Abstract
Vanadium(IV)-doped titanium dioxide (TiO2) photocatalyst powders were prepared by the sol–gel method and characterized by Brunauer–Emmett–Teller–specific surface area, scanning electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and ultraviolet-visible spectroscopy. V-doping in the TiO2 increases the crystal grain size, which decreases the specific surface areas of powders. This V-doping changes the band gap of TiO2, leading to extend the absorption to visible light regions (400–800 nm). Photocatalytic degradation of methylene blue (MB) in water was investigated as a function of the vanadium content in TiO2 and was found to follow pseudo first-order rate kinetics. Appropriate content of V-doping is an effective means to improve the photocatalytic activity of TiO2 for MB degradation under visible light irradiation.
Key words: : vanadium-doped TiO2, sol–gel, visible light, photocatalytic
Introduction
Environmental pollution has increased public concern nowadays, and decontamination of polluted water and air by photocatalysis has been attracting a lot of attention for its efficiency and promising economy (Sun et al., 2005). Semiconductor heterogeneous photocatalysis is a popular technique that has been applied to a variety of problems of environmental interest. Among various oxide semiconductor photocatalysts, titanium dioxide (TiO2), with great potential to decompose air pollutants and aqueous organic contaminants (Lee et al., 1999; Mao et al., 2005; Yin et al., 2006; Chang et al., 2008; Lu et al., 2010), has been proved to be the most suitable catalysts for widespread environmental application. This is because of its excellent capability of degrading a large variety of environmental contaminants (e.g., organics, bacteria, and viruses), nontoxicity, resistant to acid, alkali and organic solvent, as well as the rich source and low prices of titanium materials (Lee et al., 1999; Senthilkumaar and Porkodi, 2005; Zhou et al., 2005; Yin et al., 2006). Nevertheless, the application of TiO2 as a photocatalyst for visible light-induced chemical reactions was hampered by its large band-gap energy (3.2 eV for anatase TiO2), which requires ultraviolet (UV) light to activate and leads to the lower energy efficiency. To increase the activity of TiO2 and reduce the band-gap energy to the visible light range, the modification of the catalysts has also been attempted by doping them with various transition metals (Yamashita et al., 2003; Wu et al., 2004; Zhu et al., 2006; Shi et al., 2008; Surolia et al., 2010), including vanadium. Recent reports have indicated that vanadium doping provides a promising strategy to improve the photoactivity of titania under visible light. The vanadium ionic radius is almost the same as the titanium ion, so it can be easily doped into TiO2 (Klosek and Raftery, 2001; Wu and Chen, 2004). Therefore, many researchers have prepared V-doped TiO2 and studied its photocatalytic properties. Klosek and Raftery (2001) reported that the V-doped TiO2 can photodegrade ethanol under the illumination of visible light. Anpo et al. (2005) modified TiO2 catalysts by bombarding them with high-energy metal ions. The metal ion-implanted TiO2 showed a large absorption shift toward the visible light region, and the V ion had the highest effectiveness in the red shift. Wu and Chen (2004), and Zhou et al. (2007) synthesized the V-doped TiO2 with visible activity by using modified sol–gel and ion implantation methods, respectively. However, Martin et al. (1994) found that V-doped TiO2 produced by a coprecipitation method reduced the photoactivity over 4-chlorophenol due to charge–carrier recombination. Gu et al. (2008) prepared V-doped TiO2 nanoparticles through the controlled hydrolysis of tetrabutyl titanate in V ion solution and found the photocatalysts showed a very weak red shift. The utilization of visible light is still low. The significant disagreements in the vanadium doping effects in the reported literature might arise from the widely varying experimental conditions in sample preparation and the determination of photoactivity employed by different researchers. Furthermore, the photoactivity of metal ion-doped TiO2 photocatalysts strongly depends on the amounts of dopants (Yamashita et al., 2003). No matter which method is employed, the control of the dopant amount seems very sensitive. A rational distribution of dopant concentration is the key to the effect of doping (Zhu et al., 2006). Thus, the doping TiO2 with widely different amounts of V4+ was realized in our present work to search the optimal V4+ doping amount.
