Abstract
Dendrimers comprise a category of branched materials with diverse functions that can be constructed with defined architectural and chemical structures. When decorated with bioactive ligands made of peptides and saccharides through peripheral chemical groups, dendrimer conjugates are turned into nanomaterials possessing attractive binding properties with the cognate receptors. At the cellular level, bioactive dendrimer conjugates can interact with cells with avidity and selectivity, and this function has particularly stimulated interests in investigating the targeting potential of dendrimer materials for the design of drug delivery systems. In addition, bioactive dendrimer conjugates have so far been studied for their versatile capabilities to enhance stability, solubility and absorption of various types of therapeutics. This review presents a brief discussion on three aspects of the recent studies to use peptide- and saccharide-conjugated dendrimers for drug delivery: (i) synthesis methods, (ii) cell- and tissue-targeting properties and (iii) applications of conjugated dendrimers in drug delivery nanodevices. With more studies to elucidate the structure–function relationship of ligand–dendrimer conjugates in transporting drugs, the conjugated dendrimers hold promise to facilitate targeted delivery and improve drug efficacy for discovery and development of modern pharmaceutics.
Keywords: dendrimer, ligand, drug delivery, targeting, binding
1. Introduction
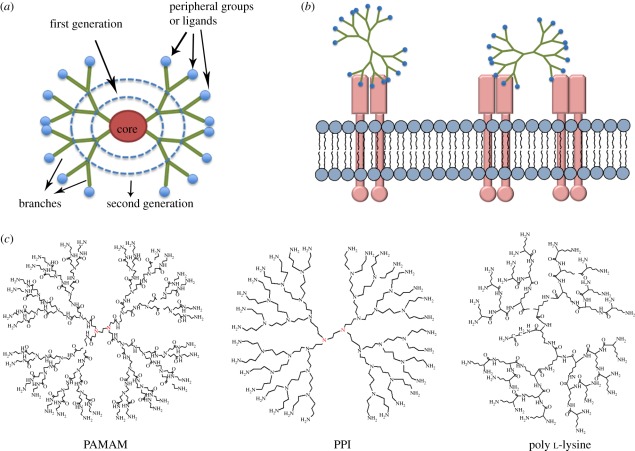
Dendrimers constitute a unique category of ‘tree-like’ materials that possess definable structural features [1,2]. In principle, dendrimers can be made with monodisperse molecular weight, tunable sizes and nanoscale starburst branches, making them ideal scaffolds for the creation of functional nano-biomaterials. As shown in figure 1a, dendrimers are typically composed of three distinct regions: the core, the interior branches and the peripheral groups. With each successive generation, the branches grow radially; so the size and functionality of the dendrimer can be simultaneously tuned. The core and the interior branches of a dendrimer can be synthetic or based on natural peptide or saccharide structures. When decorated with peptide or carbohydrate ligands through surface functional groups, dendrimers are endowed with the bioactivity to mediate the interaction with cell surface receptors (figure 1b). There are a large number of dendrimeric structures that have been reported in the literature (figure 1c), among them, the commercially available polyamidoamine (PAMAM) is one of the most widely explored dendrimers for biomaterial applications.
Figure 1.
(a) Schematic of a dendrimer structure; (b) bioactive dendrimers can interact with specific receptors on cell membrane; (c) three types of commonly used dendrimer materials, as illustrated by G3.0 PAMAM, G3.0 poly (propylene)imine (PPI) and G2.0 dendritic poly l-lysine.
Notably, multiple copies of ligands grafted on a dendrimer may coordinate the receptor binding in a multivalent way, and the ligand interaction with receptors or cells is often manifested with enhanced avidity and selectivity [3–6]. Ligand-conjugated dendrimers by virtue of recapitulating the natural multivalent mechanisms, therefore, hold great promise for use in a broad array of biomedical applications, as has been shown in the development of artificial proteins, anti-microbial/anti-viral reagents, and drug delivery and release materials [6–9]. Moreover, new applications of dendrimer conjugates are also emerging in the fields of cell labelling, diagnostic imaging and tissue engineering. In the field of drug delivery, dendrimers and bioactive ligands combined together may offer additional advantages besides mediating specific binding. Notably, dendrimer scaffolds may provide extra functional groups for drug conjugation; hydrophilic dendrimer conjugates may enhance the solubility of drugs or drug-loaded devices.
In this review, we aim to provide a concise review on the use of peptide- and saccharide-conjugated dendrimers for developing biomaterials for drug delivery systems. The content is organized into three sections: (i) the conjugation chemistry to graft peptide, and saccharide ligands to dendrimer scaffolds, (ii) the cell- and tissue-targeting properties of peptide- and saccharide ligand-conjugated dendrimers and (iii) the drug delivery efficiency of dendrimer conjugates. Readers should bear in mind that dendrimers themselves have been directly used in many biomedical applications without the presence of bioactive ligands. For more general and background information, readers can refer to previous reviews on dendrimeric materials [10–15].
2. Conjugation chemistry to prepare peptide- and saccharide–dendrimer conjugates
The conjugation chemistry to decorate the surface of dendrimer scaffolds has been extensively investigated in the past, and numerous methods have been established to couple peptide-, saccharide- and glycopeptide-based ligands to various dendrimeric scaffolds. We do not intend to be exhaustive with all of the chemistries that have been developed thus far, but will mainly provide examples to illustrate the practical methods that can be adopted by laboratories interested in studying the applications of dendrimer conjugates. The reviews by Roy and Jezek et al. can provide more comprehensive information on the chemistry of synthesizing peptide- and saccharide–dendrimer conjugates [6,10,16,17]. It should be noted that the term ‘glycopeptide dendrimers’ can refer to two categories of dendrimer conjugate materials in the literature: (i) α-amino acid-based polypeptide dendrimers grafted with saccharides and (ii) non-amino acid dendrimers grafted with glycopeptide ligands.
Multivalent binding between ligands and receptors often exhibits temporal and spatial complexity at molecular and supramolecular levels. Therefore, understanding the effectiveness and limitations of different conjugation methods, and applying them appropriately to synthesizing bioactive dendrimers are essential steps for controlling the structure and property of the final delivery material. When attaching peptides or carbohydrates, the common ligation strategies can be applied directly to generating bioactive dendrimer conjugates. Nevertheless, there are at least two factors characteristically associated with the ligation of dendrimer scaffolds: the type and generation of dendrimer scaffolds that would determine the shape and size of final products; and the number of peripheral branches and modification level that could affect the multivalent spatial arrangement and receptor-binding properties of bioactive ligands.
2.1. Synthesis of peptide–dendrimer conjugates
To conjugate peptide ligands to a dendrimeric scaffold, various conjugation techniques have been adopted in the past. The difference of these approaches may be illustrated by the study of Mihov et al., in which three methods were described to conjugate an oligolysine to the polyphenylene dendrimers (PPDs) with four, eight and 16 terminal branches [13]. First, the oligolysine was assembled directly on the PPD surface from protected lysine monomers by employing α-amino acid N-carboxyanhydride polymerization. This process resulted in PPD grafted with protected oligolysines that were deprotected afterwards in a trifluoroacetic acid solution. Second, the protected pentalysine moieties were coupled through the C-terminal to the PPD scaffolds functionalized with amine groups. The carbodiimide coupling chemistry was used and resulted in amide linkages. Third, the surface of PPD was decorated with maleimide groups, and the cysteine-terminated peptides were grafted via Michael-type addition.
Among these methods, directly conjugating unprotected peptides are most commonly practised in laboratories that develop biomaterials. Conjugates of regular shapes and a controlled degree of peptide modification level can often be purified and obtained [18–22]. However, as the generation and the number of branches of a dendrimer scaffold grow, it may become difficult to achieve uniform and controllable conjugation level owing to the steric hindrance of dense branches. In one study by Waite & Roth [21], generation 5 (G5) polyamidoamine (PAMAM) was reacted with heterobifunctional cross-linker sulphosuccinimidyl 6-[3′-(2-pyridyldithio)propionamido]hexanoate in various molar equivalents of dendrimer, and a cyclic arginine–glycine–aspartic acid (RGD) peptide was subsequently grafted. The average modification level was reported as two, three, seven and 10 RGD ligands per dendrimer according to a UV spectroscopic analysis. Our laboratory tested a common strategy to graft linear RGD peptides to amine-terminated G2, G4 and G5 PAMAM (theoretically containing 16, 64 and 128 terminal groups, respectively). A heterobifunctional cross-linker, NHS-PEG2-maleimide (SM (PEG)2), was used, which was first reacted with the amine groups of PAMAM and then linked to the sulphydryl of CGRGDS oligopeptides. By NMR analysis, the average molar percentage of peptide modification on the periphery groups was found to decrease from 59 per cent on G2 PAMAM to 52 per cent on G4 PAMAM, and to 48 per cent on G5 PAMAM (B. Lv & Y. Luo 2011, unpublished data). These modification levels correspond to an average of nine, 33 and 61 RGD ligands per G2, G4 and G5 PAMAM, respectively.
In the study by Baker and co-workers [18–20], cyclic RGD was conjugated to PAMAM scaffold. The peripheral amine groups were partially acetylated to prevent non-specific binding to cell membranes and intermolecular interaction/aggregation, and the remaining amines were directly reacted with fluorescein isothiocyanate (FITC) or converted to carboxylic acid to react with the N-terminus of cyclic RGD peptides. By regulating the feed ratio between RGD ligand and PAMAM dendrimer, PAMAM–RGD conjugates with different modification levels were obtained and purified. For instance, 2–3 or 12–13 RGD ligands could be conjugated to one dendrimer when RGD was reacted in five or 10 molar equivalents of G5 PAMAM scaffolds, respectively. And G3 PAMAM could be modified with an average of 4.1 peptides per dendrimer when RGD was reacted with PAMAM in 12 : 1 molar ratio.
Peptide ligands grafted to dendrimer scaffolds may show different degrees of stability depending on the chemical properties of the linkages. Shao & Tam [23,24] described a general conjugation approach using a dendrimer having a lysine core with four aldehyde peripheral groups. The tetravalent scaffolds were reacted with three types of side-chain unprotected peptides, of which the N-terminus were functionalized with weak nucleophilic bases: aminooxy, hydrazide and cysteine 1,2-aminothiol (generated during the solid-phase peptide synthesis). The direct conjugation resulted in oxime, hydrazone and thiazolidine linkages, respectively. Among them, the thiazolidine appeared to be superior in terms of reaction rate and product stability compared with oxime or hydrazone linkages.
Although direct conjugation of unprotected peptides is a convenient way to prepare dendrimer conjugates, it is difficult to achieve region-selective modification when the peptides contain side groups that may interfere with the conjugation reaction. Using protected peptides could avoid this problem, and peptides can be selectively ligated via the C- or N-terminus. This strategy would be particularly useful for conjugating long or complex peptide ligands. As shown by Ozawa et al. [25], a glycopeptide derived from an extracellular matrix metalloproteinase inducer, synthesized in a partially protected form with the side-chain, and the N-terminus protected by tert-butoxycarbonyl groups was coupled to G1 PAMAM through the C-terminus via a thioester coupling reaction catalysed by silver ions.
2.2. Synthesis of saccharide–dendrimer conjugates
Owing to the important applications of glycodendrimers in the development of pharmaceutical reagents, the chemistry of conjugating saccharides to dendrimer scaffolds has been intensively studied in the past and comprehensive reviews on the synthetic aspect of saccharide–dendrimer conjugates are available in the literature [6,10,16,17]. Here, we briefly summarize the current methods for preparing saccharide-modified PAMAM conjugates. As shown in table 1, PAMAM dendrimers decorated with different types of saccharide ligands were obtained in various studies. Typically, the synthesis involves preparation of a dendrimeric scaffold with surface functional groups and conjugation of glycans pre-derived with complementary reactive groups. Saccharides that are derived from isothiocyanate and carboxylic acid groups can directly react with the surface amine groups of PAMAM, resulting in thiourea and amide linkages, respectively, offering the convenience of requiring no chemical modification of native PAMAM scaffolds.
Table 1.
