Abstract
Bioceramics, because of its excellent biocompatible and mechanical properties, has always been considered as the most promising materials for hard tissue repair. It is well know that an appropriate cellular response to bioceramics surfaces is essential for tissue regeneration and integration. As the in vivo implants, the implanted bioceramics are immediately coated with proteins from blood and body fluids, and it is through this coated layer that cells sense and respond to foreign implants. Hence, the adsorption of proteins is critical within the sequence of biological activities. However, the biological mechanisms of the interactions of bioceramics and proteins are still not well understood. In this review, we will recapitulate the recent studies on the bioceramic–protein interactions.
Keywords: bioceramics, protein adsorption, interface
1. Introduction
The word ceramic was developed a long time ago; it is said to come from a Greek word for pottery [1]. A traditional ceramic is an inorganic solid prepared generally by heating and subsequent cooling. Ceramics may be crystalline, partly crystalline or amorphous [2]. Because most common ceramics are crystalline [3], the definition of traditional ceramic often referred to inorganic crystalline materials, as opposed to glass [4–6]. With the rapid development of interdisciplinary, including materials, medicinal and ecological sciences, bioceramics also emerged and developed rapidly [7,8], and the definition of bioceramics is far beyond traditional ceramics. As a multi-disciplinary outcome, the relative products of bioceramics have reached a flourishing stage [9,10].
Bioceramics arouse great interest because of their excellent biocompatibility [11,12]. During recent decades, a vast number of bioceramics have been developed and applied [13,14], such as calcium phosphate (Ca-P)-based ceramics, titania, alumina, zirconia, bioglass, etc. Among these bioceramics, the Ca-P-based ones, e.g. hydroxyapatite (HA), tricalcium phosphate (TCP), biphase calcium phosphate (BCP) are the most studied [15–17]. Because of similar chemical components, these ceramics have excellent biocompatibility and bioactivity [18,19]. However, medical applications of the Ca-P-based ceramics are limited to small filler implants, grains and coatings owing to their poor mechanical properties [20]. Titania, alumina and zirconia are the most frequently used inorganic metal-based ceramics [21,22]. These metal-based bioceramics exhibit high mechanical strength, excellent corrosion and wear resistance and good biocompatibility [10,23]. They are, therefore, frequently used in high load-bearing sites such as the pygal, dental and submaxillary implant.
To fabricate the bioceramics, the chemical, physical and mechanical properties of these materials have been extensively studied in previous research [24–27]. Since the osteoinductivity and bone regeneration of bioceramics are well-known and proved [28,29], research is further focused on understanding the mechanisms of bioactivity [30–33]. The works that are in progress mainly cover two aspects. One focuses on studying the biological events that occur in vivo between bioceramics and living tissues [34–38]. The other tries to reveal the mechanisms of apatite layer formation or the regeneration process on the implanted bioceramics [18,39]. However, both works are closely related to protein adsorption on the bioceramic surface.
Protein adsorption is a unique property of bioceramics [11,40]. When bioceramics are implanted into a living body, proteins from the surrounding body fluids will be spontaneously adsorbed onto their surfaces, and then cellular attachment, proliferation and migration occurs [41–43]. Thus, the protein-adsorption behaviour plays a vital role during bone tissue regeneration [44,45].
In this paper, we primarily focus on the protein adsorption of bioceramics; in particular we divide the bioceramics into two classes: Ca-P and non-Ca-P ceramics. Because the chemical elements of Ca-P ceramics are similar to natural bones, Ca-P as bone substitute materials show excellent biocompatibility, bioactivity and osteoconductivity. However, their applications are limited to small non-bearing implants, grains or as coatings of metals because of their poor mechanical properties. Another category is generally classified into non-Ca-P, such as titania, alumina, zirconia, etc. These ceramics are traditional and have been widely used in hard tissue repair. The phenomenon of protein adsorption has attracted much attention in the field of tissue engineering. However, the protein-adsorption characteristics of different biomaterials and the mechanisms on the effect of adsorption efficiency need to be further clarified. An intensive knowledge of protein adsorption is not only beneficial to the optimization of the surface structure of biomaterials, but also helpful to develop specific applications within the field of biomedicine.
2. Evolution of bioceramics in hard tissue engineering
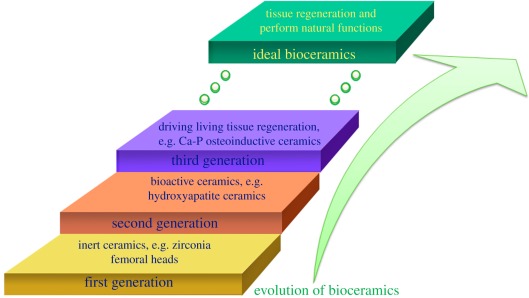
To review the development of bioceramics [22,46–48], we can generally classify it into three stages. Figure 1 shows the schematic of the bioceramics evolution. The first generation is called inert ceramics, which aimed to substitute natural bone. For example, zirconia [49–52], titanium [53–55] and alumina [56–58] are primarily used in fabrication of femoral heads to substitute the damaged bones [59]. Although these ceramics are biocompatible, the living body usually reacts against the implants because they are foreign, and these implants themselves are likely to never transform into bone. The second generation is called bioactive ceramics, which aimed to mimic some biomineralization-related functions. Bioactive ceramics yielded promising results in the 1970s. These ceramics can react with physiological fluids and form a biological-type apatite. In the presence of living cells, this apatite can form new bone. HA and some other Ca-P composites are typical bioactive ceramics [60–62]. These bioceramics show excellent biocompatibility properties, but medical applications are limited to small fillers [63,64], grains and coatings [65–68] due to poor mechanical properties. The third generation of bioceramics aimed to provide an adequate scaffolding system which can help drive the living tissue regeneration [69]. By optimizing the biomaterials and controlling the implant–tissue interface, the sophisticated implant system can induce tissue regeneration and help to recover it. Some reported Ca-P ceramics show osteoinductivity and are considered to have the capacity of bone regeneration to a certain degree. The final purpose of tissue engineering attempts to develop artificial materials that are able to replace the function of biological tissues in situations where the living body is damaged and cannot perform by itself [70]. Ideal bioceramics [69,71] not only need the ability to recover or regenerate the damaged tissue, but also request to perform their natural functions.
Figure 1.
A schematic of the evolution of bioceramics.
3. Protein adsorption on Ca-P ceramics
As the main inorganic composition of a body's hard tissue, Ca-P bioceramics have excellent bioactivity and good capability in osteoconduction or osteoinduction [8,72–79], and have been widely adopted in bone repair or orthopaedic application. Table 1 lists most of the Ca-P compounds and their calcium to phosphorous molar ratio (Ca/P ratio) and stability [78]. It is well known that protein adsorption plays an important role in determining the biological properties of Ca-P. Many studies about protein adsorption behaviours have been done in order to better understand the mechanism of protein adsorption and the reasons that Ca-P bioceramics have excellent biological properties. The results and conclusions have greatly expanded our knowledge of bioactivity and osteoinductivity.
Table 1.
The Ca-P bioceramics [78].
| name | symbol | formula | Ca/P | stability |
|---|---|---|---|---|
| monocalcium phosphate monohydrate | MCPM | Ca(H2PO4)2·H2O | 0.5 | stable |
| dicalcium phosphate dihydrate | DCPD | CaHPO4 ·2H2O | 1.0 | sub-stable |
| dicalcium phosphate anhydrate | DCPA | CaHPO4 | 1.0 | stable |
| octacalcium phosphate | OCP | Ca8(HPO4)2(PO4)4·5H2O | 1.33 | sub-stable |
| tricalcium phosphate | TCP | Ca3(PO4)2 | 1.5 | sub-stable |
| amorphous calcium phosphate | ACP | Cax(PO4)y ·nH2O | 1.2–2.2 | sub-stable |
| hydroxyapatite | HA | Ca10(PO4)6(OH)2 | 1.67 | stable |
| tetracalcium phosphate | TTCP | Ca4(PO4)2O | 2.0 | sub-stable |
In recent years, the biological, physical–chemical methodologies and their combination have been wildly adopted in the research of protein adsorption on materials. With the rapid development of computer technology, the simulation method has been increasingly applied in this area and provided us much adsorption information at the atomic level. Many facets in protein adsorption have attracted much attention. For example, the effect of different material properties on adsorption behaviour, such as chemical component and surface properties; the effect of different protein properties and environments on the adsorption behaviour, such as the pH of a protein solution, the acidity/basicity or electric charge of proteins and the conformation change of protein upon adsorption onto the surface. Dee et al. [80] have shown the main properties of proteins and surfaces that affect adsorption in tables 2 and 3.
Table 2.
Properties of proteins that affect protein adsorption [80].
| property | effect |
|---|---|
| size | larger molecules can have more sites of contact with the surface |
| charge | molecules near their isoelectric point generally adsorb more readily |
| structure stability | less stable proteins, such as those with less intra-molecular cross-linking, can unfold to a greater extent and form more contact points with the surface |
| unfolding rate | molecules that rapidly unfold can form contacts with the surface more quickly |
Table 3.
Properties of surfaces that affect protein adsorption [80].
| feature | effect |
|---|---|
| topography | greater texture exposes more surface area for interaction with proteins |
| composition | chemical makeup of a surface will determine the types of intermolecular forces governing interaction with proteins |
| hydrophobicity | hydrophobic surfaces tend to bind more protein |
| heterogeneity | non-uniformity of surface characteristics results in domains that can interact differently with proteins |
| potential | surface potential will influence the distribution of ions in solution and interaction with proteins |
We will review protein adsorption on Ca-P bioceramics based on these properties in detail in the following sections.
3.1. The effect of Ca-P–protein properties on protein adsorption
3.1.1. Surface features of Ca-P
Topography
The physical–chemical feature of material surface is one of the decisive factors of protein adsorption. Surface topography, such as roughness, porosity, pore size and particle size, etc., determines the scale of the surface area that interacts with the protein molecules. More exposed surface area can provide more interaction sites for protein adsorption. These sites bond protein molecules through different ways, such as electrostatic force, hydrophobicity and so on. It is generally accepted that the higher surface area/specific surface area (SSA), the higher the quantity of protein adsorption, based on lots of experimental results. Essentially, modulating the roughness, porosity, pore size and particle size of material creates more surface area that can benefit protein adsorption. Note that increasing surface area is not only valid for improving protein adsorption on Ca-P bioceramics, but also valid for other materials.
Greater roughness could lead to a greater surface area, but this may not be invariable at the nano scale. Dos Santos et al. [81] found that the albumin and fibronectin adsorption on HA (Au-coated or not) with lower nano-roughness (32 ± 6 nm) was higher than that observed on β-TCP (Au-coated or not) over time. Cai et al. [82] also showed that the nanoscale roughness on titanium surfaces had little effect on the structure and the amount of adsorbed albumin and fibrinogen. However, the adsorption of larger molecules like collagen can be influenced by the different degrees of roughness on polymer surfaces [83]. Differences were observed not only in the amount of proteins but also in their structure. The reason may be that when the roughness scale increases from nanometres to micrometres, the topography may appear smooth to the protein, considering the protein size, and have little effect on the adsorption process [84,85]. Presently, it is known that nanoscale roughness of Ca-P can affect the protein-adsorption process, but more studies need to be done to understand the influencing trends, especially for different protein adsorption on different Ca-P surfaces.
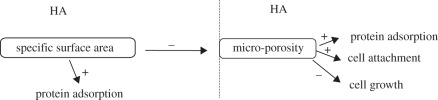
Porosity, pore size/distribution and particle size also impact protein adsorption through regulating the surface area. The presence of porosity greatly increases the surface area of materials and improves the protein adsorption. Zhu et al. [86] reported that the amount of the total adsorbed proteins on porous biphasic Ca-P (BCP (HA/TCP = 7 : 3)) was far beyond that on dense BCP. Lots of pores with the size distribution from 100 to 500 μm diameter presented on porous BCP, and many micropores distributed on the wall of the macropores. The increased surface area of materials is mainly attributed to the presence of macropores and micropores. Higher porosity leads to higher surface area. The porosity could further increase protein adsorption and the subsequent cell attachment [87]. Many other studies also proved the effect of porosity on protein adsorption on Ca-P [88–90]. Most proteins would suffer structural or conformational rearrangement after adsorption on the substrate surface [80]. The behaviour of proteins adsorbed on porous Ca-P is a multi-layer adsorption process and that on dense Ca-P is a monolayer one. It could be attributed to the hold-back effect of porous structures [86]. Furthermore, the effect of porous structures on protein adsorption has been considered as an interpretation of the osteoinductive potential of Ca-P bioceramics after implanting to ectopic sites [91,92]. Ca-P bioceramics can adsorb and enrich native bone morphogenetic proteins (BMPs) from body fluid, which induce bone formation in a dose-dependent manner [93]. If the threshold of BMP local concentration for triggering osteoinduction is satisfied, the Ca-P bioceramics could have the potential to be osteoinductive. The porous structure of Ca-P increases the SSA, which leads Ca-P to bond more BMPs, and plays a vital role for the osteoinductivity of Ca-P bioceramics. Meanwhile, pore size is another factor that controls protein adsorption. It should correlate with protein size and cell size. If the nano/meso-pore is smaller than the protein, the protein could not be adsorbed in the pores and thus the efficient surface area for this protein adsorption must be decreased. Contrarily, the protein is easily trapped in the meso-pores and improves adsorption. Many experimental results have validated this phenomenon. Fujii et al. [94] reported that zinc-substituted HA (Zn-HA) nano crystals with some Zn content was more appropriate for β2-microglobulin (β2-MG) adsorption than for bovine serum albumin (BSA) adsorption, which is attributed to the pore size presented on Zn-HA being suitable for β2-MG adsorption. The same story also happened on carbonate HA and other Ca-P [95–97]. Meanwhile, the cell size is a key benchmark for macro-pore size selection. The average size of body cells is about 50 μm, thus 0–100 μm micro-pores were adopted in porous Ca-P bioceramics for benefiting cell adhesion, and 100–500 μm macro-pores benefiting tissue ingrowth [91,92]. As for the particle size, it mainly correlated with the SSA. Smaller particle size leads to higher SSA, which enhances protein adsorption [96,98]. Rouahi et al. [88] reported that HA powder with 100 nm particles led to higher adsorption of proteins than that on HA powder with 1 μm particles. This was attributed to the higher SSA of nano-scale HA powder compared with the micro-scale HA. Thus, the quantity of proteins adsorbed on powders was positively correlated with their SSA. Combining the effect of porosity, it should be that the higher the SSA of Ca-P particles, the higher their protein adsorption, the lower the micro-porosity of ceramics, the lower their protein adsorption, and the lower the initial cell attachment as shown in figure 2.
Figure 2.
Schematic of the inverse correlation existing between SSA, protein adsorption capacity of HA powder and protein adsorption and cell attachment and growth on sintered HA ceramics [88].
It should be noted that increasing SSA does not mean the size of particle is as small as possible. Because, when the size is on the order of nanometres, many factors are likely to change (e.g. the surface defects increase in inverse proportion to the particle size) [99]. Certain specific effects of nanomaterials could impact on protein adsorption. Unfortunately, there are few studies of the nano effect on protein adsorption on the Ca-P surface.
