Abstract
Although remarkable progress has been made on biomaterial research, the ideal biomaterial that satisfies all the technical requirements and biological functions is not available up to now. Surface modification seems to be a more economic and efficient way to adjust existing conventional biomaterials to meet the current and ever-evolving clinical needs. From an industrial perspective, plasma immersion ion implantation and deposition (PIII&D) is an attractive method for biomaterials owing to its capability of treating objects with irregular shapes, as well as the control of coating composition. It is well acknowledged that the physico-chemical characteristics of biomaterials are the decisive factors greatly affecting the biological responses of biomaterials including bioactivity, haemocompatibility and antibacterial activity. Here, we mainly review the recent advances in surface modification of biomaterials via PIII&D technology, especially titanium alloys and polymers used for orthopaedic, dental and cardiovascular implants. Moreover, the variations of biological performances depending on the physico-chemical properties of modified biomaterials will be discussed.
Keywords: plasma immersion ion implantation and deposition, surface modification, physico-chemical characteristics, bioactivity, haemocompatibility, antibacterial activity
1. Introduction
The development of biomaterials has been an evolving process [1–3]. Over the past few decades, the field of biomaterials began to shift in emphasis from achieving a bioinert tissue response to instead stimulating specific cellular responses at the molecular level [3]. This new generation of biomaterials, also called third-generation biomaterials, are proposed and expected to direct cell responses in a predictable manner by regulating material characteristics [3–5]. The deeper understanding of peri-implant–tissue interactions created opportunities for material scientists to achieve specific biological responses by designing and adjusting material properties.
It has long been recognized that an ideal biomaterial should possess mechanical properties matched with injured tissues for stress–strain balance, establish strong chemical bondings at the biomaterials–tissues interface to ensure implant anchorage and exhibit high antibacterial activities to prevent bacterial infection during implant surgery [6–10]. For cardiovascular biomaterials, they also should possess excellent haemocompatibility to inhibit thrombus formation [11]. All of these desirable properties of biomaterials are closely related to overall implant efficacy and can be regulated and tuned by physico-chemical characteristics of biomaterials, such as bulk and surface properties [12,13]. The bulk properties of biomaterials, especially mechanical properties, should mimic the injured tissues replaced to bear mechanical load. The most significant surface properties of biomaterials, including chemical composition, microstructure, roughness and hydrophilicity or hydrophobicity, have been extensively studied and proved to be capable of directing cellular responses [14–17]. When immersed in a given medium, effective surface charge, stability and released ions are also important features affecting in vitro and in vivo biological performances [18,19].
Although considerable progress has been made to improve the biological responses of biomaterials, long-term clinical success has still not been achieved. Because developing new biomaterials is costly and time-consuming, selectively modifying existing conventional biomaterials is a more economic and efficient way to meet particular needs. Surface modification can be carried out by a lot of methods usually classified as mechanical machining, chemical and physical treatments. Plasma-surface modification techniques such as plasma spray, plasma polymerization and plasma immersion ion implantation & deposition (PIII&D), which are proven to be economic and effective, have already been widely applied in the modification of semiconductors and biomedical industry. The unique advantage of plasma modification over the other methods is the ability of selectively enhancing surface properties (approx. several hundred nanometres), whereas the bulk contributions remain unchanged [20–23]. Among the various plasma-based techniques, PIII&D is a versatile technique that can conduct multiple processes, such as simultaneous and consecutive implantation, deposition and etching owing to it combining the advantages of conventional plasma and ion beam technologies. Another major advantage of PIII&D is the omnidirectional processing capability to tailor the surface properties of many biomaterials, including metals, ceramics and polymers, by introducing a myriad of different kinds of elements and functional groups into the materials with complex shapes [24,25]. PIII&D technique is usually applied in areas such as microelectronics, aerospace engineering and precision manufacturing. In the past 10 years, applications of PIII&D technique in biomedical implant modification have attracted increasing interest because of its controllability and versatility [26–32].
In this study, recent outstanding work on applying PIII&D technique to improve mechanical, chemical and biological properties such as bioactivity, haemocompatibility and antibacterial activity of biomaterials is reviewed. In addition, original opinions will be emphasized and discussed.
2. Concepts and fundamentals of plasma immersion ion implantation and deposition
Plasma is the fourth state of matter. Under certain conditions, a solid substrate decomposes into freely moving charged particles and enters the plasma state. Thus, the plasma can be considered as a collection of electrons, single- or multi-charged positive or negative ions accompanied by neutral atoms, excited particles, electromagnetic radiation, molecules and some molecular fragments. In plasma, the densities of excited particles, ions and electrons as well as the intensity of the electromagnetic radiation far exceed those that are found in more mundane situations encountered elsewhere [33]. The most important feature of plasma is that the positive- and negative-charged particles are in a state of charge equilibrium, and the sum of the positive and negative charges in a sufficiently large volume is equal to zero. In fact, plasma technology is a dry, green and economic technique in a variety of applications.
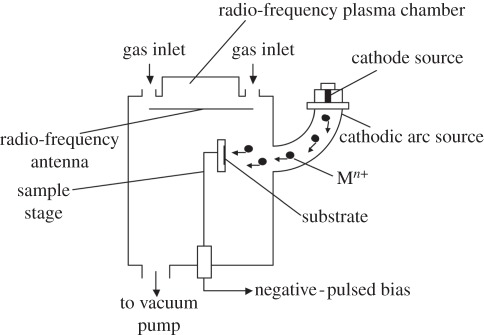
To produce plasma, electron separation from atoms or molecules in the gas state, or ionization, is required. In most practical situations, the plasma is produced by an electrical discharge. Generally, a PIII&D system comprises a vacuum chamber with a workpiece stage, plasma source and high-voltage pulse modulator. During the PIII&D processing, a workpiece is immersed in plasma under a high bias voltage, which repels electrons away from the workpiece while driving the positive ions of the plasma toward it, creating a plasma sheath around the workpiece. The plasma sheath plays an important role in PIII&D because it dictates the implantation process and can be used to predict process parameters and results, including the implantation current, implantation dose and impurities profile. Positive ions will be accelerated by the electric field and implanted vertically into the negative potential surfaces, as illustrated in figure 1. As the workpiece is covered by the plasma sheath, PIII&D can be used in processing devices with complex shapes. However, when a high negative potential is applied to insulating samples, the surface potential of insulators may not reach the full applied bias potential owing to the dielectric surface charging effect, leading to an irregular plasma sheath. The charging effects will be mainly determined by pulse duration, pulse frequency, dielectric coefficient and thickness of samples. Moreover, for insulators, the deformation caused by heat formation generated by ion collision in the process of PIII&D is much more severe than for conductors owing to the poor thermal conductivity, which is a critical problem limiting the modification of polymers.
Figure 1.
Schematic of ion implantation and deposition processes.
The fundamental difference between PIII&D and conventional ‘line of sight’ ion implantation is that, in PIII&D, the workpiece is directly placed in the plasma and biased to high negative voltage as an active part of the system, while the workpiece is isolated from the ion beam generation in conventional ion implantation process. So from the industrial view, PIII&D is more attractive because of the low cost and capability of modifying complicated shapes. Since PIII&D was first introduced in the 1980s, many applications have already been developed. Modifications on many metals such as titanium and titanium alloys, magnesium alloys and aluminium as well as polymers have been carried out to enhance the mechanical properties, bioactivity, biocompatibility, blood compatibility and antibacterial activity. These investigations will be fully reviewed and discussed in the following.
3. Mechanical and chemical properties of plasma immersion ion implantation and deposition-treated biomaterials
Today, most biomedical devices available in the marketplace are made from titanium and its alloys, stainless steel, polymers and ceramics. Although considerable advances have already been made in improving the biological performances of these biomaterials, the ideal long-term stability is still not achieved. The PIII&D technique is now regarded as an attractive method to enhance the physico-chemical characteristics and mechanical properties, which profoundly impact the function of a biomaterial once it is placed in a biological environment. In this section, we mainly discuss the decisive properties, such as hardness, wear behaviour and corrosion resistance, all of which have great significance in ensuring application safety and elongating useful life of biomedical implants [34,35].
3.1. Mechanical properties
For longevity of hip and other joint replacements, the leading cause of implant failure is the aseptic osteolysis occurring because of a chronic inflammatory response to implant-derived wear particles. It has been reported that cells incubated with wear particles could produce cytokines that play a key role in osteoclast differentiation and activation, finally resulting in bone lysis. Moreover, the wear debris is found to accelerate the corrosion process and may cause genetic damage [36]. Recent study has proved that a graphitic layer formed on a metal-on-metal hip replacement, which reduces friction as well as wear and corrosion, indicating a route for the design of improved implants [37]. Hence, there has been a constant attempt by material scientists to improve the wear resistance so as to minimize the loosening of implants from bone. During the past two decades, PIII&D technology has been considered as an effective and easy process to fabricate a protective coating on orthopaedic implants to delay the wear process.