Different coupling methods to graft saccharide ligands to PAMAM dendrimers.
| type of dendrimer surface groups and conjugation linkages | saccharide ligands | generation of PAMAM (percentage modification level) | reference | |
|---|---|---|---|---|
| gluco-, manno-, galacto-, cellobio-, lacto-configurated glycosyl isothiocyanate | G0 and G1 (−)a | [26] | ||
| P-isothiocyanatophenyl α-d-mannopyranoside |  |
G0–G3 (−) | [27] | |
| P-isothiocyanatophenyl β-d-lactoside |  |
G0–G5 (−) | [28] | |
| phenylisothiocyanate derivatized Galβ1-3GalNacβ1-4(Neu5Acα(2-3) Galβ(1-4)Glcβ1-1Cer (oligo-GM1)  R: galβ1-3galNACβ1-4-(sialic acid α2-3)galβ1-4 portion acid |
G2 (−) | [29] | ||
 linkage: thiourea linkage: thiourea |
P-isothiocyanatophenyl α-sialoside |  |
G0–G3 (−) | [30] |
| isothiocyanate-derivatized sialate |  |
G4 (approx. 100%) | [31] | |
| 4-isothiocyanatophenyl α-d-mannopyranoside |  |
G4–G6 (83–87%) | [32] | |
| isothiocyanate-derivatized dimannose |  |
G3 (81%) and G4 (75%) | [33] | |
 linkage: secondary amine (through reductive amination) linkage: secondary amine (through reductive amination) |
P-formylphenyl α-sialoside |  |
G1–G5 (−) | [34,35] |
 linkage: amide linkage: amide |
O-β-d-galactopyranosyl-(l-4)-d-glucono-l,5-lactone |  |
G2–G4 (approx. 100%) | [36] |
| O-(tetra-O-acetyl-β-d-glucopyranosyl)-l-serine N-carboxyanhydride O-(2-aeetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-giucopyranosyl)-l-serine N-carboxyanhydride |
 X=OAc or NHAc X=OAc or NHAc |
G3 (105 s.r/d)b and G5 (389 s.r./d) | [37] | |
| T-Ag-acid (βGal-(1-3)-αGalNAc) |  |
G0–G3 (−) | [38] | |
| extended acid derivative of 3-aminopropyl α-d-mannopyranoside |  |
G1 and G2 (−) | [39] | |
 linkage: amide linkage: amide |
amine-tethered Man-6-P derivative |  |
G0.5 and G1.5 (100%), G2.5 (94–100%), G3.5 (88–91%) | [39] |
amine-functionalized heparin oligosaccharides
|
G2.5 (25%) | [40] | ||
 linkage: halogenoacetamide linkage: halogenoacetamide |
thiolated carbohydrate derived sialoside |  |
G0 and G2 (25–26%) | [41–43] |
 linkage: 2,3-triazol (through click chemistry) linkage: 2,3-triazol (through click chemistry) |
5′-N-methyluronamide (N)-methanocarba 2-alkynyl triazoles |  |
G4 (100%) | [44] |
 linkage: hydrazone linkage: hydrazone |
glucose, mannose, galactose, GlcNAc, lactose | G1–G5 (varied modification level between 45% and 99%) | [45,46] | |
aSpecific saccharide modification level not reported.
bNumber of saccharide residues per dendrimer.
As is noted, the derivation of saccharide ligands can be laborious and time-consuming as it necessitates protection/deprotection of hydroxyl groups in the sugar ring. The inconvenience and technical difficulty may limit the accessibility of glycodendrimers to laboratories without the expertise and experience in glycochemistry. Functional derivation of saccharide molecules may also be impractical when large numbers of different saccharide–dendrimers need to be prepared such as in high-throughput studies [47–49]. To overcome this issue, we recently reported a general approach to synthesize saccharide-modified dendrimers via direct conjugation of underivatized reducing saccharides to hydrazide-functionalized dendrimers [45]. This new approach takes advantage of the native reducing ends of glycans to form N-glycoside with hydrazide groups and leads to glycodendrimers with average modification ranging from 45 per cent to 99 per cent on G1–G5 PAMAM scaffolds. Despite the convenience, problems related to better controlling the modification level and improving the purification of the conjugate products remain to be fully addressed for this method.
3. Cell- and tissue-targeting properties of ligand-conjugated bioactive dendrimers
Multivalent interactions underlie numerous biological and pathological events, and play an important role in cellular recognition, trafficking, signalling and infection [50–52]. For example, the monomeric binding between glycan epitopes and a lectin receptor is usually weak at the molecular scale—with the disassociation constant (Kd) falling in the range of 10−3 to 10−6 M; instead, glycobiological processes involving multivalent ligand–receptor interactions show Kd at the order of 10−9 M or below [10,53–55]. In contrast to saccharide ligands, less-pronounced affinity enhancement in terms of magnitude has been observed in studies involving multivalent peptide conjugates [56,57].
When presenting bioactive ligands using dendrimeric scaffolds, the specific multivalent effects on the avidity of ligand–receptor interactions are determined by a number of factors that are associated with structural and spatial characteristics, which are generated through the ligand-conjugation process and the inherent structures of dendrimer scaffolds. These factors—often intrinsically correlated—typically include ligand valency, density and topology. Although numerous mechanistic studies on the receptor-binding behaviour of dendrimer conjugates have been carried out at the molecular level, investigation at the cell, tissue and systemic levels would provide more relevant information regarding the targeting and delivery capabilities of the dendrimer conjugates. The following sections discuss in vitro and in vivo fundamental work that elucidates basic cell- and tissue-binding properties of peptide- and saccharide–dendrimer conjugates.
3.1. Cell-binding properties of bioactive dendrimer conjugates in vitro
To profile the cell- and tissue-binding properties of dendrimer conjugates, imaging agents such as fluorophore molecules are often co-immoblized with bioactive ligands to the surface of dendrimer scaffolds. Notably, upon binding to cells, internalization of dendrimer materials is often observed and imaged in many studies. One of the most intensively studied ligand is RGD, a peptide sequence that can effectively bind integrin receptors and induce adhesion responses [58,59]. In drug delivery studies, cyclized RGD peptides were found to bind with high affinity to cells expressing the αvβ3 integrins and are useful ligands to target tumour vasculature [60,61]. By decorating the surface of G5 PAMAM dendrimers with a double cyclized RGD peptide and a fluorophore, a series of in vitro studies by Baker group demonstrated the effectiveness of RGD–PAMAM to interact with both normal and tumour cells, which include human dermal microvessel endothelial cells, human umbilical vein endothelial cells (HUVEC), odontoblast-like MDPC-23 cells and human glioblastoma cells (U87-MG) cells [18–20]. In these studies, quantitative evaluation indicated increased amounts of cell-bound dendrimers in response to increased conjugate dosage in cell culture, with no obvious saturation levels observed. The dendrimer conjugates also showed preferential binding to different types of cells. In one study [18], dendrimer RGD–PAMAM conjugates were observed to bind with high efficiency to HUVECs in vitro and confirmed previous findings that cyclic RGD peptide binds JURKAT T lymphocyte cells [18–20]; dendrimer RGD–PAMAM conjugates bound to JURKAT cells at about 10 per cent less efficiency than to HUVECs. In contrast, the modified dendrimers showed only moderate binding to KB cells (about 20% binding efficiency compared with HUVECs) and virtually no binding to L1210 mouse lymphocyte cells (about 2% compared with HUVECs). The authors postulated that the variable uptake of the dendrimer was based on integrin receptor expression levels of different cell types, though receptor expression levels in the cell lines were not quantified in the study.
The internalization of RGD–PAMAM conjugates was also observed and found to be time-dependent. For example, the cytoplasmic distribution of RGD–PAMAM conjugates in a punctate pattern was visible only 6 h after the material was incubated with MDPC-23 cells [19]. It is noted that in most studies on conjugated dendrimers, potentially erroneous conclusions may have been drawn regarding vehicle uptake; evaluation methods failed to distinguish between dendrimers that merely bound to the cell membrane and those that actually cross the membrane to reach the cytoplasm. One major mechanism for internalization is likely through receptor-mediated endocytosis. For drug delivery applications, understanding the fundamental mechanism of cellular uptake of dendrimers and correlating the process with drug delivery efficacy would be essential for improving the design of delivery systems. This topic is further discussed in §3.2.
To understand the influence of RGD valency on the binding behaviour of RGD–PAMAM conjugates, Waite et al. conjugated cyclic RGD peptides to G5 PAMAM [62]. Dendrimers with two, three, seven and 10 copies of RGD ligands were obtained to study how these conjugates interact with U87-MG glioblastoma cells. It was demonstrated by surface plasmon resonance experiments and competitive cell adhesion assays that dendrimers with two or three copies of RGD ligands may actually have higher affinity towards the integrin receptors and cells compared with those with higher valencies. The authors proposed that the high density of ligand presentation and possibly steric hindrance due to the high valency might actually impede the effective binding of RGD with integrins. In a spheroid three-dimensional tumour model, however, the conjugate with a higher density such as 10-mer RGD–PAMAM showed higher rates of internalization by tumour cells. When kinetic data were fit into a transport mathematical model, dendrimers with higher RGD density support more homogeneous distribution in the tumour spheroids. In another in vitro study, anti-epithelial cell adhesion molecule (aEpCAM) was linked to G7 PAMAM scaffolds, and the multivalent conjugates exhibited significantly higher affinity with EpCAM and the immobilized conjugates showed the ability to capture various tumour cells [63]. However, at the cellular level, the correlation of the ligand modification level on PAMAM scaffolds with the cell-binding strength was not reported in this study. Besides showing higher affinity and uptake, clustering of ligands on branched scaffolds may enhance the selectivity of the multivalent conjugates in binding with receptors. For instance, in one study conducted by Jin et al., a linear RGD-dendrimer conjugated with mono- and tri-valency contained in the sequence AVTGRGDSY was tested on transgenic human embryonic kidney (HEK) 293 and U87-MG cells showing different expression patterns of integrin subtypes [64]. In contrast to the monomeric ligand that showed no differential binding with cells expressing β1 and β3 integrins, di-meric and tri-meric peptide ligands exhibited a specific high affinity for αvβ3 integrin positive cells. This study was conducted using only branched conjugates with low valencies; no similar in vitro studies have been reported for the dendrimer conjugates with hyperbranched structures.
Saccharide ligands have also decorated surface dendrimer scaffolds to develop cell-targeting materials. Hepatic cells express asialoglycoprotein receptors that bind galactose (Gal), lactose and N-acetylgalactosamine (GalNAc), which provides attractive opportunities for targeted delivery systems. Indeed, Gal, lactose and GalNAc have been exploited as ligands in delivery systems in the treatment of hepatic diseases [45]. Through hydrazone linkages, our laboratory created a small library of glycosylated dendrimers using G5 PAMAM and studied how these conjugates interact with HepG2 cells [45,46]. The results showed that Gal-, lactose- and N-acetylglucosamine (GlcNAc)-modified PAMAM had significantly higher avidity towards HepG2 cells compared with other saccharide-modified dendrimers in the library. When lactose–PAMAM conjugates with different valencies were compared, the lactose–PAMAM dendrimer with an intermediate level of lactose modification seemed to be able to attain optimal binding with cells.
In a recent study, Medina et al. coupled GalNAc, via a peptide and thiourea linkages, to a G5 PAMAM dendrimer with a diaminobutane (DAB) core [65] that had been shown to preferentially accumulate in the liver and extravasate through the leaky tumour vasculature [66,67]. The greater internal hydrophobicity of the G4 PAMAM dendrimers with DAB cores drastically increased liver accumulation compared with diaminoethane-core G4 PAMAM dendrimers (that preferentially accumulated in the kidney). Interestingly, the smaller G2 DAB-core PAMAM dendrimers exhibited accumulation in the kidney. Through in vitro studies of HepG2 and MCF-7 cells, Medina et al. found GalNAc-modified dendrimers allowed selective uptake by hepatic cells, and they showed superior properties with respect to the percentage of dendrimer-transfected cells and the rate of internalization compared with a linear HPMA polymer grafted with GalNAc ligands. When the valency of GalNAc was varied on G5 PAMAM, similar uptake into HepG2 cells at all concentrations and incubation times were observed, and a 10 per cent modification level on G5 PAMAM was found enough to enable optimal cellular uptake. In this study, the linkage between saccharides and dendrimer scaffolds seemed to affect the uptake of dendrimers kinetically, and a longer spacer via the thiourea linkage might support a higher rate of dendrimer internalization by HepG2 cells.
Macrophages exhibit a mannosyl/fucosyl receptor; so to develop a macrophage-targeting dendrimer, Fernández et al. conjugated mannose and β-cyclodextrin to dendrimers, followed by inclusion of a fluorophore within the cyclodextrin [68]. To synthesize the dendrimer, reactivity between isothiocyanate and amine functionalities was exploited to form dendrimeric structures with thioureido links. The group explored the multivalent effect of vehicle activity by varying the level of decoration from one to six mannose molecules and found an amplification of lectin-binding strength (22-fold greater for hexavalent versus monovalent vehicle). In measuring the macrophage-binding properties of this vehicle, the group treated the cells at 4°C to inhibit non-specific phagocytosis and found a 20-fold preferential association of the mannosylated versus non-mannosylated vehicle.