Chemical properties of Ca-P
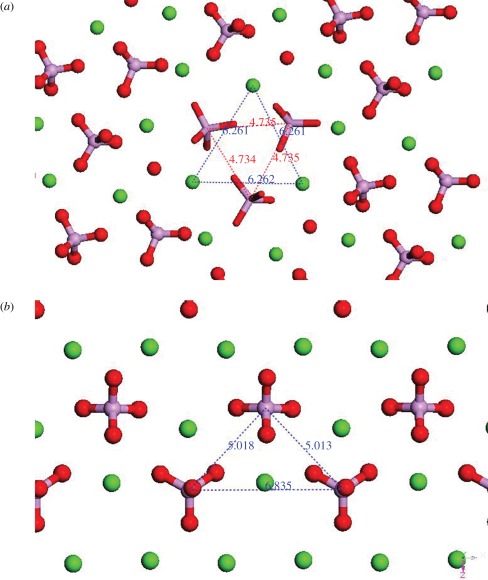
It is well known that protein-adsorption behaviours can be controlled by substrate surface parameters [100–103]. The chemical properties of the material surface play an important role on determining the efficiency of protein adsorption and the amount of protein adsorbed by interaction between the functional groups on substratum and proteins, and even the conformation of adsorbed proteins. The chemical nature of the surface can induce greater protein–surface interactions through either electrostatic or hydrophobic interactions [104]. It is generally accepted that electrostatic force played a vital role in the protein adsorption process and has been proved by lots of experimental and computer simulation studies [98,105–107]. The charged ions or groups on the substrate surface can bond the charged functional groups, including amino group, carbonyl group, carboxyl group and aromatic group, etc., on the protein molecules to dominate the protein adsorption. The bonded ions or groups on substrate surface or proteins are generally called adsorption sites. Much research has focused on the effect of chemical component or solubility/degradation rate and zeta potential of material on protein adsorption. Essentially, these effects are just to regulate the charge density, charge distribution or adsorption sites distribution on the substrate surface so as to benefit protein binding. Ca2+ and PO43− are believed to be the protein binding sites on Ca-P surfaces and provide the major driving force for protein adsorption [105,108,109]. Different component substrate materials have different structures that can lead to different charge/adsorption site distribution on the surfaces. For instance, the distribution of charged ions or groups (mainly Ca2+ and PO43−) on HA is far different from that on OCP (figure 3). The same story also happened on other Ca-P. This difference could induce the discrepancy of net charge on the substrate surface. It is known that proteins can be divided into two types generally, one is acidic protein whose isoelectric point (pI) < 7 and the other is basic protein whose pI > 7. When the pH is 7.4, which is equal to that of the physiological environment, acidic protein and basic protein carry a negative charge and positive charge, respectively. The electrostatic interaction between substrate surface and proteins could be affected by the surface charge and protein net charge in different solutions. Zhu et al. [98] reported that HA, BCP and TCP had negative surface charge, and preferred to adsorb more basic protein lysozyme (LSZ) than acidic protein BSA in pH 7.4 phosphate-buffered saline (PBS) solution. HA with higher surface net charge and thus higher value of zeta potential exhibited higher LSZ adsorption owing to the stronger electrostatic attraction between them. Another basic protein, transforming growth factor-β1 (TGF-β1), which can promote the proliferation and differentiation of bone-forming cells [110], also preferred to adsorb on the porous BCP with higher zeta potential than that on dense BCP in rat serum and in vivo [86]. Ohta et al. [111] reported that positive charge Ca2+ sites adsorbed acidic proteins and negative PO43− sites bonded basic proteins. The order of the ratios of Ca to P sites was estimated to be DCPD > OCP > HA ≫ DCPA ≫ β-TCP, which is in agreement with the order of the surface zeta potentials. And the amount of the adsorbed proteins is proportional to the surface charge. The type of adsorbed proteins is dependent on the distribution of Ca, P sites. The effect of distribution of charged ions or groups on protein adsorption is relatively obvious on the amorphous Ca-P. There is no significant difference in protein adsorption found between amorphous HA and fluorapatite since their surface structure is not highly ordered with respect to the position of Ca2+, PO43−, OH− and F− ions. Thus, the difference in the number of binding sites and the adsorption strength that also depends on the proteins is minimized [112].
Figure 3.
The top view of charged ions or groups distribution on (a) HA (001) and (b) OCP (100) planes. Ca, green; P, violet; O, red.
The vacancies and defects in the Ca-P crystal surface also impact protein adsorption [109]. Webster et al. [113] and Ergun et al. [114] reported that the amount of adsorbed BSA was decreased by the Zn2+ substituted into the HA crystal. Fujii et al. [94] found that the SSA of Zn-HA increased with increasing Zn content and the amounts of BSA adsorbed on Zn-HA decreased with increasing Zn content in spite of the increase in SSA. It could be attributed to the special arrangement of ions or groups on the substituted HA, which led Zn-HA to a highly selective adsorption of β2-MG combining with the topography effect. Elangovan et al. [115] reported that less proline-rich acidic salivary protein (PRP1) was adsorbed onto carbonated HA (CHA) than onto HA, and a smaller degree of BSA adsorption on CHA than HA with increasing carbonate content, citing changes in crystal morphology and texture as a possible cause [116]. However, Takemoto et al. [96] found that higher carbonate content of CHA adsorbed more β2-MG than lower ones. Segvich et al. [89] also reported the different adsorption behaviours of three artificial peptides on CHA and HA. Accordingly, these differences in adsorption caused by material defect/substitution are attributed to the difference in bonding site distribution or surface charge density/distribution on the substrate surfaces. Considering the variation of charge groups on proteins, the selective adsorption of proteins on different component/structure Ca-P is easy to understand.
The incubation environment is also an important factor that impacts on protein adsorption. Different kinds of ions in the solution are redistributed under control of substrate surface charges, which results in the properties of solution around the surface changing. The counter ions in the solution are attracted to the surface of substrate and make the nearby water molecules more orderly. These differences in the surface charge distribution caused by incubation in the solution have the potential to improve or inhibit protein adsorption on the material surfaces. Therefore, different solutions could induce different protein adsorption behaviours. However, for Ca-P bioceramics, the incubation solutions have similar properties according to the Ca-P application field. PBS, Hanks' balanced salt solution (HBSS), serum and in vivo are mostly employed for the investigation of protein adsorption on Ca-P. But differences in different solutions are rarely reported. The pH is an important factor that affects the electrical properties of the incubation solution. Studies showed that a decrease in pH led to an increase in acidic protein adsorption and binding affinity [117]. Meanwhile, the solubility of Ca-P is an important parameter for influencing the properties of the solution. A representative example on protein adsorption on BCP and HA occurred. It is known that β-TCP has a higher solubility than HA [118] and the dissolution of Ca2+, PO43− and other ions from β-TCP would lead to increasing ionic strength of the solution. Higher ionic strength in the solution could induce the protein to expose more polar-ionized residues to the solvent [117,119]. Thus, the amount of the protein adsorbed on BCP could be increased by the stronger interaction between protein and the surface-binding sites of BCP, which always has a higher ability to adsorb proteins than HA, considering the effect of topography at the same time [97]. This also could be the reason that BCP has better osteoinductivity than HA. For bioceramics, the sintering temperature has a great effect on the solubility of Ca-P in the incubation solution according to the differences in their thermodynamic properties [88]. Higher temperature leads to higher crystallinity, therefore lower solubility [25,120–122].
Hydrophobic properties of Ca-P
Besides the electrostatic force, the hydrophobic interaction is another important way to induce greater protein–surface affinity. It is generally true that a hydrophobic surface will adsorb proteins more strongly than a neutrally charged hydrophilic surface and thus adsorb a greater amount of proteins [123–125]. The proteins will tend to adsorb on the hydrophobic surface by hydrophobic patches of residues present in the protein's amphiphilic structure. Protein would unfold and spread its hydrophobic core over the surface owing to the thermodynamic driving force to reduce the net hydrophobic surface area of the system exposed to the solvent [125,126]. The charged and polar functional groups of proteins will tend to interact with the hydrophilic surface. For Ca-P bioceramics, BCP has a higher hydrophobicity than that of HA, but lower than that of β-TCP according to the contact angle measuring results [127,128]. This could be another reason that BCP has a higher ability to adsorb proteins than HA.
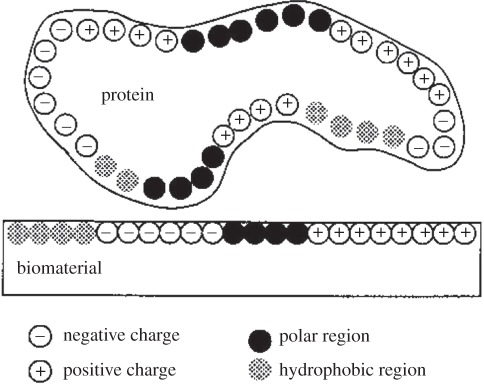
Overall, the effect of chemical and hydrophobic properties of biomaterial on protein adsorption can be illustrated in figure 4 [80]. All the variation in parameters of substrate materials, such as component, zeta potential, defect of crystalline and solubility, etc. are just to regulate the chemical properties or hydrophobicity of materials so as to obtain suitable surface charge, binding sites and polar site distribution to improve or inhibit protein adsorption.
Figure 4.
Diagram illustrating the importance of chemical and hydrophobic properties in protein adsorption [80].
Based on the above review, it is clear that Ca-P bioceramics also obey the general regulation of protein adsorption on substrate materials. In summary, the higher porosity and SSA, relative smaller particle size, suitable surface charge distribution, binding and hydrophobic/polar sites distribution for different kinds of proteins and incubation environments benefit protein adsorption on Ca-P bioceramics.
3.1.2. Properties of proteins and its conformational changes up on adsorption onto the Ca-P surface
The structure properties of proteins
The structure properties of proteins that influence surface activity are related to the primary structure of the protein, meaning that the sequence of amino acids affects protein–surface interactions. The bigger proteins have more binding sites to interact with the substrate surface. Thus, larger molecules have the potential to be adsorbed more on the surface. However, for a multi-component system, the mass transfer rate of solute molecule to a surface is directly related to its concentration and inversely to its molecular weight [126,129]. Accordingly, for multi-protein systems such as serum, the more concentrated and smaller proteins that would have greater diffusion rate tend to adsorb onto the surface first, and then are displaced by larger, more strongly interacting proteins that may be later adsorbed. This is known as the Vroman effect [130,131]. Meanwhile, the hydrophobicity-charged amino acids are generally located on the outside of proteins and are mainly responsible for adsorbing on surfaces. The same as the surface properties of Ca-P, the charge/binding site distribution on proteins also plays an important role in protein adsorption. Interestingly, proteins often show greater surface activity near their isoelectric point [80,132–134]. This could be attributed to the weaker interaction between protein molecules. Unfolding/spreading properties of the protein and its stability also impact on adsorption. Unfolding/spreading of a protein is likely to expose more sites for protein-surface contact. Less stable proteins are likely to unfold more, or faster. Although hydrophilic polar and charged amino acids are generally located on the exterior of the molecule and hydrophobic residues on the interior, hydrophobic amino acids also have the chance to interact with the surfaces. At the same time, unfolding/spreading of proteins can expose hydrophobic regions and allow interaction with the surface [80].
Considering the application field of Ca-P bioceramics, the most adopted target proteins in the investigation include acidic proteins, such as serum albumin, fibronectin, fibrinogen and bone-related phosphoproteins, and basic proteins, such as lysozyme and TGF-β1. But systematic research for kinds of protein adsorption on Ca-P is rarely reported. However, more detailed studies must be carried out. Recently, artificial peptides have been employed to further investigate the meticulous behaviour of protein adsorption [89].
The conformation changes of proteins on adsorption onto the Ca-P surface
Many of the studies have shown that the conformation of proteins would be changed when they are adsorbed onto the substrate surface, and the extent is mainly related to the surface properties of substrate and features of protein solution [135–140]. Conformation changes in adsorbed proteins have great effects on the biological activity of substrate materials and cellular interactions greatly depend on the nature of conformational change [141]. Note that not all the changes are beneficial for cell attachment: denatured fibronectin will no longer support the adhesion and the growth of cells [112]. In particular it is a vital factor for influencing the bioactivity of Ca-P bioceramics. For instance, Gibbons et al. [142] reported that there were significant differences in the affinity of oral bacteria for salivary proteins adsorbed on HA versus proteins in solution. On the other hand, if the conformation is altered, different amino acids could be exposed on the surface of the protein, which could consequently change the way the molecule binds to the substrate. Atomic force microscopy (AFM), Fourier transform infrared spectroscopy (FTIR)/attenuated total internal reflectance (ATR) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) are the most adopted methods for the investigation of protein conformation [83,112,138,140,142–145].
Zeng et al. [112] showed that the higher increase in the Amide I/Amide II ratio of BSA was seen on BSA adsorbed on a Ca-P surface, rather than on titanium (Ti) and germanium (Ge) surfaces. A greater amount of protein was adsorbed on the Ca-P surface. This result indicated that BSA lost α-helix structure owing to adsorption on all the surfaces and the greatest loss happened on the Ca-P surfaces. Still, it is difficult to know what the α-helix structure has transformed into owing to the adsorption. The great conformational changes of BSA adsorbed on the Ca-P could also be attributed to the electrostatic interactions. The distributions of binding sites play an important role in this process. Xie et al. [145] showed that the conformation of the adsorbed BSA changed during the brushite transformation to HA. In this process, the FTIR/ATR results showed that the percentage area of the dominant band (Amide I) at 1650 cm−1 was decreased and that of 1630 cm−1 was increased. Elangovan et al. [115] studied the conformational changes of acidic PRP1 upon adsorbing onto HA and CHA. In solution, large portions of PRP1 have the hydrated polyproline-type II (PPII) helical structure in addition to the random coil structure. After adsorbing onto HA and CHA, PRP1 loses a considerable portion of hydrated PPII and random coil domain, indicating that a large proportion of the proteins is composed of β turns. The conformational changes were greater in PRP1 adsorbed on HA than that on CHA, and less protein adsorbed onto CHA than onto HA. It is also attributed to the different distribution of binding sites or electrostatic ions/groups on CHA and HA. At the same time, the carbonate content of Ca-P could have a notable effect on the extent of conformational changes of proteins. On the other hand, the conformation and the structure of residue, peptide and protein also affect the Ca-P precipitate nucleation behaviour. A molecular dynamics (MD) simulation of nucleation of Ca-P on model peptides, from bone sialoprotein (BSP), with a different conformation presented, indicated that highly conserved contiguous Glu sequences have been demonstrated to be the nucleating domains of Ca-P, which is consistent with experimental results. In some simulations of the α-helical conformation, the possibility of promoting template nucleation of HA was seen, but not in random coil conformation [146].
The conformation of proteins is very important for the bioactivity or osteoinductivity of Ca-P bioceramics; however, there have not many studies focussing on this area for Ca-P. Importantly, further research for the effect of conformational changes of proteins on the biological response for Ca-P are rarely reported. The factors that could impact the conformation of proteins and the extent of its influence are still unclear. A systematic investigation of the relationship among Ca-P bioceramics properties, protein conformation and their biological response is very necessary.
