Abstract
Introduction
The prescription of physical activity for hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) can be complicated by the presence of comorbidities. The current research aimed to synthesize the relevant literature on the benefits of exercise for people with multimorbidities who experience an AECOPD, and ask: What are the parameters and outcomes of exercise in AECOPD and in conditions that are common comorbidities as reported by systematic reviews (SRs)?
Methods
An SR was performed using the Cochrane Collaboration protocol. Nine electronic databases were searched up to July 2011. Articles were included if they (1) described participants with AECOPD, chronic obstructive pulmonary disease (COPD), or one of eleven common comorbidities, (2) were an SR, (3) examined aerobic training (AT), resistance training (RT), balance training (BT), or a combination thereof, (4) included at least one outcome of fitness, and (5) compared exercise training versus control/sham.
Results
This synthesis examined 58 SRs of exercise training in people with AECOPD, COPD, or eleven chronic conditions commonly associated with COPD. Meta-analyses of endurance (aerobic or exercise capacity, 6-minute walk distance – 6MWD) were shown to significantly improve in most conditions (except osteoarthritis, osteoporosis, and depression), whereas strength was shown to improve in five of the 13 conditions searched: COPD, older adults, heart failure, ischemic heart disease, and diabetes. Several studies of different conditions also reported improvements in quality of life, function, and control or prevention outcomes. Meta-analyses also demonstrate that exercise training decreases the risk of mortality in older adults, and those with COPD or ischemic heart disease. The most common types of training were AT and RT. BT and functional training were commonly applied in older adults. The quality of the SRs for most conditions was moderate to excellent (>65%) as evaluated by AMSTAR scores.
Conclusion
In summary, this synthesis showed evidence of significant benefits from exercise training in AECOPD, COPD, and conditions that are common comorbidities. A broader approach to exercise and activity prescription in pulmonary rehabilitation may induce therapeutic benefits to ameliorate clinical sequelae associated with AECOPD and comorbidities such as the inclusion of BT and functional training.
Keywords: pulmonary disease, chronic obstructive, comorbidity, exercise, physical fitness
Introduction
Chronic obstructive pulmonary disease (COPD) is the fifth leading cause of death in the world and the mortality rate is expected to increase more than 30% during the next 10 years.1 Acute exacerbations of COPD (AECOPD) are common and are a key predictor of increased morbidity, health care costs, and mortality. The Global Initiative for Lung Disease (GOLD)1 defines a COPD exacerbation as “an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough and/or sputum that is beyond normal day-to-day variation, is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD”. Most patients with COPD have at least one exacerbation per year; a substantial proportion of patients (17%) have three or more episodes per year.2,3
Many exacerbations of COPD are managed on an outpatient basis, but if the patient is hospitalized, the length of stay (LOS) is prolonged: the median LOS in Canadian hospitals is 13 days,4 which translates to an estimated cost of CAD$9500 per hospitalization.5 Premature discharge resulting in shorter LOS, however, is associated with increased readmission and mortality.6
Hospitalization to treat acute illness can have a detrimental effect on muscle performance.7 Muscle impairment and functional decline have been well-documented in older hospitalized patients, and have been attributed to the consequences of hospitalization rather than the admitting illness.8–11 In a previous study on 1181 hospitalized patients with acute medical illnesses (circulatory, 27%; respiratory, 20%; gastrointestinal, 15%; cancer, 6%), 17% of patients required assistance with mobility on discharge, despite being able to walk independently prior to admission.12 Pitta et al reported that patients who are hospitalized with an acute exacerbation of COPD spend the majority of their time sitting, and do not return to their baseline level of activity even after 1 month post-discharge.13
Caring for hospitalized patients with AECOPD can be complicated by the presence of other chronic conditions, which can also influence the severity of the exacerbation and the health outcomes of the patient. Individuals with COPD have a higher risk of multimorbidity14–27 (Table 1) than individuals with other chronic illnesses. Despite this, there has been widespread failure to address the complexities inherent in living with multiple chronic illnesses simultaneously, especially in the hospitalized, acutely ill population. Devising exercise plans for hospitalized AECOPD patients living with multimorbidity is complicated by the limitations imposed by the acute exacerbation and by the synergistic effect of the multiple conditions upon the person. While recent reviews have examined evidence regarding the effectiveness of exercise therapy for people with multimorbidity in community settings,28,29 none have focused on hospitalized patients with COPD who also experience multi-morbidity. National physical activity guidelines fail to address this issue as well.30,31
Table 1.
Prevalence of chronic diseases in the population and in people with COPD
| Chronic disease | Prevalence in population | Prevalence in COPD |
|---|---|---|
| Heart failure | <1% age 50–59; ~7% age 80–8922 | 20%24 |
| Ischemic heart disease | 5% | Severe and very severe COPD two-fold risk25 |
| Peripheral arterial disease | 4.3% at age 40, 14.5% at age 7020 | Smokers OR 4.4620 |
| Hypertension | 15%; ~50% ≥50 years18 | 1.6-fold risk25 |
| Obesity | 67% over age 3014 | 54%26 |
| OA | 13% at age 50; ~40% at age 7523 | |
| Osteoporosis/osteopenia | 15% over age 5021 | 60%–70%27 |
| Diabetes mellitus – type 2 | 2.8% in 2000; 6.4% in 2010 with a 10.2% in the Western Pacific16 | Increased relative risk 1.5–1.825 |
| Depression | 4.7% persistent depression or anxiety17 | Depressive symptoms: 10%–80%25 Depression that requires treatment: 19%–42%25 |
Systematic reviews (SRs) provide a high level of evidence regarding the efficacy of therapeutic intervention, but the sheer number of available SRs on a given topic can make it difficult for the clinician to distill the important messages. Systematic reviews of systematic reviews have been purported as a way to both evaluate and summarize the key messages on important therapies, and are commonly published in areas of health care.32–36 The aim of the current research was to synthesize the relevant literature on the benefits of exercise for people with multimorbidity who are experiencing an AECOPD. Thus we posed the question: What are the parameters and outcomes of exercise in AECOPD and in commonly associated comorbidities as reported by SRs?
