Abstract
More and more applications of nanomaterials have been achieved in the biomedicine field. Numerous nanomedical devices, such as bone grafts with nano-hydroxyapatite and the silver-based anti-bacteria products, have been developed and have been trying to enter into the Chinese market. The State Food and Drug Administration of China (SFDA) is facing a critical challenge of how to explore and supervise the safety assessment of the nanomedical products. This paper briefly introduces the approval status of nanomedical products and the current advances of the safety assessment of nanomaterials in China.
Keywords: nanomaterials, nanomedical devices, safety assessment
1. Current state of the nanomedical products in china
Recently, with the intense interest in nano-biotechnology, more and more nanomaterials have been widely involved in biomedical studies. A large number of nanomedical devices have been successfully developed. So far, there are two categories of device with nanomaterials that have been approved for clinic applications in China by the State Food and Drug Administration of China (SFDA). One is nano-hydroxyapatite as bone repair materials, and the other is the silver-based anti-bacteria product for wound-protection etc.
Regarding the above two categories of nanomaterials, there are already 190 medical devices approved to enter the market by the SFDA, in which there are six products imported from other countries while the rest are all made in China. There are 53 nanomaterial products in the approval process of the SFDA. Among the 190 medical products, about 60 per cent are silver-based anti-bacteria products and 40 per cent are bone repair materials in which one main component is nano-hydroxyapatite. The SFDA-approved nanomedical devices are listed in table 1.
Table 1.
Nanomedical devices approved for clinical applications by SFDA.
| production place (province) | number | product description |
|---|---|---|
| Guangdong | 33 | silver-based anti-bacteria products |
| Inner Mongolia | 29 | silver-based anti-bacteria products |
| Jilin | 25 | silver-based anti-bacteria products |
| Henan | 24 | silver-based anti-bacteria products |
| Jiangsu | 12 | silver-based anti-bacteria products |
| Hubei | 11 | silver-based anti-bacteria products |
| Heilongjiang | 8 | nano-treatment instrument |
| Guizhou | 8 | silver-based anti-bacteria products, nano energy for pain relief card |
| Hunan | 8 | silver-based anti-bacteria products |
| abroad | 6 | NANOSIT, Ceram X, Ketac N100 Light-Curing Nano-Ionomer Restorative, 3M ESPE FiltekTM Z350 XT |
| Shanghai | 5 | nano-acupoint application |
| Sichuan | 5 | infrared-cured pain post; hydroxyapatite/polyamide 66 composite bone graft |
| Beijing | 4 | nano-functional magnets, mineralized collagen bone graft |
| Jiangxi | 2 | nano-oxygen running water |
| Shanxi | 2 | anti-bacteria nano-film |
| Guangxi | 2 | silver-based anti-bacteria products |
| Liaoning | 2 | silver-based anti-bacteria products |
| Zhejiang | 2 | silver-based anti-bacteria products |
| Shandong | 2 | silver-based anti-bacteria products |
| Tianjin | 2 | nanocomposite antibacterial condoms |
| Anhui | 1 | silver-based anti-bacteria products |
| Fujian | 1 | silver-based anti-bacteria products |
| Shanxi | 1 | silver-based anti-bacteria products |
| Ningxia | 1 | silver-based anti-bacteria products |
The volume of nanomedical devices made in China has been more than one billion Chinese Yuan per year since 2010, which is shared by approximately 100 enterprises. As many enterprises have been involved in the research and design of nanomedical devices, a big challenge to the SFDA nowadays is how to explore and supervise the safety assessment of these nanomedical devices.
2. Current state of the safety assessment of nanomaterials in china
According to the number of patents filed, China currently ranks second to the United States in the field of nanoscience and nanotechnology research [1]. Concerning the increase in applications of nanomaterials in medicine, China has begun to pay more attention to the risk analysis and safety evaluation of nanomaterials. Herein, we try to review the latest advances in the safety assessment of some typical nano-biomaterials in China, including carbon nanomaterials, metallic and metal oxide nanomaterials, polymer nanomaterials, nano-hydroxyapatite, etc.
