Abstract
Background/Aims
Eradication of Helicobacter pylori is widely accepted as initial therapy for low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma. However, approximately 20% of patients with this disease are not responsive to H. pylori eradication therapy. The aim of this study was to assess remission and relapse rates of low-grade gastric MALT lymphoma after H. pylori eradication and identify the clinical factors that affect remission.
Methods
Thirty-nine patients diagnosed with gastric MALT lymphoma (May 2003 to May 2010) were retrospectively analyzed.
Results
Of the 39 patients, 30 (77%) had a H. pylori infection. There were 35/39 (90%) patients with stage I. Among stage I, 25 patients with the infection underwent eradication therapy and 22/25 (88%) achieved remission. The total regression rate with eradication only in stage I was 24/28 (86%). The median time to remission was 98 days (range, 22 to 397 days). Age, tumor location, invasion depth, H. pylori burden, and severity of mononuclear leukocyte and neutrophil infiltration were not related to remission. However, patients with less neutrophil infiltration were more likely to achieve a successful first H. pylori eradication (p=0.049).
Conclusions
The results show that the rate of low-grade gastric MALT lymphoma regression (86%) with H. pylori eradication alone was higher than that in Western studies (77.8%) and that neutrophil infiltration was inversely related to success of the first H. pylori eradication procedure.
Keywords: Mucosa-associated lymphoid tissue, Lymphoma, Helicobacter pylori, Eradication, Remission
INTRODUCTION
Primary gastric lymphoma is a rare disease accounting 1-7% of gastric malignant tumors, but accounts 60% among gastrointestinal tract lymphoma.1-3 The prevalence of primary gastric lymphoma is increasing compared to the decreasing prevalence of gastric adenocarcinoma.4,5 Around 80% of primary gastric lymphoma cases are B-cell non-Hodgkin lymphoma, which is divided by mucosa-associated lymphoid tissue (MALT) lymphoma and high-grade diffuse large cell lymphoma. Since 1983 when Isaacson and Wright6 first established the concept of mucosa-associated lymph originating from MALT, and the term MALT lymphoma became widely used since then. MALT lymphoma most commonly occurring in the stomach can be divided into a low grade MALT lymphoma which has dense nuclear chromatin and low grade cell division or a high grade MALT lymphoma which has the opposite findings. Low grade gastric MALT lymphoma occurs worldwide,7-9 and is mostly found in gastric antrum and gastric body. Endoscopic features vary from shallow ulcer to simple color change of mucosa, nodularity, and mass. Low grade gastric MALT lymphomas with such nonspecific endoscopic findings are often difficult to differentiate from benign diseases, and follow-up findings after treatment also vary greatly. Low grade gastric MALT lymphoma was revealed to be caused by Helicobacter pylori as an immune response on the gastric mucosa. Patients with low grade gastric MALT lymphoma are reported to have increased H. pylori infection rate as high as 90%.10,11 H. pylori eradication has now become the treatment of choice for low grade gastric MALT lymphoma, which was often treated by surgery in the past. The indication of follow-up after eradiation, the time point of diagnosing complete remission and future treatment plan when the eradication was not led to the tumor regression are not clearly established yet. We investigated factors associated with H. pylori infection rate and eradication success rate in patients with low grade gastric MALT lymphoma, and compared clinicopathological characteristics of patients who received eradication treatment to find a predictive factor of complete remission of the disease.
MATERIALS AND METHODS
Patients and methods
The study was performed in 39 patients who received gastrofibroscopy at least once among 47 patients confirmed as low grade gastric MALT lymphoma by endoscopic biopsy performed at Seoul National University Bundang Hospital between May 2003 and May 2010. Once low grade gastric MALT lymphoma was confirmed by mucosal biopsy, the severity of the infiltration in gastric mucosa and nodal invasion were confirmed by gastric endoscopic ultrasound and the stage was established by abdominal computerized tomography and bone marrow biopsy. H. pylori infection was confirmed by positive histology, rapid urease test, or urea breath test. Tumor remission was determined by follow-up upper gastrointestinal tract endoscopy, mucosal biopsy, and other imaging studies. The follow-up endoscopy was performed with biopsy in several tissues including from lesions presumed abnormal and from areas with normal findings. The clinical courses, endoscopic findings, operation records, pathologic findings, imaging results, treatment course of the patients were retrospectively analyzed. The locations of lesions were termed proximal part from gastric fundus to mid body, and distal part below the lower body. The study was approved by the Ethics Committee at Seoul National University Bundang Hospital.