Methods
Search strategy
A systematic review was performed using the methodology outlined by the Cochrane Collaboration protocol.37 Electronic databases were searched up to July 2011 including the Cochrane Controlled Trials Register, Cochrane-Systematic Reviews, MEDLINE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), SPORTDiscus, EMBASE, PEDro (Physiotherapy Evidence Database), PsycINFO, and EBM reviews. Gray literature and reference lists from relevant articles were also reviewed to identify additional articles. Search terms were exemplified by the MEDLINE search strategy (Appendix 1) and were modified accordingly to fit the requirements of the other databases.
Study criteria
Articles were included if they (1) described participants with AECOPD or COPD, or had one or more of the following conditions that are common comorbidities: anxiety/depression, atherosclerosis, ischemic heart disease, peripheral vascular disease, heart failure, hypertension, diabetes mellitus, human immunodeficiency virus (HIV/AIDS), osteoarthritis (OA), osteoporosis, obesity/overweight, or older adults; (2) were SRs that included at least one randomized controlled trial (RCT); (3) examined an exercise intervention of aerobic training (AT), resistance training (RT), balance training (BT), or a combination thereof; (4) included at least one outcome of fitness; (5) had comparison groups of exercise training versus control/sham (that consisted of usual treatment, no exercise, or attention placebo); (6) were published in English. Articles were excluded if they (1) were published before 2000, or (2) investigated tai chi, yoga, or qi gong due to the multiple forms of these exercises and the difficulty in defining the expected training response to fitness outcomes. If two SRs reported on the same question and the majority of papers included were the same, only the SR with more articles and a more comprehensive review was included. Also, several SRs did not contain meta-analyses. These were reviewed if they met the criteria above, but data were extracted only if other reviews with meta-analyses were not available on the outcomes of interest.
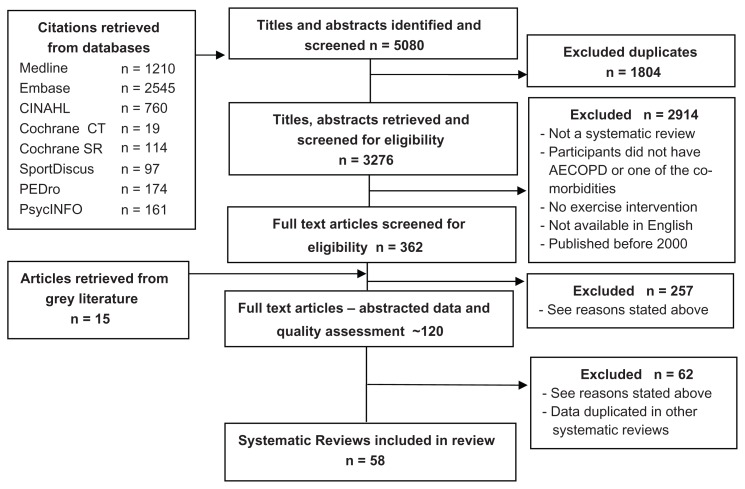
Two individuals independently screened all titles and abstracts retrieved. Any discrepancies were discussed and resolved. From the selected abstracts, one person screened 362 full text articles to determine if they met the inclusion criteria using a screening form. A second person was consulted to confirm agreement on all articles to be excluded. The flow chart of the search strategy and study selection is summarized in Figure 1.
Figure 1.
Flow chart of retrieval, screening and inclusion of systematic reviews.
For each condition, one of the coauthors abstracted data and performed the quality assessment, which was double-checked by at least one person. All discrepancies were discussed and reconciled. Data abstracted, when available, included (1) condition, age, and gender of participants, (2) the modality (Mo), frequency (Fr), intensity (I), time of session (T), and duration of program (D) of the exercise intervention, (3) descriptors of the control, placebo, or sham group, and (4) outcomes of fitness, disease control or prevention, quality of life, and function.
Data related to functional training was abstracted. Functional training can be defined as mobility exercises that are functionally- or task-based rather than focused on strength training of particular muscle groups or endurance training to improve cardiovascular or aerobic status. Examples are stair climbing, repetitive sit to stand, and transfers. Such training often includes a combination of strength, endurance, and balance.
Quality assessment
All SRs were assessed using the AMSTAR quality assessment tool.38 Scores for each item and totals are reported. Item 11 was scored as “yes” if the conflict of interest was stated for the authors of the SR.
Data synthesis
Data was synthesized in tables that described the study quality (Table 2), the participants and interventions (Table 3), and outcomes (Table 4). An outcome was assigned to one of two categories based on whether it was measured in several conditions or if it was applied to evaluate a disease specific marker: (1) generic fitness (eg, exercise capacity, strength), quality of life, and functional outcomes (eg, timed up-and-go test, stair climbing), and (2) disease specific fitness, control, and prevention outcomes (eg, dyspnea, mortality, pain, ejection fraction, cholesterol levels).
Table 2.
AMSTAR score
| Condition | Author | AMSTAR scores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | ||
| AECOPD | Puhan et al39 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Chronic obstructive pulmonary disease | Lacasse et al42 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| O’Shea et al43 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 8 | |
| Salman et al44 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | |
| Chavannes et al40 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 5 | |
| Vieira et al45 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 6 | |
| Janaudis-Ferreira et al41 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 5 | |
| Older adults | Liu and Latham50 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Howe et al49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 10 | |
| Gillespie et al48 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | |
| Weening-Dijksterhuis et al52 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | |
| Forster et al47 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | |
| Chin et al46 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 8 | |
| Rydwik et al51 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 6 | |
| Heart failure | Davies et al55 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Hwang et al54 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | |
| Hwang and Marwick57 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | |
| Chien et al53 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 7 | |
| Haykowsky et al56 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 8 | |
| van Tol et al58 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | |
| Spruit et al61 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 6 | |
| Cahalin et al60 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Benton59 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Ischemic heart disease | Haykowsky et al65 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Valkeinen et al68 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | |
| Cortes et al64 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 9 | |
| Jolliffe et al66 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| Clark et al62 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |
| Cornish et al63 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | |
| Oliveira et al67 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | |
| PVD | Watson et al69 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Wind and Koelemay70 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | |
| Hypertension | Dickinson et al73 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 8 |
| Cornelissen and Fagard72 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 4 | |
| Whelton et al74 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 8 | |
| Kelley et al71 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 7 | |
| Obesity | Shaw et al76 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 |
| Witham and Avenell75 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 8 | |
| Osteoarthritis | Devos-Comby et al79 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Lange et al80 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 7 | |
| Ottawa Panel77 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 8 | |
| Brosseau et al78 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 7 | |
| Pelland et al81 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 9 | |
| Osteoporosis | Li et al83 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
| de Kam et al82 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 7 | |
| Diabetes mellitus | Chudyk and Petrella85 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 6 |
| Umpierre et al90 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| Irvine and Taylor86 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 7 | |
| Thomas et al89 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | |
| Kelley and Kelley87 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 9 | |
| Snowling and Hopkins88 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 6 | |
| Boulé et al 84 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 6 | |
| HIV | O’Brien et al91 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| Depression | Krogh et al93 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Herring et al94 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Mead et al96 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | |
| Rethorst et al95 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 8 | |
| Lawlor and Hopker92 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 9 | |
| Tallies for 58 reports | 58 | 38 | 54 | 34 | 15 | 49 | 48 | 41 | 42 | 26 | 29 | 434 | |
| Percentage of total (58 reports) | 100 | 66 | 93 | 59 | 26 | 84 | 83 | 71 | 72 | 45 | 50 | ||
Table 3.