3. Carbon nanomaterials
Carbon nanomaterials such as carbon nanotubes, fullerene, graphene and their derivatives have a broad range of potential applications in multiple biomedical fields, especially in cancer therapy and diagnosis [2,3]. Recently, the potential toxic effects of carbon nanomaterials have attracted much attention. Carbon nanotubes were found to cause oxidative damage in living cells [4], produce a series of multiple lesions in rats [5] and also cause an indirect cytotoxicity through affecting immune functions [6]. In the mean time, Bai et al. [7] showed that carbon nanotubes only cause reversible testis damage without affecting the fertility in mice. Many efforts were also made to reduce the cytotoxicity of carbon nanotubes by functionalizing carbon nanotubes [8] or binding human serum proteins with carbon nanotubes [9].
The cytotoxicity and bioactivity of carbon nanomaterials presented a structure-dependent property. With different geometric structures, the cytotoxicity is different. For example, the cytotoxicity of fullerene is much lower than that of carbon nanotubes for lung macrophage cells [10]. Chang et al. [11] also found that fullerene derivatives C60(C(COOH)2)2 have no cytotoxicity to the Rh35 and HepG2 cells. However, Su et al.'s [12] research indicated that the cytotoxicity of C60(OH)x was cell type-specific.
Graphene, a two-dimensional sp2-carbon nanomaterial, has attracted more attention in many more fields than carbon nanotubes and fullerene in recent years. However, in China, the potential toxicity of graphene has been relatively less explored until recently. A number of researchers demonstrated that graphene oxides (GO) showed very good biocompatibility with several types of cells and animals at low concentration [13,14], and even the ability to promote neurite sprouting of mouse hippocampal cells [15,16]. However, owing to its high accumulation and long-term retention, graphene oxides can cause oxidative stress to cells and animals and induce cell apoptosis and lung granuloma formation at high concentration (at a dose of 10 mg kg−1) [13,17]. On the other hand, Hu et al. [18] found that foetal bovine serum can reduce the cytotoxicity of GO, owing to the high protein adsorption capacity of GO. Yang et al. [19] demonstrated that poly(ethylene glycol)(PEG)-ylated graphene oxides showed significantly improved biocompatibility, not causing appreciable toxicity at a dose of 20 mg kg−1 to mice. The toxicity of carbon nanomaterials is a subject of ongoing debate, and much work should be done before carbon nanomaterials can be applied in clinic.
4. Metallic and oxide nanomaterials
Metallic and metal oxide nanomaterials such as quantum dots (QDs), gold and silver nanoparticles (NPs), magnetic NPs, TiO2 and SiO2, have been widely used in biosensors, cell tracking, molecular imaging, photothermal therapy and drug delivery [20]. The research of potential toxic effects of metallic compound nanomaterials has become a critical issue in China.
QDs, which are also known as semiconductor nanocrystals, are generally composed of metal elements such as Cd, Se, Zn, Te, Ag. CdSe and CdTe QDs are the most widely studied, owing to their unique optical properties. The in vitro cytotoxic effects of QDs in numerous cells have been studied by many groups. Su et al. [21] showed both the release of free Cd2+ and the specific properties of NPs contribute to the cytotoxicity of CdTe QDs. Yan et al. [22] reported that mercaptosuccinic acid-capped CdTe QDs can cause direct toxic effects on human vascular endothelial cells both by impairing mitochondria and inducing endothelial apoptosis. Furthermore, Li et al. [15,16] found microRNAs as participants in cytotoxicity of CdTe QDs in NIH/3T3 cells by using the SOLiD sequencing method. Potential toxicity of QDs in animals has also been investigated by several groups. Liu et al. [23] showed that CdSe QDs can promote the production of intracellular reactive oxygen species and induce significant impairments to the liver in mice. Appropriate surface modification can enhance the stability of QDs in physiological solutions and improve the biocompatibility of QDs. Su et al. [24] found that ZnS layer coating can greatly reduce cytotoxicity and improve the biocompatibility of CdTe QDs. Guo et al. [25] showed surface modification with F-68 and sodium dodecyl sulphate could reduce the cytotoxicity of CdSe QDs. Li et al. [26] found a chirality-dependent cytotoxicity of QDs, that is, d-glutathione (d-GSH)-coated QDs showed less cytotoxicity than l-GSH-coated QDs.