The sol–gel process (Brinker and Scherer, 1990) is a versatile method to prepare nanosize materials. This technique does not require complicated processes such as chemical vapor deposition. It provides a simple and easy means of synthesizing nanosize particles, and has advantages of flexible control of pore structures and dopant concentration and a high level of chemical purity. The incorporation of an active metal in the sol during the gelation stage allows the metal to have a direct interaction with support; therefore, the material possesses special catalytic properties.
In this work, we dealt with the design of visible light-sensitive photocatalyst through the TiO2 doped with vanadium (IV) using the sol–gel process. A systematic characterization was conducted. The photodegradation of methylene blue (MB) was chosen as a model reaction to evaluate the photocatalytic activity of the as-prepared samples. The effects of vanadium loading content on the performances of the photocatalysts for MB degradation were investigated.
Materials and Methods
Preparation of vanadium-doped TiO2 photocatalysts
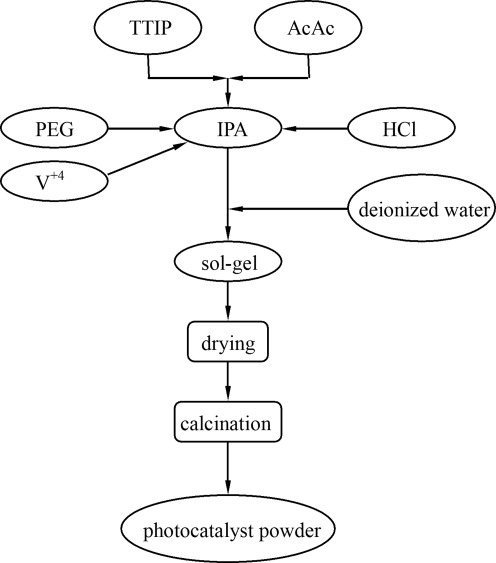
V-doped TiO2 photocatalyst powders were prepared by sol–gel route using titanium tetraisopropoxide (TTIP, Acros; >98%) and vanadium(IV) sulfate oxide (VOSO4, Alfa; 99.9%) as the precursors, isopropanol (Tedia; 99.96%) as the solvent, acetylacetone (AcAc, Tedia; 99.8%) as the stabilizer, polyethylene glycol (Merk) for synthesis as the structure-directing agent, and hydrochloric acid (HCl, Nihon Shiyaku; 37%) as a catalyst (Pecchi et al., 2001). In a typical preparation procedure, 10 mL TTIP, 6.86 mL AcAc, and 20 mL IPA were added into a Pyrex reactor under magnetic stirring, and then 1 g PEG, 2 mL 2 N HCl, and the required amount of VOSO4 were added sequentially. Finally, 5 mL deionized water was added drop-wise into the above solution with a burette under stirring. The resultant alkoxide solution was kept refluxing at the temperature of 50°C–60°C for 2 h, resulting in the titania sol. After the consecutive procedures of drying, calcination, and grinding, the desired photocatalysts were obtained. For convenience sake, the samples were labeled as mV-TiO2, where “m” refers to the molar percentage concentration of V. For example, a sample labeled as 0.01V-TiO2 implies that the sample had been doped with 1 mol% V. For comparison, pure TiO2 was also prepared without adding VOSO4 in the same way. The preparation scheme is shown in Fig. 1.
FIG. 1.
Preparation scheme of V-doped titanium dioxide (TiO2) photocatalyst powders using sol–gel method.