Characteristics of participants and interventions
| Author | RCTs | n (% M or F) | Age (years) | Characteristics of exercise intervention |
|---|---|---|---|---|
| AECOPD | ||||
| Puhan et al39 | 9 | 427 (64% M) | Range 62–70 | Mo: AT and RT; Fr: Twice daily – 3×/week; T: NR except one study stated 2 hours/session; D: 10 days–6 months |
| COPD | ||||
| Lacasse et al42 | 31 | 1322 | NR | Mo: AT and RT, U/E, L/E, and/or respiratory muscle training; Fr and I: NR; D: 4–52 weeks |
| O’Shea et al43 | 9 of 18 | 296 of 679 (70% M) | Range 48.5–72 | Mo: RT using free weights, machine, Theraband; Fr: 2×/week–daily; I: variable; T: 5–12 reps, 2–4 sets; D: 6–12 weeks |
| Salman et al44 | 20 | 999 | 59–73 | Mo: AT, RT: U/E, L/E, and/or respiratory muscle training; Fr: ≥3 ×/week; D: 6 weeks–12 months; other details NR |
| Chavannes et al40 | 4 of 5 | 210 (~55% M) | 49–63 | Mo: AT + RT (four studies), AT (one study); Fr: 2–7×/week; D: 8 weeks–18 months; other details NR |
| Vieira et al45 | 8 of 12 | 370 of 728 | Range 38–78 | Mo: Home-based AT and RT; Fr: 2×/week – twice daily; I: 90% of velocity of 6 MWD, ≥70% of maximal speed of SWT, cycle ergometer: 30 W, cycling: 70% of work rate; T: 30–60 minutes; D: 4–52 weeks |
| Janaudis-Ferreira et al41 | 5 | 157 | NR | Mo: Arm AT and RT; Fr: 3×/week – 2×/day; I: NR; T: 4–10 reps 1–3 sets; D: 6–8 weeks |
| Older adults | ||||
| Liu and Latham50 | 83 of 121 | 3059 of 6700 | >60 | Mo: RT in gym or against Theraband; Fr: 2–3×/week, except one was daily; I: ranged from low to high intensity; D: most 8–12 weeks but ranged from 2–104 weeks |
| Howe et al49 | 34 | 2883 (<50% M) | 60–75 years | Mo: RT, gait, balance, co-ordination, and functional tasks, tai chi, qi gong, dance, yoga; other details NR |
| Gillespie et al48 | 14 of 111 | 55303 (<50% M) | >60 | Mo: AT, RT, gait, balance and functional training, flexibility training, tai chi, and square stepping; general physical activity (walking groups); other details NR |
| Weening- Dijksterhuis et al52 | 27 | 6459 | >70 | Mo: RT (strength, resistance), ROM, balance, functional, gait, tai chi, flexibility; other details NR |
| Forster et al47 | 37 of 49 | 3611 (33% M) | Mean 82 | Mo: RT, AT (walking) and general daily living skills eg, eating, dressing, and climbing stairs, flexibility, balance; Fr: mean of 3.5×/week T: ≤30 minutes/week; D: 4 weeks–2 years |
| Chin et al46 | 20 | 2515 (majority F) | 77–88 | Mo: AT, RT, flexibility, balance, tai chi, and/or multi-component training; Fr: usually 3×/week; D: range 10–28 weeks |
| Rydwik et al51 | 16 | 1269 | >70 | Mo: RT, gait, and ROM training; Fr: usually 2–3×/week T: variable; D: 9 weeks–2 years |
| Heart failure | ||||
| Davies et al55 | 19 | 3647 (majority M) | 51–72 | Mo: AT or AT + RT; Fr: 2–7×/week; I: 40% max HR or 85% VO2 max; T: 15–120 minutes; D: 24 weeks–3 years |
| Hwang et al54 | 4 of 8 | 103 (60% M) | 62–77 | Mo: RT for U/E and L/E; Fr: 2–3×/week; I: 60%–80% 1RM; T: 12–60 minutes; D: 8–22 weeks |
| Hwang and Marwick57 | 19 | 1069 (85% M) | 59.05 (95% CI 55–63) | Mo: AT or AT + RT: cycle ergometer, walking, calisthenics, ball games, strength training; Fr: NR; I: AT: −10% of AT, 60%–80% HRmax, 70% VO2 max RT: 80% 1RM, 70% HRmax; T: 40–400 minutes/week; D: 8–52 weeks |
| Chien et al53 | 10 | 648 (79% M) | 52–81 | Mo: Home based AT (walking program, and/or cycle ergometer) and RT (added in 3/10 studies); Fr: 2–5×/week; I: 40%–70% HRmax, or 70% HR at VO2 max; T: 20–60 minutes; D: 6–52 weeks. All home programs |
| Haykowsky et al56 | 9 of 14 | 538 of 812 (83% M) | 52–61 | Mo: AT: cycle ergometer, rowing, swimming, walking, arm ergometer, treadmill, interval training; Fr: 2–7 days/week; I: 50%–80% VO2 peak, 60%–85% HRpeak, or 50%–70% max work load; T: 20–60 minutes; D: 2–14 months |
| van Tol et al58 | 35 | 1486 (76% M) | Mean 60.6 (SD 7.5) | Mo: AT + RT; Fr: range 1–7×/week, mean 3.7×/week; I: 50%–80% VO2 peak, 60%–80% HRmax, 60%–80% HRR; T: 15–96 minutes, 50 minutes (mean); D: 3–26 weeks, 13.0 weeks (mean) |
| Spruit et al61 | 4 of 10 | 114 (72% M) | Mean 57–77 | Mo: RT alone Fr: 2–3×/week; I: 40%–80% 1 RM; T: 15–60 reps in 1–3 sets; D: 8–20 weeks; progression described in some reports |
| Cahalin et al60 | 11 of 22 | 387 of 633 (70% M) | 30–80 | Mo: RT: variety of strength exercises either alone or with short or long bursts of AT; Fr: 2–5×/week; I: RT: 20%–80% 1RM; AT: up to 80% VO2 peak, or 70% HRpeak, or 50% peak workload; T: 5–60 minutes; D: 4–26 weeks |