Owing to the wide applications of gold nanoparticles (AuNPs), the cytotoxicity of AuNPs has become a big issue in China. Investigators have observed that AuNPs can induce oxidative stress in blood serum [27] and cause cytotoxicity in human K562 cells at a high concentration [28]. Lin et al. [29] found that the cytotoxicity of AuNPs increases as the percentage of ammonium-functionalized ligands on an AuNP increases. Yi et al. [30] reported that AuNPs exposure-induced osteogenic differentiation and inhibited adipogenic differentiation of mesenchymal stem cells by activating the p38 mitogen-activated protein kinase signalling pathway.
The cytotoxicity of silver nanoparticles (AgNPs) has also been well studied in China owing to their wide applications in biomedicine as antivirus and antibacterial reagents. Wei et al. [31,32] reported that AgNPs could be phagocytized into the cells, thus inducing cell apoptosis, and smaller nanoparticles may cause higher toxic effects than larger ones owing to their easier entry into cells. AgNPs may also alter the action potential of hippocampal CA1 neurons by depressing the voltage-gated sodium current [33]. Moreover, Yang et al. [34] showed AgNPs can interfere with DNA replication fidelity and bind with DNA. However, there are some conflicting results owing to the difficulty of obtaining different-sized AgNPs as standards for their safety evaluation. More recently, Li et al. [35] prepared a series of monodispersed AgNPs and standardized their size- and dose-dependent cytotoxicity.
Fe2O3 and Fe3O4 nanoparticles are the most widely used magnetic nanoparticles. Both of them were found to generate oxidative stress in human umbilical endothelial cells [36]. In addition, Wei et al. [37] found that Fe3O4, oleic acid-coated Fe3O4 and carbon-coated Fe could affect the viability of human hepatoma BEL-7402 cells via cell arrest and induce apoptosis through the mitochondrial-dependent pathway. The potential toxicity of magnetic nanoparticles in animals has also been explored. Zhu et al. [38] found potential lung and systemic cumulative toxicity of Fe2O3 nanoparticles in rats, even at a low concentration. Moreover, smaller Fe2O3 nanoparticles could induce more severe oxidative stress and nerve cell damage in the brain than larger particles [39]. On the other hand, Song et al. [40] showed Fe nanowires had a good biocompatibility and a very low cytotoxicity with HeLa cells.
The toxic effects of other metallic and metallic compound nanomaterials such as TiO2, SiO2, copper and zinc NPs have also been explored in China. For TiO2 NPs, much research focused on the potential neurotoxicological effects. TiO2 NPs could be translocated into the brain and cause brain injury in mice [41,42]. Additionally, the cytotoxicity of TiO2 NPs was found to be dependent on their size, shape and surface modification [43,44]. For SiO2 NPs, Wang et al. [45] showed that SiO2 NPs can cause a dose-dependent cytotoxicity in cultured HEK293 cells by increasing oxidative stress. Moreover, SiO2 NPs have a potential risk for neurodegenerative diseases [46]. On the other hand, Liu et al. [47] showed mesoporous hollow SiO2 NPs had a low toxicity in mice with intravenous injection, and could be excreted from the body over four weeks. For copper and zinc NPs, research found the toxicity of these NPs was size-dependent [48,49].