Characterization of photocatalysts
The specific surface area of the as-prepared V-TiO2 powders was determined by a BET analyzer (Micromeritics, ASAP 2101), and the surface morphologies and particle size were observed by a scanning electron microscopy (SEM; Hitachi, S4800-I). The surface chemical states of the V-TiO2 powders were detected by X-ray photoelectron spectroscopy (XPS; Ulvac-Phi, Model ES 650). The crystalline phase for the photocatalyst powders obtained was identified by an X-ray diffractometer (XRD; Rigaku, Rint-2000). A spectrophotometer (Hitachi, U-3010) equipped with an integrating sphere was used to record the UV-visible (UV-Vis) absorption spectra of the powder samples. The baseline correction was performed using a calibrated sample of barium sulfate, and the spectra were recorded at room temperature in air in the range of 200–800 nm.
Photocatalytic degradation of dyes
The photocatalytic degradation of MB in water was carried out in a double-walled cylindrical Pyrex glass reactor. The reactor was equipped with water circulation in the outer jacket to maintain a constant temperature of 25°C. A 300 mL of 20 ppm MB solution and 0.1 g photocatalyst powders were introduced into the reactor. The reaction mixture was mixed at a constant rate during irradiation by a magnetic stirrer. The visible light source is a 23 W fluorescent lamp that placed 40 cm on the top of the reactor. This lamp has an emission range between 390 and 620 nm, with the strongest emission at 545 nm. A suitable cut-off filter (ZUL0400; Asahi Spectra Co.) was placed to ensure that only visible light entered the reactor. The light intensity was estimated to be approximately 4.89 mWcm−2 from the light source incident to the top of the solution. Before irradiation, the suspension was stirred in the dark for 1 h to reach adsorption equilibrium. The blank experiment was also conducted without using photocatalysts. The concentration of aqueous MB was sampled once per hour and determined with a UV-Vis spectrophotometer (Hitachi, U-3010).
Results and Discussion
Characterization of photocatalysts
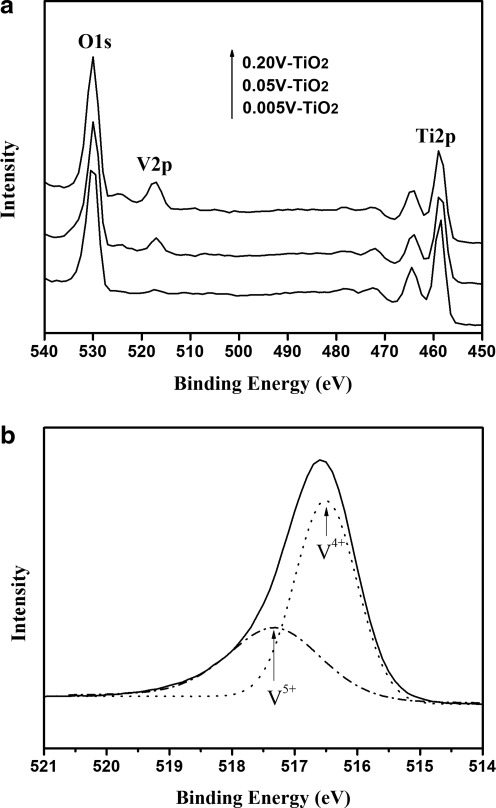
Figure 2a shows the XPS spectra of V-doped TiO2 samples. As seen, the presence of Ti2p, V2p, and O1s signals in the spectra of the samples demonstrates the as-synthesized powders consist of Ti, V, and O elements. Figure 2b shows the high-resolution V2p3/2 spectrum of the 0.05V-TiO2 powders. Using the Gaussian peak fitting, the V2p3/2 XPS spectrum could be deconvoluted into two subpeaks, one located at 516.5 eV, characteristic of V4+, and the other centered at 517.3 eV, characteristic of V5+ (Li et al., 2009). This suggests that V species exist in the forms of V4+ and V5+ in the V-doped TiO2 matrix. Obviously, some V4+ ions were oxidized into V5+ in the preparation process, because vanadium existed only as V4+ in the precursor; the formation of V5+ possibly occurred during annealing.