3.2. Interactions between proteins and Ca-P
3.2.1. Interactions
All the protein-adsorption behaviours on Ca-P are the result of the interaction between proteins and Ca-P. Different interaction behaviours at the organic–inorganic interface can lead to different properties of protein adsorption and structure, morphology, size, orientation, nucleation and growth of Ca-P precipitates. Investigations of interaction between proteins and Ca-P, combining the theoretical analysis method, are helpful to better understand the mechanism of protein adsorption, even biomineralization.
Protein interaction with Ca-P mainly depends on the electrostatic force, and sometimes on the hydrogen bond, which was proved by experimental and computer simulation results [106,107,117]. Proteins adsorbed on the surfaces of Ca-P mainly through positive Ca sites, binding negative carboxylate groups, and negative P/OH sites, binding positive amino groups in protein, while other groups such as the charged guanido group, neutral amino and hydroxyl groups, have relatively weak interaction with the surfaces. Different proteins have different arrangements of charged groups that lead to different adsorption behaviours. As the Ca-P crystals have different structures on their planes, considering the pattern recognition between crystal surfaces and molecules [147], proteins could have the property of selective adsorption on Ca-P surface planes, which could be the reason for protein effects on morphology, size and orientation of Ca-P crystals. For instance, acidic proteins are preferentially adsorbed on the (100) face of HA and OCP crystals [148]. Whereas the strength of interaction between amelogenin and the crystal faces of OCP was in the order of (010) > (001) > (100), which indicated that amelogenin adsorption on OCP should block the growth of (010) face [149]. Different arrangements of carboxylate groups and amino groups results in different types of residues in protein, called acidic residue and basic residue that are negative and positive, respectively. Thus, based on the electrostatic attraction, the acidic proteins should preferably be adsorbed on the Ca site-based surfaces, basic proteins preferentially adsorbed on the P/OH site-based surfaces; acidic residues preferably bonded to the Ca sites, basic residues preferentially bonded to the P/OH sites. Overall, many of the mineralization-related proteins are acidic and phosphorylated, and are believed to play a key role in biomineralization [150]. The acidic phosphoproteins are obviously rich in acidic residues, aspartic acid (Asp) and glutamic acid (Glu), but also contain basic residues, arginine (Arg) and lysine (Lys). Glu preferred to adsorb strongly onto the HA (001) face, which resulted in the formation of plate-like HA; glycine (Gly) did not show any significantly preferential adsorption on HA surfaces, which resulted in rod-like HA [86,111,151].
3.2.2. Molecular dynamics simulation of protein adsorption
With the rapid development of computer technology, the simulation method has been increasingly applied in the field of protein adsorption and provided us with much of the Ca-P—proteins interaction information at the atomic level. The MD simulation method is mostly employed and has been widely applied in chemistry and biology. MD simulation is a technique that is based on classical mechanics, and calculates the time-dependent behaviours of a molecular system. In this computational methodology, the atoms are described as soft bodies. Combining suitable force-field parameters for proteins, the MD simulation has been widely applied in the research of protein adsorption on solid substrate [152,153]. In the research of biomaterials, it has also begun to play an increasingly prominent role. For example, The oligopeptide, Arg-Gly-Asp (RGD) tripeptides, fibronectin and HSA adsorption behaviour on rutile (110) surface have been studied by MD based on the Amber force field [154,155] and the Charmm force field [156]. The conformational change of HSA in the process of adsorption on a carbon nanotube surface also is simulated by the MD method based on the Charmm force field [157]. The interactive behaviours between different subdomains of HSA and graphite surfaces with or without water [158,159], and adsorption behaviour of certain oligopeptides on quartz surfaces have been evaluated by MD based on the CVFF force field [160].
Meanwhile, lots of MD work of the interaction between protein and Ca-P has been done. The Charmm force field-based simulations of fibronectin-type III with different orientations and BMP-7 adsorption on HA (001) surface indicated that electrostatic energy plays a dominant role in the interaction; the charged –COO− and –NH3+ are the strongest groups that interact with the HA surface [106,107]. The CVFF force field-based simulations of polyacrylic acid adsorption on HA surface indicated that potential sites for chelation and hydrogen bond formation between HA and polyacrylic acid exist, which depend on the exposed surface of HA. The COO− group strongly attached to calcium atoms and is a more prominent site for HA mineralization than the COOH group [161,162]. The Amber force field-based simulation of interaction between Hyp-Pro-Gly tri-peptide in collagen protein and the HA surfaces indicated that this tri-peptide interacted primarily with HA (1 0), rather than the HA (001) plane according to the results of adsorption energy, which is in agreement with an experiment that in natural bone the (1
0), rather than the HA (001) plane according to the results of adsorption energy, which is in agreement with an experiment that in natural bone the (1 0) surface grows preferentially from a collagen matrix [163–165]. Adsorption of Gly and Glu amino acids on the HA surface has been investigated by MD based on a mixed BHM and the Lennard_Jones force field. Its results indicated that the amino acids adsorbed on the HA (001) and (100) surfaces, with their positive amino groups occupying vacant calcium sites, and their negative carboxylate groups occupying vacant P or OH sites, precisely and formed an ordered adsorption layer; Glu preferred to adsorb strongly onto the HA (001) surface, which resulted in the formation of plate-like HA. However, Gly did not show any significantly preferential adsorption between these two HA surfaces [151]. Moreover, the issue of how BSP promotes the nucleation of HA was also investigated by MD simulation based on the Charmm force field, which simulated the interaction between different conformational peptides in BSP and Ca/P ions in aqueous solution. The results showed that a Ca2+ equilateral triangle formed around the surface of a peptide with α-helical conformation, which matched the distribution of Ca2+ on the (001) surface of the HA crystal. It indicated that the highly conserved contiguous Glu sequences should be the nucleating domains [146].
0) surface grows preferentially from a collagen matrix [163–165]. Adsorption of Gly and Glu amino acids on the HA surface has been investigated by MD based on a mixed BHM and the Lennard_Jones force field. Its results indicated that the amino acids adsorbed on the HA (001) and (100) surfaces, with their positive amino groups occupying vacant calcium sites, and their negative carboxylate groups occupying vacant P or OH sites, precisely and formed an ordered adsorption layer; Glu preferred to adsorb strongly onto the HA (001) surface, which resulted in the formation of plate-like HA. However, Gly did not show any significantly preferential adsorption between these two HA surfaces [151]. Moreover, the issue of how BSP promotes the nucleation of HA was also investigated by MD simulation based on the Charmm force field, which simulated the interaction between different conformational peptides in BSP and Ca/P ions in aqueous solution. The results showed that a Ca2+ equilateral triangle formed around the surface of a peptide with α-helical conformation, which matched the distribution of Ca2+ on the (001) surface of the HA crystal. It indicated that the highly conserved contiguous Glu sequences should be the nucleating domains [146].
Although MD simulation has been used in many research fields, we should note the limitation of MD calculations when applying the results to experiments. The simulation scales of time and space cannot match the real environments for the limitation of computer power. Moreover, differences between the parameters of force fields which were calculated based on quantum mechanics and practical results also existed. Thus, there is still a degree of discrepancy between computational results and experimental data. Nevertheless, the general trend and detailed information at the atomic level are useful in predicting and explaining the adsorption phenomena and are sufficient to narrow the experimental tests or reduce the analysis cost, even though the simulation results may not be the exact results.
4. Protein adsorption on non-Ca-P ceramics
Beyond Ca-P ceramics, protein adsorption on non-Ca-P ceramics is more complicated because the adsorbent substrates are uncertain [166,167]. Unlike Ca-P ceramics, the physical and chemical characters of non-Ca-P ceramics surface are different, and they are more sensitive to protein adsorption [168].
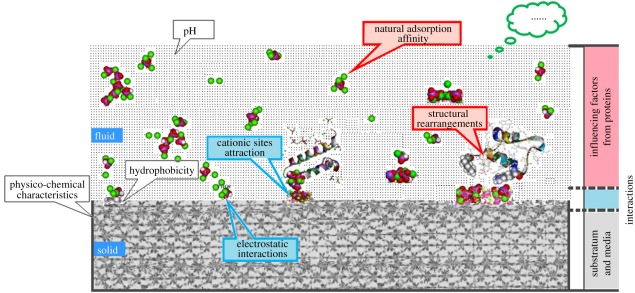
Many factors could affect protein adsorption. The dominant factors in non-Ca-P ceramics are different. However, protein adsorption is primarily determined by three categories: the properties of the proteins [169,170], the properties of the substrates and media [171–173], and the protein–substratum interactions [37,174–177]. Figure 5 shows a schematic of the influencing factors with respect to protein adsorption.
Figure 5.
The influencing factors of the protein adsorptions.
4.1. Influencing factors from proteins
The properties of the materials are natural characteristics to decide their adsorption affinity [178,179]. The adsorption phenomenon, firstly, is highly affected by the nature of the adsorbent proteins themselves. Norde & Anusiem [180] divided the proteins into two classes, ‘hard’ and ‘soft’ proteins. ‘Hard’ proteins have strong internal coherence and structural rearrangements that only make small contributions to the adsorption process. These proteins prefer to be adsorbed onto hydrophobic surfaces, whereas they can also be adsorbed on hydrophilic surfaces if they are electrostatically attracted [181,182]. ‘Soft’ proteins, which have lower structural stability, can be adsorbed even under more rigorous conditions. For example, on hydrophilic, electrostatically repelling surfaces, these proteins also show a large driving force for adsorption as a result of their structural rearrangements [183]. On this point, the famous ‘Vroman effect’ [184–186] also supports that the properties of proteins can greatly decide their adsorption. That is, on the same adsorbent surface, the natural property of protein can be critical factors for adsorption.
A study by Jachimska & Pajor [187] observed that the contact angle is very sensitive to the degree of protein adsorption on a solid surface. The maximum contact angle was observed when BSA and mica were oppositely charged. They also observed that higher positive zeta potential of BSA correlates with a higher contact angle. And at a higher positive zeta potential, BSA exhibited a higher binding affinity. This agrees with the opinion that hydrophobic surface shows better protein-adsorption affinity [188,189]. Jachimska also observed that even when the effective zeta potential of BSA was negative, which showed poor hydrophobic properties, a certain amount of BSA was also adsorbed. This phenomenon may originate from the heterogeneous charge distribution across the BSA molecules. Because the distribution of charge across a BSA molecule is heterogeneous and contains positive and negative plots, the positive plots would be the effective bonding area, so as to help the adsorption.
4.2. Influence factors from the substrates and media
The physico-chemical characteristics of adsorbent substrates and media are the other category of factors responsible for protein-binding capacity. Many influence factors from adsorbents should take into account, for example, chemical composition [190], dissolution behaviour or pH [79,191–193], degree of crystallization [194], microstructure, hydrophobicity [195–198], ζ-potential [199], surface roughness [200–202] surface reactivity [203], etc.
Obviously, different types of adsorbent substrates would greatly affect the protein-adsorption results. In research from Rosengren et al. [204], two bioactive glass-ceramics, RKKP and AP40, which differ only with respect to their Lanthanun and Tantalum content, have shown great differences in osteo-integrative ability when implanted into osteogenic rats [205,206]. Their investigation reveals that the differences of the osteo-integrative ability presented by two materials are because the two materials attract either different sets of proteins or different amounts of specific proteins, which can activate or suppress host response. Van Oss et al. [207] presented research about albumin adsorption on different inorganic oxide surfaces. They compared the adsorption of HSA on SiO2, SnO2 and ZrO2, and the corresponding results showed that the adsorption of HSA was 500.8 μg m2 on the SiO2 surface, whereas it was 968.2 μg m2 and 1157.9 μg m2 on the SnO2 and ZrO2 surface, respectively. SiO2 was quite hydrophilic and bears a higher negative charge, both the hydrophilicity and the strong negative ζ-potential resulted in the silica surfaces being repulsed by the adsorbed proteins at neutral pH. Rosengren et al. [204] revealed that the amount of plasma proteins adsorbed on the different materials could be very different. After the same adsorbed treatment, the binding plasmas were 0.42 mg m−2 on hydroxyapatite, 0.22 mg m−2 on zirconia and 0.20 mg m−2 on alumina. In addition, different forms of the materials also influence the adsorption, e.g. dense, solid plate, porous structure or powder depositions, etc.
A hydrophobic surface is ideal to promote protein bonding. Tanaka et al. [208] and Jeyachandran et al. [209] observed that BSA would rather adsorb to the hydrophobic surfaces than the hydrophilic ones. According to their report, the electrostatic force of attraction or repulsion between the BSA molecules and the surface does not play a primary role on determining the adsorption behaviour of the protein.
Demaneche et al. [210] studied the BSA adsorption onto a mica surface, observing that BSA protein-adsorption patterns are a function of pH. Bergers et al. [211] also revealed that the adsorption of protein was pH-sensitive. They studied the role of electrostatics and pH effect in the process of protein adsorption, and a result was observed that at low ionic strength a set of model proteins is responsible for the pH of the incubation medium. This pH dependency could be ascribed to the mean positive charge of the protein.
According to some previous studies, the protein-bonding capacity on Ca-P ceramics and non-Ca-P ceramics is different. For example, bioactive glass only can absorb a small amount or a few kinds of proteins. In contrast, many more proteins and greater amounts can be adsorbed on Ca-P ceramics [86,212–214]. Accordingly, it was confirmed that the bone-like apatite deposition was one of the factors responsible for the acceleration of bone regeneration. Therefore, researchers tried to modify the surface properties of the bioceramics to improve the protein adsorption and cell attachment. Pre-formation of a layer of apatite is a prevailing idea [215,216]. Biomimetic and plasma-spraying techniques are two popular methods used to fabricate the hydroxyapatite layer on the ceramics surface [217–219]. The biomimetic technique treats the substrates within simulated body fluid (SBF) to form an apatite layer on the surface. Both these methods have shown good results for promoting protein adsorption and cell attachment.
4.3. The protein-adsorbent substratum interactions
Bioceramics do not work alone during protein adsorption. The proteins, on the other hand, also play vital roles on determining the protein-adsorption process [220]. The mechanism of protein-adsorbent substrate interactions has been widely investigated. It was found that although most proteins are subject to macro-scale repulsion when approaching the hydrophilic surfaces in a neutral pH environment, they also undergo local micro-scale attraction onto a variety of sites, with the hydrophilic surfaces driven by the plurivalent cations embedded in these sites. Some studies found that cationic exchange (for example, the bioceramics are treated by the Ca2+ and La3+ ions) can make the negatively charged surface much more hydrophobic, thereby strongly promoting adsorption of various proteins.
When hydrophilicity and hydrophobicity are close to each other on the bioceramics surface, the hydrophobic effect does not play an important role in protein adsorption. The electrostatic interactions between ceramics and proteins may take the dominant role. From the protein adsorption results presented in the work of Beurer et al. [221] and Wierenga et al. [222], it can be concluded that the amount of adsorbed protein strongly corresponds to the net charge of the protein and ceramics surface. Rezwan et al. [220] studied the adsorption of lysozyme and BCA on silica and AlOOH-coated silica particles, where the uncoated and AlOOH-coated silica particles represent negatively and positively charged oxide surfaces. It was found that at the same pH (at pH 7), a protein oppositely charged to the oxide surface adsorbed much higher amounts. In contrast, proteins of the same charge did not, or only in very low amounts, adsorb on the oxide surface. During the absorption process, proteins with charged amino acid side chains may experience repulsive and attractive coulomb interactions, which could play an important role on protein adsorption.