3.2. Cell- and tissue-targeting of bioactive dendrimer conjugates in vivo
In addition to in vitro studies elucidating the kinetic and dynamic profile of dendrimer conjugates in interacting with cells, evaluation of these bioactive materials in vivo would shed light on their pharmacological properties that are valuable for developing therapeutics and drug delivery systems. Indeed, discrepancies have often been observed between in vitro and in vivo studies regarding cell-binding behaviour of conjugated dendrimers in vivo.
Liskamp's group conjugated 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid to a dendrimer featuring multivalent cyclic RGD to develop a vehicle that could target and image tumours [56]. Following complexation of targeted vehicle with 111In and in vitro validation of binding to αvβ3 integrin, the group injected the radiolabelled dendrimer into SK-RC-52 tumour-bearing mice and found enhanced uptake of the RGD-targeted vehicle in tumours. Their analysis on the effect of multivalency indicated that tetrameric RGD-dendrimer exhibited better tumour-targeting than dendrimers decorated with dimeric or monomeric RGD peptides. However, in other studies, dendrimers conjugated to Gd(III)-chelating agents and cyclized RGD demonstrated effective binding to melanoma cells only in vitro, whereas in vivo tumour uptake was negligible [69].
Phage display technology has become a popular tool for screening ligands that can target cells and tissues with high affinity and selectivity. After a peptide ligand is screened from in vivo phage display, multivalent scaffolds are often used to design conjugates that either enhance the binding property and/or enable extra loading of imaging or pharmaceutical compounds for diagnostic and therapeutic purposes [70–72]. Recent studies have shown that dendrimers can provide a well-defined, highly versatile platform for multimeric presentation of ligands [73,74]. Liu et al. [75] performed in vivo phage display to identify peptide sequences that targeted lung cancer xenografts in a mouse model. After conjugating G4 PAMAM dendrimers with FITC and the phage display-identified targeting sequence RCPLSHSLICY, the group found that the peptide-conjugated dendrimers enriched three times more in NCI-460 than 293T cells in a time- and dose-dependent manner. In the biodistribution assay, via intravenous injection, it was found that while the targeting and non-targeting vehicles were both found in other organs, animals treated with PAMAM conjugates with targeting peptide ligands exhibited 30 per cent greater fluorescence intensity in the lung cancer xenograft than non-targeting materials.
Meijer's group conducted a side-by-side study of two dendrimer-ligated tumour-homing peptides tethered to a pentavalent polyamide dendrimeric scaffold [74]. One peptide they investigated, CREKA, had previously been shown by in vivo phage display to home to the blood vessels and stroma of tumours [76]. Additionally, CREKA had been used to image atherosclerotic plaques ex vivo and in vivo [77]. The other homing peptide investigated was a cyclic nonapeptide, LyP-1 (CGNKRTRGC), that recognizes tumour lymphatic cells. By tethering these peptides and fluorescein to a pentavalent dendrimeric scaffold, the ability of these peptides to target tumours was elucidated: they found that CREKA-functionalized dendrimers localized to the vasculature of a prostate cancer xenograft vasculature, whereas LyP-1-modified dendrimers extravasated out of the blood vessels and into areas of the tumour that are difficult to reach via passive diffusion from blood.
4. Applications of peptide- and saccharide-conjugated dendrimers for drug delivery
4.1. General applications of dendrimer materials in drug delivery
Dendrimers provide a unique category of scaffolds for biological applications because of the well-defined size, multivalency and chemical composition. In fact, all of these parameters are determined during the chemical synthesis of the dendrimer. Some of the structural characteristics—and the associated homogeneity—are definable to such a degree that would be difficult to achieve with multivalent polymers and nanoparticles. For example, with peptide or saccharide decoration, the diameter of a conjugated dendrimer is theoretically in the order of a few to tens of nanometres if the intermolecular aggregation was prevented. This size—on the scale of a protein—may be advantageous for extravasation and binding properties during systemic delivery. An exact number of ligands of definable density can be conveniently achieved through conjugation, and allows for systematic profiling and screening for optimal cell-binding properties for drug delivery. In addition, the conformational stability of the nano-geometry offered by dendrimer scaffolds may be superior to some of the nanoparticles (e.g. linear polymers and liposomes) that may deform or dissociate during the transport process in vivo.
Dendrimeric conjugates containing multiples copies of saccharides or peptides may themselves exhibit therapeutic benefits and be developed as anti-prion, anti-microbial and anti-viral agents. Positively charged dendrimers, owing to either native PAMAM terminal amines or guanidine-modification, have shown the ability to prevent prion folding and even induce prion unfolding with decreased cytotoxicity due to dendrimer glycosylation [78,79]. For anti-microbial application, dendrimers modified with largely positive peptides show strong toxicity and selectivity for bacteria [80]. Glycodendrimers have demonstrated anti-viral properties by presenting saccharides to either directly bind to viruses or to saturate cell surface receptors [81–85]; both methods inhibit virus–cell interaction.
For drug delivery purposes, dendrimers may serve at least two functions in the drug delivery process: to carry drug molecules and transport them to the diseased tissue; and to present bioactive ligands to enable targeted cellular interactions. This allows the cargo to overcome biological barriers encountered in drug transport in vivo.
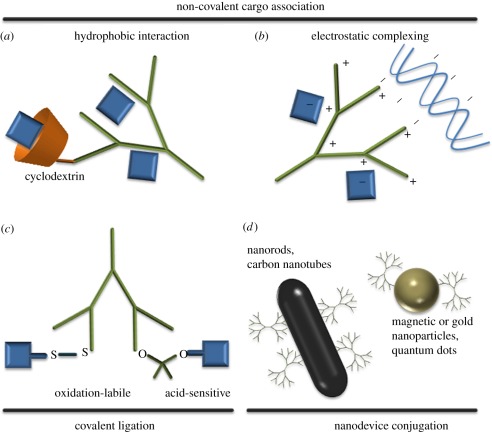
As illustrated in figure 2a–c, there are, in general, three methods to load the dendrimer scaffolds with therapeutics. First, therapeutic compounds may associate with the interior branches of dendrimers through hydrophobic interactions. The drug–dendrimer association can also be enhanced when cyclodextrin is introduced to enable host–guest interactions through inclusion of the drug molecule into the pit of the cyclic oligosaccharides. This method has provided for the solubilization of a broad range of hydrophobic small molecules involved in anti-cancer, -depressant, -inflammatory and -microbial applications [14,86]. The second association is through electrostatic interaction; charged therapeutics can directly form complexes with dendrimers containing counter-charged groups. This method has been widely studied for nucleic acid-based therapeutics and other negatively charged therapeutic compounds [87,88]. Release of these non-covalently bound drugs may be driven by concentration gradients or by pH-triggered conformational change of the dendrimer [89]; as pH changes from high or neutral to a low pH (such as in the endosome), PAMAM dendrimers undergo a conformational change from a ‘dense core’ to a ‘dense shell’. The third approach is through covalent bonding, which is convenient when the therapeutic contains functional groups that are readily ligated. This can be beneficial when drugs cannot associate with dendrimers through physical interactions and/or stable association of drugs is desired to prevent drugs from leaching out of dendrimer to cause side effects [90,91]. In the case where a therapeutic agent should be free from the vehicle to be fully active, consideration should be given to how the agent will be released, such as by conjugation via acid-labile linkage that can be cleaved in the mature endosome.
Figure 2.
Different types of dendrimer–therapeutic cargo associations. (a) Hydrophobic interactions between drug molecules and dendrimer backbone or constituent. (b) Ionic binding. (c) Covalent ligation, including degradable linkages. (d) Dendrimers can also be used to functionalize and/or enhance the solubility and biocompatibility of other nanodevices.
In other applications, dendrimers can be used to modify other nanodevices, such as carbon nanotubes [92–95], gold nanoparticles [84,96–100] and nanorods [101], magnetic particles [102,103] and quantum dots [104–106] (figure 2d). Modification by bioactive dendrimer conjugates can particularly serve to improve biocompatibility, enhance solubility and provide a mechanism for tissue- or cell-specific targeting. We focus our discussion on studies primarily relying on peptide- and carbohydrate-conjugated dendrimers to carry and deliver drugs, as shown in figure 2a–c.
4.2. Delivery via peptide-conjugated dendrimers
When dendrimer conjugates are designed to serve multiple functions, drug molecules and ligands may both need to associate with the dendrimer scaffold through its surface functional groups. This entails special considerations in regard to the spatial arrangement of drug molecules and ligands in the dendrimeric system.
In linking the chemotherapeutic drug methotrexate (MTX) to the G3 PAMAM–RGD conjugates, a dendron body contains two types of functional groups: an alkyne and 16 carboxylic acids groups. The focal-point alkyne allowed for regio-specific binding with MTX-azide through ‘click chemistry’ and the branched carboxylic acids presented c(RGDyK) peptide in multivalent fashion [20]. When tested on HUVECs, MTX linked to RGD–PAMAM dendrons showed similar levels of cytotoxicity compared to the free MTX drug in vitro.
Dendrimers conjugated with targeting peptides have also been used for gene delivery. In the study by Pandita et al., G5 and G6 PAMAMs were partially modified with RGD ligands with varied densities (i.e. four, eight and 16 RGDs per dendrimer), and the resulting conjugates were used to complex with plasmid DNA for gene delivery into bone marrow-derived mesenchymal stem cells [107]. Ligand conjugation seemed to improve the cellular uptake of nucleic acids and upregulate the expression of the target genes, with the optimal delivery efficiency observed for the conjugates with a median modification level. In another study, Roth's group also demonstrated in vitro transfection efficiency of RGD-decorated PAMAM using a three-dimensional tumour model constructed from multicellular spheroids [21]. In this study, partially decorated G5 PAMAM dendrimers were electrostatically complexed with a cy3-labelled anti-GFP siRNA. Through a confocal analysis, the siRNA loaded in RGD–PAMAM dendrimers was found to penetrate into the core of the tumour spheroids more effectively compared with the siRNA carried by undecorated dendrimers. This result was likely due to the dynamic interaction among RGD–PAMAM, cells and ECM in the spheroid model. However, it was observed that the ligand decoration on the delivery vehicle did not improve the silencing effects. Taking into consideration the aforementioned studies, it is noted that although ligand-conjugated dendrimers can lead to enhanced cell binding, whether they can really increase the intracellular drug delivery efficiency may need to be further investigated. An improved imaging method or strategy to accurately locate delivery material and drug inside and outside cells may help us understand whether this is due to deficient cell internalization or inefficient intracellular release of the cargo, which could lead to strategies to improve the design of delivery systems-based bioactive dendrimers.
Despite the question on delivery efficacy in vitro, the peptide ligand on the dendrimer can be beneficial in transporting therapeutics to targeted tissue in vivo. One peptide derivative with potential for targeting the brain is Angiopep-2, a 19-amino acid peptide derived from the Kunitz domain of aprotinin, which accumulates in the parenchyma, binds to lipoprotein receptor-related protein 1 and exhibits a high propensity to transcytose across the blood–brain barrier [108]. Jiang's group conjugated Angiopep-2 to the terminal groups of a PEGylated G5 PAMAM and determined that the vehicle was internalized by brain capillary endothelial cells through clathrin- and caveolae-mediated endocytosis and, to a small extent, macropinocytosis [109]. The group found that in a mouse model brain uptake of targeting vehicles loaded with fluorescent-labelled DNA was up to eightfold greater than non-targeting vehicle. In a later study, the group found the biodistribution of the targeting vehicle in the brain—especially within the tumour—was greater than controls and demonstrated the ability of Angiopep-2-modified dendrimer to target glioma [110]. Furthermore, they loaded the dendrimers with TNF-related apoptosis-inducing factor and, in a glial xenograft mouse model, found greater apoptosis in the tumours owing to targeting dendrimer treatment groups than controls, including commercial temozolomide. The results that the targeted delivery vehicle can perform better than a clinical standard are promising for future applications in treating brain tumours.