| Benton59 | 4 of 16 | 115 of 379 (80% M) | Mean 30–76 | Mo: RT: chair stands, heel lifts, weights, pulleys, hydraulic fitness; Fr: 2–3×/week, I: 60% 1 RM; AT: cycling, arm ergometer, stair climbing, walking; T: 2–60 minutes; D: 2–5 month study duration; reps and progression: variable |
| Ischemic heart disease | ||||
| Haykowsky et al65 | 12 | 647 | 55 | Mo: AT: Cycle, walk, jogging, calisthenics; Fr: 3–7×/week; I: 60%–80% VO2 max or peak HR; T: 30–180 minutes; D: 1–6 months |
| Valkeinen et al68 | 18 | 922 (majority M) | 59.9 ± 4.9 | Mo: AT: walking, jogging, cycling, and arm cranking; Fr: 3.1 ± 0.4×/week; I: 70%–80% HRmax, HRR, HR of anaerobic threshold or 25%–70% VO2 max; T: 20–60 minutes; D: 14.2 ± 13.5 weeks Mo: RT: Fr: 3.5 ± 0.7×/week; I: 50%–80% 1 RM or 40%–60% MVC; T: 20–60 minutes; D: 22.0 ± 11.1 weeks |
| Cortes et al64 | 11 of 14 | 3148 (≥70% M) | 55–65 | Mo: Early mobilization: sitting on bed/chair, walking, moving legs, climbing stairs, any movement out of bed, dangling legs, progressive activity while in hospital; settings descriptions: CCU, hospital wards, in-hospital |
| Jolliffe et al66 | 32 | 8440 (majority M) | 55 | Mo: AT: walking, running, cycling, skipping, swimming, stairs, and rowing; Fr: 1–5×/week; I: 70%–85% HRmax or predicted HRmax, other 75% max work capacity; T: 20–60 minutes; D: 4 weeks to 30 months Mo: RT: strengthening exercises for U/E and L/E; Fr: 3×/week; I: Target HR 85% HRmax; T: 60 minutes; D: 12 weeks |
| Clark et al62 | 41 of 63 | 8460 of 21295 (majority M) | Mean 49–71 | AT, RT: no details on intervention |
| Cornish et al63 | 2 of 7 | 120 of 213 | 57 ± 14–71.5 ± 7.8 | Mo: AT: interval training: aerobic dance movements, upper/lower body exercises, cycle ergometer, or treadmill walking/running; Fr: 2–3×/week; I: 3 intervals of 5–10 minutes at RPE 15–18 in one study and 3 minutes at 60%–70% HRmax + 4 minutes at 90%–95% HRmax in another; T: 50–60 minutes; D: 16–26 weeks |
| Oliveira et al67 | 4 of 11 | 126 | most <60 | Mo: RT: free weights in one study, NR in others; I: 40%–60% MVC or 50%–80% 1 RM; D: 10 weeks to 6 months; Other details NR |
| PVD | ||||
| Watson et al69 | 16 of 22 | 783 of 1200 (>50% M) | 45–89 | Mo: AT, RT: polestriding, cycling, and U/E and L/E exercises. Fr: 2–7×/week; T: 30–120 minutes; D: 3–12 months |
| Wind and Koelemay70 | 10 of 15 | 625 of 761 (>50% M) | 60–76 | Mo: AT: crank exercise, walking, others not detailed. Fr: 2–3×/week; T: 30–60 minutes; D: 12–26 week except one study was 104 weeks |
| Hypertension | ||||
| Dickinson et al73 | 21 of 105 | 1518 of 6805 (~57% M) | 52 (30–67) | Mo: AT: brisk walking, jogging, cycling; Fr: 3–5×/week; T: 30–60 minutes; D: 8–52 week (median 12). 2 trials received RT, but details NR; 1 trial offered advice to participants |
| Cornelissen and Fagard72 | 28 of 72 | 492 in exercise group | 52.7 ± 11.8 | Mo: AT: walking, jogging, running, cycling; Fr: 2–7×/week (median 3); I: 30%–87% HRR (median 65); T: 25–60 minutes (median 40); D: 4–52 weeks (median 12) |
| Whelton et al74 | 15 of 54 | 872 (majority M) | Mean 40–69 Range 18–86 |
Mo: AT: biking, walking, jogging; Fr: 1–7×/week; I: 40%–70% VO2 max, 60%–85% HRmax, 40%–70% Wmax; T: 20–60 minutes; D: 3–26 weeks |
| Kelley et al71 | 47 | 2543 >50% M |
48 | Mo: AT: walking, jogging, cycling, aerobic dance, and swimming; Fr: 1–5×/week; I: 45%–86% VO2 max; T: 15–60 minutes; D: 360–9360 minutes |
| Obesity | ||||
| Shaw et al76 | 4 of 41 | 440 of 3476 (majority M) | 30–64 | Mo: AT: walking/jogging, circuit training; Fr: 2–3×/week; I: 60%–80% HRmax; T: 30–60 minutes; D: 26–52 weeks |
| Witham and Avenell75 | 2 of 9 | 173 (100% F) | 60 | Mo: supervised AT, no details; Fr: 3×/week; I: aiming for 70% VO2 max; T: NR; D: 3 months; Transition to home: exercise 4×/week plus weekly supervised exercise |
| OA | ||||
| Devos-Comby et al79 | 16 | 2154 | Mean 65.8, range 29–89 | Mo: AT + RT: knee muscles strength training, low/medium intensity exercise, and light physical activity (walking, aerobic exercise, balance or flexibility exercises); Fr: 2–5×/week (sometimes 2×/day); I: AT: 50%–85% HRR, RT: 6 set × 5 max contraction (others NR); T: 20 minutes – 1.5 hour; D: 4–12 weeks (different follow-up periods) |
| Lange et al80 | 18 | 2723 | Range 55–74 | Mo: RT: dynamic or isotonic training mainly targeting the quadriceps using resistance machines, free weights, or Theraband; Fr: 2–7×/week; I: light to maximal; T: 10–60 minutes, 5–12 reps, 1–10 sets (most 3 sets); D: 1–30 months |
| Ottawa Panel77 | 26 | 2486 | >18 | Mo: AT: walking or cycle ergometer; RT: strengthening (eg, isometric, isotonic, isokinetic, eccentric, concentric, aerobic), general physical activity, combination of exercises; Fr: 1×/week–10×/day (depends on phase); I: AT: 50%–70% HRR when described, RT: variable. T: 5 minutes–1.5 hours; D: 4 weeks–18 months |