Van Oss et al. [207] studied discrete cationic sites and electrostatic interactions in plurivalent cation-containing particle solution. They observed that washing with Na2EDTA can desorb proteins from inorganic particle surfaces. Na2EDTA comprises many discrete sites with local excess positive charge owing to the presence of plurivalent cations. These local positively charged sites attract negatively charged proteins not only through electrostatic attraction, but also as a consequence of the fact that they are electron acceptors, which will bind to proteins that are electron donors.
5. Multi-protein competition adsorption and the Vroman effect
Another interesting aspect of protein adsorption to bioceramics is competition adsorption between two or more proteins on the same adsorbent surface [223–225]. Leo Vroman firstly observed that fibrinogen preferentially adsorbed onto tantalum surfaces from blood plasma. From then on, many researchers have found and proved this phenomenon [186,226]. This kind of multi-protein adsorption phenomenon is finally named as ‘the Vroman effect’. The Vroman effect describes that in a mixed protein system, protein adsorption usually involves a series of adsorption-displacement steps, in which the proteins with low molecular weight are adsorbed on the surface first and then are displaced by those proteins with relatively higher molecular weight [227,228]. For example, some researchers [186] found that the high molecular weight of kininogen would displace the low molecular weight of fibrinogen. However, certain proteins, such as human serum albumin, are observed to be relatively resistant to displacement at hydrophobic surfaces.
The competitive adsorption of proteins is highly dependent on the concentrations and competing components [223,226]. Brash & Lyman [229] demonstrated that protein adsorption is in direct proportion to the protein concentration. Hyeran Noh et al. [230,231] performed the standard depletion method to measure multi-protein adsorption by using SDS-gel electrophoresis as a separation and a quantification tool. They observed the competitive adsorption behaviour of proteins on the same hydrophobic adsorbent surface, and thereby they concluded that the change of protein size (mainly molecular weight of proteins) affected protein-adsorption kinetics. In addition, it seems that the Vroman effect, at least in part, is due to a purely physical process unrelated to protein biochemistry or protein-adsorption kinetics.
Actually, the competitive adsorption of proteins is more realistic to the situation in the living body. However, nowadays, the Vroman effect is a visible phenomenon but the underlying molecular mechanisms with respect to this process have not yet been well explored.
6. Conclusions
Protein adsorptions are highly complicated matters and more research into the mechanisms regarding the nature of the adsorption is needed. Especially, under the requirement of regenerative medicine, more efforts need be paid to explore the adsorbed proteins on the surface of bioceramics, such as adsorbed protein species, protein conformation and protein–protein interactions. These factors are likely to definitively regulate adhered cells, especially stem cells, to differentiate special tissues. Additionally, up to now available and feasible methods and devices have been lacking. Thus, another important task is to develop rational methods and devices to precisely analyse the adsorbed proteins.
Acknowledgments
This work was supported by the National High Technology Research and Development Programme of China (2011AA030105), National Natural Science Foundation of China (No. 31170928), National 973 Programme (Grant 2011CB606201) and Youth Scholar Fund (20090181120078).
References
- 1.Dorozhkin S. V. 2010. Bioceramics of calcium orthophosphates. Biomaterials 31, 1465–1485 10.1016/j.biomaterials.2009.11.0 (doi:10.1016/j.biomaterials.2009.11.0) [DOI] [PubMed] [Google Scholar]
- 2.Best S. M., Porter A. E., Thian E. S., Huang J. 2008. Bioceramics: past, present and for the future. J. Eur. Ceram. Soc. 28, 1319–1327 10.1016/j.jeurceramsoc.2007.12.001 (doi:10.1016/j.jeurceramsoc.2007.12.001) [DOI] [Google Scholar]
- 3.Williams D. F. 2009. On the nature of biomaterials. Biomaterials 30, 5897–5909 10.1016/j.biomaterials.2009.07.027 (doi:10.1016/j.biomaterials.2009.07.027) [DOI] [PubMed] [Google Scholar]
- 4.Allan B. 1999. Closer to nature: new biomaterials and tissue engineering in ophthalmology. Br. J. Ophthalmol. 83, 1235–1240 10.1136/bjo.83.11.1235 (doi:10.1136/bjo.83.11.1235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doremus R. H. 1992. Bioceramics. J. Mater. Sci. 27, 285–297 10.1007/BF00543915 (doi:10.1007/BF00543915) [DOI] [Google Scholar]
- 6.Jandt K. D. 2007. Evolutions, revolutions and trends in biomaterials science-a perspective. Adv. Eng. Mater. 9, 1035–1050 10.1002/adem.200700284 (doi:10.1002/adem.200700284) [DOI] [Google Scholar]
- 7.Kawahara H. 1979. Today and tomorrow of bioceramics. J. Oral. Implantol. 8, 411–432 [PubMed] [Google Scholar]
- 8.Cao W., Hench L. L. 1996. Bioactive materials. Ceram. Int. 22, 493–507 10.1016/0272-8842(95)00126-3 (doi:10.1016/0272-8842(95)00126-3) [DOI] [Google Scholar]
- 9.Salinas A. J., Vallet-Regi M. 2007. Evolution of ceramics with medical applications. Z. Anorg. Allg. Chem. 633, 1762–1773 10.1002/zaac.200700278 (doi:10.1002/zaac.200700278) [DOI] [Google Scholar]
- 10.Hench L. L. 1991. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 74, 1487–1510 10.1111/j.1151-2916.1991.tb07132.x (doi:10.1111/j.1151-2916.1991.tb07132.x) [DOI] [Google Scholar]
- 11.Ducheyne P., Qiu Q. 1999. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials 20, 2287–2303 10.1016/S0142-9612(99)00181-7 (doi:10.1016/S0142-9612(99)00181-7) [DOI] [PubMed] [Google Scholar]
- 12.Porter A. E., Patel N., Skepper J. N., Best S. M., Bonfield W. 2003. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 24, 4609–4620 10.1016/S0142-9612(03)00355-7 (doi:10.1016/S0142-9612(03)00355-7) [DOI] [PubMed] [Google Scholar]
- 13.Dorozhkin S. V. 2008. Green chemical synthesis of calcium phosphate bioceramics. J. Appl. Biomater. Biomech. 6, 104–109 [PubMed] [Google Scholar]
- 14.Huang Y., Jin X., Zhang X., Sun H., Tu J., Tang T., Chang J., Dai K. 2009. In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration. Biomaterials 30, 5041–5048 10.1016/j.biomaterials.2009.05.077 (doi:10.1016/j.biomaterials.2009.05.077) [DOI] [PubMed] [Google Scholar]
- 15.Rucker M., Laschke M. W., Junker D., Carvalho C., Tavassol F., Mulhaupt R., Gellrich N. C., Menger M. D. 2008. Vascularization and biocompatibility of scaffolds consisting of different calcium phosphate compounds. J. Biomed. Mater. Res. A. 86, 1002–1011 10.1002/jbm.a.31722 (doi:10.1002/jbm.a.31722) [DOI] [PubMed] [Google Scholar]
- 16.Sinikovic B., Kramer F. J., Swennen G., Lubbers H. T., Dempf R. 2007. Reconstruction of orbital wall defects with calcium phosphate cement: clinical and histological findings in a sheep model. Int. J. Oral. Maxillofac. Surg. 36, 54–61 10.1016/j.ijom.2006.07.014 (doi:10.1016/j.ijom.2006.07.014) [DOI] [PubMed] [Google Scholar]
- 17.Xin R., Leng Y., Chen J., Zhang Q. 2005. A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials 26, 6477–6486 10.1016/j.biomaterials.2005.04.028 (doi:10.1016/j.biomaterials.2005.04.028) [DOI] [PubMed] [Google Scholar]
- 18.Combes C., Rey C. 2002. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials 23, 2817–2823 10.1016/S0142-9612(02)00073-X (doi:10.1016/S0142-9612(02)00073-X) [DOI] [PubMed] [Google Scholar]
- 19.Kim H. W., Kim H. E. 2006. Nanofiber generation of hydroxyapatite and fluor-hydroxyapatite bioceramics. J. Biomed. Mater. Res. B Appl. Biomater. 77, 323–328 10.1002/jbm.b.30376 (doi:10.1002/jbm.b.30376) [DOI] [PubMed] [Google Scholar]
- 20.Suchanek W., Yoshimura M. 1998. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 13, 94–117 10.1557/JMR.1998.0015 (doi:10.1557/JMR.1998.0015) [DOI] [Google Scholar]
- 21.Fischer H., Weiss R., Telle R. 2008. Crack healing in alumina bioceramics. Dent. Mater. 24, 328–332 10.1016/j.dental.2007.06.001 (doi:10.1016/j.dental.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 22.Marti A. 2000. Inert bioceramics (Al2O3, ZrO2) for medical application. Injury 31(Suppl 4), 33–36 10.1016/S0020-1383(00)80021-2 (doi:10.1016/S0020-1383(00)80021-2) [DOI] [PubMed] [Google Scholar]
- 23.Hong J. J., Yang S. M. 1996. Adsorption of tricarboxylic acid biosurfactant derived from spiculisporic acid on titanium dioxide surface. Colloids Surf. B Biointerf. 7, 221–233 10.1016/0927-7765(96)01302-1 (doi:10.1016/0927-7765(96)01302-1) [DOI] [Google Scholar]
- 24.Hsu Y. H., Turner I. G., Miles A. W. 2007. Mechanical characterization of dense calcium phosphate bioceramics with interconnected porosity. J. Mater. Sci. Mater. Med. 18, 2319–2329 10.1007/s10856-007-3136-0 (doi:10.1007/s10856-007-3136-0) [DOI] [PubMed] [Google Scholar]
- 25.Legeros R. Z., Lin S., Rohanizadeh R., Mijares D., Legeros J. P. 2003. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J. Mater. Sci. Mater. Med. 14, 201–209 10.1023/A:1022872421333 (doi:10.1023/A:1022872421333) [DOI] [PubMed] [Google Scholar]
- 26.Rangavittal N., Landa-Canovas A. R., Gonzalez-Calbet J. M., Vallet-Regi M. 2000. Structural study and stability of hydroxyapatite and beta-tricalcium phosphate: two important bioceramics. J. Biomed. Mater. Res. 51, 660–668 (doi:10.1002/1097-4636(20000915)51:4<660::AID-JBM14>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- 27.Kokubo T. 1991. Bioactive glass ceramics: properties and applications. Biomaterials 12, 155–163 10.1016/0142-9612(91)90194-F (doi:10.1016/0142-9612(91)90194-F) [DOI] [PubMed] [Google Scholar]
- 28.Frayssinet P., Mathon D., Lerch A., Autefage A., Collard P., Rouquet N. 2000. Osseointegration of composite calcium phosphate bioceramics. J. Biomed. Mater. Res. 50, 125–130 (doi:10.1002/(SICI)1097-4636(200005)50:2<125::AID-JBM5>3.0.CO;2-#) [DOI] [PubMed] [Google Scholar]
- 29.Langstaff S., Sayer M., Smith T. J., Pugh S. M., Hesp S. A., Thompson W. T. 1999. Resorbable bioceramics based on stabilized calcium phosphates. I. Rational design, sample preparation and material characterization. Biomaterials 20, 1727–1741 10.1016/S0142-9612(99)00086-1 (doi:10.1016/S0142-9612(99)00086-1) [DOI] [PubMed] [Google Scholar]
- 30.Langstaff S., Sayer M., Smith T. J., Pugh S. M. 2001. Resorbable bioceramics based on stabilized calcium phosphates. II. Evaluation of biological response. Biomaterials 22, 135–150 10.1016/S0142-9612(00)00139-3 (doi:10.1016/S0142-9612(00)00139-3) [DOI] [PubMed] [Google Scholar]
- 31.Radin S. R., Ducheyne P. 1994. Effect of bioactive ceramic composition and structure on in vitro behavior. III. Porous versus dense ceramics. J. Biomed. Mater. Res. 28, 1303–1309 10.1002/jbm.820281108 (doi:10.1002/jbm.820281108) [DOI] [PubMed] [Google Scholar]
- 32.Kotani S., Yamamuro T., Nakamura T., Kitsugi T., Fujita Y., Kawanabe K., Kokubo T. 1992. Enhancement of bone bonding to bioactive ceramics by demineralized bone powder. Clin. Orthop. Relat. Res. 278, 226–234 [PubMed] [Google Scholar]
- 33.Fujita Y., Yamamuro T., Nakamura T., Kitsugi T., Kotani S., Ohtsuki C., Kokubo T. 1992. Mechanism and strength of bonding between two bioactive ceramics in vivo. J. Biomed. Mater. Res. 26, 1311–1324 10.1002/jbm.820261005 (doi:10.1002/jbm.820261005) [DOI] [PubMed] [Google Scholar]
- 34.Lu J. X., Flautre B., Anselme K., Hardouin P., Gallur A., Descamps M., Thierry B. 1999. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J. Mater. Sci. Mater. Med. 10, 111–120 10.1023/A:1008973120918 (doi:10.1023/A:1008973120918) [DOI] [PubMed] [Google Scholar]
- 35.Gunther K., Scharf H. P., Puhl W. 1993. In vitro toxicity tests of bioceramics and bone transplants in fibroblast culture. Biomed. Technol. (Berl). 38, 249–254 10.1515/bmte.1993.38.10.249 (doi:10.1515/bmte.1993.38.10.249) [DOI] [PubMed] [Google Scholar]
- 36.Wu H., Zhu T. B., Du J. Y., Hong G. X., Sun S. Z., Xu X. H. 1992. New bone formation in the in vivo implantation of bioceramics. A quantitative analysis. Chin. Med. J. (Eng). 105, 753–757 [PubMed] [Google Scholar]
- 37.Daculsi G., Hartmann D. J., Heughebaert M., Hamel L., Le Nihouannen J. C. 1988. In vivo cell interactions with calcium phosphate bioceramics. J. Submicrosc. Cytol. Pathol. 20, 379–384 [PubMed] [Google Scholar]
- 38.Ducheyne P. 1987. Bioceramics: material characteristics versus in vivo behavior. J. Biomed. Mater. Res. 21, 219–236 [PubMed] [Google Scholar]