The wear performance of plasma-treated titanium and its alloys has been extensively investigated. Alonso et al. [38] fabricated nitrogen (N2)-implanted Ti-6Al-4V by PIII, and they found that the load-bearing capacity seems to depend more on the N2 implantation dose rather than on the hardness and no obvious hardening was observed after PIII treatment. Besides, the role of oxygen (O2) in N2-implantation was further exploited by Wan et al. [39]. They prepared TiN- and Ti-O/TiN-gradient films into Ti-6Al-4V by single implantation of N2 and sequentially dual ion implantation of N2/O2, respectively. The results show that Ti-O/TiN-coated sample gave the best wear performance compared with the untreated and TiN-coated samples. Besides N2 and O2, hydrogen (H2) and methane also have been employed to improve the wear resistance of orthopaedic implants [40,41].
When it comes to polymers, much more severe situations may occur. Some polymers have been the principal materials for joint replacement prostheses, such as ultra-high molecular weight polyethylene (UHMWPE) and poly(ether ether ketone) (PEEK). It is suggested that hardness and elastic modulus are the two primary material properties that define wear resistance [42]. These properties of UHMWPE and PEEK have been shown to be improved using PIII&D. In the process of PIII, energetic ions transfer energy to the substrate as they collide with the atoms of the material, which will cause the alteration of polymer structure in the near-surface region. The hardness and elastic modulus of treated polymer surfaces are increased owing to the effects of densification and cross-linking. Shi et al. [43] treated UHMWPE with N2-PIII and found that the nanohardness and elastic modulus doubled, whereas the wear coefficients reduced by as much as three times compared with the untreated samples. Powles et al. [44] fabricated hydrogenated amorphous carbon (a-C : H) layers on PEEK using H2-PIII. Their result suggested that the modified a-C : H region possesses better hardness and greater elastic recovery than the unmodified material, indicating a possible improvement of wear resistance.
Compared with the gradient-modified layer fabricated during implantation process, the mismatch in hardness between deposited film and substrate remains a hidden danger that may cause the cracking of the thin hard coating owing to the deformation of the substrate (the eggshell phenomenon) and needs to be further studied. Although a variety of gaseous implantations have been proved effective in improving the mechanical properties of orthopaedic implants, all the parameters, such as ion source, pulse frequency, bias voltage, temperature and time, should be carefully selected according to the usage and composition of a specific implant device to achieve its best wear resistance. For example, the toxic ions, such as cobalt, nickel, vanadium and aluminium, which are present in titanium alloys or stainless steel could be damaging when exposed to the biological environment in the long term.
3.2. Corrosion resistance
Once implanted, corrosion occurs when a metallic implant is placed in the hostile human body via an electrochemical mechanism. Corrosion is critical because it can adversely affect the biocompatibility and mechanical properties. There is clinical evidence for the release of corrosion products (corroded particles and metal ions) from the implants, which will obviously lead to adverse biological reactions and in turn accelerate the corrosion process [45]. Therefore, creating an appropriate passive layer on biomaterial surfaces to reduce the corrosion rate and impede the corrosion products from leaching is considered to be an effective way to delay the process of corrosion. Several studies have reported on the use of PIII&D technology for surface modification of biomaterials to enhance their corrosion resistance.
Nickel–titanium (Ni–Ti) shape memory alloys are useful orthopaedic biomaterials because of their superelastic and shape memory properties. However, the problem associated with out-diffusion of harmful nickel ions in prolonged use inside the human body raises a critical safety concern. Ray et al. [46] have conducted a series of surface modifications by PIII&D technology to improve the whole corrosion resistance and impede nickel ions leaching from Ni–Ti alloys. Their studies provided a complete discussion on the factors greatly influencing anti-corrosion efficacy of barrier layers, including film quality and composition, process parameters, and post-treatment after PIII&D. In a comparison study, the anti-corrosion efficacies of oxide films produced by atmospheric-pressure oxidation (O2-oxidation) and oxygen-PIII (O2-PIII) have been investigated [47]. The potentiodynamic polarization tests and electrochemical impedance spectroscopy studies showed that the O2-PIII exhibited higher corrosion potential (Ecorr) and breakdown potential (Eb) than both of the untreated control and O2-oxidation sample, indicating the best pitting corrosion resistance of O2-PIII sample. Although atmosphere oxidation produced a thicker oxide film, its lower corrosion resistance may be attributed to the diffuse and non-uniform oxide structure throughout its depth as well as the contaminants introduced during the oxidation process. Therefore, from the perspective of corrosion resistance, the quality of oxide film is more important than its thickness, and the introduction of energetic plasma oxygen ions could yield a more effective corrosion-resistant layer.
Recent studies found that vacuum annealing is an effective way to increase the film quality. In a related study, Ray et al. investigated the effects of different post-annealing temperatures on the anti-efficacy of N2-implanted Ni–Ti alloys [48,49]. The electrochemical results showed a significant improvement in the corrosion resistance of Ni–Ti alloys modified by N2 ion implantation followed with vacuum annealing at 450°C compared with that of N2-PIII sample. The X-ray photoelectron spectroscopy (XPS) analysis revealed that annealing at 450°C consolidates the N2 atoms to a narrower and better-defined region, thus producing a more compact and dense nitride structure that could effectively impede out-diffusion of Ni in addition to offering better mechanical properties. A follow-up study was conducted to explore the corrosion resistances of Ni–Ti alloys modified by N2 ion implantations at different pulsing frequencies, including 50, 100 and 200 Hz [50]. The 50 Hz N2-PIII sample exhibited better electrochemical stability at open circuit than the samples implanted at higher frequencies, because the heat produced by higher frequencies gave rise to instability of the TiN layer. Before immersion, 50 Hz N2-PIII sample showed a slightly higher Eb that became lower than those of 100 and 200 Hz samples after 14 days immersion. This inconsistent result may be caused by greater damage to the thinner TiN layers of 50 Hz N2-PIII sample. Therefore, both the barrier layer quality and thickness are essential building blocks to obtain a better corrosion resistance.
There is no doubt that PIII&D technique provides an effective approach for a number of biomaterials to enhance their corrosion resistance and improve their mechanical properties at the same time.
4. Bioactivity of plasma immersion ion implantation and deposition-treated biomaterials
The motivation to create bioactive surfaces for biomedical implants to control their biological functions comes from increasing clinical demands. Prosthetic loosening is the most common cause of long-term failure in hip and knee replacements [51]. To address this issue, researchers have created cell-specific materials surfaces to form strong interactions between cells and implants to improve osseointegration for long-term success.
Recently, many studies have attempted to enhance the osseointegration of implants by various surface modifications. The aim is to provide implant surfaces with biological properties for the adsorption of proteins, the adhesion and differentiation of cells, and integration of tissues. These biological properties are closely related to chemical composition, wettability and roughness of implant surfaces. Although ceramic coatings could provide excellent bioactivity, the weak bonding strength between substrates and coatings is still the biggest issue owing to the mismatched coefficients of thermal expansion. In contrast, PIII&D technology can combat this problem by forming in situ coatings onto the material surface without changing its bulk properties. Meanwhile, there is no obvious boundary between modification layers and substrates.
Over the past decade, a range of gases and nutritious elements have been introduced to aid in osseointegration by PIII&D, which will be discussed in detail in the following.
4.1. Titanium and its alloys
Titanium and its alloys are often used as biomedical implants owing to their relatively low modulus, excellent fatigue strength and formability [52]. However, their relatively poor bioactivity imposes tight limits on the long-term stability. To solve this problem, various elements have been implanted to enhance the bioactivity of biomaterial surfaces.