To design targeted systems to gene delivery, dendronized structures may also be used to enable the loading of DNA or RNA therapeutics. Whereas most bioactive dendrimer conjugates rely on surface functionalization of dendrimers, Hammond's group ligated a clinically relevant anti-tumour peptide (WIFPWIQL) to a PAMAM-based dendron for gene therapy [111]. This was accomplished by reducing the disulphide bond of a G4 cystamine-core PAMAM dendrimer, followed by conjugation to the peptide through a heterobifunctional linker. This vehicle showed a propensity to condense plasmid DNA into small structures (greater than 210 nm) and transfected prostate carcinoma cells in vitro to express luciferase. The group demonstrated up to fivefold greater transfection with this asymmetrical vehicle than control groups, and even greater transfection than PEI, a transfection standard. Preliminary in vivo studies successfully targeted EFG43-FGF4 tumours in a mouse model. As only one copy of the targeting ligand was incorporated into the dendron scaffold in this material, it may be worthwhile exploring how dendrons carrying multivalent ligands could be combined with unmodified cationic dendrons to improve the structural design and transfection efficiency of the gene delivery system.
Besides targeting ligands, cell-penetrating peptides have been widely studied for increasing the cross-membrane capability of various deliver systems [112–115]. As one of the major cell-penetrating peptides, Tat is derived from the HIV virus and can cross the cell membrane of many cell types, potentially due to its net cationic charge [116–118]. Dendrimers conjugated with Tat, however, have seen varied success as transfection vectors. In an early paper by Juliano's group, G5 PAMAM dendrimers conjugated to Tat (15.9 peptides per dendrimer) showed no improvement in the delivery efficiency of siRNA oligonucleotides against the transmembrane protein p-glycoprotein MDR1 in 3T3 cells [119]. In a more recent paper, however, Chang and co-workers [120] found that bacterial magnetic nanoparticles (BMPs) decorated with Tat-functionalized PAMAM dendrimers exhibited high transfection levels of anti-epidermal growth factor receptor (EGFR) psiRNA; in fact, they demonstrated that the targeted dendrimer performed as well as the Lipofectamine 2000 treatment group. In vitro, EGFR knockdown in U251 glioma cells by the targeting dendrimer was slightly better than lipofectamine, whereas apoptosis was similar between the treatment groups. Furthermore, in vitro cell invasion was inhibited to a greater degree by the targeted delivery vehicle than lipofectamine (66% versus 62%). In a U251 subcutaneous tumour mouse model, the group described significant apoptosis, downregulation of EGFR expression and tumour suppression by the targeted vehicle. The difference in delivery efficiency observed from TAT attached dendrimers can partially attribute to the density and distribution of TAT peptide on delivery vehicles; as in the previous study, there are nearly 16 TAT on each PAMAM molecule while in each Tat–BMPs–PAMAM particle the peptide number reaches 3190.
4.3. Delivery via saccharide-conjugated dendrimers
As many glycan moieties are hydrophilic and neutral, glycosylated dendrimers often show good water solubility and biocompatibility and have been explored for use in drug delivery systems. Colchicine is a current standard treatment to treat gout, but has potential as an anti-cancer agent owing to its inhibition of mitosis. In an attempt to develop a targeted vehicle for colchicine to cancer cells, Reymond's group conjugated the drug to glycopeptide dendrimers via a thioether bond and screened a small collection of glycopeptide dendrimers with varied glycan ligands (glucose, Gal, GalNAc and lactose) and peptide building blocks (based on amino acids including serine, threonine, histidine, aspartic acid, glutamic acid, leucine, valine and phenylalanine) [121,122]. In in vitro cytotoxicity tests on HeLa cells, all colchicine-loaded glycodendrimeric vehicles were found able to cause extensive cell death. Conversely, non-transformed mouse embryonic fibroblasts experienced extensive cell death only from treatment with highly GalNAc-decorated vehicles, and not other glycodendrimer treatments. These findings suggest that several saccharides may serve as effective ligands for selective anti-tumour dendrimeric delivery vehicles while not targeting other cell types; glucose-conjugated dendrimers demonstrated a high selectivity for HeLa cells by inducing cell death in 163-fold more HeLa cells than fibroblasts. It should be noted that whereas many dendrimeric vehicles are based on branched polymers, the structure of Reymond's dendrimers consisted solely of amino acids. Their reasoning was based on the aspect that unless the vehicle is cleared from the body, dendrimeric drug carriers should undergo intracellular biodegradation after delivery, and that a dendrimeric structure consisting of peptides could exploit endogenous proteases to enable biodegradation.
One group used Gal-conjugated dendrimers as a delivery vehicle for anti-malarial therapy [90,91]. Primaquine is a common anti-malarial medication, but in regular formulation can exhibit severe side effects, such as haemolysis. To target the drug to the site of metabolism in the liver and shield it from inducing side effects in side cell populations, the researchers conjugated Gal to poly (propylene)imine (PPI) dendrimers and complexed primaquine within the carrier via the hydrophobic effect. Two hours after intravenous injection of the loaded vehicle, 50 per cent of the initial dose was found in the liver, compared with 26 per cent of the non-targeted vehicle, demonstrating the targeting ability of Gal. Besides targeting to the liver, coating of the dendrimer with Gal had three beneficial effects: prolonged drug release up to 6 days, compared with 2 days for uncoated dendrimer; 15-fold greater entrapment of primaquine; and drastically reduced haemolysis owing to surface neutralization by glycosylation. In a later paper, the group loaded a related anti-malarial drug, chloroquine, into Gal-decorated poly-l-lysine dendrimers and found fivefold decreased uptake of the vehicle by macrophages compared with uncoated dendrimers [91]. This characteristic would allow for shielding of the delivery system from macrophages while targeting the liver. Similar to previous findings, coating of the lysine dendrimers with Gal reduced haemolytic toxicity and immunogenicity of the conjugates while extending the release of the anti-malarial drug.
Uekama's and co-workers [123–125] demonstrated gene transfer via mannosylated dendrimers in other cell types. Similar to Fernández's group, they confirmed uptake by and transfection of macrophages, but they also found that mannosylated G3 PAMAM dendrimers, complexed with luciferase pDNA, provided for high gene transfer activity in NR8383, NIH3T3, HepG2 and especially A549 cells. These findings illustrate how the same ligand can target multiple cell types; so development of delivery vehicles should include specificity evaluation, especially when the cargo drug can induce negative effects in unintended cell types.
Saccharide ligand-conjugated dendrimers can also be used to develop targeted vehicle for RNAi therapy. Recently, our groups introduced a new paradigm for designing siRNA delivery systems based on neutral conjugated dendrimers [46]. By replacing the terminal amines with hydrazide groups and GalNAc ligands according a method described above [45], cationic PAMAM dendrimers were transformed into neutral glycosylated carriers. Anti-luciferase siRNA was complexed to these protonatable dendrimers at pH 5 via electrostatic interaction [46]. The hydrazides on the PAMAM periphery were then cross-linked with homobifunctional glutaraldehyde to retain encapsulated siRNA following pH neutralization. Both intra- and inter-particle cross-linking may happen during this process, while the inter-particle cross-linking can be reduced to a minimal degree with the appropriate glutaraldehyde concentration and GlcNAc modification. This loaded GalNAc-conjugated dendrimer successfully induced RNAi in luciferase-expressing HepG2 cells in vitro. This demonstrated the potential for charge-neutral cross-linked dendrimeric vehicles in drug delivery applications, which could overcome the cytotoxic effects owing to dendrimer cationic charge and improve the vehicle stability through chemical cross-linking.
5. Toxicity of bioactive dendrimer conjugates
Safety is one of the crucial concerns for developing drug delivery systems based on dendrimer materials [126]. Although dendrimers are in general considered biocompatible, how to ensure the safe use of dendrimer materials may be case-specific and vary with the specific composition and structure of each type of material. In vitro assays have indicated that dendrimers may exhibit haemolytic toxicity, cytotoxicity and haematological toxicity in a time-, concentration- and cell-type-dependent manner [127–130]. The peripheral groups on dendrimers seem to be an important factor in determining the degree of toxicity. Studies have shown that many dendrimers with cationic groups at the periphery (e.g. PAMAM, PPI, DAB and dendrimeric poly-l-lysine) are much more toxic than those with anionic or neutral groups in haemolysis and cytotoxicity assays [128,131,132], while dendrimers with neutral hydrophilic peripheral groups may show better biocompatibility compared with dendrimers decorated with hydrophobic moieties [128]. It was speculated that the positive charges—which result from the presence of cationic amine groups on dendrimers—could interact with the cell membrane through electrostatic interactions to cause membrane destabilization [133], apoptosis pathway activation [134] and medium depletion [135]. Consequently, substitution of peripheral amines, at least partially, of these cationic dendrimers may reduce potential toxicity and improve their biocompatibility. It was shown that acetylation [133,136–138] or PEGylation [133,139,140] of terminal amines significantly decreased the in vitro and in vivo cytotoxicity of PAMAM and PPI. In addition to the peripheral chemistry, the interior scaffold of a dendrimer also affects its toxicity. For example, with the same amount of surface amines, DAB dendrimer displayed slightly higher toxicity than PAMAM [128]. Cationic dendrimers, especially PAMAM, often display generation-dependent toxicity. For instance, the concentrations of dendrimer to induce 90 per cent cell death of V79 Chinese hamster lung fibroblasts after 24 h were 1 mM, 10 μM and 100 nM for G3, G5 and G7 PAMAMs, respectively [127]. The positive correlation of toxicity with dendrimer generation is likely due to the increasing number and density of surface cationic charges.
The toxicity levels of ligand-decorated dendrimers may vary with the structural characteristics of the peptide and saccharide ligands. By modifying G5 PPI with neutral amino acids (phenylalanine and glycine), the haemolytic activity and cytotoxicity of amino acid-dendrimer conjugates were significantly lower compared with PPI [129]. In contrast, PAMAM decorated with the cationic amino acid arginine showed increased cytotoxicity: twice as much cell death resulted from decorated dendrimers than from G4 PAMAM dendrimers when tested on HepG2 and HEK 293 cells in the concentration range between 0.01 to 0.36 mg ml−1 [141]. In Yang and Kao's study, although the conjugation of RGD peptide on G4 PAMAM increased the viability of fibroblasts upon dendrimer treatment, RGD-G4 PAMAM still caused more than 50 per cent mortality after 96 h treatment of cells at 77 μM, showing time- and concentration-dependent toxicity [51].
Many glycans are neutral and hydrophilic, and glycosylated dendrimers often exhibit enhanced biocompatibility compared with native cationic dendrimers. This has been demonstrated in studies on many types of glycosylated dendrimers, including PAMAM, PPI and polylysine [45,90,91,129,142]. For example, compared with the nearly 40 per cent mortality rate of HepG2 cells upon treatment of 70 μM G5 PAMAM, glycodendrimers with glucose, mannose, GlcNAc, lactose and Gal derivation on the surface exhibited no significant difference in cell viability to the non-treated cell group [45]. In a study by Agashe et al. [129] on saccharide-modified PPI in the range 0.001–1 mg ml−1, uncoated G5 PPI led to 86 per cent haemolysis after 4 h incubation and around 98 per cent mortality in HepG2 and Cos-7 cells, but lactose- and mannose-derived PPI showed less than 3 per cent haemolysis and above 95 per cent cell viability. It is noted that in these experiments, saccharide-conjugated dendrimers could exhibit slight toxicity, presumably owing to unmodified cationic residues on the dendrimer surface. Owing to their biocompatibility and hydrophilicity, glycodendrimers have also been used to mitigate the toxicity and increase solubility of other delivery devices, such as single-walled carbon nanotubes and boron nitride nanotubes [94,143].
Compared with in vitro studies, fewer investigations have been conducted at the systemic level to evaluate the toxicity of dendrimer-based materials. In one study by Heiden et al., treatment of 1 μM unmodified G4 PAMAM lead to 95 per cent mortality in zebrafish embryos 120 h post-fertilization, whereas RGD-decorated G4 dendrimers showed only 5 per cent mortality at a concentration of 2 μM [144]. However, decoration with RGD did not completely eliminate toxicity, as 20 μM RGD–PAMAM caused 99 per cent mortality. Additionally, sub-lethal concentrations of G4 PAMAM induced interference in developmental progression (including reduced body growth, bent trunk and smaller head and eyes), but the anionic intermediate G3.5 neither affected nor arrested development. Such results illustrate that the toxicity of peptide–dendrimer conjugates likely lies on the surface properties of the dendrimer. One thing that should be noted is that, unlike in exhaustive toxicity studies, many application-based studies apply the dendrimer conjugates at relatively low concentrations, such as several hundreds of nanomolar, at which toxicity has not been exhibited to a large degree. Therefore, safety risks still exist for dendrimer materials. More studies need to be carried out to understand how to ensure safe use of dendrimers and dendrimer conjugates for in vivo applications.