FIG. 2.
(a) X-ray photoelectron spectroscopy (XPS) spectra of V-doping TiO2 samples and (b) V2p3/2 XPS deconvoluion of the 0.05V-TiO2 powders.
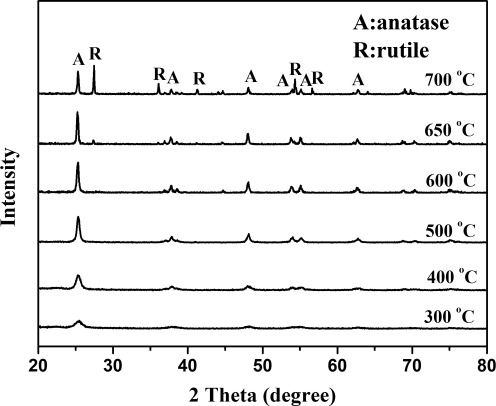
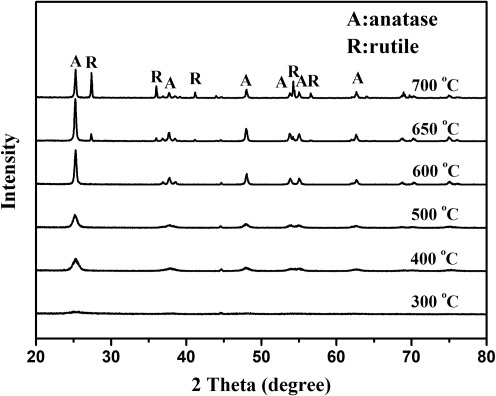
The XRD patterns of undoped (pure) and V-doped TiO2 photocatalyst powders prepared in this study are shown in Figs. 3 and 4, respectively. The crystal phase of pure nanocrystalline TiO2 would transform from anatase to rutile phase at the temperatures above 600°C. As can be seen from Fig. 4, there is no crystalline phase produced when the 0.01V-TiO2 particles were calcined at 300°C. The crystalline phases of pure anatase produced at the temperatures between 400°C and 600°C. To prevent the V-doped TiO2 photocatalyst from forming rutile crystal and losing its decomposition ability to pollutants, the temperature of 600°C was adopted as the selected calcination temperature in the subsequent preparation experiments. However, the XRD patterns of undoped/V-doped TiO2 samples reported by Zhou et al. (2010) show an increase in the rutile phase at the temperatures above 450°C. This could be because the phase transformation of TiO2 powders depends on many factors, including materials used (precursors and stabilizers), dopants, synthesis method, and time of calcination.
FIG. 3.
X-ray diffractometer (XRD) patterns of undoped TiO2 calcined at different temperature.
FIG. 4.
XRD patterns of 0.01V-TiO2 calcined at different temperature.
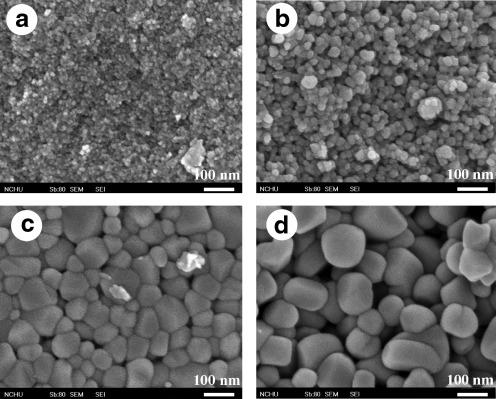
Figure 5 shows the SEM micrographs of some V-doped TiO2 samples calcined at 600°C. As can be seen, the morphologies of samples present anomalistic sphericity and the particle size range of the V-doped TiO2 is about 10–100 nm (crystallite size calculated using Scherrer's equation is 12.7–52.9 nm), whereas the particle size of undoped pure TiO2 particles obtained from the same preparation conditions is 10–30 nm (crystallite size calculated using Scherrer's equation is 11.7–26.4 nm). The crystallite size of V-doped TiO2 increases as compared to undoped TiO2 calcined at the same temperature, and increases with increasing vanadium loading on the catalysts, which accorded with the results observed by Liu et al. (2009a). Crystallite size increase of V-doped nanoparticles leads to the decrease of specific surface areas.