It is widely acknowledged that the hydroxyl groups on the outermost surface of biomaterials, such as Ti–OH, Si–OH, Zr–OH and Ta–OH, can create a negatively charged surface when soaked in simulated body fluid (SBF), thereby inducing bone-like apatite formation [53]. This apatite layer has been found to provide excellent bone-bonding ability for biomaterials in the human body. Therefore, establishing a hydroxyl layer on the Ti alloy surface is considered to be an effective strategy to enhance its bioactivity [54]. Although NaOH and heat treatment is a much easier and traditional way to form Ti–OH groups on the surface, there is a potential risk of destruction of the passive layer after chemical etching process. PIII has been reported to be an efficient solution to solve this problem by various plasma implantations. A recent study by Xie et al. suggested that a highly active surface with Ti–OH groups can be achieved by H2O and subsequent H2 implantations into a Ti surface (represented as H2O + H2-PIII) but not by either H2O or H2 implantation (represented as H2O-PIII or H2-PIII) [55]. The in vitro biological test further demonstrated that human osteoblastic OCP-1 cells exhibited better adhesion and spreading on Ti–H2O + H2 surface than on the others. These observed phenomena indicated the importance of dual H2O + H2 implantation process. As reported by Farshi et al. [56], H2 tends to accumulate in the dislocation or defect layer produced by ion implantation. Therefore, in this experiment, the ‘H2O-PIII first’ procedure was designed to introduce near-surface damages which could trap H2 in the subsequent implantation step so as to maintain the bioactivity on the outermost surface. In another study, Liu et al. [57] also obtained Ti–OH-functionalized titanium surface by implanting H2 to the surface of plasma sprayed TiO2 coating. During this PIII process, the reaction between energetic H2 ions and outermost bridge oxygen species greatly contributed to the formation of Ti–OH bondings. And the TiO2 coating also can be considered as a H2 diffusion barrier. In this regard, constructing bioactive surfaces of Ti alloys with abundant Ti–OH groups can be achieved by combination of various oxygen-containing films on Ti alloy substrate with H2 implantation.
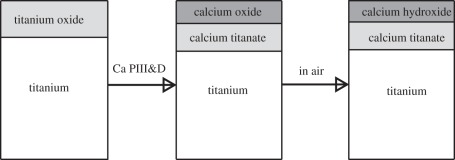
Interestingly, Maitz et al. [58] found that sodium (Na) implantation combined with a wet processing was an alternative synthetic approach to form Ti–OH bondings on Ti surface (Na-PIII). In this experiment, the Na-implanted titanium was oxidized to form sodium titanate in boiling water, and then sodium dissolved and exchanged with protons, leading to the formation of Ti–OH groups. Although comparable bioactivities of Na-PIII and Ti–OH were observed, released sodium ions were believed to be harmful to cells for a long-term culture, leading to more pronounced cytolytic cells and obviously suppressed cellular activity after two weeks. This fact highlights the significance of choosing biocompatible metallic elements for implantation to enhance the bioactivity. Nutritious element implantation seems like a good choice for improving bioactivity of Ti and Ti alloys owing to their necessity for the human body and various biological functions, including calcium (Ca) and magnesium (Mg) [59]. Liu et al. [60] implanted Ca ions into titanium and the results showed that Ca PIII-modified titanium samples could induce significant precipitation of apatite or calcium phosphorus (CaP) composites. This might be ascribed to the high activity of Ca, which is easily oxidized to form calcium oxide, followed by adsorption of H2O and CO2 to produce calcium hydroxide and calcium carbonate, as illustrated in figure 2. At the same time, the existence of micro-galvanic couple Ti–Ca would accelerate the release of calcium ions, resulting in supersaturation of calcium ions in SBF and eventually promoting apatite precipitation.
Figure 2.
Schematic of the surface reaction (cross section of the Ca PIII-D sample shown). Adapted from Liu et al. [60] with permission.
Recently, tantalum (Ta)-based implants have attracted considerable attention owing to the low modulus of elasticity, relatively high frictional characteristics, less peri-implant stress shielding and excellent corrosion resistance of Ta. However, its wide applications in load-bearing implants have been limited because of its high density and price. One simple solution is coating Ta film on Ti surface by the PIII&D method. The fabrication of Ta PIII&D and subsequent in vitro cell biological responses as well as in vivo animal tests are in progress in our group. Apart from the elements we mentioned, other bioactive and biocompatible elements, such as zinc, boron and strontium, have great potential in improving the bioactivity, which needs to be further explored.
Although the specific regulating role of introduced ions is far from elucidated, as is true for many studies in such an interdisciplinary field, physical and chemical factors seem to influence the biological outcomes by affecting the microenvironment surrounding the biomaterials surfaces, such as variations in surface charges as above mentioned. Finally, advances in biomaterials have to be supported by material innovations and fundamental understanding of the crosstalk between artificial implants and human biological systems.
4.2. Biomedical polymers
Synthetic polymers are the most versatile class of biomaterials being extensively applied in surgical devices, artificial organs, and orthopaedic and vascular prostheses. Their synthetic flexibility makes it possible to develop polymers having a wide spectrum of properties with excellent reproducibility to meet particular requirements. However, the inadequate interaction between polymer and peri-implant hampers their application as bone substitutes. It is notable that plasma activation offers a green and convenient way to address this problem by altering surface properties, such as bioactivity and hydrophilicity or hydrophobicity. Compared with plasma polymerization, PIII&D can introduce both functional groups (–NH2, –OH and –COOH) and nutritious elements (most of them are metallic) into the polymeric materials and form in situ-modified layers, which make PIII&D a more desirable method to modify surface properties while retaining the favourable bulk properties regardless of biostability or biodegradability of the polymer itself.
In a recent study, Zhang et al. reported on the surface modification of polyvinyl chloride using O2 and H2O implantation. The XPS analyses and Fourier transform infrared spectra showed that both processes could significantly enhance the O/C ratio and oxygen-containing functional groups on the surface, thereby increasing the surface hydrophilicity and surface energy of implanted samples [61].
In another study, Wang et al. investigated the effects of H2O-PIII and NH3-PIII on the bioactivity of biodegradable polybutylene succinate (PBSu) surface. After implantation process, C–OH and C–NH2 functional groups emerged from the PBSu surface, respectively. The results of biological tests demonstrated that both osteoblast compatibility and apatite formability are enhanced by either H2O-PIII-PBSu or NH3-PIII-PBSu, although H2O-PIII-PBSu is more effective in rendering PBSu suitable for bone-replacement implants [62].
Studies on surface modification of polytetrafluoroethylene (PTFE) using O2 PIII were further carried out by Wang and co-workers. In this case, they compared the biological efficacy of three sets of implantation parameters including long pulse, long frequency O2 PIII (L-PTFE), conventional short pulse, low frequency O2 PIII (S-PTFE) and O2 plasma immersion (P-PTFE). Compared with the untreated PTFE, the modified samples, especially L-PTFE, exhibited less oxygen-containing, rougher and more hydrophobic surfaces, providing better osteoblast adhesion and differentiation. However, only L-PTFE could upregulate the osteocalcin expression of the seeded osteoblast, indicating this novel O2 PIII treatment is better than conventional O2 PIII and O2 plasma immersion in the development of PTFE for bone or cartilage replacements [63].
The series of examples mentioned above suggest that the PIII&D technique is of great advantage in surface modification of polymers, especially those which are resistant to chemical etching and thus difficult to be modified using chemical methods.
5. Haemocompatibility of plasma immersion ion implantation and deposition-treated biomaterials
Referring to the commercial products of cardiovascular devices, such as artificial heart valves, rotary heart pumps and stents, the crucial requirement of biocompatibility is the bioinertness to blood. In other words, the implants must avoid the occurrence of thrombogenesis. In practice, the haemocompatibility of the devices is not adequate, and patients should continuously take anticoagulation medication after receiving implants. Hence, the development of a blood-contacting material with better blood compatibility is necessary. Diamond-like carbon (DLC) and Ti-O thin films were prepared using PIII&D technology for improving the haemocompatibility of implants in City University of Hong Kong and Southwest Jiaotong University, China [64–76].
5.1. Diamond-like carbon thin films
DLC thin films have been widely studied and used for many industrial purposes in the past 20 years owing to their superior chemical, optical, electrical and tribological properties. Their particularly favourable attributes include low friction coefficient, high hardness and wear resistance [74,75,77]. Recent study shows that DLC-coated PEEK possesses better surface hardness and elastic modulus, which are much closer to cortical bones, indicating a possible way to alleviate osteanabrosis caused by stress shielding effects [78]. Another study shows that phosphorus-doped DLC thin films could even be used to produce patterned neuron networks, suggesting that DLC is a candidate coating material for implants in the human nervous system [79]. Commercially, DLC thin films have been used as the coating materials in many applications, for instance, shavers, cutting and drilling tools, protective coating for magnetic media, and optical lenses. In addition to their good tribological qualities, DLC thin films are well known for their biocompatibility and improved cell adhesion as well as for chemical inertness, which enhance their utility as a coating candidate for cardiovascular devices [80].