5. Nano-hydroxyapatite-containing biomaterials
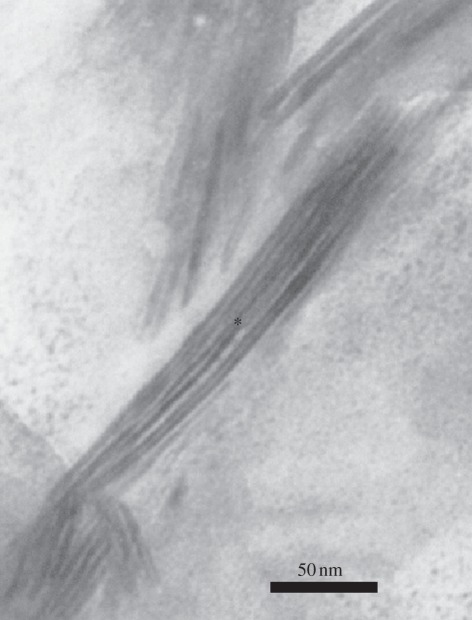
Hydroxyapatite (HA) and its derivatives have been widely studied to explore their biocompatibility with tissue. Cui et al. developed a nano-hydroxyapatite/collagen composite and tested its ability in bone repairing. The composite showed some features of natural bone in both composition and microstructure. The inorganic phase in the composite was carbonate-substituted HA with low crystallinity and nanometre size. HA crystals grew on the surface of these fibrils in such a way that their c-axes were oriented along the longitudinal axes of the fibrils. The mineralized collagen fibrils aligned parallel to each other to form fibres, and the hierarchical fibres of collagen–hydroxyapatite composite were observed under transmission electron microscopy [50]. For example, Figure 1 shows the microstructure of the composite of bone graft that had been used in clinics. At the interface of this implant and marrow tissue, solution-mediated dissolution and macrophage-mediated resorption led to the degradation of the composite, followed by interfacial bone formation by osteoblasts. The process of implant degradation and bone substitution was reminiscent of bone remodelling. The composite can be incorporated into bone metabolism instead of being a permanent implant [51].
Figure 1.
Higher magnification of the mineralized collagen fibrils. Selected area electron diffraction pattern of the mineralized collagen fibrils in the area of the asterisk in this figure revealed that nano-HA crystals are ordered assembled with the collagen fibres.
Wang et al. [52] found that nano-HA/polyamide composite scaffolds exhibit good biocompatibility and extensive osteoconductivity with host bone. Xu et al. found that polyvinyl alcohol hydrogel modified with nano-HA can improve biocompatibility [53]. Lin et al. [54] showed a nano-grade HA/collagen composite which had an excellent biocompatibility in mice.
6. Polymer nanomaterials
Polymer NPs are generally designed for targeted delivery of drugs. The toxic effects of several polymers have been explored in China. Hu et al. [55] found polymeric NP–aptamer bioconjugates exhibited little toxicity and could diminish the toxicity of mercury. Huang et al. [56] showed poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) nanomaterials did not cause any acute toxicity and genotoxicity. He et al. [57] synthesized a family of novel MeO-poly (ethylene glycol)-poly (d,l-lactic-co-glycolic acid)-poly (ethylene glycol)-OMe triblock copolymer nanoparticles, and showed the cytotoxicity and haemocompatibility of copolymer were dependent on the molecular weight of PEG used in the synthesis of polymers.
7. Prospect on the safety assessment of nanomaterials in china
Special attention should be paid to nanomaterials when they are used for implants in human body, because they could invade through three body barriers of lung, intestines and skin and directly enter the circulation system.
Based on the research listed above, nanomaterials present two distinct biological characteristics. First, in the level of the whole body, nanomaterials tend to target and accumulate in certain organs, such as the liver, spleen and kidney. Second, in the cellular level, nanomaterials sized in the range of smaller than about 40 nm can easily enter cells in different ways and cause changes and even the loss of cellular functions. Therefore, for the clinical applications of nanomaterials, more detailed systematic investigations on the nanomaterials performance, in terms of transformation, distribution, transference, metabolism, drainage and accumulation inside the human body, are seriously needed. The SFDA will make efforts to work together with the scientists to continue exploring and supervising the safe clinical applications of nanobiomaterials in medicine.
Acknowledgments
This work is in part supported by NSFC grant 50573044, and Chinese National Hi-tech programme No. 2011AA030105.