Immunogenicity is another important aspect of biocompatibility. Roberts et al. [127] studied the immunogenicity of PAMAM dendrimers (G3, G5 and G7) by immunoprecipitation and Ouchterlony double-diffusion assay, and no signs of immunogenicity were observed at a dose range of 0.1 nM to 0.1 μM. As studied by Agashe et al. [129] dendrimers or conjugated dendrimers were unable to elicit any detectable humoral immune response under their experimental conditions in Balb/C mice. However, the immune response to conjugated dendrimers could be dependent on the specific type of ligands, and saccharides could especially impose the risk of immunogenicity. As shown by Ortega-Munoz et al. [145], multivalent structures including glycopolymers, glycocyclodextrin and glycocluster can activate cell adhesion and stimulate monocytes and macrophages.
6. Concluding remarks
Amid the bulk of biomedical research in applications for dendrimers, peptide- and saccharide-conjugated dendrimers have emerged due in part to their contribution to nanoparticle solubility, biocompatibility and stability, and also for cell selectivity and delivery efficiency. Other drug delivering or therapeutic dendrimer conjugates have used larger proteins as ligands, especially antibodies or proteins that bind to overexpressed receptors in diseased cells. This approach allows for high selectivity, but may be limited by the size increase of the vehicle owing to relatively large ligands, short half-life because of proteolytic cleavage and necessary a priori knowledge of ligand. Short peptide ligands, on the other hand, can be discovered through phage display or derived from the binding domains of large proteins. Short peptide strands, like peptides of all lengths, are susceptible to proteolysis, but their abbreviated structures provide for fewer sites of recognition by proteases compared to larger peptides and proteins. It should be noted that—although largely unexplored—oligosaccharide ligands for dendrimeric drug delivery applications hold promise owing to the sheer number of oligosaccharide permutations; for example, there are 206 (6.4×107) possible peptide hexamers, but drastically more hexasaccharide permutations (1.44×1015). The large number of possible oligosaccharides—as compared to peptides of the same length—could lead to the development of saccharide ligands of high selectivity [10].
In particular, the multivalent presentation of bioactive ligands through dendrimeric scaffolds provides exciting opportunities to probe how nanoscale characteristics of ligand arrangement would affect the cell–material interaction and the drug delivery efficiency. Through in vitro binding assays, the kinetic and dynamic profile of ligand–dendrimer conjugates to interact with cells begin to unfold, with dendrimeric conjugates showing efficiency to enhance the uptake and the ligand density observed to modulate the cell-binding property. From the perspective of molecular design, it remains to be elucidated how the structure and bioactivity of dendrimers are fundamentally correlated. A number of intrinsically woven factors, including the dendrimer scaffold structure, the ligand valency and density, and spacer should be taken into consideration when designing and analysing the property of ligand-conjugated dendrimers.
To employ bioactive dendrimers for fabrication of drug delivery systems, new methodologies to accommodate the therapeutic cargoes in delivery systems without compromising the cell-targeting and -delivery capability of bioactive dendrimers would be valuable. Although receptor-mediated endocytosis is thought to be involved in internalization of ligand-conjugated vehicle, how the uptake mechanism is linked to the delivery efficiency on the cellular level in terms of membrane penetration of bioactive dendrimers and cytoplasmic release of drug molecules remains largely unexplored. More studies on this aspect would lead to theoretical principles to guide the rational design of targeted drug delivery systems. Despite the debatable effect on enhancing drug efficacy, ligand-conjugated dendrimers may improve the targeting and specificity of drug delivery, as shown by many in vitro and in vivo studies. Exploiting new technologies to screen appropriate ligands would be essential to identify peptide and saccharides for optimal design of drug delivery systems. As reviewed herein, peptides screened from phage display have been conjugated to dendrimer scaffolds and demonstrate delivery benefits in vivo. Glycan arrays exist that can identify saccharides capable of interacting with specific proteins [146,147], and this provides opportunities for high-throughput study of saccharide–cell interactions and screening of glycosylated dendrimers suitable for drug delivery. Whether the ligand-conjugated dendrimers can facilitate drugs to reach the targeted tissue/cells and induce therapeutic effects in vivo could be difficult to predict without significant in vivo tests owing to the complex tissue environment and physiological conditions. Significant proof-of-principle research has been conducted with these bioactive dendrimers, i.e. the majority of research articles describe the uptake of fluorophore-conjugated dendrimers, while relatively few articles demonstrate therapeutic effects of drug-loaded vehicles. Obviously, for proposed drug delivery vehicles to reach the clinic, these next steps of testing must be taken.
In biomedical applications, safety and biocompatibility are among high-priority issues relating to the clinical potential of a biomaterial. Comprehensive understanding of the toxicity and developing strategies to improve biocompatibility would accelerate the developmental process towards clinical application of dendrimer-based materials. Safety issues could be more complex and beyond our current understanding of bioactive dendrimers owing to the possible unknown bioactivity of these materials. It is noted that bioactive dendrimers may participate in roles beyond cell binding, possibly including mediation of cellular signalling activities. For example, one study demonstrated that the integrin-mediated c-Jun N-terminal kinase (JNK) signaling pathway could be activated by an RGD–PAMAM conjugate to modulate the differentiation of dental pulp cells [148]. Only with more studies carried out to address both fundamental and practical issues to further our understanding of cell–material interactions and drug delivery problems would bioactive dendrimers see translation into clinical applications in the near future.
Acknowledgments
The research was supported by National High-Tech R&D Programme of China (project no. 2009AA03Z314), the Beijing Natural Science Foundation (Project no. 7102088), a Whitaker International Fellowship for W.D.G., and the GT-Emory-PKU Coulter Seed grant.
References
- 1.Tomalia D. A., Baker H., Dewald J., Hall M., Kallos G., Martin S., Roeck J., Ryder J., Smith P. 1985. A new class of polymers: starburst-dendritic macromolecules. Polym. J. 17, 117–132 10.1295/polymj.17.117 (doi:10.1295/polymj.17.117) [DOI] [Google Scholar]
- 2.Frechet J. M. 2002. Dendrimers and supramolecular chemistry. Proc. Natl Acad. Sci. USA 99, 4782–4787 10.1073/pnas.082013899 (doi:10.1073/pnas.082013899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee R. T., Lee Y. C. 2000. Affinity enhancement by multivalent lectin–carbohydrate interaction. Glycoconj. J. 17, 543–551 10.1023/A:1011070425430 (doi:10.1023/A:1011070425430) [DOI] [PubMed] [Google Scholar]
- 4.Lundquist J. J., Toone E. J. 2002. The cluster glycoside effect. Chem. Rev. 102, 555–578 10.1021/cr000418f (doi:10.1021/cr000418f) [DOI] [PubMed] [Google Scholar]
- 5.Boas U., Heegaard P. M. 2004. Dendrimers in drug research. Chem. Soc. Rev. 33, 43–63 10.1039/b309043b (doi:10.1039/b309043b) [DOI] [PubMed] [Google Scholar]
- 6.Chabre Y. M., Roy R. 2008. Recent trends in glycodendrimer syntheses and applications. Curr. Top. Med. Chem. 8, 1237–1285 10.2174/156802608785848987 (doi:10.2174/156802608785848987) [DOI] [PubMed] [Google Scholar]
- 7.Clayton R., Hardman J., LaBranche C. C., McReynolds K. D. 2011. Evaluation of the synthesis of sialic acid-PAMAM glycodendrimers without the use of sugar protecting groups, and the anti-HIV-1 properties of these compounds. Bioconjug. Chem. 22, 2186–2197 10.1021/bc200331v (doi:10.1021/bc200331v) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Cheng Y., Xu T. 2007. Design, synthesis and potent pharmaceutical applications of glycodendrimers: a mini review. Curr. Drug Discov. Technol. 4, 246–254 10.2174/157016307783220503 (doi:10.2174/157016307783220503) [DOI] [PubMed] [Google Scholar]
- 9.Kojima C., Tsumura S., Harada A., Kono K. 2009. A collagen-mimic dendrimer capable of controlled release. J. Am. Chem. Soc. 131, 6052–6053 10.1021/ja809639c (doi:10.1021/ja809639c) [DOI] [PubMed] [Google Scholar]
- 10.Sebestik J., Niederhafner P., Jezek J. 2011. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids 40, 301–370 10.1007/s00726-010-0707-z (doi:10.1007/s00726-010-0707-z) [DOI] [PubMed] [Google Scholar]
- 11.Mintzer M. A., Grinstaff M. W. 2011. Biomedical applications of dendrimers: a tutorial. Chem. Soc. Rev. 40, 173–190 10.1039/b901839p (doi:10.1039/b901839p) [DOI] [PubMed] [Google Scholar]
- 12.Lee C. C., MacKay J. A., Frechet J. M., Szoka F. C. 2005. Designing dendrimers for biological applications. Nat. Biotechnol. 23, 1517–1526 10.1038/nbt1171 (doi:10.1038/nbt1171) [DOI] [PubMed] [Google Scholar]
- 13.Mihov G., Grebel-Koehler D., Lübbert A., Vandermeulen G. W. M., Herrmann A., Klok H.-A., Müllen K. 2005. Polyphenylene dendrimers as scaffolds for shape-persistent multiple peptide conjugates. Bioconjugate Chem. 16, 283–293 10.1021/bc049839k (doi:10.1021/bc049839k) [DOI] [PubMed] [Google Scholar]
- 14.Svenson S. 2009. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 71, 445–462 10.1016/j.ejpb.2008.09.023 (doi:10.1016/j.ejpb.2008.09.023) [DOI] [PubMed] [Google Scholar]
- 15.Svenson S., Tomalia D. A. 2005. Dendrimers in biomedical applications: reflections on the field. Adv. Drug Deliv. Rev. 57, 2106–2129 10.1016/j.addr.2005.09.018 (doi:10.1016/j.addr.2005.09.018) [DOI] [PubMed] [Google Scholar]
- 16.Roy R. 2003. A decade of glycodendrimer chemistry. Trends Glycosci. Glycotechnol. 15, 291–310 10.4052/tigg.15.291 (doi:10.4052/tigg.15.291) [DOI] [Google Scholar]
- 17.Chabre Y. M., Roy R. 2010. Design and creativity in synthesis of multivalent neoglycoconjugates. Adv. Carbohydr. Chem. Biochem. 63, 165–393 10.1016/S0065-2318(10)63006-5 (doi:10.1016/S0065-2318(10)63006-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukla R., Thomas T. P., Peters J., Kotlyar A., Myc A., Baker J. R. 2005. Tumor angiogenic vasculature targeting with PAMAM dendrimer–RGD conjugates. Chem. Commun. 46, 5739–5741 10.1039/b507350b (doi:10.1039/b507350b) [DOI] [PubMed] [Google Scholar]
- 19.Hill E., Shukla R., Park S. S., Baker J. R., Jr 2007. Synthetic PAMAM–RGD conjugates target and bind to odontoblast-like MDPC 23 cells and the predentin in tooth organ cultures. Bioconjug. Chem. 18, 1756–1762 10.1021/bc0700234 (doi:10.1021/bc0700234) [DOI] [PubMed] [Google Scholar]