Different kinds of undoped and doped DLC thin films have been fabricated using PIII&D since the confirmation of its biocompatibility. In the fabrication process of DLC thin films, acetylene (C2H2) gas is usually used to produce the carbon plasma for film deposition, whereas argon gas always is blended to enhance the cohesion between substrate and films. The dopants are introduced into the vacuum chamber simultaneously via thermal or electron induced evaporation. By using the PIII&D system with C2H2, amorphous H2-coated carbon films can be produced. For doped DLC film fabrication, however, a dopant source is added to the system to provide additional ions for film deposition. For instance, Sui & Cai [81] coated Ni–Ti shape memory alloys with un-doped DLC films. The platelet adhesion and protein adsorption tests showed that the coated sample exhibits positive consequence on haemocompatibility without sacrificing the superelasticity. They further investigated fluorine-doped DLC films and found that the corrosion resistance and hydrophobicity of modified samples are well improved, indicating a potential for anti-thrombogenicity [82]. Besides, calcium- and phosphorus-doped DLC thin films have been successfully deposited on different substrates using PIII&D [64–67,76]. The results indicated that both the P-DLC and Ca-DLC films show smaller numbers of adhered and unactivated platelets than low temperature isotropic carbon (LTIC).
5.2. Titanium oxide thin films
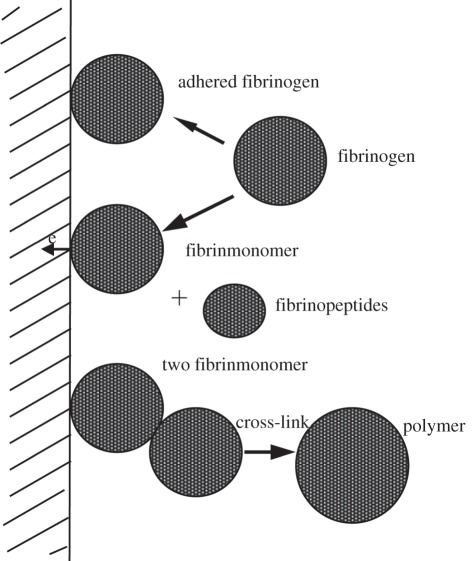
Titanium oxide is widely used in optical and electrical applications because of its high refractive index and dielectric constant. It is also very attractive as a biocompatible protective coating on medical implants, where a protective surface layer of TiO2 increases the wear resistance and corrosion resistance considerably, as mentioned in §3. Titanium oxide films used in artificial heart valves have also been synthesized using PIII&D technology [68–73]. In vitro blood compatibility investigation indicates that the TiO2 film has longer clotting time, lower haemolytic rate, less amounts of adherent platelets, less aggregation and less pseudopodium of the adherent platelets [73]. In vivo tests also demonstrate that the TiO2 film has much better haemocompatibility than LTIC [68]. In order to improve the mechanical properties, Leng et al. [69] fabricated Ti-O/Ti-N duplex coatings on biomedical titanium alloys by metal plasma immersion ion implantation and reactive plasma nitriding/oxidation. The presence of Ti-O improves the blood compatibility, and the main effect of Ti-N is to improve the mechanical properties. Blood compatibility investigation reveals that the Ti-O/Ti-N duplex coatings are better than LTIC. Studies have shown that TiO2 has good blood compatibility owing to the n-type semiconductivity with a wider band gap of 3.2 eV, low surface energy and low critical surface force. The good surface physical properties preclude fibrinogen from denaturation and consequently prevent the blood coagulation process [70–72]. The activation of fibrinogen on the solid surface can be correlated with the electrochemical reaction between the protein and material surface. It is postulated that the denaturing of fibrinogen is related to the charges of fibrinogen transferred to the material, as shown in figure 3. During this process, fibrinogen decomposes and transforms into fibrinmonomer and fibrinopeptides, followed by cross-linking to form the irreversible thrombus [70].
Figure 3.
Interaction of fibrinogen with a solid via the charge transfer process.
6. Antibacterial activity of plasma immersion ion implantation and deposition-treated biomaterials
Bacterial infection has been recognized as a serious problem during implant surgery, which may lead to implant failure, revision surgery and even member amputation, all associated with extremely high medical costs not to mention the pain and suffering of the patients [83]. Although antibiotics are still the main treatments for clinical infections, their widespread abuse has given rise to a breed of super bacteria that are resistant to common antibiotics, which is becoming more severe nowadays [84]. One exciting direction is to fabricate antibacterial biomaterials by incorporating broad-spectra bactericidal agents into surfaces or modifying surface physico-chemical properties to prevent bacterial growth or adhesion.
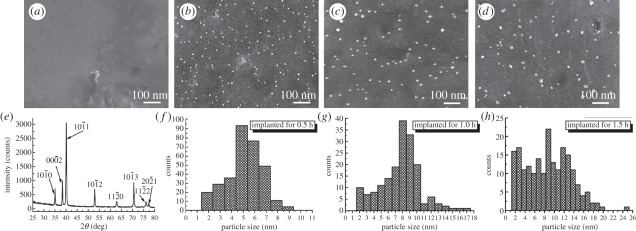
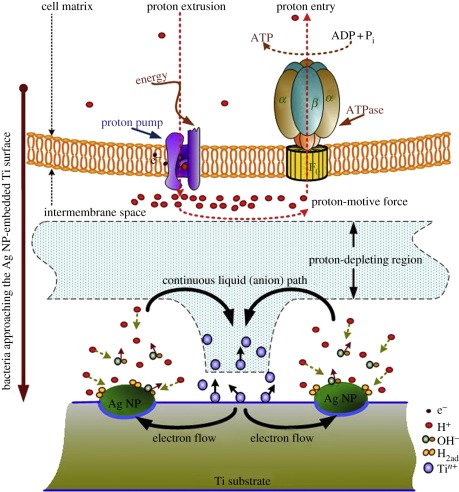
As well-known broad-spectra antimicrobial agents, silver (Ag) and copper (Cu) have been implanted into various materials surfaces using PIII&D to offer excellent antibacterial activities. Liu's group has recently prepared Ag nanoparticle-embedded Ti via single Ag PIII&D (Ag-PIII-Ti, as shown in figure 4) and proposed a new antibacterial mechanism for Ag-PIII-Ti [85]. It is believed that Ag and Ti will constitute one microgalvanic couple owing to the different potentials when immersed in an electrolyte solution. The cathodic reaction will form a proton-depleted region between bacterial membrane and titanium substrate, which probably disrupts the synthesis of adenosine triphosphate and leads to bacteria death. It is notable that proliferation of bacteria is inhibited on the Ag-PIII-Ti surface where that of osteoblasts is promoted. The inconsistent results are probably due to the larger size of human cells and the different synthetic and growth mechanisms between bacteria and human cells. Hence, this finding provides important clues on how to achieve specific biological responses to different species on the same surface. The schematic of this proposed mechanism is illustrated in figure 5.
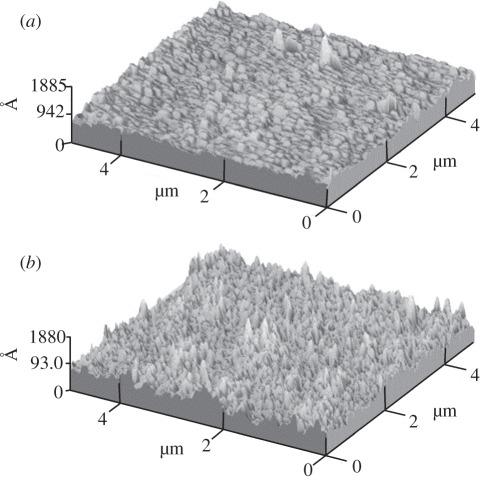
Figure 4.
Surface morphology of the titanium surfaces after Ag-PIII examined by scanning electron microscopy: (a) control pure Ti; (b) 0.5 h-Ag-PIII; (c) 1.0 h-Ag-PIII; (d) 1.5 h-Ag-PIII. (e) X-ray diffraction pattern of control pure Ti surface, indexed as a-Ti with hexagonal close-packed structure. Size distributions of the particles on (f) 0.5 h-Ag-PIII. (g) 1.0 h-Ag-PIII and (h) 1.5 h-Ag-PIII. Adapted from Cao et al. [85] with permission.
Figure 5.
Illustration for the possible toxicity mechanism on the Ag nanoparticle (NP)-embedded surface. Adapted from Cao et al. [85] with permission.