- 39.Klein C. P., Patka P., Den Hollander W. 1989. Macroporous calcium phosphate bioceramics in dog femora: a histological study of interface and biodegradation. Biomaterials 10, 59–62 10.1016/0142-9612(89)90011-2 (doi:10.1016/0142-9612(89)90011-2) [DOI] [PubMed] [Google Scholar]
- 40.Yan W. Q., Oka M., Nakamura T. 1998. Bone bonding in bioactive glass ceramics combined with bone matrix gelatin. J. Biomed. Mater. Res. 42, 258–265 (doi:10.1002/(SICI)1097-4636(199811)42:2<258::AID-JBM10>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 41.Puleo D. A., Nanci A. 1999. Understanding and controlling the bone–implant interface. Biomaterials 20, 2311–2321 10.1016/S0142-9612(99)00160-X (doi:10.1016/S0142-9612(99)00160-X) [DOI] [PubMed] [Google Scholar]
- 42.Low S. B. 1998. Bioactive ceramics in implant, grafting, and periodontal treatment. Dent. Implantol. Update. 9, 65–67 [PubMed] [Google Scholar]
- 43.Laczka O. A., Laczka M., Kasugai S., Ohya K. 1998. Behavior of bone marrow cells cultured on three different coatings of gel-derived bioactive glass-ceramics at early stages of cell differentiation. J. Biomed. Mater. Res. 42, 433–442 (doi:10.1002/(SICI)1097-4636(19981205)42:3<433::AID-JBM13>3.0.CO;2-H) [DOI] [PubMed] [Google Scholar]
- 44.Sawyer A., Hennessy K., Bellis S. 2005. Regulation of mesenchymal stem cell attachment and spreading on hydroxyapatite by RGD peptides and adsorbed serum proteins. Biomaterials 26, 1467–1475 10.1016/j.biomaterials.2004.05.008 (doi:10.1016/j.biomaterials.2004.05.008) [DOI] [PubMed] [Google Scholar]
- 45.Radin S., Ducheyne P. 1996. Effect of serum proteins on solution-induced surface transformations of bioactive ceramics. J. Biomed. Mater. Res. 30, 273–279 (doi:10.1002/(SICI)1097-4636(199603)30:3<273::AID-JBM1>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 46.Troczynski T. 2004. Bioceramics: a concrete solution. Nat. Mater. 3, 13–14 10.1038/nmat1039 (doi:10.1038/nmat1039) [DOI] [PubMed] [Google Scholar]
- 47.Daculsi G., Laboux O., Malard O., Weiss P. 2003. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 14, 195–200 10.1023/A:1022842404495 (doi:10.1023/A:1022842404495) [DOI] [PubMed] [Google Scholar]
- 48.Rehak L., Makai F., Bakos D., Vanis M. 2002. Calcium phosphate bioceramics in orthopedic implants. Acta Chir. Orthop. Traumatol. Cech. 69, 103–107 [PubMed] [Google Scholar]
- 49.Roy M. E., Whiteside L. A., Magill M. E., Katerberg B. J. 2011. Reduced wear of cross-linked UHMWPE using magnesia-stabilized zirconia femoral heads in a hip simulator. Clin. Orthop. Relat. Res. 469, 2337–2345 10.1007/s11999-011-1800-7 (doi:10.1007/s11999-011-1800-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukatsu K., Pezzotti G., Hayaishi Y., Sugano N. 2009. Evaluation of phase stability in zirconia femoral heads from different manufacturers after in vitro testing or in vivo retrieval. J. Arthroplasty. 24, 1225–1230 10.1016/j.arth.2009.06.026 (doi:10.1016/j.arth.2009.06.026) [DOI] [PubMed] [Google Scholar]
- 51.Cohn R. M., Gonzalez Della Valle A., Peterson M., Cornell C. N. 2008. Similar wear in total hip arthroplasties with metallic or zirconia femoral heads. HSS. J. 4, 107–111 10.1007/s11420-008-9084-5 (doi:10.1007/s11420-008-9084-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy M. E., Whiteside L. A., Katerberg B. J., Steiger J. A. 2007. Phase transformation, roughness, and microhardness of artificially aged yttria-and magnesia-stabilized zirconia femoral heads. J. Biomed. Mater. Res. A. 83, 1096–1102 10.1002/jbm.a.31438 (doi:10.1002/jbm.a.31438) [DOI] [PubMed] [Google Scholar]
- 53.Cotogno G., Holzwarth U., Franchi M., Rivetti S., Chiesa R. 2006. Tribological characterization of surface-treated commercially pure titanium for femoral heads in total hip replacement: a feasibility study. Int. J. Artif. Organs. 29, 1174–1184 [DOI] [PubMed] [Google Scholar]
- 54.Barouk P., Maynou C., Hildebrand H. F., Aubertin F., Breme J., Cassagnaud X., Mestdagh H. 2004. High incidence of total hip arthroplasty aseptic loosening with ion-coated titanium femoral heads. Rev. Chir. Orthop. Repar. Appar. Mot. 90, 26–32 10.1016/S0035-1040(04)70003-3) (doi:10.1016/S0035-1040(04)70003-3)) [DOI] [PubMed] [Google Scholar]
- 55.Raimondi M. T., Pietrabissa R. 2000. The in vivo wear performance of prosthetic femoral heads with titanium nitride coating. Biomaterials 21, 907–913 10.1016/S0142-9612(99)00246-X (doi:10.1016/S0142-9612(99)00246-X) [DOI] [PubMed] [Google Scholar]
- 56.Huet R., Sakona A., Kurtz S. M. 2011. Strength and reliability of alumina ceramic femoral heads: review of design, testing, and retrieval analysis. J. Mech. Behav. Biomed. Mater. 4, 476–483 10.1016/j.jmbbm.2010.12.010 (doi:10.1016/j.jmbbm.2010.12.010) [DOI] [PubMed] [Google Scholar]
- 57.Bal B. S., Rahaman M. N., Aleto T., Miller F. S., Traina F., Toni A. 2007. The significance of metal staining on alumina femoral heads in total hip arthroplasty. J. Arthroplasty. 22, 14–19 10.1016/j.arth.2006.02.155 (doi:10.1016/j.arth.2006.02.155) [DOI] [PubMed] [Google Scholar]
- 58.Tanaka K., Tamura J., Kawanabe K., Shimizu M., Nakamura T. 2003. Effect of alumina femoral heads on polyethylene wear in cemented total hip arthroplasty. Old versus current alumina. J. Bone Joint Surg. Br. 85, 655–660 [PubMed] [Google Scholar]
- 59.Sollazzo V., Pezzetti F., Scarano A., Piattelli A., Bignozzi C. A., Massari L., Brunelli G., Carinci F. 2008. Zirconium oxide coating improves implant osseointegration in vivo. Dent. Mater. 24, 357–361 10.1016/j.dental.2007.06.003 (doi:10.1016/j.dental.2007.06.003) [DOI] [PubMed] [Google Scholar]
- 60.Adenis J. P., Camezind P., Petit B., Pilon F., Robert P. Y., Boncoeur-Martel M. P., Camezind-Vidal M. A., Rayanachekir N. B., Labrousse F. 2003. Bone formation in hydroxyapatite tricalcium phosphate ceramic implants used in the treatment of the postenucleation socket syndrome. Orbit. 22, 183–191 10.1076/orbi.22.3.183.15615 (doi:10.1076/orbi.22.3.183.15615) [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez-Lorenzo L. M., Vallet-Regi M., Ferreira J. M., Ginebra M. P., Aparicio C., Planell J. A. 2002. Hydroxyapatite ceramic bodies with tailored mechanical properties for different applications. J. Biomed. Mater. Res. 60, 159–166 10.1002/jbm.1286 (doi:10.1002/jbm.1286) [DOI] [PubMed] [Google Scholar]
- 62.Adenis J. P., Bertin P., Lasudry J. G., Boncoeur-Martel M. P., Leboutet M. J., Robert P. Y. 1999. Treatment of the postenucleation socket syndrome with a new hydroxyapatite tricalcium phosphate ceramic implant. Ophthal. Plast. Reconstr. Surg. 15, 277–283 10.1097/00002341-199907000-00009 (doi:10.1097/00002341-199907000-00009) [DOI] [PubMed] [Google Scholar]
- 63.Overgaard S., Lind M., Josephsen K., Maunsbach A. B., Bunger C., Soballe K. 1998. Resorption of hydroxyapatite and fluorapatite ceramic coatings on weight-bearing implants: a quantitative and morphological study in dogs. J. Biomed. Mater. Res. 39, 141–152 (doi:10.1002/(SICI)1097-4636(199801)39:1<141::AID-JBM16>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 64.Barth E., Ronningen H., Solheim L. F. 1986. Bone fixation of ceramic-coated and fiber titanium implants. A study in weight-bearing rats. Acta Orthop Scand. 57, 25–29 10.3109/17453678608993209 (doi:10.3109/17453678608993209) [DOI] [PubMed] [Google Scholar]
- 65.Tachibana Y., Ninomiya S., Kim Y. T., Sekikawa M. 2003. Tissue response to porous hydroxyapatite ceramic in the human femoral head. J. Orthop. Sci. 8, 549–553 10.1007/s00776-003-0678-y (doi:10.1007/s00776-003-0678-y) [DOI] [PubMed] [Google Scholar]
- 66.Miyake H., Ohta T., Tanaka H. 2000. A new technique for cranioplasty with L-shaped titanium plates and combination ceramic implants composed of hydroxyapatite and tricalcium phosphate (Ceratite). Neurosurgery. 46, 414–418 10.1097/00006123-200002000-00028 (doi:10.1097/00006123-200002000-00028) [DOI] [PubMed] [Google Scholar]
- 67.Nimb L., Jensen J. S., Gotfredsen K. 1995. Interface mechanics and histomorphometric analysis of hydroxyapatite-coated and porous glass-ceramic implants in canine bone. J. Biomed. Mater. Res. 29, 1477–1482 10.1002/jbm.820291203 (doi:10.1002/jbm.820291203) [DOI] [PubMed] [Google Scholar]
- 68.Takahashi A., Koshino T. 1995. Compressive and bone-bonding strength of hydroxyapatite thermal decomposition product implanted in the femur of rabbit as a bioactive ceramic bone cement. Biomaterials 16, 937–943 10.1016/0142-9612(95)93119-X (doi:10.1016/0142-9612(95)93119-X) [DOI] [PubMed] [Google Scholar]
- 69.Hench L. L., Polak J. M. 2002. Third-generation biomedical materials. Science 295, 1014–1017 10.1126/science.1067404295/5557/1014 (doi:10.1126/science.1067404295/5557/1014) [DOI] [PubMed] [Google Scholar]
- 70.Langer R., Vacanti J. P. 1993. Tissue engineering. Science 260, 920–926 10.1126/science.8493529 (doi:10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- 71.Hench L. L. 1980. Biomaterials. Science 208, 826–831 10.1126/science.6246576 (doi:10.1126/science.6246576) [DOI] [PubMed] [Google Scholar]
- 72.Chow L. C., Eanes E. D. 2001. Calcium phosphate cements, vol. 18 Basel, Switzerland: Karger [Google Scholar]
- 73.Hench L. L. 1998. Bioceramics. J. Am. Ceram. Soc. 81, 1705–1711 10.1111/j.1151-2916.1998.tb02540.x (doi:10.1111/j.1151-2916.1998.tb02540.x) [DOI] [Google Scholar]
- 74.Legeros R. Z. 1991. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 15, 1–6 [PubMed] [Google Scholar]
- 75.Oonishi H., Kushitani S., Yasukawa E., Iwaki H., Hench L. L., Wilson J., Tsuji E., Sugihara T. 1997. Particulate bioglass compared with hydroxyapatite as a bone graft substitute. Clin. Orthop. Relat. Res. 334, 316–325 10.1097/00003086-199701000-00041 (doi:10.1097/00003086-199701000-00041) [DOI] [PubMed] [Google Scholar]
- 76.Sepulveda P., Jones J. R., Hench L. L. 2002. Bioactive sol gel foams for tissue repair. J. Biomed. Mater. Res. 59, 340–348 10.1002/jbm.1250 (doi:10.1002/jbm.1250) [DOI] [PubMed] [Google Scholar]
- 77.Silva G. A., Coutinho O. P., Ducheyne P., Reis R. L. 2007. Materials in particulate form for tissue engineering. II. Applications in bone. J. Tissue Eng. Regen. Med. 1, 97–106 10.1002/term.1 (doi:10.1002/term.1) [DOI] [PubMed] [Google Scholar]
- 78.Vallet-Regí M., González-Calbet J. M. 2004. Calcium phosphates as substitution of bone tissues. Prog. Solid Stat. Chem. 32, 1–31 10.1016/j.progsolidstchem.2004.07.001 (doi:10.1016/j.progsolidstchem.2004.07.001) [DOI] [Google Scholar]
- 79.Zhang Z., Dalgleish D. G., Goff H. D. 2004. Effect of pH and ionic strength on competitive protein adsorption to air/water interfaces in aqueous foams made with mixed milk proteins. Colloids Surf. B Biointerf. 34, 113–121 10.1016/j.colsurfb.2003.11.009 (doi:10.1016/j.colsurfb.2003.11.009) [DOI] [PubMed] [Google Scholar]
- 80.Dee K. C., Puleo D. A., Bizios R. 2002. Tissue–biomaterial interactions. New Jersey, NJ: John Willey and Sons Inc. press [Google Scholar]
- 81.Dos Santos E., Farina M., Soares G., Anselme K. 2008. Surface energy of hydroxyapatite and β-tricalcium phosphate ceramics driving serum protein adsorption and osteoblast adhesion. J. Mater. Sci. Mater. Med. 19, 2307–2316 10.1007/s10856-007-3347-4 (doi:10.1007/s10856-007-3347-4) [DOI] [PubMed] [Google Scholar]
- 82.Cai K., Frant M., Bossert J., Hildebrand G., Liefeith K., Jandt K. D. 2006. Surface functionalized titanium thin films: zeta-potential, protein adsorption and cell proliferation. Colloids Surf. B Biointerf. 50, 1–8 10.1016/j.colsurfb.2006.03.016 (doi:10.1016/j.colsurfb.2006.03.016) [DOI] [PubMed] [Google Scholar]
- 83.Dufrêne Y. F., Marchal T. G., Rouxhet P. G. 1999. Influence of substratum surface properties on the organization of adsorbed collagen films: in situ characterization by atomic force microscopy. Langmuir 15, 2871–2878 10.1021/la981066z (doi:10.1021/la981066z) [DOI] [Google Scholar]
- 84.Yap F. L., Zhang Y. 2007. Protein and cell micropatterning and its integration with micro/nanoparticles assembly. Biosens. Bioelectron. 22, 775–788 10.1016/j.bios.2006.03.016 (doi:10.1016/j.bios.2006.03.016) [DOI] [PubMed] [Google Scholar]
- 85.Han M., Sethuraman A., Kane R. S., Belfort G. 2003. Nanometer-scale roughness having little effect on the amount or structure of adsorbed protein. Langmuir 19, 9868–9872 10.1021/la030132g (doi:10.1021/la030132g) [DOI] [Google Scholar]
- 86.Zhu X. D., Fan H. S., Xiao Y. M., Li D. X., Zhang H. J., Luxbacher T., Zhang X. D. 2009. Effect of surface structure on protein adsorption to biphasic calcium-phosphate ceramics in vitro and in vivo. Acta Biomater. 5, 1311–1318 10.1016/j.actbio.2008.11.024 (doi:10.1016/j.actbio.2008.11.024) [DOI] [PubMed] [Google Scholar]
- 87.Rouahi M., Gallet O., Champion E., Dentzer J., Hardouin P., Anselme K. 2006. Influence of hydroxyapatite microstructure on human bone cell response. J. Biomed. Mater. Res. A. 78A, 222–235 10.1002/jbm.a.30682 (doi:10.1002/jbm.a.30682) [DOI] [PubMed] [Google Scholar]