References
- 1.Tang L., Carley S., Porter A. 2011. Charting nano environmental, health, and safety research trajectories: is China convergent with the United States? J. Sci. Policy Gov. 1, 1–16 [Google Scholar]
- 2.Li X. M., Fan Y. B., Watari F. 2010. Current investigations into carbon nanotubes for biomedical application. Biomed. Mater. 5, 022001. 10.1088/1748-6041/5/2/022001 (doi:10.1088/1748-6041/5/2/022001) [DOI] [PubMed] [Google Scholar]
- 3.Feng L. Z., Liu Z. A. 2011. Graphene in biomedicine: opportunities and challenges. Nanomed. UK 6, 317–324 10.2217/nnm.10.158 (doi:10.2217/nnm.10.158) [DOI] [PubMed] [Google Scholar]
- 4.Cheng W. W., et al. 2011. Single-walled carbon nanotube induction of rat aortic endothelial cell apoptosis: reactive oxygen species are involved in the mitochondrial pathway. Int. J. Biochem. Cell B 43, 564–572 10.1016/j.biocel.2010.12.013 (doi:10.1016/j.biocel.2010.12.013) [DOI] [PubMed] [Google Scholar]
- 5.Liu A. H., Sun K. N., Yang J. F., Zhao D. 2008. Toxicological effects of multi-wall carbon nanotubes in rats. J. Nanopart. Res. 10, 1303–1307 10.1007/s11051-008-9369-0 (doi:10.1007/s11051-008-9369-0) [DOI] [Google Scholar]
- 6.Sun Z., et al. 2011. Carbon nanotubes enhance cytotoxicity mediated by human lymphocytes in vitro. PLoS ONE 6, e21073. 10.1371/journal.pone.0021073 (doi:10.1371/journal.pone.0021073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y., Zhang Y., Zhang J., Mu Q., Zhang W., Butch E. R., Snyder S. E., Yan B. 2010. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat. Nano 5, 683–689 10.1038/nnano.2010.153 (doi:10.1038/nnano.2010.153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H. Y., et al. 2008. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity, and immune response. Nano Lett. 8, 859–865 10.1021/nl0730155 (doi:10.1021/nl0730155) [DOI] [PubMed] [Google Scholar]
- 9.Ge C. C., et al. 2011. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl Acad. Sci. USA 108, 16 968–16 973 10.1073/pnas.1105270108 (doi:10.1073/pnas.1105270108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia G., Wang H. F., Yan L., Wang X., Pei R., Yan T., Zhao Y., Guo X. 2005. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 39, 1378–1383 10.1021/es048729l (doi:10.1021/es048729l) [DOI] [PubMed] [Google Scholar]
- 11.Ye C., et al. 2006. In situ observation of C60(C(COOH)2)2 interacting with living cells using fluorescence microscopy. Chinese Sci. Bull. 51, 1060–1064 10.1007/s11434-006-1060-1 (doi:10.1007/s11434-006-1060-1) [DOI] [Google Scholar]
- 12.Su Y. Y., et al. 2010. Cellular uptake and cytotoxic evaluation of fullerenol in different cell lines. Toxicology 269, 155–159 10.1016/j.tox.2009.11.015 (doi:10.1016/j.tox.2009.11.015) [DOI] [PubMed] [Google Scholar]
- 13.Zhang X. Y., Yin J. L., Peng C., Hu W., Zhu Z., Li W., Fan C., Huang Q. 2011. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 49, 986–995 10.1016/j.carbon.2010.11.005 (doi:10.1016/j.carbon.2010.11.005) [DOI] [Google Scholar]
- 14.Chang Y. L., Yang S. T., Liu J. H., Dong E., Wang Y., Cao A., Liu Y., Wang H. 2011. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 200, 201–210 10.1016/j.toxlet.2010.11.016 (doi:10.1016/j.toxlet.2010.11.016) [DOI] [PubMed] [Google Scholar]
- 15.Li N., et al. 2011. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 32, 9374–9382 10.1016/j.biomaterials.2011.08.065 (doi:10.1016/j.biomaterials.2011.08.065) [DOI] [PubMed] [Google Scholar]