To study the antibacterial ability of polyethylene (PE) surfaces, Zhang et al. have conducted a series of Ag or Cu ion implantations. The initial studies demonstrated that either Ag- or Cu-implanted PE sample (Ag-PIII-PE or Cu-PIII-PE) could offer excellent instantaneous antibacterial activity due to the release of Ag ions or Cu ions [86]. Further studies found that various gases, including NH3, O2 and N2, co-implanted with Cu into PE surfaces induced slower release of Cu ions and higher antibacterial activity compared to Cu-PIII-PE, indicating enhancement in long-term antibacterial performance [87]. Similar observation was also found for Ag/N2 co-implanted sample [88]. Chemical state analyses showed that the gas plasma implantation led to dehydrogenation and produced unsaturated C=O, C=C−, −C≡N and −C=N bonds, which are believed to be capable of adjusting the release rate of Cu or Ag and have an appreciable effect on bacteria killing. In particular, more −C≡N and −C=N bonds in Cu/N2 co-PIII&D are the most effective in prolonging the antibacterial properties. The role of unsaturated bonds in offering antibacterial activity has been further confirmed by a more recent study, in which N2 plasma-modified PBSu was reported to exhibit high antibacterial activity against both Staphylococcus aureus (S. aureus) and Escherichia coli, with antibacterial ratio as high as 91.41 per cent and 90.34 per cent, respectively [62].
In a separate study, Wang et al. have fabricated three thin a-C : H films (as shown in figure 6) with different structures and chemical bonds on polyethylene terephthalate (PET) using C2H2 PIII&D at varying working pressures (0.5, 1 and 2 Pa) [89]. The adhesion tests revealed similar bacterial adhesion efficiencies between S. aureus on the a-C : H film deposited at 0.5 Pa and Staphylococcus epidermidis on the film deposited at 1.0 Pa, which were about one-sixth of those bacteria on the untreated PET surface. The reduction of bacteria can be explained by the free energy of adhesion (ΔFadh), which is positive for bacterial strains on the PET surfaces fabricated at 0.5 and 1.0 Pa (ΔFadh > 0), suggesting energetically unfavourable on these surfaces. Except the free energy theory, the author proposed that the bacterial adhesion on a-C : H films appeared to be also relevant to the structure of the film, and with decreasing sp3/sp2, the number of bacteria adhered on the carbon film diminishes. This is consistent with the research finding described in the previous paragraph.
Figure 6.
Atomic force microscopy photomicrographs. (a) The polyethylene terephthalate (PET) control and (b) PIII-PET films. Adapted from Wang et al. [89] with permission.
A critical problem associated with biomaterials is that the improvement in antibacterial activity is always accompanied with compromised biocompatibility, which cannot satisfy the clinical needs. To solve this issue, more comprehensive studies and deeper understanding of biophysiochemical interactions at the implant–bio interface will be required to achieve distinct bacteria/eukaryotic cell responsive surfaces.
7. Conclusion and future perspectives
From the first appearance in the 1980s to now, PIII&D has become a popular physical technique suitable for surface modification of various materials. PIII&D can offer omnidirectional processing capability to tailor the surface properties of many biomaterials by introducing a myriad of different kinds of elements and functional groups into the materials. This study reviewed the development and recent progress for improvement of mechanical and biological properties of biomaterials (Ti, Ti alloys and biopolymers) by PIII&D method in China.
Apart from the composition and physical and chemical properties, the nano- and microstructure of surfaces has been established as a key factor affecting a number of cellular responses, such as cell morphology, adhesion and differentiation. However, the creation of versatile nano- or microstructures cannot be achieved only by PIII&D technology. To ensure optimal performance of biomedical implants and to be compatible with the specific requirements of biological molecules, combination of PIII&D with other fabrication method may be required. Moreover, interdisciplinary approaches will be critical for designing biomaterial surfaces. Ultimately, advances in emerging biomedical applications with precisely controlled physical, chemical and biological properties remain a great opportunity for future research.
Acknowledgements
This work was jointly supported by the National Basic Research Programme of China (973 Programme, 2012CB933601), National Natural Science Foundation of China (31100675 and 51071168) and the Young Scholar Project of State Key Laboratory of High Performance Ceramics Superfine Microstructure.
References
- 1.Hench L. L. 1980. Biomaterials. Science 208, 826–831 10.1126/science.6246576 (doi:10.1126/science.6246576) [DOI] [PubMed] [Google Scholar]
- 2.Hench L. L., Wilson J. 1984. Surface-active biomaterials. Science 226, 630–636 10.1126/science.6093253 (doi:10.1126/science.6093253) [DOI] [PubMed] [Google Scholar]
- 3.Hench L. L., Polak J. M. 2002. Third-generation biomedical materials. Science 295, 1014–1017 10.1126/science.1067404 (doi:10.1126/science.1067404) [DOI] [PubMed] [Google Scholar]
- 4.Narayan R. J. 2010. The next generation of biomaterial development. Phil. Trans. R. Soc. A 368, 1831–1837 10.1098/rsta.2010.0001 (doi:10.1098/rsta.2010.0001) [DOI] [PubMed] [Google Scholar]
- 5.Griffith L. G., Naughton G. 2002. Tissue engineering: current challenges and expanding opportunities. Science 295, 1009–1014 10.1126/science.1069210 (doi:10.1126/science.1069210) [DOI] [PubMed] [Google Scholar]
- 6.Vandamme K., Holy X., Bensidhoum M., Logeart-Avramoglou D., Naert I. E., Duyck J. A., Petite H. 2011. In vivo molecular evidence of delayed titanium implant osseointegration in compromised bone. Biomaterials 32, 3547–3554 10.1016/j.biomaterials.2011.01.056 (doi:10.1016/j.biomaterials.2011.01.056) [DOI] [PubMed] [Google Scholar]
- 7.Park J.-W., An C.-H., Jeong S.-H., Suh J.-Y. 2012. Osseointegration of commercial microstructured titanium implants incorporating magnesium: a histomorphometric study in rabbit cancellous bone. Clin. Oral Implant. Res. 23, 294–300 10.1111/j.1600-0501.2010.02144.x (doi:10.1111/j.1600-0501.2010.02144.x) [DOI] [PubMed] [Google Scholar]
- 8.Lee J. H., Ryu H. S., Lee D. S., Hong K. S., Chang B. S., Lee C. K. 2005. Biomechanical and histomorphometric study on the bone–screw interface of bioactive ceramic-coated titanium screws. Biomaterials 26, 3249–3257 10.1016/j.biomaterials.2004.08.033 (doi:10.1016/j.biomaterials.2004.08.033) [DOI] [PubMed] [Google Scholar]
- 9.Hetrick E. M., Schoenfisch M. H. 2006. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 35, 780–789 10.1039/b515219b (doi:10.1039/b515219b) [DOI] [PubMed] [Google Scholar]
- 10.Berry C. W., Moore T. J., Safar J. A., Henry C. A., Wagner M. J. 1992. Antibacterial activity of dental implant metals. Implant Dent. 1, 59–65 10.1097/00008505-199200110-00006 (doi:10.1097/00008505-199200110-00006) [DOI] [PubMed] [Google Scholar]