FIG. 5.
Scanning electron microscopy images of (a) 0.005V-TiO2; (b) 0.05V-TiO2; (c) 0.09V-TiO2; (d) 0.2V-TiO2 (magnification 150,000×).
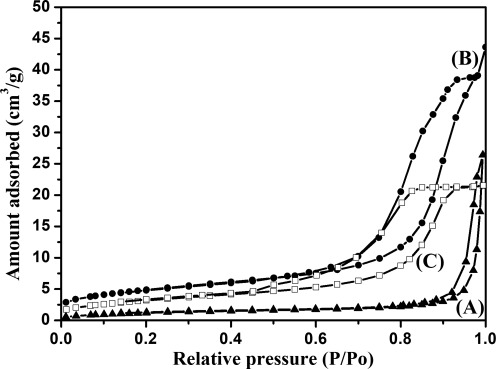
The specific surface areas of the V-doped TiO2 powders obtained in this work are 4.6–16.5 m2g−1 from BET analyses, whereas that of undoped TiO2 is around 16.5 m2g−1. A close observation reveals that the surface areas of V-doped samples are always less than that of undoped TiO2. There is no doubt that the decrease of surface area of V-doped samples is due to the increase of the grain size, which is consistent with the results of SEM. Figure 6 shows the nitrogen adsorption–desorption isotherms of 0.005V-TiO2, 0.05V-TiO2, and 0.2V-TiO2. They showed the type IV adsorption–desorption isotherm because they had a typical H2 hysteresis loop, which indicates the appearance of mesoporous materials (Sing et al., 1985).
FIG. 6.
N2 adsorption–desorption isotherm of (Curve A) 0.005V-TiO2, (Curve B) 0.05V-TiO2, and (Curve C) 0.2V-TiO2.
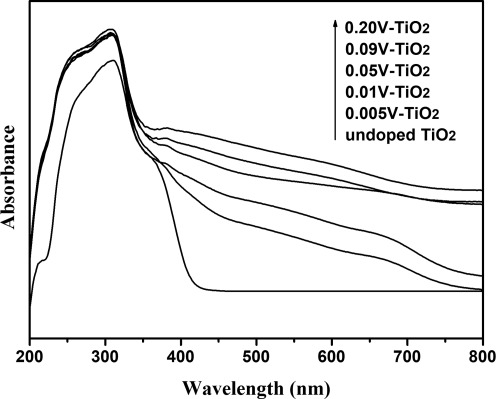
The UV-Vis absorption spectra of the samples (Fig. 7) show that the onset of absorption is shifted to longer wavelengths (red shift) with increasing vanadium content in the TiO2 lattice. This visible light absorption due to the formation of the impurity energy levels within the band gap of TiO2 was also increased with further increasing the vanadium contents. These results clearly indicated that the visible light-sensitive TiO2 was briefly prepared by controlling the doping amount of vanadium species into TiO2 lattice.
FIG. 7.
Ultraviolet–visible absorption spectra of the undoped and V-doped TiO2 powders.