- 20.McNerny D. Q., Kukowska-Latallo J. F., Mullen D. G., Wallace J. M., Desai A. M., Shukla R., Huang B., Banaszak Holl M. M., Baker J. R., Jr 2009. RGD dendron bodies; synthetic avidity agents with defined and potentially interchangeable effector sites that can substitute for antibodies. Bioconjug. Chem. 20, 1853–1859 10.1021/bc900217h (doi:10.1021/bc900217h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waite C. L., Roth C. M. 2009. PAMAM–RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjug. Chem. 20, 1908–1916 10.1021/bc900228m (doi:10.1021/bc900228m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H., Kao W. J. 2007. Synthesis and characterization of nanoscale dendritic RGD clusters for potential applications in tissue engineering and drug delivery. Int. J. Nanomed. 2, 89–99 10.2147/nano.2007.2.1.89 (doi:10.2147/nano.2007.2.1.89) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao J., Tam J. P. 1995. Unprotected peptides as building blocks for the synthesis of peptide dendrimers with oxime, hydrazone, and thiazolidine linkages. J. Am. Chem. Soc. 117, 3893–3899 10.1021/ja00119a001 (doi:10.1021/ja00119a001) [DOI] [Google Scholar]
- 24.Rao C., Tam J. P. 1994. Synthesis of peptide dendrimer. J. Am. Chem. Soc. 116, 6975–6976 10.1021/ja00094a078 (doi:10.1021/ja00094a078) [DOI] [Google Scholar]
- 25.Ozawa C., Hojo H., Nakahara Y., Katayama H., Nabeshima K., Akahane T., Nakahara Y. 2007. Synthesis of glycopeptide dendrimer by a convergent method. Tetrahedron 63, 9685–9690 10.1016/j.tet.2007.07.023 (doi:10.1016/j.tet.2007.07.023) [DOI] [Google Scholar]
- 26.Lindhorst T. K., Kieburg C. 1996. Glycocoating of oligovalent amines: synthesis of thiourea-bridged cluster glycosides from glycosyl isothiocyanates. Angew. Chem. Int. Ed. 35, 1953–1956 10.1002/anie.199619531 (doi:10.1002/anie.199619531) [DOI] [Google Scholar]
- 27.Page D., Roy R. 1997. Synthesis and biological properties of mannosylated starburst poly(amidoamine) dendrimers. Bioconjug. Chem. 8, 714–723 10.1021/bc970126u (doi:10.1021/bc970126u) [DOI] [PubMed] [Google Scholar]
- 28.Andre S., Ortega P. J. C., Perez M. A., Roy R., Gabius H. J. 1999. Lactose-containing starburst dendrimers: influence of dendrimer generation and binding-site orientation of receptors (plant/animal lectins and immunoglobulins) on binding properties. Glycobiology 9, 1253–1261 10.1093/glycob/9.11.1253 (doi:10.1093/glycob/9.11.1253) [DOI] [PubMed] [Google Scholar]
- 29.Thompson J. P., Schengrund C. L. 1997. Oligosaccharide-derivatized dendrimers: defined multivalent inhibitors of the adherence of the cholera toxin B subunit and the heat labile enterotoxin of E. coli to GM1. Glycoconj. J. 14, 837–845 10.1023/A:1018590021762 (doi:10.1023/A:1018590021762) [DOI] [PubMed] [Google Scholar]
- 30.Zanini D., Roy R. 1998. Practical synthesis of starburst PAMAM α-thiosialodendrimers for probing multivalent carbohydrate–lectin binding properties. J. Org. Chem. 63, 3486–3491 10.1021/jo972061u (doi:10.1021/jo972061u) [DOI] [Google Scholar]
- 31.Landers J. J., Cao Z. Y., Lee I., Piehler L. T., Myc P. P., Myc A., Hamouda T., Galecki A. T., Baker J. R. 2002. Prevention of influenza pneumonitis by sialic acid-conjugated dendritic polymers. J. Infect. Dis. 186, 1222–1230 10.1086/344316 (doi:10.1086/344316) [DOI] [PubMed] [Google Scholar]
- 32.Woller E. K., Cloninger M. J. 2001. Mannose functionalization of a sixth generation dendrimer. Biomacromolecules 2, 1052–1054 10.1021/bm015560k (doi:10.1021/bm015560k) [DOI] [PubMed] [Google Scholar]
- 33.Mangold S. L., Morgan J. R., Strohmeyer G. C., Gronenborn A. M., Cloninger M. J. 2005. Cyanovirin-N binding to Manα1–2Man functionalized dendrimers. Org. Biomol. Chem. 3, 2354–2358 10.1039/b417789d (doi:10.1039/b417789d) [DOI] [PubMed] [Google Scholar]
- 34.Sashiwa H., Shigemasa Y., Roy R. 2000. Chemical modification of chitosan. 3. Hyperbranched chitosan-sialic acid dendrimer hybrid with tetraethylene glycol spacer. Macromolecules 33, 6913–6915 10.1021/ma0005769 (doi:10.1021/ma0005769) [DOI] [Google Scholar]
- 35.Sashiwa H., Shigemasa Y., Roy R. 2001. Highly convergent synthesis of dendrimerized chitosan-sialic acid hybrid. Macromolecules 34, 3211–3214 10.1021/ma001534n (doi:10.1021/ma001534n) [DOI] [Google Scholar]
- 36.Aoi K., Itoh K., Okada M. 1995. Globular carbohydrate macromolecule sugar balls. 1. Synthesis of novel sugar-persubstituted poly(amido amine) dendrimers. Macromolecules 28, 5391–5393 10.1021/ma00119a037 (doi:10.1021/ma00119a037) [DOI] [Google Scholar]
- 37.Aoi K., Tsutsumiuchi K., Yamamoto A., Okada M. 1997. Globular carbohydrate macromolecule ‘sugar balls’ 0.3. ‘Radial-growth polymerization’ of sugar-substituted alpha-amino acid N-carboxyanhydrides (GlycoNCAs) with a dendritic initiator. Tetrahedron 53, 15 415–15 427 [Google Scholar]
- 38.Baek M. G., Roy R. 2002. Synthesis and protein binding properties of T-antigen containing GlycoPAMAM dendrimers. Biorg. Med. Chem. 10, 11–17 10.1016/S0968-0896(01)00248-6 (doi:10.1016/S0968-0896(01)00248-6) [DOI] [PubMed] [Google Scholar]
- 39.Appeldoorn C. C. M., Joosten J. A. F., el Maate F. A., Dobrindt U., Hacker J., Liskamp R. M. J., Khan A. S., Pieters R. J. 2005. Novel multivalent mannose compounds and their inhibition of the adhesion of type 1 fimbriated uropathogenic E. coli. Tetrahedron-Asymmetry 16, 361–372 10.1016/j.tetasy.2004.11.014 (doi:10.1016/j.tetasy.2004.11.014) [DOI] [Google Scholar]
- 40.de Paz J. L., Noti C., Bohm F., Werner S., Seeberger P. H. 2007. Potentiation of fibroblast growth factor activity by synthetic heparin oligosaccharide glycodendrimers. Chem. Biol. 14, 879–887 10.1016/j.chembiol.2007.07.007 (doi:10.1016/j.chembiol.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 41.Llinares M., Roy R. 1997. Multivalent neoglycoconjugates: solid-phase synthesis of N-linked alpha-sialodendrimers. Chem. Commun. 21, 2119–2120 10.1039/a703679e (doi:10.1039/a703679e) [DOI] [Google Scholar]
- 42.Zanini D., Roy R. 1997. Synthesis of new alpha-thiosialodendrimers and their binding properties to the sialic acid specific lectin from Limax flavus. J. Am. Chem. Soc. 119, 2088–2095 10.1021/ja963874n (doi:10.1021/ja963874n) [DOI] [Google Scholar]
- 43.Zanini D., Roy R. 1996. Novel dendritic α-sialosides: synthesis of glycodendrimers based on a 3,3′-iminobis(propylamine) core. J. Org. Chem. 61, 7348–7354 10.1021/jo961047z (doi:10.1021/jo961047z) [DOI] [PubMed] [Google Scholar]
- 44.Tosh D. K., et al. 2010. Polyamidoamine (PAMAM) dendrimer conjugates of ‘clickable’ agonists of the A3 adenosine receptor and coactivation of the P2Y14 receptor by a tethered nucleotide. Bioconjug. Chem. 21, 372–384 10.1021/bc900473v (doi:10.1021/bc900473v) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X., Liu J., Luo Y. 2012. Facile glycosylation of dendrimers for eliciting specific cell–material interactions. Polym. Chem. 3, 310–313 10.1039/c1py00404b (doi:10.1039/c1py00404b) [DOI] [Google Scholar]
- 46.Liu J., Zhou J., Luo Y. 2012. siRNA delivery systems based on neutral crosslinked dendrimers. Bioconjug. Chem. 23, 174–183 10.1021/bc200433s (doi:10.1021/bc200433s) [DOI] [PubMed] [Google Scholar]
- 47.Hook A. L., Anderson D. G., Langer R., Williams P., Davies M. C., Alexander M. R. 2010. High throughput methods applied in biomaterial development and discovery. Biomaterials 31, 187–198 10.1016/j.biomaterials.2009.09.037 (doi:10.1016/j.biomaterials.2009.09.037) [DOI] [PubMed] [Google Scholar]
- 48.Potyrailo R., Rajan K., Stoewe K., Takeuchi I., Chisholm B., Lam H. 2011. Combinatorial and high-throughput screening of materials libraries: review of state of the art. ACS Comb. Sci. 13, 579–633 10.1021/co200007w (doi:10.1021/co200007w) [DOI] [PubMed] [Google Scholar]
- 49.Godula K., Bertozzi C. R. 2010. Synthesis of glycopolymers for microarray applications via ligation of reducing sugars to a poly(acryloyl hydrazide) scaffold. J. Am. Chem. Soc. 132, 9963–9965 10.1021/ja103009d (doi:10.1021/ja103009d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulson J. C., Blixt O., Collins B. E. 2006. Sweet spots in functional glycomics. Nat. Chem. Biol. 2, 238–248 10.1038/nchembio785 (doi:10.1038/nchembio785) [DOI] [PubMed] [Google Scholar]
- 51.Marth J. D., Grewal P. K. 2008. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 10.1038/nri2417 (doi:10.1038/nri2417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertozzi C. R., Kiessling L. L. 2001. Chemical glycobiology. Science 291, 2357–2364 10.1126/science.1059820 (doi:10.1126/science.1059820) [DOI] [PubMed] [Google Scholar]
- 53.Mammen M., Choi S. K., Whitesides G. M. 1998. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2755–2794 [DOI] [PubMed] [Google Scholar]
- 54.Kiessling L. L., Gestwicki J. E., Strong L. E. 2000. Synthetic multivalent ligands in the exploration of cell–surface interactions. Curr. Opin. Chem. Biol. 4, 696–703 10.1016/S1367-5931(00)00153-8 (doi:10.1016/S1367-5931(00)00153-8) [DOI] [PubMed] [Google Scholar]
- 55.Niederhafner P., Sebestik J., Jezek J. 2008. Glycopeptide dendrimers. I. J. Pept. Sci. 14, 2–43 10.1002/psc.931 (doi:10.1002/psc.931) [DOI] [PubMed] [Google Scholar]
- 56.Dijkgraaf I., et al. 2007. Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes. Org. Biomol. Chem. 5, 935–944 10.1039/b615940k (doi:10.1039/b615940k) [DOI] [PubMed] [Google Scholar]
- 57.Welsh D. J., Smith D. K. 2011. Comparing dendritic and self-assembly strategies to multivalency-RGD peptide-integrin interactions. Org. Biomol. Chem. 9, 4795–4801 10.1039/c1ob05241a (doi:10.1039/c1ob05241a) [DOI] [PubMed] [Google Scholar]
- 58.Pasqualini R., Koivunen E., Ruoslahti E. 1997. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat. Biotechnol. 15, 542–546 10.1038/nbt0697-542 (doi:10.1038/nbt0697-542) [DOI] [PubMed] [Google Scholar]
- 59.Almutairi A., et al. 2009. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc. Natl Acad. Sci. USA 106, 685–690 10.1073/pnas.0811757106 (doi:10.1073/pnas.0811757106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pierschbacher M. D., Ruoslahti E. 1984. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 10.1038/309030a0 (doi:10.1038/309030a0) [DOI] [PubMed] [Google Scholar]
- 61.Assa-Munt N., Jia X., Laakkonen P., Ruoslahti E. 2001. Solution structures and integrin binding activities of an RGD peptide with two isomers. Biochemistry 40, 2373–2378 10.1021/bi002101f (doi:10.1021/bi002101f) [DOI] [PubMed] [Google Scholar]
- 62.Waite C. L., Roth C. M. 2011. Binding and transport of PAMAM-RGD in a tumor spheroid model: the effect of RGD targeting ligand density. Biotechnol. Bioeng. 108, 2999–3008 10.1002/bit.23255 (doi:10.1002/bit.23255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myung J. H., Gajjar K. A., Saric J., Eddington D. T., Hong S. 2011. Dendrimer-mediated multivalent binding for the enhanced capture of tumor cells. Angew. Chem. Int. Ed. 50, 11 769–11 772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin Z. H., Furukawa T., Waki A., Akaji K., Coll J. L., Saga T., Fujibayashi Y. 2010. Effect of multimerization of a linear Arg–Gly–Asp peptide on integrin binding affinity and specificity. Biol. Pharm. Bull. 33, 370–378 10.1248/bpb.33.370 (doi:10.1248/bpb.33.370) [DOI] [PubMed] [Google Scholar]