- 16.Li S. C., Wang Y., Wang H. T., Bai Y., Liang G., Wang Y., Huang N., Xiao Z. 2011. MicroRNAs as participants in cytotoxicity of CdTe quantum dots in NIH/3T3 cells. Biomaterials 32, 3807–3814 10.1016/j.biomaterials.2011.01.074 (doi:10.1016/j.biomaterials.2011.01.074) [DOI] [PubMed] [Google Scholar]
- 17.Wang K., Ruan J., Song H., Zhang J., Wo Y., Guo S., Cui D. 2011. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 6, 8. 10.1007/S11671-010-9751-6 (doi:10.1007/S11671-010-9751-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W. B., Peng C., Lv M., Li X., Zhang Y., Chen N., Fan C., Huang Q. 2011. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 5, 3693–3700 10.1021/nn200021j (doi:10.1021/nn200021j) [DOI] [PubMed] [Google Scholar]
- 19.Yang K., Wan J. M., Zhang S. A., Zhang Y., Lee S. T., Liu Z. 2011. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 5, 516–522 10.1021/nn1024303 (doi:10.1021/nn1024303) [DOI] [PubMed] [Google Scholar]
- 20.Kumar C. 2009. Metallic nanomaterials. Weinheim, Germany: Wiley [Google Scholar]
- 21.Su Y. Y., Hu M., Fan C. H., He Y., Li Q. N., Li W. X., Wang L. H., Shen P. P., Huang Q. 2010. The cytotoxicity of CdTe quantum dots and the relative contributions from released cadmium ions and nanoparticle properties. Biomaterials 31, 4829–4834 10.1016/j.biomaterials.2010.02.074 (doi:10.1016/j.biomaterials.2010.02.074) [DOI] [PubMed] [Google Scholar]
- 22.Yan M., Zhang Y., Xu K. D., Fu T., Qin H., Zheng X. 2011. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology 282, 94–103 10.1016/j.tox.2011.01.015 (doi:10.1016/j.tox.2011.01.015) [DOI] [PubMed] [Google Scholar]
- 23.Liu W., et al. 2011. CdSe quantum dot (QD)-induced morphological and functional impairments to liver in mice. PLoS ONE 6, e24406. 10.1371/journal.pone.0024406 (doi:10.1371/journal.pone.0024406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su Y. Y., et al. 2009. The cytotoxicity of cadmium based, aqueous phase—synthesized, quantum dots and its modulation by surface coating. Biomaterials 30, 19–25 10.1016/j.biomaterials.2008.09.029 (doi:10.1016/j.biomaterials.2008.09.029) [DOI] [PubMed] [Google Scholar]
- 25.Guo G. N., Liu W., Liang J. G., He Z., Xu H., Yang X. 2007. Probing the cytotoxicity of CdSe quantum dots with surface modification. Mater. Lett. 61, 1641–1644 10.1016/j.matlet.2006.07.105 (doi:10.1016/j.matlet.2006.07.105) [DOI] [Google Scholar]
- 26.Li Y. Y., Zhou Y. L., Wang H. Y., Perrett S., Zhao Y., Tang Z., Nie G. 2011. Chirality of glutathione surface coating affects the cytotoxicity of quantum dots. Angew. Chem. Int. Ed. 50, 5860–5864 10.1002/anie.201008206 (doi:10.1002/anie.201008206) [DOI] [PubMed] [Google Scholar]
- 27.Jia H. Y., Liu Y., Zhang X. J., Han L., Du L. B., Tian Q., Xu Y. C. 2009. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J. Am. Chem. Soc. 131, 40–41 10.1021/ja808033w (doi:10.1021/ja808033w) [DOI] [PubMed] [Google Scholar]
- 28.Zhang X. D., Guo M. L., Wu H. Y., Sun Y. M., Ding Y. Q., Feng X., Zhang L. A. 2009. Irradiation stability and cytotoxicity of gold nanoparticles for radiotherapy. Int. J. Nanomed. 4, 165–173 10.2147/IJN.S6723 (doi:10.2147/IJN.S6723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J. Q., Zhang H. W., Chen Z., Zheng Y. 2010. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 4, 5421–5429 10.1021/nn1010792 (doi:10.1021/nn1010792) [DOI] [PubMed] [Google Scholar]