- 11.Macnair R., Underwood M. J., Angelini G. D. 1998. Biomaterials and cardiovascular devices. J. Eng. Med. 212, 465–471 10.1243/0954411981534222 (doi:10.1243/0954411981534222) [DOI] [PubMed] [Google Scholar]
- 12.Roach P., Eglin D., Rohde K., Perry C. C. 2007. Modern biomaterials: a review—bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 18, 1263–1277 10.1007/s10856-006-0064-3 (doi:10.1007/s10856-006-0064-3) [DOI] [PubMed] [Google Scholar]
- 13.Thevenot P., Hu W., Tang L. 2008. Surface chemistry influences implant biocompatibility. Curr. Topics Med. Chem. 8, 270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divya Rani V. V., Vinoth-Kumar L., Anitha V. C., Manzoor K., Deepthy M., Shantikumar V. N. In press Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 10.1016/j.actbio.2012.01.021 (doi:10.1016/j.actbio.2012.01.021) [DOI] [PubMed] [Google Scholar]
- 15.Kou P. M., Schwartz Z., Boyan B. D., Babensee J. E. 2011. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater. 7, 1354–1363 10.1016/j.actbio.2010.10.020 (doi:10.1016/j.actbio.2010.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gittens R. A., McLachlan T., Olivares-Navarrete R., Cai Y., Berner S., Tannenbaum R., Schwartz Z., Sandhage K. H., Boyan B. D. 2011. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 32, 3395–3403 10.1016/j.biomaterials.2011.01.029 (doi:10.1016/j.biomaterials.2011.01.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J. Y., Shaughnessy M. C., Zhou Z., Noh H., Vogler E. A., Donahue H. J. 2008. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials 29, 1776–1784 10.1016/j.biomaterials.2007.12.026 (doi:10.1016/j.biomaterials.2007.12.026) [DOI] [PubMed] [Google Scholar]
- 18.Kim S., English A. E., Kihm K. D. 2009. Surface elasticity and charge concentration-dependent endothelial cell attachment to copolymer polyelectrolyte hydrogel. Acta Biomater. 5, 144–151 10.1016/j.actbio.2008.07.033 (doi:10.1016/j.actbio.2008.07.033) [DOI] [PubMed] [Google Scholar]
- 19.Mikulewicz M., Chojnacka K. 2011. Cytocompatibility of medical biomaterials containing nickel by osteoblasts: a systematic literature review. Biol. Trace Elem. Res. 142, 865–889 10.1007/s12011-010-8798-7 (doi:10.1007/s12011-010-8798-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders A. 1997. Metal plasma immersion ion implantation and deposition: a review. Surf. Coat. Technol. 93, 158–167 10.1016/s0257-8972(97)00037-6 (doi:10.1016/s0257-8972(97)00037-6) [DOI] [Google Scholar]
- 21.Chu P. K. 2010. Recent applications of plasma-based ion implantation and deposition to microelectronic, nano-structured, and biomedical materials. Surf. Coat. Technol. 204, 2853–2863 10.1016/j.surfcoat.2010.01.045 (doi:10.1016/j.surfcoat.2010.01.045) [DOI] [Google Scholar]
- 22.Chu P. K., Chan C. 2001. Applications of plasma immersion ion implantation in microelectronics: a brief review. Surf. Coat. Technol. 136, 151–156 10.1016/s0257-8972(00)01046-x (doi:10.1016/s0257-8972(00)01046-x) [DOI] [Google Scholar]
- 23.Tian X. B., Chu P. K., Fu R., Yang S. Q. 2004. Hybrid processes based on plasma immersion ion implantation: a brief review. Surf. Coat. Technol. 186, 190–195 10.1016/j.surfcoat.2004.04.049 (doi:10.1016/j.surfcoat.2004.04.049) [DOI] [Google Scholar]
- 24.Anders A. 2002. From plasma immersion ion implantation to deposition: a historical perspective on principles and trends. Surf. Coat. Technol. 156, 3–12 10.1016/s0257-8972(02)00066-x (doi:10.1016/s0257-8972(02)00066-x) [DOI] [Google Scholar]
- 25.Anders A. 2002. Energetic deposition using filtered cathodic arc plasmas. Vacuum 67, 673–686 10.1016/s0042-207x(02)00260-9 (doi:10.1016/s0042-207x(02)00260-9) [DOI] [Google Scholar]
- 26.Armitage D. A., Mihoc R., Tate T. J., McPhail D. S., Chater R., Hobkirk J. A., Shinawi L., Jones F. H. 2007. The oxidation of calcium implanted titanium in water: a depth profiling study. Appl. Surf. Sci. 253, 4085–4093 10.1016/j.apsusc.2006.09.006 (doi:10.1016/j.apsusc.2006.09.006) [DOI] [Google Scholar]
- 27.Chu P. K. 2006. Bioactivity of plasma implanted biomaterials. Nucl. Instrum. Methods Phys. Res. B 242, 1–7 10.1016/j.nimb.2005.08.015 (doi:10.1016/j.nimb.2005.08.015) [DOI] [Google Scholar]
- 28.Chu P. K. 2007. Plasma surface treatment of artificial orthopedic and cardiovascular biomaterials. Surf. Coat. Technol. 201, 5601–5606 10.1016/j.surfcoat.2006.07.003 (doi:10.1016/j.surfcoat.2006.07.003) [DOI] [Google Scholar]
- 29.Chu P. K. 2009. Applications of plasma-based technology to microelectronics and biomedical engineering. Surf. Coat. Technol. 203, 2793–2798 10.1016/j.surfcoat.2009.02.131 (doi:10.1016/j.surfcoat.2009.02.131) [DOI] [Google Scholar]
- 30.Krischok S., et al. 2007. Influence of ion implantation on titanium surfaces for medical applications. Surf. Sci. 601, 3856–3860 10.1016/j.susc.2007.04.060 (doi:10.1016/j.susc.2007.04.060) [DOI] [Google Scholar]
- 31.Liang H., Wan Y. Z., He F., Huang Y., Xu J. D., Li J. M., Wang Y. L., Zhao Z. G. 2007. Bioactivity of Mg-ion-implanted zirconia and titanium. Appl. Surf. Sci. 253, 3326–3333 10.1016/j.apsusc.2006.07.038 (doi:10.1016/j.apsusc.2006.07.038) [DOI] [Google Scholar]
- 32.Liu B., Zheng Y. F. 2011. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 7, 1407–1420 10.1016/j.actbio.2010.11.001 (doi:10.1016/j.actbio.2010.11.001) [DOI] [PubMed] [Google Scholar]
- 33.Anders A. 2004. Fundamentals of pulsed plasmas for materials processing. Surf. Coat. Technol. 183, 301–311 10.1016/j.surfcoat.2003.09.049 (doi:10.1016/j.surfcoat.2003.09.049) [DOI] [Google Scholar]
- 34.Garcia J. A., Rodriguez R. J. 2011. Ion implantation techniques for non-electronic applications. Vacuum 85, 1125–1129 10.1016/j.vacuum.2010.12.024 (doi:10.1016/j.vacuum.2010.12.024) [DOI] [Google Scholar]
- 35.Maendl S., Sader R., Thorwarth G., Krause D., Zeilhofer H. F., Horch H. H., Rauschenbach B. 2002. Investigation on plasma immersion ion implantation treated medical implants. Biomol. Eng. 19, 129–132 10.1016/S1389-0344(02)00025-4 (doi:10.1016/S1389-0344(02)00025-4) [DOI] [PubMed] [Google Scholar]
- 36.Daley B., Doherty A. T., Fairman B., Case C. P. 2004. Wear debris from hip or knee replacements causes chromosomal damage in human cells in tissue culture. J. Bone Joint Surg. Br. 86B, 598–606 10.1302/0301-620x.86b4.14368 (doi:10.1302/0301-620x.86b4.14368) [DOI] [PubMed] [Google Scholar]
- 37.Liao Y., Pourzal R., Wimmer M. A., Jacobs J. J., Fischer A., Marks L. D. 2011. Graphitic tribological layers in metal-on-metal hip replacements. Science 334, 1687–1690 10.1126/science.1213902 (doi:10.1126/science.1213902) [DOI] [PubMed] [Google Scholar]
- 38.Alonso F., Rinner M., Loinaz A., Onate J. I., Ensinger W., Rauschenbach B. 1997. Characterization of Ti-6Al-4V modified by nitrogen plasma immersion ion implantation. Surf. Coat. Technol. 93, 305–308 10.1016/s0257-8972(97)00065-0 (doi:10.1016/s0257-8972(97)00065-0) [DOI] [Google Scholar]
- 39.Wan G. J., Huang N., Leng Y. X., Yang P., Chen J. Y., Wang J., Sun H. 2004. TiN and Ti–O/TiN films fabricated by PIII-D for enhancement of corrosion and wear resistance of Ti–6A1–4V. Surf. Coat. Technol. 186, 136–140 10.1016/j.surfcoat.2004.04.037 (doi:10.1016/j.surfcoat.2004.04.037) [DOI] [Google Scholar]