Statistical analysis
Fisher's exact test and Wilcoxon rank sum test were performed for between-group comparison. Logistic regression analysis was performed to find factors associated with remission of lymphoma. Factors associated with the time to remission were analyzed by Kaplan-Meier method. p-value less than 0.05 were considered statistically significant. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) were used for statistical analysis.
RESULTS
Clinicopathological characteristics of patients
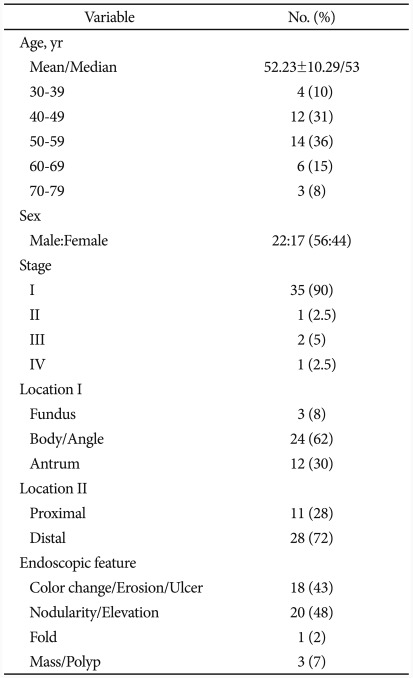
There were 22 male patients and 17 female patients among a total of 39 patients, their mean age was 52.23±10.29 years. Four of them were in their 30s (10%), 12 in their 40s (31%), 14 in their 50s (36%), 6 in their 60s (15%), and 3 in their 70s (8%), with 50s accounting for the biggest part. Stage 1 was the most common in 35 cases (90%), followed by stage 2, stage 3, and stage 4 in 1 case (2.5%), 2 cases (5%), and 1 case (2.5%), respectively (Table 1).
Table 1.
Clinicopathological Characteristics of 39 Patients
Endoscopic findings
Lesions with mucosal nodule or elevated lesion from mucosa were most common in 20 cases (48%) while flat or depressed lesion with erosion, shallow ulcer, or color change of the mucosa were found in 18 cases (43%). Other findings included hypertrophic fold in 1 case (2%) and mass forming lesion in 3 cases (7%). Lymphoma was found mostly commonly at gastric fundus and angle in 24 cases (62%), followed by gastric antrum in 12 cases (30%) and gastric fundus in 3 cases (8%). Lesions were more commonly detected at the distal part when divided by proximal and distal parts, each accounting for 28% (11 cases) and 72% (28 cases). The first impression of lesions by endoscopic findings were benign disease in 15 cases (38%), early gastric cancer in 9 cases (23%), advanced gastric cancer in 2 cases (5%), gastric MALT lymphoma in 12 cases (31%) and other findings 1 case (3%). Seven cases (78%) among 9 cases with early gastric cancer were determined as flat type early gastric cancer (early gastric cancer IIc) by impression. Gastric MALT lymphoma could be differentiated by endoscopic findings only in 41% (16/39).
Rates of positive H. pylori and eradication
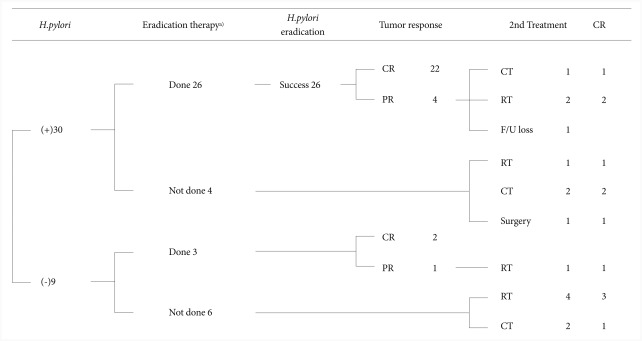
Thirty (77%) among the total 39 patients were H. pylori positive, 26 patients among them received a triple therapy using proton pump inhibitors. Rabeprazole and esomeprazole were each used in 8 cases, pantoprazole in 6 cases, lansoprazole in 2 cases, and omeprazole in 1 case for proton pump inhibitor. The eradication rate of first-line eradication therapy was 69% (18/26). Second-line eradication using proton pump inhibitors, tetracycline, metronidazole, and bismuth was successful in all 8 patients who received the second-line therapy (Fig. 1).