- 65.Medina S. H., Tekumalla V., Chevliakov M. V., Shewach D. S., Ensminger W. D., El-Sayed M. E. H. 2011. N-acetylgalactosamine-functionalized dendrimers as hepatic cancer cell-targeted carriers. Biomaterials 32, 4118–4129 10.1016/j.biomaterials.2010.11.068 (doi:10.1016/j.biomaterials.2010.11.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato N., Kobayashi H., Hiraga A., Saga T., Togashi K., Konishi J., Brechbiel M. W. 2001. Pharmacokinetics and enhancement patterns of macromolecular MR contrast agents with various sizes of polyamidoamine dendrimer cores. Magn. Reson. Med. 46, 1169–1173 10.1002/mrm.1314 (doi:10.1002/mrm.1314) [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi H., Brechbiel M. W. 2005. Nano-sized MRI contrast agents with dendrimer cores. Adv. Drug Del. Rev. 57, 2271–2286 10.1016/j.addr.2005.09.016 (doi:10.1016/j.addr.2005.09.016) [DOI] [PubMed] [Google Scholar]
- 68.Benito J. M., Gomez-Garcia M., Ortiz Mellet C., Baussanne I., Defaye J., Garcia Fernandez J. M. 2004. Optimizing saccharide-directed molecular delivery to biological receptors: design, synthesis, and biological evaluation of glycodendrimer–cyclodextrin conjugates. J. Am. Chem. Soc. 126, 10 355–10 363 [DOI] [PubMed] [Google Scholar]
- 69.Boswell C. A., Eck P. K., Regino C. A., Bernardo M., Wong K. J., Milenic D. E., Choyke P. L., Brechbiel M. W. 2008. Synthesis, characterization, and biological evaluation of integrin αvβ3-targeted PAMAM dendrimers. Mol. Pharm. 5, 527–539 10.1021/mp800022a (doi:10.1021/mp800022a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarthy J. R., Weissleder R. 2008. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 60, 1241–1251 10.1016/j.addr.2008.03.014 (doi:10.1016/j.addr.2008.03.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrenko V. 2008. Evolution of phage display: from bioactive peptides to bioselective nanomaterials. Expert Opin. Drug Deliv. 5, 825–836 10.1517/17425247.5.8.825 (doi:10.1517/17425247.5.8.825) [DOI] [PubMed] [Google Scholar]
- 72.Helms B. A., Reulen S. W., Nijhuis S., de Graaf-Heuvelmans P. T., Merkx M., Meijer E. W. 2009. High-affinity peptide-based collagen targeting using synthetic phage mimics: from phage display to dendrimer display. J. Am. Chem. Soc. 131, 11 683–11 685 10.1021/ja902285m (doi:10.1021/ja902285m) [DOI] [PubMed] [Google Scholar]
- 73.Bastings M. M. C., Helms B. A., van Baal I., Hackeng T. M., Merkx M., Meijer E. W. 2011. From phage display to dendrimer display: insights into multivalent binding. J. Am. Chem. Soc. 133, 6636–6641 10.1021/ja110700x (doi:10.1021/ja110700x) [DOI] [PubMed] [Google Scholar]
- 74.Lempens E. H., Merkx M., Tirrell M., Meijer E. W. 2011. Dendrimer display of tumor-homing peptides. Bioconjug. Chem. 22, 397–405 10.1021/bc100403e (doi:10.1021/bc100403e) [DOI] [PubMed] [Google Scholar]
- 75.Liu J., Liu J., Chu L., Wang Y., Duan Y., Feng L., Yang C., Wang L., Kong D. 2011. Novel peptide–dendrimer conjugates as drug carriers for targeting nonsmall cell lung cancer. Int. J. Nanomed. 6, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simberg D., et al. 2007. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl Acad. Sci. USA 104, 932–936 10.1073/pnas.0610298104 (doi:10.1073/pnas.0610298104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters D., Kastantin M., Kotamraju V. R., Karmali P. P., Gujraty K., Tirrell M., Ruoslahti E. 2009. Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci. USA 106, 9815–9819 10.1073/pnas.0903369106 (doi:10.1073/pnas.0903369106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Supattapone S., Nguyen H. O. B., Cohen F. E., Prusiner S. B., Scott M. R. 1999. Elimination of prions by branched polyamines and implications for therapeutics. Proc. Natl Acad. Sci. USA 96, 14 529–14 534 10.1073/pnas.96.25.14529 (doi:10.1073/pnas.96.25.14529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soto C. et al. 2000. Reversion of prion protein conformational changes by synthetic b-sheet breaker peptides. Lancet 355, 192–197 10.1016/S0140-6736(99)11419-3 (doi:10.1016/S0140-6736(99)11419-3) [DOI] [PubMed] [Google Scholar]
- 80.Pini A., et al. 2005. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob. Agents Chemother. 49, 2665–2672 10.1128/AAC.49.7.2665-2672.2005 (doi:10.1128/AAC.49.7.2665-2672.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bourne N., Stanberry L., Kern E., Holan G., Matthews B., Bernstein D. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 44, 2471–2474 10.1128/AAC.44.9.2471-2474.2000 (doi:10.1128/AAC.44.9.2471-2474.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carlescu I., Scutaru D., Popa M., Uglea C. V. 2009. Synthetic sialic-acid-containing polyvalent antiviral inhibitors. Med. Chem. Res. 18, 477–494 10.1007/s00044-008-9139-7 (doi:10.1007/s00044-008-9139-7) [DOI] [Google Scholar]
- 83.Jiang Y. H., Emau P., Cairns J. S., Flanary L., Morton W. R., McCarthy T. D., Tsai C. C. 2005. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89. 6P in macaques. AIDS Res. Hum. Retroviruses 21, 207–213 10.1089/aid.2005.21.207 (doi:10.1089/aid.2005.21.207) [DOI] [PubMed] [Google Scholar]
- 84.Martínez-Ávila O., Hijazi K., Marradi M., Clavel C., Campion C., Kelly C., Penadés S. 2009. Gold manno-glyconanoparticles: multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chem. Eur. J. 15, 9874–9888 10.1002/chem.200900923 (doi:10.1002/chem.200900923) [DOI] [PubMed] [Google Scholar]
- 85.Sakamoto J. I., et al. 2009. Systematic syntheses of influenza neuraminidase inhibitors: a series of carbosilane dendrimers uniformly functionalized with thioglycoside-type sialic acid moieties. Biorg. Med. Chem. 17, 5451–5464 10.1016/j.bmc.2009.06.036 (doi:10.1016/j.bmc.2009.06.036) [DOI] [PubMed] [Google Scholar]
- 86.Zhou Y., Guo Z., Zhang Y., Huang W., Yan D. 2009. Hyperbranched polyamidoamines containing β-cyclodextrin for controlled release of chlorambucil. Macromol. Biosci. 9, 1090–1097 10.1002/mabi.200900110 (doi:10.1002/mabi.200900110) [DOI] [PubMed] [Google Scholar]
- 87.Dutta T., Jain N. K., McMillan N. A., Parekh H. S. 2010. Dendrimer nanocarriers as versatile vectors in gene delivery. Nanomedicine 6, 25–34 [DOI] [PubMed] [Google Scholar]
- 88.D'Emanuele A., Attwood D. 2005. Dendrimer–drug interactions. Adv. Drug Deliv. Rev. 57, 2147–2162 10.1016/j.addr.2005.09.012 (doi:10.1016/j.addr.2005.09.012) [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., Bryantsev V. S., Diallo M. S., Goddard W. A., III 2009. PAMAM dendrimers undergo pH responsive conformational changes without swelling. J. Am. Chem. Soc. 131, 2798–2799 10.1021/ja8100227 (doi:10.1021/ja8100227) [DOI] [PubMed] [Google Scholar]
- 90.Bhadra D., Yadav A. K., Bhadra S., Jain N. K. 2005. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int. J. Pharm. 295, 221–233 10.1016/j.ijpharm.2005.01.026 (doi:10.1016/j.ijpharm.2005.01.026) [DOI] [PubMed] [Google Scholar]
- 91.Agrawal P., Gupta U., Jain N. K. 2007. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials 28, 3349–3359 10.1016/j.biomaterials.2007.04.004 (doi:10.1016/j.biomaterials.2007.04.004) [DOI] [PubMed] [Google Scholar]
- 92.Pan B., et al. 2009. Synthesis and characterization of polyamidoamine dendrimer-coated multi-walled carbon nanotubes and their application in gene delivery systems. Nanotechnology 20, 125 101–125 109 [DOI] [PubMed] [Google Scholar]
- 93.Wu P., Chen X., Hu N., Tam U. C., Blixt O., Zettl A., Bertozzi C. R. 2008. Biocompatible carbon nanotubes generated by functionalization with glycodendrimers. Angew. Chem. Chem. Int. 120, 5100–5103 10.1002/ange.200705363 (doi:10.1002/ange.200705363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Y.-P., Huang W., Lin Y., Fu K., Kitaygorodskiy A., Riddle L. A., Yu Y. J., Carroll D. L. 2001. Soluble dendron-functionalized carbon nanotubes: preparation, characterization, and properties. Chem. Mater. 13, 2864–2869 10.1021/cm010069l (doi:10.1021/cm010069l) [DOI] [Google Scholar]
- 95.Campidelli S., Sooambar C., Diz E. L., Ehli C., Guldi D. M., Prato M. 2006. Dendrimer-functionalized single-wall carbon nanotubes: synthesis, characterization, and photoinduced electron transfer. J. Am. Chem. Soc. 128, 12 544–12 552 10.1021/ja055379 (doi:10.1021/ja055379) [DOI] [PubMed] [Google Scholar]
- 96.Shi X., Wang S. H., Lee I., Shen M., Baker J. R., Jr 2009. Comparison of the internalization of targeted dendrimers and dendrimer-entrapped gold nanoparticles into cancer cells. Biopolymers 91, 936–942 10.1002/bip.21279 (doi:10.1002/bip.21279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun Y., Pan B., Gao F., Ao L., Tian H., He R., Cui D. 2005. Controlled self-assembly of thiol-terminated poly (amidoamine) dendrimer and gold nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 259, 89–94 10.1016/j.colsurfa.2005.02.009 (doi:10.1016/j.colsurfa.2005.02.009) [DOI] [Google Scholar]
- 98.Rojo J., Díaz V., de la Fuente J. M., Segura I., Barrientos A. G., Riese H. H., Bernad A., Penadés S. 2004. Gold glyconanoparticles as new tools in antiadhesive therapy. ChemBioChem 5, 291–297 10.1002/cbic.200300726 (doi:10.1002/cbic.200300726) [DOI] [PubMed] [Google Scholar]
- 99.Alexandridis P. 2011. Gold nanoparticle synthesis, morphology control, and stabilization facilitated by functional polymers. Chem. Eng. Technol. 34, 15–28 10.1002/ceat.201000335 (doi:10.1002/ceat.201000335) [DOI] [Google Scholar]
- 100.Shan J., Tenhu H. 2007. Recent advances in polymer protected gold nanoparticles: synthesis, properties and applications. Chem. Commun. 44, 4580–4598 10.1039/b707740h (doi:10.1039/b707740h) [DOI] [PubMed] [Google Scholar]
- 101.Li Z., et al. 2009. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol. Pharm. 7, 94–104 10.1021/mp9001415 (doi:10.1021/mp9001415) [DOI] [PubMed] [Google Scholar]
- 102.Pan B., Cui D., Sheng Y., Ozkan C., Gao F., He R., Li Q., Xu P., Huang T. 2007. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 67, 8156–8163 10.1158/0008-5472.CAN-06-4762 (doi:10.1158/0008-5472.CAN-06-4762) [DOI] [PubMed] [Google Scholar]
- 103.Martin A. L., Li B., Gillies E. R. 2008. Surface functionalization of nanomaterials with dendritic groups: toward enhanced binding to biological targets. J. Am. Chem. Soc. 131, 734–741 10.1021/ja807220u (doi:10.1021/ja807220u) [DOI] [PubMed] [Google Scholar]
- 104.Ghosh S., Saha A. 2009. Synthesis and spectral studies of CdTe–dendrimer conjugates. Nanoscale Res. Lett. 4, 937–941 10.1007/s11671-009-9339-1 (doi:10.1007/s11671-009-9339-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z., et al. 2010. Arginine–glycine–aspartic acid-conjugated dendrimer-modified quantum dots for targeting and imaging melanoma. J. Nanosci. Nanotechnol. 10, 4859–4867 10.1166/jnn.2010.2217 (doi:10.1166/jnn.2010.2217) [DOI] [PubMed] [Google Scholar]