| Brosseau et al78 | 9 of 12 | 1363 | 57–69.4 | Mo: AT: walking, stationary bike; Fr: 1–4 ×/week; I: 50%–85% HRR or 60%–80% HRmax. T: 30–90 minutes when reported; D: 5–12 weeks. Some changed to home-based program up to 15 months. Some studies included strengthening exercises |
| Pelland et al81 | 17 (plus 4 CCTs) | 2325 | Range 55.0–74.6 | Mo: ROM exercises, low intensity walking on treadmill, RT: quadriceps, hamstrings, and hip abduction strengthening, stationary bike; Fr: most 2–3×/week, range: 1×/week–2×/day; I: variable; T: 15–40 minutes, 3–9 exercises, 6–20 reps, 1–4 sets; D: 4 week–15 months |
| Osteoporosis | ||||
| Li et al83 | 5 groups in 4 papers | 256 (100% F) | NR | Mo: RT or combined stretch/strength/balance programs: strengthening of extremities and trunk (1 study), “agility” training (1 study), combined exercise (stretching, strengthening, balance, posture) (2 studies), trunk flexor/extensor strengthening (1 study); Fr: 3 studies 2 days/week, 1 study 7 days/week; I: NR; T: 40–60 minutes; D: 10–25 weeks |
| De Kam et al82 | 28 | 1707, some reports used the same subjects | Mean 57–82 | Mo: fast walking; aerobic exercise; weight-bearing aerobic exercise; strengthening exercises; balance exercises; posture and gait exercises; trunk extensor exercises; heel drops with impact; heel drops with no impact; whole body vibration; jumping; agility training including ball games, relay races and obstacle courses; tai chi; home-based walking, strengthening and stretching; Fr: 3–7×/week; I: not described – only one study described progression by increasing step count by 30%; T: 4–90 minutes; D: 10–104 weeks |
| Diabetes | ||||
| Chudyk and Petrella85 | 30 of 34 | NR | NR | Mo: AT; Fr: most 3×/week but range of 1–7×/week; I: 50%–85% VO2 max or 35%–85% HRmax; T: 40–75 minutes; D: 2–24 months. RT: variable |
| Umpierre et al90 | 23 of 47 | 1513 of 8538 | Range of means 52–69 | Mo: AT, RT; Fr: 2–5×/week T: AT: 30–150 minutes/week and RT: 9–27 sets; D: 12–52 weeks |
| Irvine and Taylor86 | 7 of 9 | 162 of 256 | 47–68 | Mo: RT: free weights or weight machines; Fr: 2–3×/week; I: 55%–85% 1RM or to moderate on Borg; T: 8–15 reps, 1–3 sets, 5–10 exercises; D: 8–26 weeks |
| Thomas et al89 | 14 | 377 | 45–65 | Mo: qi gong, RT, AT: cycle ergometer, walking cycling, skiing, swimming; Fr: 3–7×/week; I: AT: 50%–85% VO2 peak, 65%–75% HRR or 85% HRmax; T: 30–120 minutes; if PRT, 2–3 sets of 10–12 reps; D: 8 weeks–1 year |
| Kelley and Kelley87 | 7 | Both | 40–75 | Mo: AT: cycle ergometer, walking, jogging, cycling, swimming, skiing; Fr: 3–7×/week I: 60%–75% VO2 max; T: 30–75 minutes; D: 10–26 weeks |
| Snowling and Hopkins88 | 27 | 1003 | 55 ± 7 |
Mo: AT; Fr: 3–7×/week; I: 50%–85% VO2 max, HR 110–140 bpm, 40%–80% HRR; T: 30–120 minutes; D: 6–104 weeks. Mo: RT; Fr: 3–5×/week; I: 50%–80% of 1 RM; T: 10–20 reps, 2–3 sets, 5–10 ex; D: 5–16 weeks |
| Boulé et al84 | 27 | 266 | 55.7 | Mo: AT: continuous aerobic; walking, cycling, skiing jogging, rowing, Theraband; Fr: 3–6×/week; 60%–75%; I: VO2 max; T: 40–60 minutes; D: 8–52 weeks |
| HIV | ||||
| O’Brien et al91 | 7 of 14 | 306 of 454 (~70% M) | 18–58 | Mo: AT: interval or continuous aerobic, walking, jogging, stair stepping, ski machine, stationary bike, cross country machine. Fr: 3×/week I: AT: usually 50%–85% VO2 max and 60%–80% HRmax or RT: 60%–80% 1 RM; T: 20–60 minutes, D: 5–24 weeks |
| Depression/anxiety | ||||
| Krogh et al93 | 13 | 272 | 17–85 | Mo: AT, RT, AT + RT; Fr: 2–5×/week; D: 8–16 week; AT: I: 70%–80% HRmax, 50%–70% aerobic capacity; T: 30–60 minutes. RT: I: 80% RM; T: 20 minutes or 8 reps × 3 sets |
| Herring et al94 | 40 | NR (59% F) | 50 ± 10 | Mo: AT, RT, AT+RT; Fr: 3 ± 1×/wk; I: variable; T: 42 ± 22; D: 16 ± 10 wk |
| Mead et al96 | 23 of 25 | 640 | ≥18 | Mo: AT: running, treadmill walking or walking, stationary cycling; RT; Mixed: A + RT, qi gong exercises, individually tailored, tai chi; D: 10 days – 16 weeks |
| Rethorst et al95 | 58 | 2982 | 15–94 | Mo: AT; Fr: 3–5×/week; I: moderate to high T: 20–60 minutes; Mo: RT; F: 2–3 ×/week T: 20–60 minutes; Mo: A + RT; T: 20–90 minutes; D: acute – 52 week |
| Lawlor and Hopker92 | 11 of 14 | 479 (~34% M) | All ages | Mo: AT: running, walking; Fr: 2–5×/week; T: 20–60 minutes; D: 4–12 week |
Notes: In RCT columns, number of RCTs from total number of studies. In number of subject’s (n) columns, number of subjects that were analyzed and total number of participants.