Photocatalytic degradation of MB dye
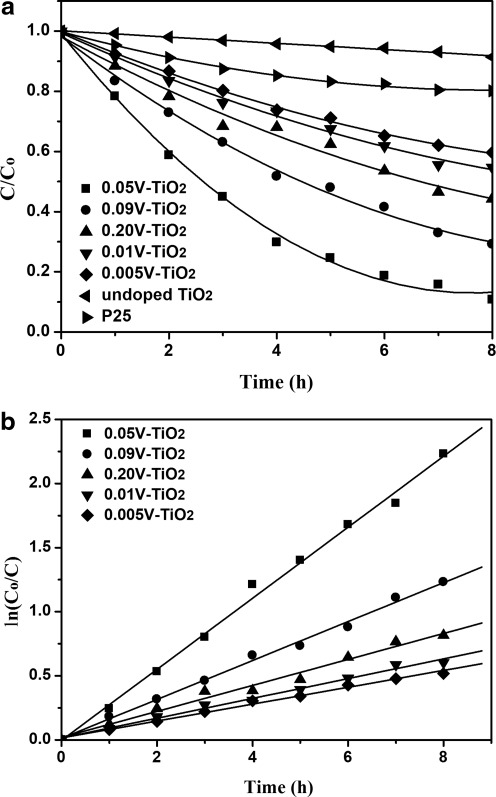
The mechanism of photocatalytic degradation of organic compounds (e.g., dyes) in water is believed to involve absorption of a UV photon by TiO2 to produce an electron–hole pair. Both hole and electron can react with water to yield hydroxyl and superoxide radicals that destroy the organic/dye molecules. MB, an organic dye, was selected as target compound because MB is ubiquitously used and the removal of the dye from wastewaters has been an acute problem (Gnaser et al., 2005). The photocatalytic activity of the V-doped TiO2 was quantitatively examined by photocatalytic degradation of MB in water for 8 h irradiation using visible light. The blank experiment showed that MB molecules were hardly photodegraded in the absence of any catalyst under the same irradiation conditions. As shown in Fig. 8a, Co and C represent the initial and remnants concentration of MB, respectively. As seen, the concentration of MB decreased with increasing irradiation time and all the V-doped TiO2 catalysts have much higher photocatalytic activity than the undoped one and the commercial Degussa P25 TiO2. Loading V4+ in TiO2 has the extension effect for the absorption spectra of the TiO2 in the visible region, leading to the increase of visible-induced catalytic activity. The photocatalytic decolorization of MB is an apparent first-order reaction verified by the linear transforms ln(Co/C)=f(t) illustrated in Fig. 8b. To enable quantitative comparison, the apparent rate constants displayed in Table 1 are calculated to represent the photoactivities. As shown in Fig. 8 and Table 1, it was found that for V-doped TiO2, the reaction rate increased with increase in the level of vanadium doped (onto TiO2) up to 5 mol% followed by a decrease in rate with further increase in vanadium level. The appearance of an optimal dopant concentration in V-doped TiO2 can be explained by the balance of an increase in trapping sites leading to efficient trapping and fewer trapped carriers leading to longer lifetimes for interfacial charge transfer (Liu et al., 2009b). The presence of V on TiO2 favors the migration of photogenerated electrons to vanadium, thus improving the electron–hole separation. On the V-doped TiO2, V clusters at a lower concentration act as a separation center; here, clusters are aggregates of species containing between three and a few thousand atoms. The photogenerated electrons are transferred from TiO2 conduction band to the dopant vanadium species and the holes accumulate in the TiO2 valence band. Hence, photogenerated electrons and holes were efficiently separated. However, V clusters at a higher concentration act as a recombination center and the recombination rate between electrons and holes increases exponentially with the increase of V concentration because the average distance between trap sites decreases by increasing the number of V clusters confined within a particle (Li and Li, 2002). Furthermore, too many V clusters on TiO2 would shield the photosensitive TiO2 surface, scatter the visible light to decrease the absorption by TiO2, and subsequently decrease the surface concentration of the electrons and holes available for further reactions.
FIG. 8.
Degradation of methylene blue on different catalysts: (a) C/Co versus visible light irradiation time and (b) first-order relation ln(Co/C)=kt.
Table 1.