Fig. 1.
Therapeutic scheme of 39 mucosa-associated lymphoid tissue (MALT) lymphoma cases at a uni-center study of Seoul National University Bundang Hospital cases from May 2003 to May 2010. Among 39 patients, 26 patients took eradication therapies. CR, complete remission; PR, partial remission; CT, chemotherapy; RT, radiotherapy; FU, follow-up. a)Include 2nd-line eradication therapy.
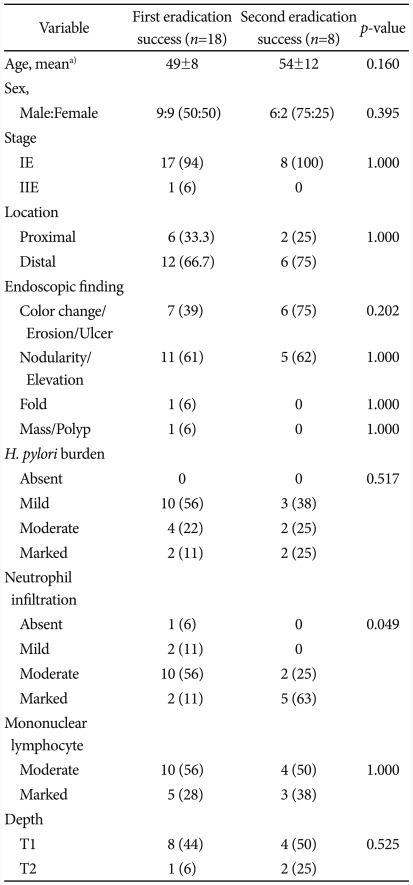
Factors associated with H. pylori eradication
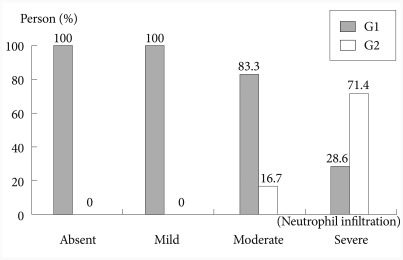
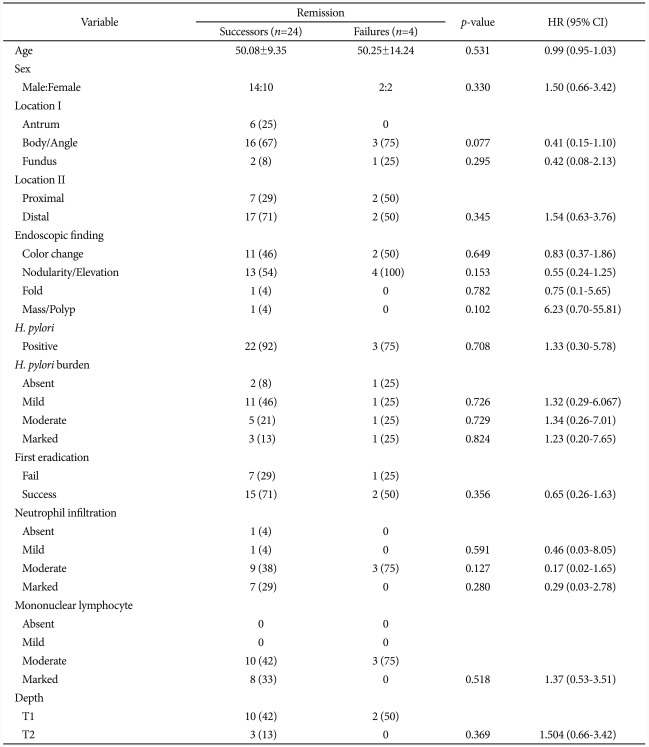
Success of the first-line eradication therapy was affected by the severity of neutrophil infiltration in mucosal tissues (p=0.049) (Table 2). When a linear-by-linear association was analyzed between the success of the first-line eradication therapy and the severity of neutrophil infiltration, the rate of success in the first-line therapy was increased in milder case of neutrophil infiltration (p=0.012) (Fig. 2). Age, stage of the disease, location of gastric MALT lymphoma, the degree of H. pylori, severity of mononuclear lymphocyte infiltration, and the depth of tissue invasion were not significantly associated with the success of H. pylori eradication.