Abbreviations: AECOPD, acute exacerbation of COPD; AT, aerobic training; D, duration of training program; Fr, frequency; HRR, heart rate reserve; I, intensity; L/E: Lower extremity; M, male; F, female; Mo, modality; mod, moderate; NR, not reported; RT, resistance training; T, session time; U/E, upper extremity; ROM, range of motion; SWT, shuttle walking test; PRT, progressive resistance training.
Table 4.
Generic and disease-specific outcomes from exercise interventions
| Author | Condition and severity | Type of training: | Generic fitness, quality of life and functional outcomes # RCTs/n/difference [95% confidence intervals] | Disease specific fitness, control and prevention outcomes # trials/n/difference [95% confidence intervals] |
|---|---|---|---|---|
| Puhan et al39 | COPD after AECOPD | AT, RT |
6MWD: 6/NR/WMD: 77.7 m [12.2, 143.2]; Shuttle walk test: 3/NR/WMD: 64.4 M [41.3, 87.4]; QoL: SGRQ: 3/NR/WMD: 9.88 [−14.40, −5.37] |
Dyspnea: 5/NR/0.97 [0.35, 1.58]; Admission to hospital: 5/250/OR: 0.22 [0.08, 0.58]; Mortality: 3/110/OR: 0.28 [0.10, 0.84] |
| Lacasse et al42 | COPD | AT, RT |
Maximal exercise capacity on cycle ergometer: 13/511/WMD: 8.4 watts [3.45, 13.41]; 6MWD: 16/669/WMD: 48.5 m [31.6, 65.3]; QoL: Fatigue of CRQ: 11/618/WMD: 0.92 [0.71, 1.13] |
QoL: Change in dyspnea of CRQ: 11/618/WMD: 1.06 [0.85, 1.26] |
| O’ Shea et al43 | Mild to severe COPD | RT |
Leg press strength: 4/77/SES: 0.96 [0.26, 1.66]; Knee extensor strength: 3/125/SES: 0.52 [0.30,0.74]; Cycling endurance: 2/52/SES: 0.87 [0.29, 1.44]. |
|
| Salman et al44 | Mild to severe COPD | AT, RT | Walking distance: 20/979/SES: 0.71 [0.43, 0.99] | Shortness of breath: 12/723/SES: 0.62 [0.26, 0.91] |
| Chavannes et al40 | Mild to mod. COPD | AT, AT + RT, | Exercise tolerance: Limited evidence of improvement. SES NR | |
| Vieira et al45 | COPD | Home based AT, RT |
Exercise capacity: 2 of 2 studies show ↑ in 6MWD or constant work rate test; QoL: 3 of 6 studies showed ↑ compared to control |
|
| Janaudis-Ferreira et al41 | Mod. to severe COPD | Arm AT, RT | Unsupported and supported arm exercise capacity: 2 (of 4) studies and 1 (of 2) showed ↑ compared to control | |
| Liu and Latham50 | Elderly | RT |
Lower limb strength: 73/3059/SES 0.84 [0.67, 1.00]; VO2 max: 18/710/WMD 1.50 mL/kg/min [0.49, 2.51]; 6MWD: 11/325/WMD 52.37 m [17.38, 87.37]; Gait speed: 24/1179/WMD 0.08 m/s [0.04, 0.12]; Timed up-and-go: 12/691/WMD −0.69 s [−1.11, −0.27]; Time to stand from a chair: 11/384/SES −0.94 [−1.49, −0.38]; Stair climbing: 8/268/−1.44 s [−2.51, −0.37]; Vitality: (SF-36) 10/611/WMD 1.33 [−0.89, 3.55]; Main function: 33/2172/SES 0.14 [0.05, 0.22] |
Death: 13/1125/RR 0.89 [0.52, 1.54]; Pain: 6/503/SES −0.30 [−0.48, −0.13] |
| Howe et al49 | Improving balance | Balance, gait, functional task |
Single leg stance time, eyes open: 4/164/MD 0.33 s [0.02, 0.64]; Berg Balance Scale 3/126/MD 2.72 [0.94, 4.50] |
|
| Gillespie et al48 | Falls prevention | Balance, gait, functional |
Rate of falls: 3/461/RR: 0.73 [0.54, 0.98] 0.036; Number of fallers: 17/2492/RR: 0.83 [0.72, 0.97] 0.018 |
|
| Weening-Dijksterhuis et al52 | Institutionalized frail elderly | AT, RT, balance and functional training |
Strength: ↑ in 8 of 9 studies; 6MWD: ↑ in 3 of 3 studies; Balance: ↑ in 10 of 10 studies; Psychological function/perceived health: some effect; Function: ↑ in 4 of 4 on depression and activity measures |
|
| Forster et al47 | Elderly in long term care | AT, RT and balance |
Mobility (variety of tests): ↑ in 24 of 35 trials; Strength: ↑ in 18; Balance: ↑ in 12 of 16 studies |
|
| Chin et al46 | Frail, Elderly | AT, RT and balance |
Physical Performance Test: ↑ in 3 of 4 studies; 6MWD: ↑ in 9 of 17 studies |
|
| Rydwik et al51 | Institutionalized elderly, multiple diagnoses | AT, RT, balance, mobility, gait, ADL |
Strength: ↑ in 6 of 9 studies; Mobility: ↑ in 8 of 12 studies; Range of motion: ↑ in 2 of 3 studies; Gait: ↑ in 4 of 8 studies; Activities of daily living: ↑ in 3 of 6 studies |
|
| Davies et al 55 | HF, severity not an inclusion criterion | AT, RT |
Hospital admissions related to heart failure: 7/569/RR: 0.72 [0.52, −0.99]; QoL using Minnesota Living with Heart Failure Questionnaire: 6/700/WMD −10.3 [−15.9, −4.8]; QoL using all scales: 9/779/SMD: −0.57 [0–0.83, −0.31] |
|
| Hwang et al54 | HF, diagnosis based on clinical signs or LVEF < 40% | RT | 6MWD: 2/40/52 m [19, 85] | |
| Hwang and Marwick57 | HF | AT (15) or AT + RT (4) |
VO2