Apparent First-Order Reaction Rate Constants
| |
Sample |
||||
|---|---|---|---|---|---|
| 0.005V-TiO2 | 0.01V-TiO2 | 0.05V-TiO2 | 0.09V-TiO2 | 0.2V-TiO2 | |
| k (h−1) | 0.06585 | 0.07714 | 0.27705 | 0.15188 | 0.10131 |
Conclusions
Vanadium(IV)-doped TiO2 photocatalysts have been prepared by the sol–gel method. The XRD patterns of the as-prepared V-doped TiO2 are similar to pristine TiO2, giving the typical patterns of anatase phase. The increase of vanadium doping can promote the particle growth, resulting in a decrease the specific surface areas of powders, whereas the vanadium-doping changes the band gap of this material, leading to red-shift in the UV-Vis spectra, which is benefit to generate electron and hole under visible light irradiation. Therefore, V-doping is an effective means to improve the photocatalytic activity of TiO2 for MB dye degradation under visible light irradiation. In our experiment condition, the photocatalytic activity of 5 mol% V-doped TiO2 nanoparticles reaches to optimal for all the prepared samples.
Acknowledgment
The authors gratefully acknowledge the financial support of the National Science Council of the Republic of China under grant NSC 98-2622-E-168-004-CC3.
Author Disclosure Statement
No competing financial interests exist.
References
- Anpo M. Dohshi S. Kitano M. Hu Y. Takeuchi M. Matsuoke M. The preparation and characterization of highly efficient titanium oxide-based photofunctional material. Annu. Rev. Mater. Res. 2005;35:1. [Google Scholar]
- Brinker C.J. Scherer G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. San Diego: Academic Press; 1990. [Google Scholar]
- Chang Y.P. Ma Y.S. Chiang C.L. Liu C.L. Chang C.N. Apply TiO2-catalyzed UV system to decolor dispersed Blue S-3RF wastewater. Environ. Eng. Sci. 2008;25:557. [Google Scholar]
- Gnaser H. Savina M.R. Calaway W. Tripa C.E. Veryovkin I.V. Pellin M.J. Photocatalytic degradation of methylene blue on nanocrystalline TiO2: Surface mass spectrometry of reaction intermediates. J. Mass Spectrom. 2005;245:61. [Google Scholar]
- Gu D.E. Yang B.C. Hu Y.D. V and N co-doped nanocrystal anatase TiO2 photocatalysts with enhanced photocatalytic activity under visible light irradiation. Catal. Commun. 2008;9:1472. [Google Scholar]
- Klosek S. Raftery D. Visible light driven V-doped TiO2 photocatalyst and its photooxidation of ethanol. J. Phys. Chem. B. 2001;105:2815. [Google Scholar]
- Lee B.N. Liaw W.D. Lou J.C. Photocatalytic decolorization of methylene blue in aqueous TiO2 suspension. Environ. Eng. Sci. 1999;16:165. [Google Scholar]
- Li F.B. Li X.Z. The enhancement of photodegradation efficiency using Pt-TiO2 catalyst. Chemosphere. 2002;48:1103. doi: 10.1016/s0045-6535(02)00201-1. [DOI] [PubMed] [Google Scholar]
- Li L. Liu C. Liu Y. Study on activities of vanadium (IV/V) doped TiO2(R) nanorods induced by UV and visible light. Mater. Chem. Phys. 2009;113:551. [Google Scholar]
- Liu B. Wang X. Cai G. Wen L. Song Y. Zhao X. Low temperature fabrication of V-doped TiO2 nanoparticles, structure and photocatalytic studies. J. Hazard. Mater. 2009;169:1112. doi: 10.1016/j.jhazmat.2009.04.068. [DOI] [PubMed] [Google Scholar]
- Liu S. Xie T. Chen Z. Wu J. Highly active V–TiO2 for photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2009;255:8587. [Google Scholar]