- 40.Leng Y. X., Chen J. Y., Zeng Z. M., Tian X. B., Yang P., Huang N., Zhou Z. R., Chu P. K. 2000. Properties of titanium oxide biomaterials synthesized by titanium plasma immersion ion implantation and reactive ion oxidation. Thin Solid Films 377, 573–577 10.1016/s0040-6090(00)01306-7 (doi:10.1016/s0040-6090(00)01306-7) [DOI] [Google Scholar]
- 41.Tang B. Y., Chu P. K., Wang S. Y., Chow K. W., Wang X. F. 1998. Methane and nitrogen plasma immersion ion implantation of titanium metal. Surf. Coat. Technol. 103–104, 248–251 10.1016/S0257-8972(98)00403-4 (doi:10.1016/S0257-8972(98)00403-4) [DOI] [Google Scholar]
- 42.Leyland A., Matthews A. 2000. On the significance of the H/E ratio in wear control: a nanocomposite coating approach to optimised tribological behaviour. Wear 246, 1–11 10.1016/s0043-1648(00)00488-9 (doi:10.1016/s0043-1648(00)00488-9) [DOI] [Google Scholar]
- 43.Shi W., Li X. Y., Dong H. 2001. Improved wear resistance of ultra-high molecular weight polyethylene by plasma immersion ion implantation. Wear 250, 544–552 10.1016/s0043-1648(01)00636-6 (doi:10.1016/s0043-1648(01)00636-6) [DOI] [Google Scholar]
- 44.Powles R. C., McKenzie D. R., Fujisawa N., McCulloch D. G. 2005. Production of amorphous carbon by plasma immersion ion implantation of polymers. Diam. Relat. Mater. 14, 1577–1582 10.1016/j.diamond.2005.02.013 (doi:10.1016/j.diamond.2005.02.013) [DOI] [Google Scholar]
- 45.Okabe Y., Kurihara S., Yajima T., Seki Y., Nakamura I., Takano I. 2005. Formation of super-hydrophilic surface of hydroxyapatite by ion implantation and plasma treatment. Surf. Coat. Technol. 196, 303–306 10.1016/j.surfcoat.2004.08.190 (doi:10.1016/j.surfcoat.2004.08.190) [DOI] [Google Scholar]
- 46.Poon R. W. Y., Ho J. P. Y., Liu X. Y., Chung C. Y., Chu P. K., Yeung K. W. K., Lu W. W., Cheung K. M. C. 2005. Improvements of anti-corrosion and mechanical properties of NiTi orthopedic materials by acetylene, nitrogen and oxygen plasma immersion ion implantation. Nucl. Instrum. Methods Phys. Res. B 237, 411–416 10.1016/j.nimb.2005.05.030 (doi:10.1016/j.nimb.2005.05.030) [DOI] [Google Scholar]
- 47.Poon R. W. Y., Ho J. P. Y., Liu X. Y., Chung C. Y., Chu P. K., Yeung K. W. K., Lu W. W., Cheung K. M. C. 2005. Anti-corrosion performance of oxidized and oxygen plasma-implanted NiTi alloys. Mater. Sci. Eng. A 390, 444–451 10.1016/j.msea.2004.08.061 (doi:10.1016/j.msea.2004.08.061) [DOI] [Google Scholar]
- 48.Poon R. W. Y., Ho J. P. Y., Liu X. Y., Chung C. Y., Chu P. K., Yeung K. W. K., Lu W. W., Cheung K. A. 2005. Formation of titanium nitride barrier layer in nickel-titanium shape memory alloys by nitrogen plasma immersion ion implantation for better corrosion resistance. Thin Solid Films 488, 20–25 10.1016/j.tsf.2005.04.002 (doi:10.1016/j.tsf.2005.04.002) [DOI] [Google Scholar]
- 49.Poon R. W. Y., Yeung K. W. K., Liu X. Y., Chu P. K., Chung C. Y., Lu W. W., Cheung K. M. C., Chan D. 2005. Carbon plasma immersion ion implantation of nickel-titanium shape memory alloys. Biomaterials 26, 2265–2272 10.1016/j.biomaterials.2004.07.056 (doi:10.1016/j.biomaterials.2004.07.056) [DOI] [PubMed] [Google Scholar]
- 50.Liu X. M., et al. 2008. In vitro corrosion behavior of TiN layer produced on orthopedic nickel-titanium shape memory alloy by nitrogen plasma immersion ion implantation using different frequencies. Surf. Coat. Technol. 202, 2463–2466 10.1016/j.surfcoat.2007.08.017 (doi:10.1016/j.surfcoat.2007.08.017) [DOI] [Google Scholar]
- 51.Drees P., Eckardt A., Gay R. E., Gay S., Huber L. C. 2007. Mechanisms of disease: molecular insights into aseptic loosening of orthopedic implants. Nat. Clin. Pract. Rheumatol. 3, 165–171 10.1038/ncprheum0428 (doi:10.1038/ncprheum0428) [DOI] [PubMed] [Google Scholar]
- 52.Liu X. Y., Chu P. K., Ding C. X. 2004. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. Rep. 47, 49–121 10.1016/j.mser.2004.11.001 (doi:10.1016/j.mser.2004.11.001) [DOI] [Google Scholar]
- 53.Kokubo T., Kim H. M., Kawashita M. 2003. Novel bioactive materials with different mechanical properties. Biomaterials 24, 2161–2175 10.1016/s0142-9612(03)00044-9 (doi:10.1016/s0142-9612(03)00044-9) [DOI] [PubMed] [Google Scholar]
- 54.Jonasova L., Muller F. A., Helebrant A., Strnad J., Greil P. 2004. Biomimetic apatite formation on chemically treated titanium. Biomaterials 25, 1187–1194 10.1016/j.biomaterials.2003.08.009 (doi:10.1016/j.biomaterials.2003.08.009) [DOI] [PubMed] [Google Scholar]
- 55.Xie Y. T., Liu X. Y., Huang A. P., Ding C. X., Chu P. K. 2005. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials 26, 6129–6135 10.1016/j.biomaterials.2005.03.032 (doi:10.1016/j.biomaterials.2005.03.032) [DOI] [PubMed] [Google Scholar]
- 56.Farshi M. S., Baumann H., Ruck D., Bethge K. 1997. Hydrogen accumulation in titanium, zirconium and hafnium caused by nitrogen implantation. Nucl. Instrum. Methods Phys. Res. B 127, 787–790 10.1016/S0168-583X(97)00008-6 (doi:10.1016/S0168-583X(97)00008-6) [DOI] [Google Scholar]
- 57.Liu X. Y., Zhao X. B., Fu R. K. Y., Ho J. P. Y., Ding C. X., Chu P. K. 2005. Plasma-treated nanostructured TiO2 surface supporting biomimetic growth of apatite. Biomaterials 26, 6143–6150 10.1016/j.biomaterials.2005.04.035 (doi:10.1016/j.biomaterials.2005.04.035) [DOI] [PubMed] [Google Scholar]
- 58.Maitz M. F., Poon R. W. Y., Liu X. Y., Pham M. T., Chu P. K. 2005. Bioactivity of titanium following sodium plasma immersion ion implantation and deposition. Biomaterials 26, 5465–5473 10.1016/j.biomaterials.2005.02.006 (doi:10.1016/j.biomaterials.2005.02.006) [DOI] [PubMed] [Google Scholar]
- 59.Palacios C. 2006. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 46, 621–628 10.1080/10408390500466174 (doi:10.1080/10408390500466174) [DOI] [PubMed] [Google Scholar]
- 60.Liu X. Y., Poon R. W. Y., Kwok S. C. H., Chu P. K., Ding C. X. 2005. Structure and properties of Ca-plasma-implanted titanium. Surf. Coat. Technol. 191, 43–48 10.1016/j.surfcoat.2004.08.118 (doi:10.1016/j.surfcoat.2004.08.118) [DOI] [Google Scholar]
- 61.Zhang W., Chu P. K., Ji J., Zhang Y., Jiang Z. 2006. Effects of O2 and H2O plasma immersion ion implantation on surface chemical composition and surface energy of poly vinyl chloride. Appl. Surf. Sci. 252, 7884–7889 10.1016/j.apsusc.2005.09.057 (doi:10.1016/j.apsusc.2005.09.057) [DOI] [Google Scholar]
- 62.Wang H., Ji J., Zhang W., Zhang Y., Jiang J., Wu Z., Pu S., Chu P. K. 2009. Biocompatibility and bioactivity of plasma-treated biodegradable poly(butylene succinate). Acta Biomater. 5, 279–287 10.1016/j.actbio.2008.07.017 (doi:10.1016/j.actbio.2008.07.017) [DOI] [PubMed] [Google Scholar]
- 63.Wang H., Kwok D. T. K., Wang W., Wu Z., Tong L., Zhang Y., Chu P. K. 2010. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials 31, 413–419 10.1016/j.biomaterials.2009.09.066 (doi:10.1016/j.biomaterials.2009.09.066) [DOI] [PubMed] [Google Scholar]
- 64.Yang P., Huang N., Leng Y. X., Chen J. Y., Fu R. K. Y., Kwok S. C. H., Leng Y., Chu P. K. 2003. Activation of platelets adhered on amorphous hydrogenated carbon (a-C : H) films synthesized by plasma immersion ion implantation-deposition (PIII-D). Biomaterials 24, 2821–2829 10.1016/s0142-9612(03)00091-7 (doi:10.1016/s0142-9612(03)00091-7) [DOI] [PubMed] [Google Scholar]