max: 16/733/2.86 mL/kg/min [1.43, 4.29]; Exercise duration: 7/241/2.00 min [1.43, 2.57]; 6MWD: 6/628/30.4 m [6.1, 54.7] |
|
| Chien et al53 | HF, diagnosis based on clinical signs or LVEF < 40% | Mostly AT, home-based. RT added in 3/10 studies |
VO2
max: 7/355/MD 2.71 mL/kg/min [0.67, 7.74]; 6MWD: 5/320/MD 41.09 m [19.12, 63.06] |
Hospitalization due to cardiac events: 2/143/OR 0.75 [0.19, 2.92] |
| Haykowsky et al56 | HF, severity not a criterion, clinically stable | AT | VO2 max: 9/538/WMD 2.98 mL/kg/min [2.47, 3.49] |
Ejection fraction: 9/538/WMD 2.59% [1.44, 3.74]; End-diastolic volume: 5/371/WMD −11.49 mL [−19.95, −3.02]; End-systolic volume: 5/371/WMD −12.87 mL [−17.80, −7.93] |
| van Tol et al58 | HF, severity not a criterion for inclusion | AT, RT |
VO2
max: 31/1240/MD 2.06 mL/kg/min; Watts on maximal test: 19/715/MD 14.3 W; Anaerobic threshold: 13/511/MD 1.91 mL/kg/min; 6MWD: 15/599/MD 46.2 m; HR during maximal exercise: 18/683/MD 3.5 bpm; SBP during maximal exercise: 10/382/MD 5.4 mmHg; QoL: 9/463/MD −9.7 points |
End-diastolic volume at rest: 9/527/WD −3.13 mL; Cardiac output during maximal exercise: 3/104/WD 2.51 L/min |
| Spruit et al61 | HF, severity not a criterion for inclusion | RT |
Mean peak isotonic strength of upper and lower body: 1/16/37% improvement; Muscle endurance: 1/16/299% improvement |
|
| Cahalin et al60 | HF, severity not a criterion | RT, with short or long bursts of AT | Muscle strength, muscle endurance, daily activity, forearm blood flow, performance of heel lift, and QoL increased and resting HR decreased, but no synthesis of data from more than one RCT was provided | Left ventricular ejection fraction, left ventricular fractional shortening, and insulin-stimulated glucose uptake improved, but no synthesis of data from more than one RCT was provided |
| Benton59 | HF, severity not a criterion | AT, RT | Muscle strength, muscle endurance, QoL, heart rate during exercise, and forearm blood flow improved, but no synthesis of data from more than one RCT was provided | |
| Haykowsky et al65 | Post-MI | AT | Meta-regression analysis shows that exercise training had beneficial effects on LV remodeling in clinically stable post-MI patients with greatest benefits occurring when training starts earlier following MI (from one week) and lasts longer than 3 months Ejection fraction: Q = 25.48, df = 2, P < 0.01; End systolic volume: Q = 23.89, df = 2, P < 0.005; End diastolic volume: Q = 27.42, df = 2, P < 0.01 |
|
| Valkeinen et al68 | Ischemic heart disease (MI, angina, CABG, PTCA, angioplasty, percutaneous intervention) | AT (majority), RT | VO2 max for aerobic training: 15/807/SMD 0.67 mL/kg/min [0.39, 0.94]; Longer exercise training period (>6 months) starting soon after a cardiac event (<3 months) had a significant effect on VO2 max in patients with CHD: 7/406/SMD 0.94 mL/kg/min [0.38, 1.50] and 11/647/SMD 0.77 mL/kg/min [0.44, 1.10, P < 0.001] respectively | |
| Cortes et al64 | Acute myocardial infarction | In hospital early mobilization | Trend towards decreased total mortality and non-fatal re-infarction, but n.s | |
| Jolliffe et al66 | Coronary heart disease | AT (majority), RT |
Comprehensive cardiac rehabilitation: Total cardiac death: 22/2903/OR 0.75 [0.59, 0.97]; Total cholesterol: 9/1198, −0.65 mmol/L [−0.75, −0.55]; LDL cholesterol: 6/728, −0.61 mmol/L [−0.73, −0.50]; Triglycerides: Small but significant reduction (no numbers) Exercise only: Total cardiac death: 8/2312/OR 0.70 [0.51, 0.94]; Total mortality: 12/2582/OR 0.74 [0.56, 0.98] |
|
| Clark et al62 | Ischemic heart disease | AT, RT (no details) |
Program with exercise: Recurrent MI: 12/3997/RR 0.62 [0.44, 0.87] Exercise only: Mortality: 11/2285/RR 0.72 [0.54, 0.95] |
|
| Cornish et al63 | Ischemic CAD (narrative review) | AT (interval training) | Exercise capacity: 2 studies (of 2) showed ↑ in either 6 MWD, cycle test time, VO2 max, time to fatigue and HRrest, while both showed increase in workload | |
| Oliveira et al67 | Post-MI, CABG (narrative review) | RT | Exercise capacity: 2 of 2 studies showed ↑ in 6MWD; Muscle strength: 2 studies (of 2) showed ↑ in muscle strength | |
| Watson et al69 | PVD | AT, RT |
Maximal walking time: 7/255/MD: 5.1 [4.5, 5.7]; Maximal walking distance: 6/391, MD: 113.20 M [95.0, 131.4] |
Pain-free walking time time: 3/150, MD: 2.9 min [2.5, 3.3]; Pain-free walking distance: 6/322, MD: 82.2 M [71.7, 92.7] |
