the pacemaker is on the right. Asymmetrical discharges do occur and, in the eventual response to stimulation of chemoreceptors, reflex interactions actually augment the laterality of effects. Peripheral interactions, in the sense that changes effected by one may induce another reflex, are responsible in part for the balances of autonomic activity ultimately seen as the body reacts to stimuli.
Keywords: laterality in sympathetic action, cardiac reactions, vasomotor nerve response, baroreceptor reflex
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alanís J., Defilló B., Gordón S. Changes in the efferent discharges of sympathetic and parasympathetic cardiac nerves provoked by activation of carotid chemoreceptors. Arch Int Physiol Biochim. 1968 Apr;76(2):214–235. doi: 10.3109/13813456809058701. [DOI] [PubMed] [Google Scholar]
- DE BURGH DALY M., SCOTT M. J. An analysis of the primary cardiovascular reflex effects of stimulation of the carotid body chemoreceptors in the dog. J Physiol. 1962 Aug;162:555–573. doi: 10.1113/jphysiol.1962.sp006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. S., Goldner S., McCloskey D. I. Respiratory modulation of barareceptor and chemoreceptor reflexes affecting heart rate and cardiac vagal efferent nerve activity. J Physiol. 1976 Jul;259(2):523–530. doi: 10.1113/jphysiol.1976.sp011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki M., Kozawa E. Factors controlling the regional differentiation of sympathetic outflow--influence of the chemoreceptor reflex. Brain Res. 1975 Apr 11;87(2-3):281–291. doi: 10.1016/0006-8993(75)90425-4. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Sampson S. R., Comroe J. H., Jr Carotid sinus versus carotid body origin of nicotine and cyanide bradycardia in the dog. Am J Physiol. 1971 Feb;220(2):472–476. doi: 10.1152/ajplegacy.1971.220.2.472. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Sato A. Reflex activity of single sympathetic fibres to skeletal muscle produced by electrical stimulation of somatic and vago-depressor afferent nerves in the cat. Pflugers Arch. 1972;332(4):283–301. doi: 10.1007/BF00588576. [DOI] [PubMed] [Google Scholar]
- Kollai M., Koizumi K. Differential responses in sympathetic outflow evoked by chemoreceptor activation. Brain Res. 1977 Dec 9;138(1):159–165. doi: 10.1016/0006-8993(77)90791-0. [DOI] [PubMed] [Google Scholar]
- Kollai M., Koizumi K., Yamashita H., Brooks C. M. Study of cardiac sympathetic and vagal efferent activity during reflex responses produced by stretch of the atria. Brain Res. 1978 Jul 21;150(3):519–532. doi: 10.1016/0006-8993(78)90817-x. [DOI] [PubMed] [Google Scholar]
- Korner P. I. Integrative neural cardiovascular control. Physiol Rev. 1971 Apr;51(2):312–367. doi: 10.1152/physrev.1971.51.2.312. [DOI] [PubMed] [Google Scholar]
- LANDGREN S., NEIL E. The effect of chemical stimulation of the carotid bodies on the heart rate of the cat. Acta Physiol Scand. 1952 Jun 6;25(2-3):286–290. doi: 10.1111/j.1748-1716.1952.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Little R., Oberg B. Circulatory responses to stimulation of the carotid body chemoreceptors in the cat. Acta Physiol Scand. 1975 Jan;93(1):34–50. doi: 10.1111/j.1748-1716.1975.tb05788.x. [DOI] [PubMed] [Google Scholar]
- McQueen D. S. Effects of suberyldicholine on carotid baroreceptors and chemoreceptors. Neuropharmacology. 1974 Sep;13(9):829–835. doi: 10.1016/0028-3908(74)90038-0. [DOI] [PubMed] [Google Scholar]
- NEIL E., REDWOOD C. R. M., SCHWEITZER A. Pressor responses to electrical stimulation of the carotid sinus nerve in cats. J Physiol. 1949 Sep;109(3-4):259–271. doi: 10.1113/jphysiol.1949.sp004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat W. C., Lalley P. M. Reflex sympathetic firing in response to electrical stimulation of the carotid sinus nerve in the cat. Brain Res. 1974 Nov 8;80(1):17–40. doi: 10.1016/0006-8993(74)90721-5. [DOI] [PubMed] [Google Scholar]