Table 2.
Clinicopathological Difference between the First Eradication Success and the Failure
Values are presented as number (%).
a)In years.
Fig. 2.
Trend between the neutrophil infiltration and the eradication. The less neutrophil infiltration tends to have the higher chance of success in the first eradication. G1, group which achieved first eradication success; G2, group which obtained eradication after the second eradication.
Treatment of low grade MALT lymphoma
Twenty-nine patients among 36 patients with stage 1 and 2 gastric MALT lymphoma were H. pylori positive. Among these, 26 patients received eradication therapy, and 22 of them experienced tumor regression with 84.6% (22/26) remission rate. The remission rate was increased to 88% (22/25) when considering only the patients with stage 1 disease and positive H. pylori. When considering only the H. pylori positive patients who did not receive eradication therapy as an initial treatment, 1 patient with stage 3 disease achieved remission after chemotherapy (R-CHOP). Two patients with a past history of eradication therapy for 2 years before diagnosis were considered to be resistant to eradication therapy because they showed positive H. pylori when diagnosed of low grade gastric MATL lymphoma. They finally achieved R-CHOP and radiotherapy as an initial treatment, respectively. Another 1 patient achieved remission after the administration of proton pump inhibitor but received surgery in another hospital due to recurrence. On the other hand, 7 out of 9 patients with negative H. pylori test had stage 1 disease. Three patients with stage 1 disease received eradication therapy, and 2 among them showed remission revealing 67% remission rate. The remaining 4 patients with stage 1 disease and negative H. pylori received radiotherapy for initial treatment, and 3 of them achieved remission. One patient each in stage 3 and 4 received chemotherapy for initial treatment, but the patient with the stage 4 disease failed remission (Fig. 1). The remission rate in the stage I patients regardless of H. pylori infection was 86% (24/28) when only an eradication therapy was performed.
Factors associated with remission of lymphoma after eradication
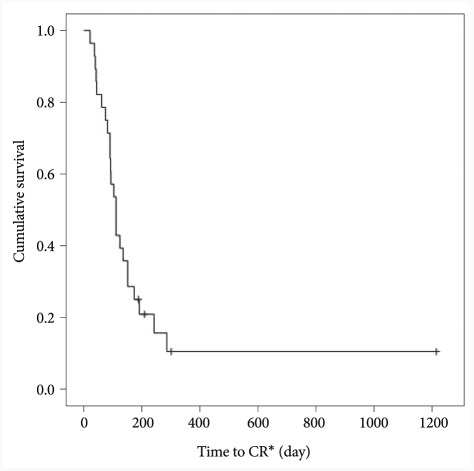
A survival curve from the onset of treatment to remission was graphed for 28 patients who received eradication therapy as the initial treatment among patients with stage 1 disease (Fig. 3). Fifty percent of them achieved remission in 111±11.78 days. These patients were divided again into a remission success group and failure group to find out analyzed factors associated with success of remission after eradication therapy. Factors such as age, disease stage, location of gastric MALT lymphoma, load of H. pylori, H. pylori eradication by first-line eradication therapy, degrees of neutrophil and mononuclear lymphocyte infiltration, and the depth of tissue invasion were not significantly different between the two groups (Table 3).
Fig. 3.
Occurrence of complete remission (CR) event. Kaplan-Meier distribution shows the half of remission occurred within 111 days.
Table 3.
Clinicopathological Difference between the Remission Successors and the Failures in Stage I Patients Who Took the Eradication Therapy as a First Therapy
Values are presented as number (%).
HR, hazard ratio; CI, confidence interval.