- 106.Pan B., Gao F., He R., Cui D., Zhang Y. 2006. Study on interaction between poly (amidoamine) dendrimer and CdSe nanocrystal in chloroform. J. Colloid Interf. Sci. 297, 151–156 10.1016/j.jcis.2005.09.068 (doi:10.1016/j.jcis.2005.09.068) [DOI] [PubMed] [Google Scholar]
- 107.Pandita D., Santos J. L., Rodrigues J., Pego A. P., Granja P. L., Tomas H. 2011. Gene delivery into mesenchymal stem cells: a biomimetic approach using RGD nanoclusters based on poly(amidoamine) dendrimers. Biomacromolecules 12, 472–481 10.1021/bm1012647 (doi:10.1021/bm1012647) [DOI] [PubMed] [Google Scholar]
- 108.Demeule M., Currie J. C., Bertrand Y., Che C., Nguyen T., Regina A., Gabathuler R., Castaigne J. P., Beliveau R. 2008. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 106, 1534–1544 10.1111/j.1471-4159.2008.05492.x (doi:10.1111/j.1471-4159.2008.05492.x) [DOI] [PubMed] [Google Scholar]
- 109.Ke W., et al. 2009. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 30, 6976–6985 10.1016/j.biomaterials.2009.08.049 (doi:10.1016/j.biomaterials.2009.08.049) [DOI] [PubMed] [Google Scholar]
- 110.Huang S., Li J., Han L., Liu S., Ma H., Huang R., Jiang C. 2011. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials 32, 6832–6838 10.1016/j.biomaterials.2011.05.064 (doi:10.1016/j.biomaterials.2011.05.064) [DOI] [PubMed] [Google Scholar]
- 111.Wood K. C., Azarin S. M., Arap W., Pasqualini R., Langer R., Hammond P. T. 2008. Tumor-targeted gene delivery using molecularly engineered hybrid polymers functionalized with a tumor-homing peptide. Bioconjug. Chem. 19, 403–405 10.1021/bc700408r (doi:10.1021/bc700408r) [DOI] [PubMed] [Google Scholar]
- 112.Gump J. M., Dowdy S. F. 2007. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol. Med. 13, 443–448 10.1016/j.molmed.2007.08.002 (doi:10.1016/j.molmed.2007.08.002) [DOI] [PubMed] [Google Scholar]
- 113.Meade B. R., Dowdy S. F. 2007. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv. Drug Deliv. Rev. 59, 134–140 10.1016/j.addr.2007.03.004 (doi:10.1016/j.addr.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 114.Murriel C. L., Dowdy S. F. 2006. Influence of protein transduction domains on intracellular delivery of macromolecules. Expert Opin. Drug Deliv. 3, 739–746 10.1517/17425247.3.6.739 (doi:10.1517/17425247.3.6.739) [DOI] [PubMed] [Google Scholar]
- 115.Wadia J. S., Dowdy S. F. 2005. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv. Drug Deliv. Rev. 57, 579–596 10.1016/j.addr.2004.10.005 (doi:10.1016/j.addr.2004.10.005) [DOI] [PubMed] [Google Scholar]
- 116.Frankel A. D., Pabo C. O. 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–1193 10.1016/0092-8674(88)90263-2 (doi:10.1016/0092-8674(88)90263-2) [DOI] [PubMed] [Google Scholar]
- 117.Green M., Loewenstein P. M. 1988. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55, 1179–1188 10.1016/0092-8674(88)90262-0 (doi:10.1016/0092-8674(88)90262-0) [DOI] [PubMed] [Google Scholar]
- 118.Patel L. N., Zaro J. L., Shen W. C. 2007. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm. Res. 24, 1977–1992 10.1007/s11095-007-9303-7 (doi:10.1007/s11095-007-9303-7) [DOI] [PubMed] [Google Scholar]
- 119.Kang H., DeLong R., Fisher M. H., Juliano R. L. 2005. Tat-conjugated PAMAM dendrimers as delivery agents for antisense and siRNA oligonucleotides. Pharm. Res. 22, 2099–2106 10.1007/s11095-005-8330-5 (doi:10.1007/s11095-005-8330-5) [DOI] [PubMed] [Google Scholar]
- 120.Han L., Zhang A., Wang H., Pu P., Jiang X., Kang C., Chang J. 2010. Tat-BMPs-PAMAM conjugates enhance therapeutic effect of small interference RNA on U251 glioma cells in vitro and in vivo. Hum. Gene Ther. 21, 417–426 10.1089/hum.2009.087 (doi:10.1089/hum.2009.087) [DOI] [PubMed] [Google Scholar]
- 121.Darbre T., Reymond J. L. 2008. Glycopeptide dendrimers for biomedical applications. Curr. Top. Med. Chem. 8, 1286–1293 10.2174/156802608785849058 (doi:10.2174/156802608785849058) [DOI] [PubMed] [Google Scholar]
- 122.Lagnoux D., Darbre T., Schmitz M. L., Reymond J. L. 2005. Inhibition of mitosis by glycopeptide dendrimer conjugates of colchicine. Chem. Eur. J. 11, 3941–3950 10.1002/chem.200401294 (doi:10.1002/chem.200401294) [DOI] [PubMed] [Google Scholar]
- 123.Arima H., Chihara Y., Arizono M., Yamashita S., Wada K., Hirayama F., Uekama K. 2006. Enhancement of gene transfer activity mediated by mannosylated dendrimer/α-cyclodextrin conjugate (generation 3, G3). J. Control. Release 116, 64–74 10.1016/j.jconrel.2006.08.026 (doi:10.1016/j.jconrel.2006.08.026) [DOI] [PubMed] [Google Scholar]
- 124.Kihara F., Arima H., Tsutsumi T., Hirayama F., Uekama K. 2002. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with alpha-cyclodextrin. Bioconjug. Chem. 13, 1211–1219 10.1021/bc025557d (doi:10.1021/bc025557d) [DOI] [PubMed] [Google Scholar]
- 125.Wada K., Arima H., Tsutsumi T., Chihara Y., Hattori K., Hirayama F., Uekama K. 2005. Improvement of gene delivery mediated by mannosylated dendrimer/α-cyclodextrin conjugates. J. Control. Release 104, 397–413 10.1016/j.jconrel.2005.02.016 (doi:10.1016/j.jconrel.2005.02.016) [DOI] [PubMed] [Google Scholar]
- 126.Duncan R., Izzo L. 2005. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 57, 2215–2237 10.1016/j.addr.2005.09.019 (doi:10.1016/j.addr.2005.09.019) [DOI] [PubMed] [Google Scholar]
- 127.Roberts J. C., Bhalgat M. K., Zera R. T. 1996. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J. Biomed. Mater. Res. 30, 53–65 (doi:10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- 128.Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weener J. W., Meijer E. W., Paulus W., Duncan R. 2000. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 65, 133–148 10.1016/S0168-3659(99)00246-1 (doi:10.1016/S0168-3659(99)00246-1) [DOI] [PubMed] [Google Scholar]
- 129.Agashe H. B., Dutta T., Garg M., Jain N. K. 2006. Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J. Pharm. Pharmacol. 58, 1491–1498 10.1211/jpp.58.11.0010 (doi:10.1211/jpp.58.11.0010) [DOI] [PubMed] [Google Scholar]
- 130.Kesharwani P., Gajbhiye V., Tekade R. K., Jain N. K. 2011. Evaluation of dendrimer safety and efficacy through cell line studies. Curr. Drug Targets 12, 1478–1497 10.2174/138945011796818135 (doi:10.2174/138945011796818135) [DOI] [PubMed] [Google Scholar]
- 131.Chen H. T., Neerman M. F., Parrish A. R., Simanek E. E. 2004. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc. 126, 10 044–10 048 10.1021/ja048548j (doi:10.1021/ja048548j) [DOI] [PubMed] [Google Scholar]
- 132.Prieto M. J., Bacigalupe D., Pardini O., Amalvy J. I., Venturini C., Morilla M. J., Romero E. L. 2006. Nanomolar cationic dendrimeric sulfadiazine as potential antitoxoplasmic agent. Int. J. Pharm. 326, 160–168 10.1016/j.ijpharm.2006.05.068 (doi:10.1016/j.ijpharm.2006.05.068) [DOI] [PubMed] [Google Scholar]
- 133.Stasko N. A., Johnson C. B., Schoenfisch M. H., Johnson T. A., Holmuhamedov E. L. 2007. Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules 8, 3853–3859 10.1021/bm7008203 (doi:10.1021/bm7008203) [DOI] [PubMed] [Google Scholar]
- 134.Hunter A. C. 2006. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 58, 1523–1531 10.1016/j.addr.2006.09.008 (doi:10.1016/j.addr.2006.09.008) [DOI] [PubMed] [Google Scholar]
- 135.Mukherjee S. P., Davoren M., Byrne H. J. 2010. In vitro mammalian cytotoxicological study of PAMAM dendrimers: towards quantitative structure activity relationships. Toxicol. In Vitro 24, 169–177 10.1016/j.tiv.2009.09.014 (doi:10.1016/j.tiv.2009.09.014) [DOI] [PubMed] [Google Scholar]
- 136.Kukowska-Latallo J. F., Candido K. A., Cao Z., Nigavekar S. S., Majoros I. J., Thomas T. P., Balogh L. P., Khan M. K., Baker J. R., Jr 2005. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 65, 5317–5324 10.1158/0008-5472.CAN-04-3921 (doi:10.1158/0008-5472.CAN-04-3921) [DOI] [PubMed] [Google Scholar]
- 137.Kolhatkar R. B., Kitchens K. M., Swaan P. W., Ghandehari H. 2007. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug. Chem. 18, 2054–2060 10.1021/bc0603889 (doi:10.1021/bc0603889) [DOI] [PubMed] [Google Scholar]
- 138.Waite C. L., Sparks S. M., Uhrich K. E., Roth C. M. 2009. Acetylation of PAMAM dendrimers for cellular delivery of siRNA. BMC Biotechnol. 9, 38–47 10.1186/1472-6750-9-38 (doi:10.1186/1472-6750-9-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo D., Haverstick K., Belcheva N., Han E., Saltzman W. M. 2002. Poly(ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules 35, 3456–3462 10.1021/ma0106346 (doi:10.1021/ma0106346) [DOI] [Google Scholar]
- 140.Yang H., Lopina S. T., DiPersio L. P., Schmidt S. P. 2008. Stealth dendrimers for drug delivery: correlation between PEGylation, cytocompatibility, and drug payload. J. Mater. Sci. Mater. Med. 19, 1991–1997 10.1007/s10856-007-3278-0 (doi:10.1007/s10856-007-3278-0) [DOI] [PubMed] [Google Scholar]
- 141.Choi J. S., Nam K., Park J. Y., Kim J. B., Lee J. K., Park J. S. 2004. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with l-arginine. J. Control. Release 99, 445–456 10.1016/j.jconrel.2004.07.027 (doi:10.1016/j.jconrel.2004.07.027) [DOI] [PubMed] [Google Scholar]
- 142.Kumar P. V., Asthana A., Dutta T., Jain N. K. 2006. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J. Drug Target 14, 546–556 10.1080/10611860600825159 (doi:10.1080/10611860600825159) [DOI] [PubMed] [Google Scholar]
- 143.Chen X., Wu P., Rousseas M., Okawa D., Gartner Z., Zettl A., Bertozzi C. R. 2009. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 131, 890–891 10.1021/ja807334b (doi:10.1021/ja807334b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heiden T. C., Dengler E., Kao W. J., Heideman W., Peterson R. E. 2008. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharmacol. 225, 70–79 10.1016/j.taap.2007.07.009 (doi:10.1016/j.taap.2007.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ortega-Munoz M., Morales-Sanfrutos J., Perez-Balderas F., Hernandez-Mateo F., Giron-Gonzalez M. D., Sevillano-Tripero N., Salto-Gonzalez R., Santoyo-Gonzalez F. 2007. Click multivalent neoglycoconjugates as synthetic activators in cell adhesion and stimulation of monocyte/machrophage cell lines. Org. Biomol. Chem. 5, 2291–2301 10.1039/b706331h (doi:10.1039/b706331h) [DOI] [PubMed] [Google Scholar]
- 146.Raman R., Venkataraman M., Ramakrishnan S., Lang W., Raguram S., Sasisekharan R. 2006. Advancing glycomics: implementation strategies at the consortium for functional glycomics. Glycobiology 16, 82R–90R 10.1093/glycob/cwj080 (doi:10.1093/glycob/cwj080) [DOI] [PubMed] [Google Scholar]
- 147.Comelli E. M., et al. 2006. A focused microarray approach to functional glycomics: transcriptional regulation of the glycome. Glycobiology 16, 117–131 10.1093/glycob/cwj048 (doi:10.1093/glycob/cwj048) [DOI] [PubMed] [Google Scholar]
- 148.Kim J. K., Shukla R., Casagrande L., Sedgley C., Nor J. E., Baker J. R., Jr, Hill E. E. 2010. Differentiating dental pulp cells via RGD–dendrimer conjugates. J. Dent. Res. 89, 1433–1438 10.1177/0022034510384870 (doi:10.1177/0022034510384870) [DOI] [PubMed] [Google Scholar]