- 88.Rouahi M., Champion E., Gallet O., Jada A., Anselme K. 2006. Physico-chemical characteristics and protein adsorption potential of hydroxyapatite particles: influence on in vitro biocompatibility of ceramics after sintering. Colloids Surf. B Biointerf. 47, 10–19 10.1016/j.colsurfb.2005.11.015 (doi:10.1016/j.colsurfb.2005.11.015) [DOI] [PubMed] [Google Scholar]
- 89.Segvich S. J., Smith H. C., Kohn D. H. 2009. The adsorption of preferential binding peptides to apatite-based materials. Biomaterials 30, 1287–1298 10.1016/j.biomaterials.2008.11.008 (doi:10.1016/j.biomaterials.2008.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu X. D., Zhang H. J., Fan H. S., Li W., Zhang X. D. 2009. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 6, 1536–1541 10.1016/j.actbio.2009.10.032 (doi:10.1016/j.actbio.2009.10.032) [DOI] [PubMed] [Google Scholar]
- 91.Yuan H., Kurashina K., De Bruijn J. D., Li Y., De Groot K., Zhang X. 1999. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 20, 1799–1806 10.1016/S0142-9612(99)00075-7 (doi:10.1016/S0142-9612(99)00075-7) [DOI] [PubMed] [Google Scholar]
- 92.Yuan H., Yang Z., Li Y., Zhang X., Bruijn J. D., Groot K. D. 1998. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 9, 723–726 10.1023/A:1008950902047 (doi:10.1023/A:1008950902047) [DOI] [PubMed] [Google Scholar]
- 93.Yuan H., Zou P., Yang Z., Zhang X., Bruijn J. D., Groot K. D. 1998. Bone morphogenetic protein and ceramic-induced osteogenesis. J. Mater. Sci. Mater. Med. 9, 717–721 10.1023/A:1008998817977 (doi:10.1023/A:1008998817977) [DOI] [PubMed] [Google Scholar]
- 94.Fujii E., Ohkubo M., Tsuru K., Hayakawa S., Osaka A., Kawabata K., Bonhomme C., Babonneau F. 2006. Selective protein adsorption property and characterization of nano-crystalline zinc-containing hydroxyapatite. Acta Biomater. 2, 69–74 10.1016/j.actbio.2005.09.002 (doi:10.1016/j.actbio.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 95.Takashima S., Kusudo Y., Takemoto S., Tsuru K., Hayakawa S., Osaka A. 2001. Synthesis of carbonate-hydroxy apatite and selective adsorption activity against specific pathogenic substances. Key Eng. Mater. 218, 175–178 10.4028/www.scientific.net/KEM.218-220.175 (doi:10.4028/www.scientific.net/KEM.218-220.175) [DOI] [Google Scholar]
- 96.Takemoto S., Kusudo Y., Tsuru K., Hayakawa S., Osaka A., Takashima S. 2004. Selective protein adsorption and blood compatibility of hydroxy-carbonate apatites. J. Biomed. Mater. Res. A. 69, 544–551 10.1002/jbm.a.30039 (doi:10.1002/jbm.a.30039) [DOI] [PubMed] [Google Scholar]
- 97.Zhu X. D., Zhang H. J., Fan H. S., Li W., Zhang X. D. 2010. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 6, 1536–1541 10.1016/j.actbio.2009.10.032 (doi:10.1016/j.actbio.2009.10.032) [DOI] [PubMed] [Google Scholar]
- 98.Zhu X. D., Fan H. S., Zhao C. Y., Lu J., Ikoma T., Tanaka J., Zhang X. D. 2007. Competitive adsorption of bovine serum albumin and lysozyme on characterized calcium phosphates by polyacrylamide gel electrophoresis method. J. Mater. Sci. Mater. Med. 18, 2243–2249 10.1007/s10856-006-0057-2 (doi:10.1007/s10856-006-0057-2) [DOI] [PubMed] [Google Scholar]
- 99.Cao G. 2004. Nanostructures and nanomaterials: synthesis, properties and applications. London, UK: Imperial College Press [Google Scholar]
- 100.Iwamoto G. K., Winterton L. C., Stoker R. S., Van Wagenen R. A., Andrade J. D., Mosher D. F. 1985. Fibronectin adsorption detected by interfacial fluorescence. J. Colloid Interf. Sci. 106, 459–464 10.1016/S0021-9797(85)80020-5 (doi:10.1016/S0021-9797(85)80020-5) [DOI] [Google Scholar]
- 101.Ta T. C., Mcdermott M. T. 2000. Mapping interfacial chemistry induced variations in protein adsorption with scanning force microscopy. Anal. Chem. 72, 2627–2634 10.1021/ac991137e (doi:10.1021/ac991137e) [DOI] [PubMed] [Google Scholar]
- 102.Sigal G. B., Mrksich M., Whitesides G. M. 1998. Effect of surface wettability on the adsorption of proteins and detergents. J. Am. Chem. Soc. 120, 3464–3473 10.1021/ja970819l (doi:10.1021/ja970819l) [DOI] [Google Scholar]
- 103.Srivastava S., Verma A., Frankamp B. L., Rotello V. M. 2005. Controlled assembly of protein–nanoparticle composites through protein surface recognition. Adv. Mater. 17, 617–621 10.1002/adma.200400776 (doi:10.1002/adma.200400776) [DOI] [Google Scholar]
- 104.Roach P., Eglin D., Rohde K., Perry C. C. 2007. Modern biomaterials: a review-bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 18, 1263–1277 10.1007/s10856-006-0064-3 (doi:10.1007/s10856-006-0064-3) [DOI] [PubMed] [Google Scholar]
- 105.Feng B., Chen J., Zhang X. 2002. Interaction of calcium and phosphate in apatite coating on titanium with serum albumin. Biomaterials 23, 2499–2507 10.1016/S0142-9612(01)00384-2 (doi:10.1016/S0142-9612(01)00384-2) [DOI] [PubMed] [Google Scholar]
- 106.Zhou H., Wu T., Dong X., Wang Q., Shen J. 2007. Adsorption mechanism of BMP-7 on hydroxyapatite (001) surfaces. Biochem. Biophys. Res. Commun. 361, 91–96 10.1016/j.bbrc.2007.06.169 (doi:10.1016/j.bbrc.2007.06.169) [DOI] [PubMed] [Google Scholar]
- 107.Shen J. W., Wu T., Wang Q., Pan H. H. 2008. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces. Biomaterials 29, 513–532 10.1016/j.biomaterials.2007.10.016 (doi:10.1016/j.biomaterials.2007.10.016) [DOI] [PubMed] [Google Scholar]
- 108.Liu Y., Layrolle P., De Bruijn J., Van Blitterswijk C., De Groot K. 2001. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J. Mater. Res. 57, 327–335 (doi:10.1002/1097-4636(20011205)57:3<327::AID-JBM1175>3.0.CO;2-J) [DOI] [PubMed] [Google Scholar]
- 109.Hay D. I., Moreno E. C. 1979. Differential adsorption and chemical affinities of proteins for apatitic surfaces. J. Dent. Res. 58, 930–942 10.1177/00220345790580024701 (doi:10.1177/00220345790580024701) [DOI] [PubMed] [Google Scholar]
- 110.Boontheekul T., Mooney D. J. 2003. Protein-based signaling systems in tissue engineering. Curr. Opin. Biotech. 14, 559–565 10.1016/j.copbio.2003.08.004 (doi:10.1016/j.copbio.2003.08.004) [DOI] [PubMed] [Google Scholar]
- 111.Ohta K., Monma H., Takahashi S. 2001. Adsorption characteristics of proteins on calcium phosphates using liquid chromatography. J. Biomed. Mater. Res. 55, 409–414 (doi:10.1002/1097-4636(20010605)55:3<409::AID-JBM1030>3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 112.Zeng H., Chittur K. K., Lacefield W. R. 1999. Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomaterials 20, 377–384 10.1016/S0142-9612(98)00184-7 (doi:10.1016/S0142-9612(98)00184-7) [DOI] [PubMed] [Google Scholar]
- 113.Webster T. J., Ergun C., Doremus R. H., Bizios R. 2002. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J. Biomed. Mater. Res. 59, 312–317 10.1002/jbm.1247 (doi:10.1002/jbm.1247) [DOI] [PubMed] [Google Scholar]
- 114.Ergun C., Webster T. J., Bizios R., Doremus R. H. 2002. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. I. Structure and microstructure. J. Biomed. Mater. Res. 59, 305–311 10.1002/jbm.1246 (doi:10.1002/jbm.1246) [DOI] [PubMed] [Google Scholar]
- 115.Elangovan S., Margolis H. C., Oppenheim F. G., Beniash E. 2007. Conformational changes in salivary proline-rich protein 1 upon adsorption to calcium phosphate crystals. Langmuir 23, 11 200–11 205 10.1021/la7013978 (doi:10.1021/la7013978) [DOI] [PubMed] [Google Scholar]
- 116.Kandori K., Saito M., Takebe T., Yasukawa A., Ishikawa T. 1995. Adsorption of bovine serum albumin on synthetic carbonate calcium hydroxyapatite. J. Colloid Interf. Sci. 174, 124–129 10.1006/jcis.1995.1373 (doi:10.1006/jcis.1995.1373) [DOI] [Google Scholar]
- 117.Wassell D. T. H., Hall R. C., Embery G. 1995. Adsorption of bovine serum albumin onto hydroxyapatite. Biomaterials 16, 697–702 10.1016/0142-9612(95)99697-K (doi:10.1016/0142-9612(95)99697-K) [DOI] [PubMed] [Google Scholar]
- 118.Daculsi G. 1998. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 19, 1473–1478 10.1016/S0142-9612(98)00061-1 (doi:10.1016/S0142-9612(98)00061-1) [DOI] [PubMed] [Google Scholar]
- 119.Yongli C., Xiufang Z., Yandao G., Nanming Z., Tingying Z., Xinqi S. 1999. Conformational changes of fibrinogen adsorption onto hydroxyapatite and titanium oxide nanoparticles. J. Colloid Interf. Sci. 214, 38–45 10.1006/jcis.1999.6159 (doi:10.1006/jcis.1999.6159) [DOI] [PubMed] [Google Scholar]
- 120.Kivrak N., Taş A. C. 1998. Synthesis of calcium hydroxyapatite-tricalcium phosphate (HA-TCP) composite bioceramic powders and their sintering behavior. J. Am. Ceram. Soc. 81, 2245–2252 10.1111/j.1151-2916.1998.tb02618.x (doi:10.1111/j.1151-2916.1998.tb02618.x) [DOI] [Google Scholar]
- 121.Osborn J. F., Newesely H. 1980. The material science of calcium phosphate ceramics. Biomaterials 1, 108–111 10.1016/0142-9612(80)90009-5 (doi:10.1016/0142-9612(80)90009-5) [DOI] [PubMed] [Google Scholar]
- 122.Bernache-Assollant I., Bouchet P., Lacassagne M. F. 2007. Spectators identification with French sports teams: a French adaptation of the sport spectator identification scale. Percept. Mot. Skills. 104, 83–90 10.2466/pms.104.1.83-90 (doi:10.2466/pms.104.1.83-90) [DOI] [PubMed] [Google Scholar]
- 123.Malmsten M. 1998. Formation of adsorbed protein layers. J. Colloid Interf. Sci. 207, 186–199 10.1006/jcis.1998.5763 (doi:10.1006/jcis.1998.5763) [DOI] [PubMed] [Google Scholar]
- 124.Grunwald C., Eck W., Opitz N., Kuhlmann J., Wöll C. 2004. Fabrication of 2D-protein arrays using biotinylated thiols: results from fluorescence microscopy and atomic force microscopy. Phys. Chem. Chem. Phys. 6, 4358–4362 10.1039/b405543h (doi:10.1039/b405543h) [DOI] [Google Scholar]
- 125.Wertz C. F., Santore M. M. 2001. Effect of surface hydrophobicity on adsorption and relaxation kinetics of albumin and fibrinogen: single-species and competitive behavior. Langmuir 17, 3006–3016 10.1021/la0017781 (doi:10.1021/la0017781) [DOI] [Google Scholar]
- 126.Latour R. A. 2005. Biomaterials: protein–surface interactions. Encyclopedia Biomater. Biomed. Eng. 1, 270–278 [Google Scholar]
- 127.Zhu X. D. 2006. Surface structure and properties of calcium phosphate bioceramics and the relation with the special adsorption of proteins. In National research center for biomaterials. China: Sichuan University [Google Scholar]
- 128.Lopes M. A., Monteiro F. J., Santos J. D., Serro A. P., Saramago B. 1999. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. 45, 370–375 (doi:10.1002/(SICI)1097-4636(19990615)45:4<370::AID-JBM12>3.0.CO;2-0) [DOI] [PubMed] [Google Scholar]
- 129.Fournier R. L. 1999. Solute transport in biological systems. Basic Trans. Phen. Biomed. Eng. 23–60 [Google Scholar]
- 130.Slack S. M., Horbett T. A. 1995. The Vroman effect: a critical review. In Proteins at interfaces II (eds Horbert T. A., Brash J. L.), pp. 112–128 Washington, DC: American Chemical Society [Google Scholar]
- 131.Vroman L., Adams A. L. 1969. Findings with the recording ellipsometer suggesting rapid exchange of specific plasma proteins at liquid/solid interfaces. Surf. Sci. 16, 438–446 10.1016/0039-6028(69)90037-5 (doi:10.1016/0039-6028(69)90037-5) [DOI] [Google Scholar]
- 132.Dee K. C., Puleo D. A., Bizios R. 2004. An Introduction to Tissue-Biomaterial Interactions. Cell Mol. Biol. 8, 419–425 [Google Scholar]
- 133.Peng Z. G., Hidajat K., Uddin M. S. 2004. Adsorption of bovine serum albumin on nanosized magnetic particles. J. Colloid Interf. Sci. 271, 277–283 10.1016/j.jcis.2003.12.022 (doi:10.1016/j.jcis.2003.12.022) [DOI] [PubMed] [Google Scholar]
- 134.Van Der Veen M., Norde W., Stuart M. C. 2004. Electrostatic interactions in protein adsorption probed by comparing lysozyme and succinylated lysozyme. Collolid Surf. B Biointerf. 35, 33–40 10.1016/j.colsurfb.2004.02.005 (doi:10.1016/j.colsurfb.2004.02.005) [DOI] [PubMed] [Google Scholar]
- 135.Norde W., Giacomelli C. E. 2000. BSA structural changes during homomolecular exchange between the adsorbed and the dissolved states. J. Biotech. 79, 259–268 10.1016/S0168-1656(00)00242-X (doi:10.1016/S0168-1656(00)00242-X) [DOI] [PubMed] [Google Scholar]
- 136.Zhou C., et al. 2004. Human immunoglobulin adsorption investigated by means of quartz crystal microbalance dissipation, atomic force microscopy, surface acoustic wave, and surface plasmon resonance techniques. Langmuir 20, 5870–5878 10.1021/la036251d (doi:10.1021/la036251d) [DOI] [PubMed] [Google Scholar]
- 137.Renner L., Pompe T., Salchert K., Werner C. 2005. Fibronectin displacement at polymer surfaces. Langmuir 21, 4571–4577 10.1021/la046801n (doi:10.1021/la046801n) [DOI] [PubMed] [Google Scholar]