- 30.Yi C. Q., Liu D. D., Fong C. C., Zhang J., Yang M. 2010. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 4, 6439–6448 10.1021/nn101373r (doi:10.1021/nn101373r) [DOI] [PubMed] [Google Scholar]
- 31.Wei L. N., Tang J. L., Zhang Z. X., Chen Y., Zhou G., Xi T. 2010. Investigation of the cytotoxicity mechanism of silver nanoparticles in vitro. Biomed. Mater. 5, 044103. [DOI] [PubMed] [Google Scholar]
- 32.Liu W., et al. 2010. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4, 319–330 10.3109/17435390.2010.483745 (doi:10.3109/17435390.2010.483745) [DOI] [PubMed] [Google Scholar]
- 33.Liu Z. W., Ren G. G., Zhang T., Yang Z. 2009. Action potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal CA1 neurons by silver nanoparticles. Toxicology 264, 179–184 10.1016/j.tox.2009.08.005 (doi:10.1016/j.tox.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 34.Yang W. J., Shen C. C., Ji Q. L., Hongjie A., Jinju W., Qingdai L., Zhizhou Z. 2009. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 20, 085102. 10.1088/0957-4484/20/8/085102 (doi:10.1088/0957-4484/20/8/085102) [DOI] [PubMed] [Google Scholar]
- 35.Li L., Sun J., Li X., Zhang Y., Wang Z., Wang C., Dai J., Wang Q. 2012. Controllable synthesis of monodispersed silver nanoparticles as standards for quantitative assessment of their cytotoxicity. Biomaterials 33, 1714–1721 10.1016/j.biomaterials.2011.11.030 (doi:10.1016/j.biomaterials.2011.11.030) [DOI] [PubMed] [Google Scholar]
- 36.Zhu M. T., Yun W., Feng W. Y., Wang M., Ouyang H., Chai Z. F. 2010. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J. Nanosci. Nanotech. 10, 8584–8590 10.1166/jnn.2010.2488 (doi:10.1166/jnn.2010.2488) [DOI] [PubMed] [Google Scholar]
- 37.Wei K., Xu X. J., Pu X. M., Hou Z. Q., Zhang Q. Q. 2011. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 6, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu M. T., Feng W. Y., Wang Y., Wang B., Wang M., Ouyang H., Zhao Y. L., Chain Z. F. 2009. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol. Sci. 107, 342–351 10.1093/toxsci/kfn245 (doi:10.1093/toxsci/kfn245) [DOI] [PubMed] [Google Scholar]
- 39.Wang B., et al. 2009. Neurotoxicity of low-dose repeatedly intranasal instillation of nano- and submicron-sized ferric oxide particles in mice. J. Nanopart. Res. 11, 41–53 10.1007/s11051-008-9452-6 (doi:10.1007/s11051-008-9452-6) [DOI] [Google Scholar]
- 40.Song M. M., Song W. J., Bi H., Wang J., Wu W. L., Sun J., Yu M. 2010. Cytotoxicity and cellular uptake of iron nanowires. Biomaterials 31, 1509–1517 10.1016/j.biomaterials.2009.11.034 (doi:10.1016/j.biomaterials.2009.11.034) [DOI] [PubMed] [Google Scholar]
- 41.Hu R. P., et al. 2010. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials 31, 8043–8050 10.1016/j.biomaterials.2010.07.011 (doi:10.1016/j.biomaterials.2010.07.011) [DOI] [PubMed] [Google Scholar]
- 42.Ma L. L., Liu J., Li N., Wang J., Duan Y., Yan J., Liu H., Wang H., Hong F. 2010. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO(2) delivered to the abdominal cavity. Biomaterials 31, 99–105 10.1016/j.biomaterials.2009.09.028 (doi:10.1016/j.biomaterials.2009.09.028) [DOI] [PubMed] [Google Scholar]
- 43.Wang J. X., et al. 2007. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 168, 176–185 10.1016/j.toxlet.2006.12.001 (doi:10.1016/j.toxlet.2006.12.001) [DOI] [PubMed] [Google Scholar]
- 44.Zhang L. L., et al. 2011. Rutile TiO2 particles exert size and surface coating dependent retention and lesions on the murine brain. Toxicol. Lett. 207, 73–81 10.1016/j.toxlet.2011.08.001 (doi:10.1016/j.toxlet.2011.08.001) [DOI] [PubMed] [Google Scholar]