- 65.Yang P., Kwok S. C. H., Fu R. K. Y., Leng Y. X., Wang J., Wan G. J., Huang N., Leng Y., Chu P. K. 2004. Structure and properties of annealed amorphous hydrogenated carbon (a-C : H) films for biomedical applications. Surf. Coat. Technol. 177, 747–751 10.1016/j.surfcoat.2003.08.016 (doi:10.1016/j.surfcoat.2003.08.016) [DOI] [Google Scholar]
- 66.Kwok S. C. H., Jin W., Chu P. K. 2005. Surface energy, wettability, and blood compatibility phosphorus doped diamond-like carbon films. Diam. Relat. Mater. 14, 78–85 10.1016/j.diamond.2004.07.019 (doi:10.1016/j.diamond.2004.07.019) [DOI] [Google Scholar]
- 67.Kwok S. C. H., Ha P. C. T., McKenzie D. R., Bilek M. M. M., Chu P. K. 2006. Biocompatibility of calcium and phosphorus doped diamond-like carbon thin films synthesized by plasma immersion ion implantation and deposition. Diam. Relat. Mater. 15, 893–897 10.1016/j.diamond.2005.10.062 (doi:10.1016/j.diamond.2005.10.062) [DOI] [Google Scholar]
- 68.Yang P., Huang N., Leng Y. X., Chen J. Y., Sun H., Wang J., Chen F., Chu P. K. 2002. In vivo study of Ti-O thin film fabricated by PIII. Surf. Coat. Technol. 156, 284–288 10.1016/s0257-8972(02)00087-7 (doi:10.1016/s0257-8972(02)00087-7) [DOI] [Google Scholar]
- 69.Leng Y. X., Yang P., Chen J. Y., Sun H., Wang J., Wang G. J., Huang N., Tian X. B., Chu P. K. 2001. Fabrication of Ti-O/Ti-N duplex coatings on biomedical titanium alloys by metal plasma immersion ion implantation and reactive plasma nitriding/oxidation. Surf. Coat. Technol. 138, 296–300 10.1016/s0257-8972(00)01172-5 (doi:10.1016/s0257-8972(00)01172-5) [DOI] [Google Scholar]
- 70.Huang N., Chen Y. R., Luo J. M., Yi J., Lu R., Xiao J., Xue Z. N., Liu X. H. 1994. In vitro investigation of blood compatibility of Ti with oxide layers of rutile structure. J. Biomater. Appl. 8, 404–412 10.1177/088532829400800406 (doi:10.1177/088532829400800406) [DOI] [PubMed] [Google Scholar]
- 71.Huang N., et al. 1998. Blood compatibility of amorphous titanium oxide films synthesized by ion beam enhanced deposition. Biomaterials 19, 771–776 10.1016/s0142-9612(98)00212-9 (doi:10.1016/s0142-9612(98)00212-9) [DOI] [PubMed] [Google Scholar]
- 72.Chen J. Y., Leng Y. X., Tian X. B., Wang L. P., Huang N., Chu P. K., Yang P. 2002. Antithrombogenic investigation of surface energy and optical bandgap and hemocompatibility mechanism of Ti(Ta+5)O2 thin films. Biomaterials 23, 2545–2552 10.1016/s0142-9612(01)00389-1 (doi:10.1016/s0142-9612(01)00389-1) [DOI] [PubMed] [Google Scholar]
- 73.Leng Y. X., et al. 2002. Structure and properties of biomedical TiO2 films synthesized by dual plasma deposition. Surf. Coat. Technol. 156, 295–300 10.1016/s0257-8972(02)00092-0 (doi:10.1016/s0257-8972(02)00092-0) [DOI] [Google Scholar]
- 74.Grill A. 2003. Diamond-like carbon coatings as biocompatible materials: an overview. Diam. Relat. Mater. 12, 166–170 10.1016/s0925-9635(03)00018-9 (doi:10.1016/s0925-9635(03)00018-9) [DOI] [Google Scholar]
- 75.Hauert R. 2003. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 12, 583–589 10.1016/s0925-9635(03)00081-5 (doi:10.1016/s0925-9635(03)00081-5) [DOI] [Google Scholar]
- 76.Chen J. Y., et al. 2002. Blood compatibility and sp3/sp2 contents of diamond-like carbon (DLC) synthesized by plasma immersion ion implantation-deposition. Surf. Coat. Technol. 156, 289–294 10.1016/s0257-8972(02)00089-0 (doi:10.1016/s0257-8972(02)00089-0) [DOI] [Google Scholar]
- 77.Cui F. Z., Li D. J. 2000. A review of investigations on biocompatibility of diamond-like carbon and carbon nitride films. Surf. Coat. Technol. 131, 481–487 10.1016/s0257-8972(00)00809-4 (doi:10.1016/s0257-8972(00)00809-4) [DOI] [Google Scholar]
- 78.Wang H., Xu M., Zhang W., Kwok D. T. K., Jiang J., Wu Z., Chu P. K. 2010. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials 31, 8181–8187 10.1016/j.biomaterials.2010.07.054 (doi:10.1016/j.biomaterials.2010.07.054) [DOI] [PubMed] [Google Scholar]
- 79.Regan E. M., Uney J. B., Dick A. D., Zhang Y., Nunez-Yanez J., McGeehan J. P., Claeyssens F., Kelly S. 2010. Differential patterning of neuronal, glial and neural progenitor cells on phosphorus-doped and UV irradiated diamond-like carbon. Biomaterials 31, 207–215 10.1016/j.biomaterials.2009.09.042 (doi:10.1016/j.biomaterials.2009.09.042) [DOI] [PubMed] [Google Scholar]
- 80.Thorwarth G., Saldamli B., Schwarz F., Juergens P., Leiggener C., Sader R., Haeberlen M., Assmann W., Stritzker B. 2007. Biocompatibility of doped diamond-like carbon coatings for medical implants. Plasma Process. Polym. 4, S364–S368 10.1002/ppap.200731001 (doi:10.1002/ppap.200731001) [DOI] [Google Scholar]
- 81.Sui J. H., Cai W. 2006. Effect of diamond-like carbon (DLC) on the properties of the NiTi alloys. Diam. Relat. Mater. 15, 1720–1726 10.1016/j.diamond.2006.03.004 (doi:10.1016/j.diamond.2006.03.004) [DOI] [Google Scholar]
- 82.Sui J. H., Zhang Z. G., Cai W. 2009. Surface characteristics and electrochemical corrosion behavior of fluorinated diamond-like carbon (F-DLC) films on the NiTi alloys. Nucl. Instrum. Methods Phys. Res. B 267, 2475–2479 10.1016/j.nimb.2009.05.049 (doi:10.1016/j.nimb.2009.05.049) [DOI] [Google Scholar]
- 83.Inman R. D., Gallegos K. V., Brause B. D., Redecha P. B., Christian C. L. 1984. Clinical and microbial features of prosthetic joint infection. Am. J. Med. 77, 47–53 10.1016/0002-9343(84)90434-0 (doi:10.1016/0002-9343(84)90434-0) [DOI] [PubMed] [Google Scholar]
- 84.Taubes G. 2008. The bacteria fight back. Science 321, 356–361 10.1126/science.321.5887.356 (doi:10.1126/science.321.5887.356) [DOI] [PubMed] [Google Scholar]
- 85.Cao H., Liu X., Meng F., Chu P. K. 2011. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 32, 693–705 10.1016/j.biomaterials.2010.09.066 (doi:10.1016/j.biomaterials.2010.09.066) [DOI] [PubMed] [Google Scholar]
- 86.Zhang W., Chu P. K. 2008. Enhancement of antibacterial properties and biocompatibility of polyethylene by silver and copper plasma immersion ion implantation. Surf. Coat. Technol. 203, 909–912 10.1016/j.surfcoat.2008.08.023 (doi:10.1016/j.surfcoat.2008.08.023) [DOI] [Google Scholar]
- 87.Zhang W., Ji J., Zhang Y., Yan Q., Kurmaev E. Z., Moewes A., Zhao J., Chu P. K. 2007. Effects of NH3, O2, and N2 co-implantation on Cu out-diffusion and antimicrobial properties of copper plasma-implanted polyethylene. Appl. Surf. Sci. 253, 8981–8985 10.1016/j.apsusc.2007.05.019 (doi:10.1016/j.apsusc.2007.05.019) [DOI] [Google Scholar]
- 88.Zhang W., Luo Y., Wang H., Jiang J., Pu S., Chu P. K. 2008. Ag and Ag/N2 plasma modification of polyethylene for the enhancement of antibacterial properties and cell growth/proliferation. Acta Biomater. 4, 2028–2036 10.1016/j.actbio.2008.05.012 (doi:10.1016/j.actbio.2008.05.012) [DOI] [PubMed] [Google Scholar]
- 89.Wang J., et al. 2004. Bacterial repellence from polyethylene terephthalate surface modified by acetylene plasma immersion ion implantation-deposition. Surf. Coat. Technol. 186, 299–304 10.1016/j.surfcoat.2004.02.046 (doi:10.1016/j.surfcoat.2004.02.046) [DOI] [Google Scholar]