Factors associated with the time to remission after eradication therapy
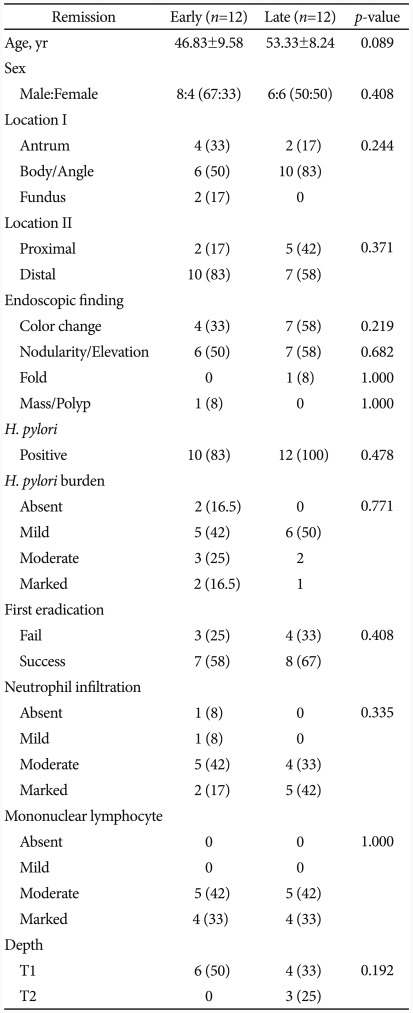
The median time from the onset of eradication therapy to the first remission diagnosis was 98 days (20 to 283 days) among 24 patients who achieved remission by eradication therapy. These patients were further divided into the early remission group and the non-early remission group based on the 98 days of median time to remission in order to find factors associated with the time to remission. Factors such as age, disease stage, location of gastric MALT lymphoma, load of H. pylori, H. pylori eradication by the first eradication therapy, degree of mononuclear lymphocyte infiltration, and the depth of tissue invasion were not significantly different between the two groups (Table 4). The patients in early remission group tended to be younger at diagnosis than those in the non-early remission group, although not statistically significant (p=0.089).
Table 4.
Clinicopathological Difference between the Early Remission and the Late Remission
Values are presented as number (%).
Follow-up and recurrence
Follow-up endoscopy was performed at about 3-month interval until remission is achieved, and every 7 months on average thereafter. The median duration of follow-up was 645 days (127 to 2,428 days). Two patients, among 24 patients with remission, experienced tumor recurrence on day 219 and day 1,327 after the first remission. These 2 patients were all confirmed of H. pylori reinfection, received eradication therapy again, and achieved remission again each on day 141 and 61 after the onset of treatment. The median duration of follow-up while remaining in remission status was 119 days (0 to 2,115 days) among 24 patients who were available for follow-up.
DISCUSSION
The remission rate of low grade gastric MALT lymphoma was 86% when using H. pylori eradication therapy only, which is higher than studies in Western countries. Furthermore, H. pylori eradication was more likely to succeed in a group with less infiltration of neutrophil. This was the first study to compare two groups divided by the median time to remission in order to find factors associated with gastric MALT lymphoma remission, and this study is important in that it attempted to minimize errors arising from subjective observations on endoscopic findings with a single center study design. Significant difference could not be detected from statistical analysis due to the small sample size, which was one of limitations of this study. Endoscopic findings of slightly elevated lesions such as mucosal nodule followed by shallow mucosal lesions including erosion, shallow ulcer, or color change were most commonly detected from low grade gastric MALT lymphoma in this study, similar to findings in previous studies.12,13 Impression or suspicion of gastric MALT lymphoma only by endoscopic findings accounted 41% of overall cases, suggesting the difficulty of differential diagnosis with other diseases.
Positive H. pylori among low grade gastric MALT lymphoma patients in this study were 77%, similar to levels in other studies.14,15 H. pylori burden or location of lymphoma were not significant factors associated with eradication of H. pylori; cases with severe neutrophil infiltration in the tumor tissues were more likely to fail the first-line eradication therapy. Such trend was not reported yet and requires further study in more patients in the future.
In a meta-analysis by Zullo et al.16 involving 1,271 patients in 34 studies reported 98.3% H. pylori eradication rate and 77.8% remission rate in positive H. pylori patients after eradication therapies including 3rd, 4th, and 5th therapies. In the present study, eradication therapy in positive H. pylori infection patients among low grade gastric MALT lymphoma of stage 1 yielded 88% complete remission rate, which is a higher level than other studies performed in Western countries.17-19 Other studies performed in Korean patients reported similar or higher rates of complete remission after eradication therapy, such as the study by Lee et al.20 which reported 82.8% of complete remission rate after H. pylori eradication therapy in 55 patients, the study by Park et al.21 which reported 72.4% in 41 patients, and the study by Hong et al.22 which reported 94.4% in positive H. pylori infection patients with stage 1 or 2 disease. Further studies such as on genetic polymorphism are required to find an explanation regarding relatively higher rate of complete remission after H. pylori eradication therapy in Korean patients compared to Western patients.