- 45.Wang F., Gao F., Lan M. B., Yuan H., Hunag Y., Liu J. 2009. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol. In Vitro 23, 808–815 10.1016/j.tiv.2009.04.009 (doi:10.1016/j.tiv.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 46.Wu J., Wang C., Sun J., Xue Y. 2011. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano 5, 4476–4489 10.1021/nn103530b (doi:10.1021/nn103530b) [DOI] [PubMed] [Google Scholar]
- 47.Liu T. L., Li L. L., Teng X., Huang X., Liu H., Chen D., Ren J., He J., Tang F. 2011. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 32, 1657–1668 10.1016/j.biomaterials.2010.10.035 (doi:10.1016/j.biomaterials.2010.10.035) [DOI] [PubMed] [Google Scholar]
- 48.Wang B., Feng W. Y., Wang T. C., Jia G., Wang M., Shi J. W., Zhang F., Zhao Y. L., Chai Z. F. 2006. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 161, 115–123 10.1016/j.toxlet.2005.08.007 (doi:10.1016/j.toxlet.2005.08.007) [DOI] [PubMed] [Google Scholar]
- 49.Meng H., Chen Z., Xing G. M., Yuan H., Chen C., Zhao F., Zhang C., Zhao Y. 2007. Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 175, 102–110 10.1016/j.toxlet.2007.09.015 (doi:10.1016/j.toxlet.2007.09.015) [DOI] [PubMed] [Google Scholar]
- 50.Zhang W., Liao S. S., Cui F. Z. 2003. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 15, 3221–3226 10.1021/cm030080g (doi:10.1021/cm030080g) [DOI] [Google Scholar]
- 51.Du C., Cui F. Z., Zhang W., Feng Q. L., Zhu X. D., de Groot K. 2000. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 50, 518–527 (doi:10.1002/(SICI)1097-4636(20000615)50:4<518::AID-JBM7>3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 52.Wang H. N., Li Y. B., Zuo Y., Li J., Ma S., Cheng L. 2007. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 28, 3338–3348 10.1016/j.biomaterials.2007.04.014 (doi:10.1016/j.biomaterials.2007.04.014) [DOI] [PubMed] [Google Scholar]
- 53.Xu F. L., Li Y. B., Deng Y. P., Xiong J. 2008. Porous nano-hydroxyapatite/poly(vinylalcohol) composite hydrogel as artificial cornea fringe: characterization and evaluation in vitro. J. Biomater. Sci. Polymer E 19, 431–439 10.1163/156856208783719473 (doi:10.1163/156856208783719473) [DOI] [PubMed] [Google Scholar]
- 54.Lin X. Y., Fan H. S., Li X. D., Tang M., Zhang L. L., Tan Y. F., Xu J. R., Zhang X. D. 2005. In vitro and in vivo biocompatibility of nano-hydroxyapatite/collagen composites. Key. Eng. Mater. 288–289, 195–198 10.4028/www.scientific.net/KEM.288-289.195 (doi:10.4028/www.scientific.net/KEM.288-289.195) [DOI] [Google Scholar]
- 55.Hu X., Tulsieram K. L., Zhou Q., Mu L., Wen J. 2012. Polymeric nanoparticle-aptamer bioconjugates can diminish the toxicity of mercury in vivo. Toxicol. Lett. 208, 69–74 10.1016/j.toxlet.2011.10.006 (doi:10.1016/j.toxlet.2011.10.006) [DOI] [PubMed] [Google Scholar]
- 56.Huang Y. N., et al. 2010. Acute toxicity and genotoxicity studies on poly(epsilon-caprolactone)-poly(ethylene glycol)- poly(epsilon-caprolactone) nanomaterials. Mut. Res. Gen. Toxicol. Environ. 696, 101–106 10.1016/j.mrgentox.2009.12.016 (doi:10.1016/j.mrgentox.2009.12.016) [DOI] [PubMed] [Google Scholar]
- 57.He L. L., Yang L. K., Duan Y. R., Deng L., Sun X., Gu Z., Zhang Z.-R. 2009. Cytotoxicity and hemocompatibility of a family of novel MeO-PEG-Poly (D,L-Lactic-co-glycolic acid)-PEG-OMe triblock copolymer nanoparticles. J. Appl. Polym. Sci. 113, 2933–2944 10.1002/app.30386 (doi:10.1002/app.30386) [DOI] [Google Scholar]