- 138.Dupont-Gillain C. C., Fauroux C. M., Gardner D. C., Leggett G. J. 2003. Use of AFM to probe the adsorption strength and time-dependent changes of albumin on self-assembled monolayers. J. Biomed. Mater. Res. A. 67, 548–558 10.1002/jbm.a.10092 (doi:10.1002/jbm.a.10092) [DOI] [PubMed] [Google Scholar]
- 139.Bergkvist M., Carlsson J., Oscarsson S. 2003. Surface-dependent conformations of human plasma fibronectin adsorbed to silica, mica, and hydrophobic surfaces, studied with use of Atomic Force Microscopy. J. Biomed. Mater. Res. A. 64, 349–356 10.1002/jbm.a.10423 (doi:10.1002/jbm.a.10423) [DOI] [PubMed] [Google Scholar]
- 140.Henry M., Dupont-Gillain C., Bertrand P. 2003. Conformation change of albumin adsorbed on polycarbonate membranes as revealed by ToF-SIMS. Langmuir 19, 6271–6276 10.1021/la034081z (doi:10.1021/la034081z) [DOI] [Google Scholar]
- 141.Candiloros H., Muller S., Ziegler O., Donner M., Drouin P. 1996. Role of albumin glycation on the erythrocyte aggregation: an in vitro study. Diabetic Med. 13, 646–650 (doi:10.1002/(SICI)1096-9136(199607)13:7<646::AID-DIA139>3.0.CO;2-J) [DOI] [PubMed] [Google Scholar]
- 142.Gibbons R. J., Hay D. I., Childs Iii W. C., Davis G. 1990. Role of cryptic receptors (cryptitopes) in bacterial adhesion to oral surfaces. Arch. Oral Biol. 35, S107–S114 10.1016/0003-9969(90)90139-2 (doi:10.1016/0003-9969(90)90139-2) [DOI] [PubMed] [Google Scholar]
- 143.Chittur K. K. 1998. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 19, 357–369 10.1016/S0142-9612(97)00223-8 (doi:10.1016/S0142-9612(97)00223-8) [DOI] [PubMed] [Google Scholar]
- 144.Cheng S. S., Chittur K. K., Sukenik C. N., Culp L. A., Lewandowska K. 1994. The conformation of fibronectin on self-assembled monolayers with different surface composition: an FTIR/ATR study. J. Colloid Interf. Sci. 162, 135–143 10.1006/jcis.1994.1018 (doi:10.1006/jcis.1994.1018) [DOI] [Google Scholar]
- 145.Xie J., Riley C., Kumar M., Chittur K. 2002. FTIR/ATR study of protein adsorption and brushite transformation to hydroxyapatite. Biomaterials 23, 3609–3616 10.1016/S0142-9612(02)00090-X (doi:10.1016/S0142-9612(02)00090-X) [DOI] [PubMed] [Google Scholar]
- 146.Yang Y., Cui Q. A., Sahai N. 2010. How does bone sialoprotein promote the nucleation of hydroxyapatite? A molecular dynamics study using model peptides of different conformations. Langmuir 26, 9848–9859 10.1021/la100192z (doi:10.1021/la100192z) [DOI] [PubMed] [Google Scholar]
- 147.Addadi L., Weiner S., Geva M. 2001. On how proteins interact with crystals and their effect on crystal formation. Z Kardiol. 90, 92–98 10.1007/s003920170049 (doi:10.1007/s003920170049) [DOI] [PubMed] [Google Scholar]
- 148.Addadi L., Moradian-Oldak J., Furedimilhofer H., Weiner S., Veis A., Slavkin H., Price P. 1992. Chemistry and biology of mineralized tissues. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 149.Iijima M., Moradian-Oldak J. 2004. Control of octacalcium phosphate and apatite crystal growth by amelogenin matrices. J. Mater. Chem. 14, 2189–2199 10.1039/b401961j (doi:10.1039/b401961j) [DOI] [Google Scholar]
- 150.George A., Sabsay B., Simonian P. A., Veis A. 1993. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 268, 12 624–12 630 [PubMed] [Google Scholar]
- 151.Pan H., Tao J., Xu X., Tang R. 2007. Adsorption processes of Gly and Glu amino acids on hydroxyapatite surfaces at the atomic level. Langmuir 23, 8972–8981 10.1021/la700567r (doi:10.1021/la700567r) [DOI] [PubMed] [Google Scholar]
- 152.Brooks B. R., Bruccoleri R. E., Olafson B. D. 1983. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 4, 187–217 10.1002/jcc.540040211 (doi:10.1002/jcc.540040211) [DOI] [Google Scholar]
- 153.Brooks B. R., et al. 2009. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 10.1002/jcc.21287 (doi:10.1002/jcc.21287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carravetta V., Monti S. 2006. Peptide-TiO2 surface interaction in solution by ab initio and molecular dynamics simulations. J. Phys. Chem. B. 110, 6160–6169 10.1021/jp056760j (doi:10.1021/jp056760j) [DOI] [PubMed] [Google Scholar]
- 155.Kang Y., Li X., Tu Y., Wang Q., Agren H. 2010. On the Mechanism of Protein Adsorption onto Hydroxylated and Nonhydroxylated TiO2 Surfaces. J. Phys. Chem. C. 114, 14 496–14 502 10.1021/jp1037156 (doi:10.1021/jp1037156) [DOI] [Google Scholar]
- 156.Skelton A. A., Liang T., Walsh T. R. 2009. Interplay of sequence, conformation, and binding at the peptide–Titania interface as mediated by water. ACS Appl. Mater. Interf. 1, 1482–1491 10.1021/am9001666 (doi:10.1021/am9001666) [DOI] [PubMed] [Google Scholar]
- 157.Shen J. W., Wu T., Wang Q., Kang Y. 2008. Induced stepwise conformational change of human serum albumin on carbon nanotube surfaces. Biomaterials 29, 3847–3855 10.1016/j.biomaterials.2008.06.013 (doi:10.1016/j.biomaterials.2008.06.013) [DOI] [PubMed] [Google Scholar]
- 158.Raffaini G., Ganazzoli F. 2003. Simulation study of the interaction of some albumin subdomains with a flat graphite surface. Langmuir 19, 3403–3412 10.1021/la026853h (doi:10.1021/la026853h) [DOI] [Google Scholar]
- 159.Ganazzoli F., Raffaini G. 2005. Computer simulation of polypeptide adsorption on model biomaterials. J. Phys. Chem. 7, 3651–3663 10.1039/B506813D (doi:10.1039/B506813D) [DOI] [PubMed] [Google Scholar]
- 160.Forte G., Grassi A., Marletta G. 2007. Molecular modeling of oligopeptide adsorption onto functionalized quartz surfaces. J. Phys. Chem. B. 111, 11 237–11 243 10.1021/jp068803h (doi:10.1021/jp068803h) [DOI] [PubMed] [Google Scholar]
- 161.Bhowmik R., Katti K. S., Katti D. 2007. Molecular dynamics simulation of hydroxyapatite–polyacrylic acid interfaces. Polymer 48, 664–674 10.1016/j.polymer.2006.11.015 (doi:10.1016/j.polymer.2006.11.015) [DOI] [Google Scholar]
- 162.Bhowmik R., Katti K. S., Verma D., Katti D. R. 2007. Probing molecular interactions in bone biomaterials: through molecular dynamics and Fourier transform infrared spectroscopy. Mater. Sci. Eng. C 27, 352–371 10.1016/j.msec.2006.05.048 (doi:10.1016/j.msec.2006.05.048) [DOI] [Google Scholar]
- 163.Austen K. F., Almora-Barrios N., De Leeuw N. H. 2009. Density functional theory study of the binding of glycine, proline, and hydroxyproline to the hydroxyapatite (0001) and (0110) surfaces. Langmuir 25, 5018–5025 10.1021/la803842g (doi:10.1021/la803842g) [DOI] [PubMed] [Google Scholar]
- 164.Almora-Barrios N., De Leeuw N. H. 2009. Modelling the interaction of a Hyp-Pro-Gly peptide with hydroxyapatite surfaces in aqueous environment. Crystengcomm 12, 960–967 10.1039/b917179g (doi:10.1039/b917179g) [DOI] [Google Scholar]
- 165.Almora-Barrios N., De Leeuw N. H. 2010. A density functional theory study of the interaction of collagen peptides with Hydroxyapatite surfaces. Langmuir 26, 14535–14542 10.1021/la101151e (doi:10.1021/la101151e) [DOI] [PubMed] [Google Scholar]
- 166.Wilson C. J., Clegg R. E., Leavesley D. I., Pearcy M. J. 2005. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 11, 1–18 10.1089/ten.2005.11.1 (doi:10.1089/ten.2005.11.1) [DOI] [PubMed] [Google Scholar]
- 167.Fang F., Szleifer I. 2001. Kinetics and thermodynamics of protein adsorption: a generalized molecular theoretical approach. Biophys. J. 80, 2568–2589 10.1016/S0006-3495(01)76228-5 (doi:10.1016/S0006-3495(01)76228-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hench L. L. 1980. Special report: the interfacial behavior of biomaterials, 1979. J. Biomed. Mater. Res. 14, 803–811 10.1002/jbm.820140611 (doi:10.1002/jbm.820140611) [DOI] [PubMed] [Google Scholar]
- 169.Chen J., Cramer S. M. 2007. Protein adsorption isotherm behavior in hydrophobic interaction chromatography. J. Chromatogr. A. 1165, 67–77 10.1016/j.chroma.2007.07.038 (doi:10.1016/j.chroma.2007.07.038) [DOI] [PubMed] [Google Scholar]
- 170.Chuang H. Y., King W. F., Mason R. G. 1978. Interaction of plasma proteins with artificial surfaces: protein adsorption isotherms. J. Lab Clin. Med. 92, 483–496 [PubMed] [Google Scholar]
- 171.Noh H., Yohe S. T., Vogler E. A. 2008. Volumetric interpretation of protein adsorption: ion-exchange adsorbent capacity, protein pI, and interaction energetics. Biomaterials 29, 2033–2048 10.1016/j.biomaterials.2008.01.017 (doi:10.1016/j.biomaterials.2008.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen J., Cramer S. M., Luo Q., Breneman C. M., Cramer S. M. 2007. Classification of protein adsorption and recovery at low salt conditions in hydrophobic interaction chromatographic systems. J. Chromatogr. A. 1139, 236–46 10.1016/j.chroma.2006.11.026 (doi:10.1016/j.chroma.2006.11.026) [DOI] [PubMed] [Google Scholar]
- 173.Allen L. T., et al. 2006. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 27, 3096–3108 10.1016/j.biomaterials.2006.01.019 (doi:10.1016/j.biomaterials.2006.01.019) [DOI] [PubMed] [Google Scholar]
- 174.Aissaoui N., Bergaoui L., Landoulsi J., Lambert J. F., Boujday S. 2011. Silane layers on silicon surfaces: mechanism of interaction, stability, and influence on protein adsorption. Langmuir 28, 656–665 10.1021/la2036778 (doi:10.1021/la2036778) [DOI] [PubMed] [Google Scholar]
- 175.Shen B. W. 1985. Lipid-protein interaction at solid-water interface. Adsorption of human apo-high density lipoprotein to amphiphilic interfaces. J. Biol. Chem. 260, 1032–1039 [PubMed] [Google Scholar]
- 176.Richarme G., Kepes A. 1983. Study of binding protein-ligand interaction by ammonium sulfate-assisted adsorption on cellulose esters filters. Biochim. Biophys. Acta. 742, 16–24 10.1016/0167-4838(83)90353-9 (doi:10.1016/0167-4838(83)90353-9) [DOI] [PubMed] [Google Scholar]
- 177.Holand W., Vogel W., Naumann K., Gummel J. 1985. Interface reactions between machinable bioactive glass-ceramics and bone. J. Biomed. Mater. Res. 19, 303–312 10.1002/jbm.820190311 (doi:10.1002/jbm.820190311) [DOI] [PubMed] [Google Scholar]
- 178.Brash J. L., Horbett T. A. 1995. Proteins at interfaces Fan overview. ACS Symp. Ser. 602, 1–23 10.1021/bk-1995-0602.ch001 (doi:10.1021/bk-1995-0602.ch001) [DOI] [Google Scholar]
- 179.Hoffman A. S. 1982. Blood–biomaterial interactions: an overview. In Biomaterials: interfacial phenomena and applications (eds Cooper S. L., Peppas N. A.), pp. 3–8 Washington, DC: ACS [Google Scholar]
- 180.Norde W., Anusiem A. C. I. 1992. Adsorption, desorption and re-adsorption of proteins on solid surfaces. Colloids Surf. 66, 73–80 10.1016/0166-6622(92)80122-I (doi:10.1016/0166-6622(92)80122-I) [DOI] [Google Scholar]
- 181.Trusova V. M., Gorbenko G. P. 2008. Electrostatically-controlled protein adsorption onto lipid bilayer: modeling adsorbate aggregation behavior. Biophys. Chem. 133, 90–103 10.1016/j.bpc.2007.12.007 (doi:10.1016/j.bpc.2007.12.007) [DOI] [PubMed] [Google Scholar]
- 182.Baio J. E., Weidner T., Baugh L., Gamble L. J., Stayton P. S., Castner D. G. 2011. Probing the orientation of electrostatically immobilized protein g b1 by time-of-flight secondary ion spectrometry, sum frequency generation, and near-edge X-ray Adsorption Fine Structure Spectroscopy. Langmuir 28, 2107–2112 10.1021/la203907t (doi:10.1021/la203907t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Norde W. 2008. My voyage of discovery to proteins in flatland … and beyond. Colloids Surf. B Biointerf. 61, 1–9 10.1016/j.colsurfb.2007.09.029 (doi:10.1016/j.colsurfb.2007.09.029) [DOI] [PubMed] [Google Scholar]
- 184.Vroman L., Adams A. L. 1969. Findings with ellipsometer suggesting rapid exchange of specific plasma proteins at liquid/solid interfaces. Surf. Sci. 16, 438–446 10.1016/0039-6028(69)90037-5 (doi:10.1016/0039-6028(69)90037-5) [DOI] [Google Scholar]
- 185.Slack S. M., Horbett T. A. 1995. The Vroman effect a critical review. ACS Symp. Ser. 602, 112–28 10.1021/bk-1995-0602.ch008 (doi:10.1021/bk-1995-0602.ch008) [DOI] [Google Scholar]
- 186.Jung S. Y., Lim S. M., Albertorio F., Kim G., Gurau M. C., Yang R. D., Holden M. A., Cremer P. S. 2003. The Vroman effect: a molecular level description of fibrinogen displacement. J. Am. Chem. Soc. 125, 12 782–12 786 10.1021/ja037263o (doi:10.1021/ja037263o) [DOI] [PubMed] [Google Scholar]
- 187.Jachimska B., Pajor A. 2011. Physico-chemical characterization of bovine serum albumin in solution and as deposited on surfaces. Bioelectrochemistry. 10.1016/j.bioelechem.2011.09.004 (doi:10.1016/j.bioelechem.2011.09.004) [DOI] [PubMed] [Google Scholar]
- 188.Bumgardner J. D., Wiser R., Elder S. H., Jouett R., Yang Y., Ong J. L. 2003. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 14, 1401–1409 10.1163/156856203322599734 (doi:10.1163/156856203322599734) [DOI] [PubMed] [Google Scholar]
- 189.Stadler H., Mondon M., Ziegler C. 2003. Protein adsorption on surfaces: dynamic contact-angle (DCA) and quartz-crystal microbalance (QCM) measurements. Anal. Bioanal. Chem. 375, 53–61 10.1007/s00216-002-1664-5 (doi:10.1007/s00216-002-1664-5) [DOI] [PubMed] [Google Scholar]