- Lu L. Zhang Y. Xiao P. Zhang X. Yang Y. Aggregation of TiO2 nanotubes as recyclable catalyst for photocatalytic degradation of methylene blue. Environ. Eng. Sci. 2010;27:281. [Google Scholar]
- Mao L. Dang H. Zhang Z. A study on the photodecomposition of active dye in a baffled fixed-bed reactor. Environ. Eng. Sci. 2005;22:660. [Google Scholar]
- Martin S.T. Morrison C.L. Hoffmann M.R. Photochemical mechanism of size-quantized vanadium-doped TiO2 particles. J. Phys. Chem. 1994;98:13695. [Google Scholar]
- Pecchi G. Reyes P. Sanhueza P. Villasenor J. Photocatalytic degradation of pentachlorophenol on TiO2 sol-gel catalysts. Chemosphere. 2001;43:141. doi: 10.1016/s0045-6535(00)00265-4. [DOI] [PubMed] [Google Scholar]
- Senthilkumaar S. Porkodi K. Heterogeneous photocatalytic decomposition of crystal violet in UV-illuminated sol-gel derived nanocrystalline TiO2 suspensions. J. Colloid Interface Sci. 2005;288:184. doi: 10.1016/j.jcis.2005.02.066. [DOI] [PubMed] [Google Scholar]
- Shi J. Zheng J. Hu Y. Zhao Y. Photocatalytic degradation of methyl orange in water by samarium-doped TiO2. Environ. Eng. Sci. 2008;25:489. [Google Scholar]
- Sing F.K.S.W. Everett D.H. Haul R.A.W. Moscou L. Pierotti R.A. Rouquerol J. Siemieniewska T. Reporting physisorption data for gas/solid systems with specific reference to the determination of surface area and porosity. Pure Appl. Chem. 1985;57:603. [Google Scholar]
- Sun B. Reddy E.P. Smirniotis P.G. Effect of the Cr6+ concentration in Cr-incorporated TiO2-loaded MCM-41 catalysts for visible light photocatalysis. Appl. Catal. B. 2005;57:139. [Google Scholar]
- Surolia P.K. Tayade R.J. Jasra R.V. Photocatalytic degradation of nitrobenzene in an aqueous system by transition-metal-exchanged ETS-10 zeolites. Ind. Eng. Chem. Res. 2010;49:3961. [Google Scholar]
- Wu C.G. Chao C.C. Kuo F.T. Enhancement of the photo catalytic performance of TiO2 catalysts via transition metal modification. Catal. Today. 2004;97:103. [Google Scholar]
- Wu J.C.S. Chen C.H. A visible-light response vanadium-doped titania nanocatalyst by sol–gel method. J. Photochem. Photobiol. A. 2004;163:509. [Google Scholar]
- Yamashita H. Harada M. Misaka J. Takeuchi M. Neppolian B. Anpo M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2. Catal. Today. 2003;84:191. [Google Scholar]
- Yin X. Xin F. Zhang F. Wang S. Zhang G. Kinetic study on photocatalytic degradation of 4BS azo dye over TiO2 in slurry. Environ. Eng. Sci. 2006;23:1000. [Google Scholar]
- Zhou J. Takeuchi M. Ray A.K. Anpo M. Zhao X.S. Enhancement of photocatalytic activity of P25 TiO2 by vanadium-ion implantation under visible light irradiation. J. Colloid Interface Sci. 2007;311:497. doi: 10.1016/j.jcis.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Zhou M. Yu J. Cheng B. Yu H. Preparation and photocatalytic activity of Fe-doped mesoporous titanium dioxide nanocrystalline photocatalysts. Mater. Chem. Phys. 2005;93:159. [Google Scholar]
- Zhou W. Liu Q. Zhu Z. Zhang J. Preparation and properties of vanadium-doped TiO2 photocatalysts. J. Phys. D Appl. Phys. 2010;43:035301. [Google Scholar]
- Zhu J. Chen F. Zhang J. Chen H. Anpo M. Fe3+-TiO2 photocatalysts prepared by combining sol-gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A. 2006;180:196. [Google Scholar]