H. pylori reinfection 6 months after successful eradication was found in 15% (4/26) in this study, higher than 2.7% in the meta-analysis by Zullo et al.16 Two of these 4 patients experienced recurrence of cancer, received the eradication therapy again, and obtained remission again within 6 months after the onset of therapy. It is suggested, from this result, that low grade gastric MALT lymphoma patients could achieve long-term remission only by H. pylori eradication therapy.23-25
Hong et al.22 reported the median time from onset of eradication therapy to remission as 3 months, which is similar with this study. Other literature reporting 5 months of time to remission recommended wait and see strategy from at least one month until 15 months.26 Three among 4 cases without remission after H. pylori eradication therapy in this study were decided as remission failure before reaching 12 months after diagnosis and result might have been different if followed for a longer time.
According to the currently available data, the remission rate after eradication therapy is about 80% when the cancer is confined to mucosa and submucosal layer. Chemotherapy, radiotherapy, or surgery should be considered when the lesion is not responsive to eradication therapy.24 R-CHOP or radiotherapy were performed in this study, each in 1 and 2 patients without response to the eradication therapy, achieved complete remission.
There were reports that eradication therapy on negative H. pylori infection might yield lower remission rate.27,28 It is now recommended, however, that eradication therapy should be first considered also for H. pylori negative low grade gastric MALT lymphoma patients because there is no established treatment of choice yet and because remission was also reported after eradication therapy for some negative H. pylori infection cases.9,13,24,27,29 We also applied eradication therapy in 3 H. pylori negative patients, and 2 patients among them achieved remission with short time to remission (36 and 88 days, respectively) and this fact supports the eradication therapy as a treatment of choice in even H. pylori negative gastric MALT lymphoma patients.
Recent literature notes negative H. pylori infection, finding of high grade lymphoma cell, and translocations of API2 and MALT genes as factors associated with remission failure after eradication therapy.13 Translocations of these genes were actually found in 67% cases of remission failure after eradication therapy in a study.29 Future studies may evaluate the validity of API2-MALT gene translocation as a predictive factor. Depth of tissue invasion or disease stage have been also selected as potential predictive factors of response to eradication therapy in gastric MALT lymphoma,28 but they did not show any significant difference in this study. On the other hand, in a retrospective study by Kim et al.30 in 95 patients with IE1 stage of disease reported that lesions in the proximal part responded significantly less to a treatment compared to lesions in the distal part. The univariate analysis in this study, however, could not find a significant difference between the two groups divided by proximal or distal part based on the location of tumor. We also attempted to compare clinical characteristics between the early remission group and the non-early remission based on the median 98 days from the onset of therapy to remission in order to find a factor associated with remission. It was found that the early remission group tended to be younger at diagnosis compared to the non-early remission group, although the difference was not statistically significant (p=0.089).
With regard to the still debated follow-up strategy after remission, Hong et al.22 reported H. pylori reinfection as a risk factor of recurrence. All the recurrent patients in this study were also reinfected by H. pylori. H. pylori reinfection is rare in general population with annual prevalence of 1.97%. There is a report the most reinfection occurs within 2 years.31 It is reasonable, therefore, to say that follow-up endoscopy is recommended at 6 month interval for at least 2 years; more clinical studies are required to determine by when the follow-up endoscopy is needed.