- 190.Verzola B., Gelfi C., Righetti P. G. 2000. Protein adsorption to the bare silica wall in capillary electrophoresis quantitative study on the chemical composition of the background electrolyte for minimising the phenomenon. J. Chromatogr. A. 868, 85–99 10.1016/S0021-9673(99)01207-8 (doi:10.1016/S0021-9673(99)01207-8) [DOI] [PubMed] [Google Scholar]
- 191.Kinnari T. J., Esteban J., Martin-De-Hijas N. Z., Sanchez-Munoz O., Sanchez-Salcedo S., Colilla M., Vallet-Regi M., Gomez-Barrena E. 2009. Influence of surface porosity and pH on bacterial adherence to hydroxyapatite and biphasic calcium phosphate bioceramics. J. Med. Microbiol. 58, 132–137 10.1099/jmm.0.002758-0 (doi:10.1099/jmm.0.002758-0) [DOI] [PubMed] [Google Scholar]
- 192.Sharpe J. R., Sammons R. L., Marquis P. M. 1997. Effect of pH on protein adsorption to hydroxyapatite and tricalcium phosphate ceramics. Biomaterials 18, 471–476 10.1016/S0142-9612(96)00157-3 (doi:10.1016/S0142-9612(96)00157-3) [DOI] [PubMed] [Google Scholar]
- 193.Wrzosek K., Polakovic M. 2011. Effect of pH on protein adsorption capacity of strong cation exchangers with grafted layer. J. Chromatogr. A 1218, 6987–6994 10.1016/j.chroma.2011.07.097 (doi:10.1016/j.chroma.2011.07.097) [DOI] [PubMed] [Google Scholar]
- 194.Hirn R., Schuster B., Sleytr U. B., Bayerl T. M. 1999. The effect of S-layer protein adsorption and crystallization on the collective motion of a planar lipid bilayer studied by dynamic light scattering. Biophys. J. 77, 2066–2074 10.1016/S0006-3495(99)77048-7 (doi:10.1016/S0006-3495(99)77048-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Silva R. A., Urzua M. D., Petri D. F., Dubin P. L. 2010. Protein adsorption onto polyelectrolyte layers: effects of protein hydrophobicity and charge anisotropy. Langmuir 26, 14 032–14 038 10.1021/la102254g (doi:10.1021/la102254g) [DOI] [PubMed] [Google Scholar]
- 196.Santos L., Rodrigues D., Lira M., Oliveira M. E., Oliveira R., Vilar E. Y., Azeredo J. 2007. The influence of surface treatment on hydrophobicity, protein adsorption and microbial colonisation of silicone hydrogel contact lenses. Cont. Lens Anterior Eye. 30, 183–188 10.1016/j.clae.2006.12.007 (doi:10.1016/j.clae.2006.12.007) [DOI] [PubMed] [Google Scholar]
- 197.Tangpasuthadol V., Pongchaisirikul N., Hoven V. P. 2003. Surface modification of chitosan films. Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 338, 937–942 10.1016/S0008-6215(03)00038-7 (doi:10.1016/S0008-6215(03)00038-7) [DOI] [PubMed] [Google Scholar]
- 198.Wu Y., Simonovsky F. I., Ratner B. D., Horbett T. A. 2005. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: a comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J. Biomed. Mater. Res. A. 74, 722–738 10.1002/jbm.a.30381 (doi:10.1002/jbm.a.30381) [DOI] [PubMed] [Google Scholar]
- 199.Zhu X., Fan H., Li D., Xiao Y., Zhang X. 2007. Protein adsorption and zeta potentials of a biphasic calcium phosphate ceramic under various conditions. J. Biomed. Mater. Res. B Appl. Biomater. 82, 65–73 10.1002/jbm.b.30706 (doi:10.1002/jbm.b.30706) [DOI] [PubMed] [Google Scholar]
- 200.Zhdanov V. P., Rechendorff K., Hovgaard M. B., Besenbacher F. 2008. Deposition at glancing angle, surface roughness, and protein adsorption: Monte Carlo simulations. J. Phys. Chem. B. 112, 7267–7272 10.1021/jp709806k (doi:10.1021/jp709806k) [DOI] [PubMed] [Google Scholar]
- 201.Rechendorff K., Hovgaard M. B., Foss M., Zhdanov V. P., Besenbacher F. 2006. Enhancement of protein adsorption induced by surface roughness. Langmuir 22, 10 885–10 888 10.1021/la0621923 (doi:10.1021/la0621923) [DOI] [PubMed] [Google Scholar]
- 202.Deligianni D. D., Katsala N., Ladas S., Sotiropoulou D., Amedee J., Missirlis Y. F. 2001. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 22, 1241–1251 10.1016/S0142-9612(00)00274-X (doi:10.1016/S0142-9612(00)00274-X) [DOI] [PubMed] [Google Scholar]
- 203.Kupas K., Ultsch A., Klebe G. 2008. Large scale analysis of protein-binding cavities using self-organizing maps and wavelet-based surface patches to describe functional properties, selectivity discrimination, and putative cross-reactivity. Proteins 71, 1288–1306 10.1002/prot.21823 (doi:10.1002/prot.21823) [DOI] [PubMed] [Google Scholar]
- 204.Rosengren A., Oscarsson S., Mazzocchi M., Krajewski A., Ravaglioli A. 2003. Protein adsorption onto two bioactive glass-ceramics. Biomaterials 24, 147–155 10.1016/S0142-9612(02)00272-7 (doi:10.1016/S0142-9612(02)00272-7) [DOI] [PubMed] [Google Scholar]
- 205.Fini M., et al. 2000. The effect of osteopenia on the osteointegration of different biomaterials: histomorphometric study in rats. J. Mater. Sci. Mater. Med. 11, 579–585 10.1023/A:1008932303913 (doi:10.1023/A:1008932303913) [DOI] [PubMed] [Google Scholar]
- 206.Belmonte M. M., et al. 1998. Bioactivity modulation of bioactive materials in view of their application in osteoporotic patients. J. Mater. Sci .Mater. Med. 9, 485–492 10.1023/A:1008827619290 (doi:10.1023/A:1008827619290) [DOI] [PubMed] [Google Scholar]
- 207.Van Oss C. J., Wu W., Giese R. F., Naim J. O. 1995. Interaction between proteins and inorganic oxides: adsorption of albumin and its desorption with a complexing agent. Coll Surf. B: Biointerf. 1, 185–189 10.1016/0927-7765(94)01170-A (doi:10.1016/0927-7765(94)01170-A) [DOI] [Google Scholar]
- 208.Tanaka M., Motomura T., Kawada M., Anzai T., Kasori Y., Shiroya T., Shimura K., Onishi M., Mochizuki A. 2000. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)–relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials 21, 1471–1481 10.1016/S0142-9612(00)00031-4 (doi:10.1016/S0142-9612(00)00031-4) [DOI] [PubMed] [Google Scholar]
- 209.Jeyachandran Y. L., Mielczarski J. A., Mielczarski E., Rai B. 2009. Efficiency of blocking of non-specific interaction of different proteins by BSA adsorbed on hydrophobic and hydrophilic surfaces. J. Colloid Interf. Sci. 341, 136–142 10.1016/j.jcis.2009.09.007 (doi:10.1016/j.jcis.2009.09.007) [DOI] [PubMed] [Google Scholar]
- 210.Demaneche S., Chapel J. P., Monrozier L. J., Quiquampoix H. 2009. Dissimilar pH dependent adsorption features of bovine serum albumin and α-chymotrypsin onmica probed by AFM. Colloids Surf. B. 70, 226–231 10.1016/j.colsurfb.2008.12.036 (doi:10.1016/j.colsurfb.2008.12.036) [DOI] [PubMed] [Google Scholar]
- 211.Bergers J. J., Vingerhoeds M. H., Van Bloois L., Herron J. N., Janssen L. H., Fischer M. J., Crommelin D. J. 1993. The role of protein charge in protein-lipid interactions. pH-dependent changes of the electrophoretic mobility of liposomes through adsorption of water-soluble, globular proteins. Biochem. 32, 4641–4649 10.1021/bi00068a023 (doi:10.1021/bi00068a023) [DOI] [PubMed] [Google Scholar]
- 212.Villarreal D. R., Sogal A., Ong J. L. 1998. Protein adsorption and osteoblast responses to different calcium phosphate surfaces. J. Oral Implantol. 24, 67–73 (doi:10.1563/1548-1336(1998)024<0067:PAAORT>2.3.CO;2) [DOI] [PubMed] [Google Scholar]
- 213.Ong J. L., Chittur K. K., Lucas L. C. 1994. Dissolution/reprecipitation and protein adsorption studies of calcium phosphate coatings by FT-IR/ATR techniques. J. Biomed. Mater. Res. 28, 1337–1346 10.1002/jbm.820281112 (doi:10.1002/jbm.820281112) [DOI] [PubMed] [Google Scholar]
- 214.Sammons R. L., Sharpe J., Marquis P. M. 1994. Use of enhanced chemiluminescence to quantify protein adsorption to calcium phosphate materials and microcarrier beads. Biomaterials 15, 842–847 10.1016/0142-9612(94)90040-X (doi:10.1016/0142-9612(94)90040-X) [DOI] [PubMed] [Google Scholar]
- 215.Touny A. H., Bhaduri S., Brown P. W. 2010. Formation of calcium deficient HAp/collagen composites by hydrolysis of alpha-TCP. J. Mater. Sci. Mater. Med. 21, 2533–2541 10.1007/s10856-010-4113-6 (doi:10.1007/s10856-010-4113-6) [DOI] [PubMed] [Google Scholar]
- 216.Tsuchiya A., Sotome S., Asou Y., Kikuchi M., Koyama Y., Ogawa T., Tanaka J., Shinomiya K. 2008. Effects of pore size and implant volume of porous hydroxyapatite/collagen (HAp/Col) on bone formation in a rabbit bone defect model. J. Med. Dent. Sci. 55, 91–99 [PubMed] [Google Scholar]
- 217.Sogo Y., Sakurai T., Onuma K., Ito A. 2002. The most appropriate (Ca+Zn)/P molar ratio to minimize the zinc content of ZnTCP/HAP ceramic used in the promotion of bone formation. J. Biomed. Mater. Res. 62, 457–463 10.1002/jbm.10200 (doi:10.1002/jbm.10200) [DOI] [PubMed] [Google Scholar]
- 218.Hendrixson D. R., St Geme J. W. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol Cell. 2, 841–850 10.1016/S1097-2765(00)80298-1 (doi:10.1016/S1097-2765(00)80298-1) [DOI] [PubMed] [Google Scholar]
- 219.Touny A. H., Laurencin C., Nair L., Allcock H., Brown P. W. 2008. Formation of composites comprised of calcium deficient HAp and cross-linked gelatin. J. Mater. Sci. Mater. Med. 19, 3193–3201 10.1007/s10856-008-3459-5 (doi:10.1007/s10856-008-3459-5) [DOI] [PubMed] [Google Scholar]
- 220.Rezwan K., Meier L. P., Gauckler L. J. 2005. Lysozyme and bovine serum albumin adsorption on uncoated silica and AlOOH-coated silica particles: the influence of positively and negatively charged oxide surface coatings. Biomaterials 26, 4351–4357 10.1016/j.biomaterials.2004.11.017 (doi:10.1016/j.biomaterials.2004.11.017) [DOI] [PubMed] [Google Scholar]
- 221.Beurer E., Venkataraman N. V., Sommer M., Spencer N. D. 2012. Protein and nanoparticle adsorption on orthogonal, charge-density-versus-net-charge surface-chemical gradients. Langmuir 28, 3159–3166 10.1021/la203964a (doi:10.1021/la203964a) [DOI] [PubMed] [Google Scholar]
- 222.Wierenga P. A., Meinders M. B., Egmond M. R., Voragen A. G., Jongh H. H. 2005. Quantitative description of the relation between protein net charge and protein adsorption to air–water interfaces. J. Phys. Chem. B. 109, 16 946–16 952 10.1021/jp050990g (doi:10.1021/jp050990g) [DOI] [PubMed] [Google Scholar]
- 223.Barnthip N., Parhi P., Golas A., Vogler E. A. 2009. Volumetric interpretation of protein adsorption: kinetics of protein-adsorption competition from binary solution. Biomaterials 30, 6495–6513 10.1016/j.biomaterials.2009.08.016 (doi:10.1016/j.biomaterials.2009.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chatterjee K., Thornton J. L., Bauer J. W., Vogler E. A., Siedlecki C. A. 2009. Moderation of prekallkrein-factor interactions in surface activation of coagulation by protein-adsorption competition. Biomaterials 30, 4915–4920 10.1016/j.biomaterials.2009.05.076 (doi:10.1016/j.biomaterials.2009.05.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nonckreman C. J., Rouxhet P. G., Dupont-Gillain C. C. 2007. Dual radiolabeling to study protein adsorption competition in relation with hemocompatibility. J. Biomed. Mater. Res. A. 81, 791–802 10.1002/jbm.a.31111 (doi:10.1002/jbm.a.31111) [DOI] [PubMed] [Google Scholar]
- 226.Noh H., Vogler E. A. 2007. Volumetric interpretation of protein adsorption: competition from mixtures and the Vroman effect. Biomaterials 28, 405–422 10.1016/j.biomaterials.2006.09.006 (doi:10.1016/j.biomaterials.2006.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Elwing H., Askendal A., Lundstrom I. 1987. Competition between adsorbed fibrinogen and high-molecular-weight kininogen on solid surfaces incubated in human plasma (the Vroman effect): influence of solid surface wettability. J. Biomed. Mater. Res. 21, 1023–1028 10.1002/jbm.820210808 (doi:10.1002/jbm.820210808) [DOI] [PubMed] [Google Scholar]
- 228.Scott C. F. 1991. Mechanism of the participation of the contact system in the Vroman effect. Review and summary. J. Biomater. Sci. Polym. Ed. 2, 173–181 [DOI] [PubMed] [Google Scholar]
- 229.Brash J. L., Lyman D. J. 1969. Adsorption of plasma proteins in solution to uncharged, hydrophobic polymer surfaces. J. Biomed. Mater. Res. 3, 175–189 10.1002/jbm.820030114 (doi:10.1002/jbm.820030114) [DOI] [PubMed] [Google Scholar]
- 230.Noh H., Vogler E. A. 2006. Volumetric interpretation of protein adsorption: mass and energy balance for albumin adsorption to particulate adsorbents with incrementally increasing hydrophilicity. Biomaterials 27, 5801–5812 10.1016/j.biomaterials.2006.08.005 (doi:10.1016/j.biomaterials.2006.08.005) [DOI] [PubMed] [Google Scholar]
- 231.Kao P., Parhi P., Krishnan A., Noh H., Haider W., Tadigadapa S., Allara D. L., Vogler E. A. 2010. Volumetric interpretation of protein adsorption: interfacial packing of protein adsorbed to hydrophobic surfaces from surface-saturating solution concentrations. Biomaterials 32, 969–978 10.1016/j.biomaterials.2010.09.075 (doi:10.1016/j.biomaterials.2010.09.075) [DOI] [PMC free article] [PubMed] [Google Scholar]