In conclusion, the remission rate of low grade gastric MALT lymphoma was 86% when using H. pylori eradication therapy only, which was a higher level than studies in Western countries. The possibility of H. pylori eradication success was increased in patients with less neutrophil infiltration.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Brooks JJ, Enterline HT. Primary gastric lymphomas. A clinicopathologic study of 58 cases with long-term follow-up and literature review. Cancer. 1983;51:701–711. doi: 10.1002/1097-0142(19830215)51:4<701::aid-cncr2820510425>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Lim FE, Hartman AS, Tan EG, Cady B, Meissner WA. Factors in the prognosis of gastric lymphoma. Cancer. 1977;39:1715–1720. doi: 10.1002/1097-0142(197704)39:4<1715::aid-cncr2820390449>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Sandler RS. Has primary gastric lymphoma become more common? J Clin Gastroenterol. 1984;6:101–107. doi: 10.1097/00004836-198404000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Severson RK, Davis S. Increasing incidence of primary gastric lymphoma. Cancer. 1990;66:1283–1287. doi: 10.1002/1097-0142(19900915)66:6<1283::aid-cncr2820660631>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Enno A, O'Rourke JL, Howlett CR, Jack A, Dixon MF, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995;147:217–222. [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacson PG, Spencer J. Gastric lymphoma and Helicobacter pylori. Important Adv Oncol. 1996:111–121. [PubMed] [Google Scholar]
- 9.Nakamura S, Aoyagi K, Furuse M, et al. B-cell monoclonality precedes the development of gastric MALT lymphoma in Helicobacter pylori-associated chronic gastritis. Am J Pathol. 1998;152:1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 10.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 11.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 12.Pinotti G, Zucca E, Roggero E, et al. Clinical features, treatment and outcome in a series of 93 patients with low-grade gastric MALT lymphoma. Leuk Lymphoma. 1997;26:527–537. doi: 10.3109/10428199709050889. [DOI] [PubMed] [Google Scholar]
- 13.Yamada T, Alpers DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW. Textbook of Gastroenterology. 5th ed. Oxford: Wiley-Blackwell; 2008. pp. 1044–1046. [Google Scholar]
- 14.Eck M, Schmausser B, Haas R, Greiner A, Czub S, Müller-Hermelink HK. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 15.Wotherspoon AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med. 1998;49:289–299. doi: 10.1146/annurev.med.49.1.289. [DOI] [PubMed] [Google Scholar]
- 16.Zullo A, Hassan C, Andriani A, et al. Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis. Am J Gastroenterol. 2009;104:1932–1937. doi: 10.1038/ajg.2009.314. [DOI] [PubMed] [Google Scholar]
- 17.Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34–37. doi: 10.1136/gut.53.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H, Saito Y, Hibi T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue (MALT) lymphoma: updated review of clinical outcomes and the molecular pathogenesis. Gut Liver. 2009;3:81–87. doi: 10.5009/gnl.2009.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018–8024. doi: 10.1200/JCO.2005.02.3903. [DOI] [PubMed] [Google Scholar]
- 20.Lee SK, Lee YC, Chung JB, et al. Endoscopic findings and results of long-term follow-up of low grade gastric MALT lymphoma. Korean J Gastrointest Endosc. 2003;26:192–198. [Google Scholar]
- 21.Park KJ, Kim DH, Kim SH, et al. A clinical study of the therapeutic outcome of low-grade gastric MALT lymphoma. Chonnam Med J. 2006;42:94–100. [Google Scholar]
- 22.Hong SS, Jung HY, Choi KD, et al. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter. 2006;11:569–573. doi: 10.1111/j.1523-5378.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 23.Fischbach W, Dragosics B, Kolve-Goebeler ME, et al. The German-Austrian Gastrointestinal Lymphoma Study Group. Primary gastric B-cell lymphoma: results of a prospective multicenter study. Gastroenterology. 2000;119:1191–1202. doi: 10.1053/gast.2000.19579. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88–95. doi: 10.7326/0003-4819-131-2-199907200-00003. [DOI] [PubMed] [Google Scholar]
- 25.Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut. 2002;50(Suppl 3):III19–III24. doi: 10.1136/gut.50.suppl_3.iii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung JB. Gastroenterology. 1st ed. Seoul: Koonja; 2009. pp. 322–323. [Google Scholar]
- 27.Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001;357:39–40. doi: 10.1016/S0140-6736(00)03571-6. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Inagaki H, Seto M, Nakamura S. Gastric low-grade B-cell MALT lymphoma: treatment, response, and genetic alteration. J Gastroenterol. 2003;38:921–929. doi: 10.1007/s00535-003-1234-4. [DOI] [PubMed] [Google Scholar]
- 29.Sleisenger MH, Fordtran JS, Feldman ML, Scharschmidt B. Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 6th ed. Philadelphia: Saunders; 1998. pp. 450–452. [Google Scholar]
- 30.Kim SJ, Yang S, Min BH, et al. Helicobacter pylori eradication for stage IE1 gastric mucosa-associated lymphoid tissue lymphoma: predictive factors of complete remission. Korean J Gastroenterol. 2010;55:94–99. doi: 10.4166/kjg.2010.55.2.94. [DOI] [PubMed] [Google Scholar]
- 31.Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther. 2006;23:275–280. doi: 10.1111/j.1365-2036.2006.02741.x. [DOI] [PubMed] [Google Scholar]