| Wind and Koelemay70 | PVD | AT | Walking distance: 9/499, WMD: 155.8 M [80.8, 230.7] | Pain free walking distance: 8/409, WMD: 81.3 M [35.5, 127.1] |
| Dickinson et al73 | Hypertension | AT |
SBP: 21/1346/MD: −6.1 mmHg [−10.1, −2.1; I2 = 87%]; DBP: 21/1346/MD: −3.0 mmHg [−4.9, −1.1; I2 = 74%] |
|
| Cornelissen and Fagard72 | Hypertension | AT |
VO2
max: 17/279/WMD 4.4 mL/kg/min [3.7, 5.1]; HR: 23/340/WMD −4.5 bpm [−6.5; −2.6]; SBP: 30/492/WMD −6.9 mmHg [−9.1; −4.6]; DBP: 30/492/WMD −4.9 mmHg [−6.5; −3.3]. |
|
| Whelton et al74 | Hypertension | AT |
SBP: 15/NR/ES −4.94 mmHg [−7.17, −2.70]; DBP: 13/NR/ES −3.73 mmHg [−5.69, −1.77] |
|
| Kelley et al71 | Hypertension | AT |
SBP: −6 mmHg [−8, −3] (number of trials/subjects NR); DBP: −5 mmHg [−7, −3] (number of trials/subjects NR) |
|
| Shaw et al76 | Obesity | AT | DBP: 2/259/WMD −2.09 mmHg [−3.68, −0.51] |
Triglycerides: 3/348/WMD −0.18 mmol/l [−0.31, −0.05]; Fasting glucose: 2/273/WMD −0.17 mmol/l [−0.30, −0.05]; HDL: 3/348/WMD 0.06 mmol/l [0.03, 0.09] |
| Witham and Avenell75 | Obese postmenopausal women | AT, RT | VO2 max: increase by 11.7% in the intervention group and 0.7% in the control group at 12 months (P < 0.001) | |
| Devos-Comby et al79 | OA | AT, RT, and balance | Direct measures of impairment (walking distance test, timed chair rise, time getting out of a car, balance tests, or gait): 11/740/SES 0.15 [0.08, 0.23] | Combined physical outcomes (Scales of physical disability, discomfort, pain, function, mobility ie, AIMS): 12/808/SES: 0.29 [0.23, 0.36]. |
| Lange et al80 | Knee OA | RT | Strength: ↑ in 9 of 14 studies; Maximal gait speed: ↑ in 4 of 4 studies; Maximal stair climb/descent: ↑ in 3 of 5 studies | Pain: ↑ in 10 of 18 studies; Physical disability: ↑ in 11 of 14 studies; Physical self efficacy: ↑ in 2 of 2 studies |
| Ottawa Panel77 | OA | AT, RT | Strength, aerobic capacity, and functional status: different levels of evidence support various types of strengthening, mobility and flexibility exercises based on RCTs but no synthesis of data from more than one RCT was provided | Pain: different levels of evidence from RCTs show that different types of exercise decrease pain. No synthesis of data from more than one RCT was provided |
| Brosseau et al78 | OA | AT | Aerobic capacity, timed walk distance, walk velocity: ↑ in RCTs but no synthesis of data from more than one RCT was provided | |
| Pelland et al81 | OA – most knee or hip | Mainly RT | Strength, function and QoL: improves, but no synthesis of data from more than one RCT was provided | Pain: decreases, but no synthesis of data from more than one RCT was provided |
| Li et al83 | Osteoporosis or osteopenia; severity not an inclusion criteria | RT or combined stretch/strength/balance programs |
QoL: All domains of SF36 were significantly improved for all 4 studies. Scores out of 100. Physical function: 5/288/WMD 2.77 [2.27, 3.37]; Pain: 5/288/WMD 4.95 [3.52, 8.70]; Role Physical: 2/78/WMD 12.41 [0.35, 24.46]; Vitality: 2/78/WMD 11.11 [3.99, 18.22] Subgroup analysis showed that programs that combined programs improved QoL physical function and pain scores more than strengthening alone |
|
| De Kam et al82 | Osteoporosis or osteopenia | AT, RT, Balance, Gait | Improvements in: TUG, standing up and walking around cones, U/E strength, posturagraphy; figure 8 walking; L/E strength; trunk strength; step test; lateral reach; walking velocity; balance performance | Improvements in: spine BMD, hip BMD, femur BMD, fall-related fractures; radius BMD, calcaneus BMD, fall risk reduction; tibia BMD, falls incidence; vertebral height |
| Chudyk and Petrella85 | Type 2 DM | AT 21 RCT AT + RT |
SBP: AT: −6.1 mmHg [−10.8, −1.4]; AT + RT: −3.6 mmHg [−6.9, −0.2] |
HbA1c AT: WMD: −0.62% [−0.98, −0.27]; AT + RT: WMD: −0.67% [−0.93, −0.40]; Triglycerides AT: WMD: −0.29 mmol/L [−0.48, −0.11]; AT + RT: WMD: −0.30 mmol/L [−0.57, −0.02]. |
| Umpierre et al90 | Type 2 DM | AT, RT, AT + RT |
HbA1c AT: 18/848/WMD: −0.73% [−1.06, −0.40]; RT: 4/261/WMD: −0.57% [−1.14, −0.01]; AT + RT: 7/404/WMD: −0.51% [−0.79, −0.23]. |
|
| Irvine and Taylor86 | Type 2 DM | RT | Strength: 4/NR/SES: 0.95 [0.58, 1.31] | HbA1c: 7/NR/SES: −0.25 [−0.47,−0.03] |
| Thomas et al89 | Type 2 DM | AT or RT | VO2 max: 3/95/MD: 4.8 mL/kg/min [2.6, 7.1] | HbA1c: 13/361/MD: −0.62% [−0.91,−0.33]. |
| Kelley and Kelley87 | Type 2 DM | AT | Low density lipoprotein: WMD: −6.4 mg/dl [−11.8, −1.1] | |
| Snowling and Hopkin88 | Type 2 DM | AT, RT, or A + RT | A + RT: SBP: 5/NR/WMD: −5.6 mmHg [−9.3, −1.8]; DBP: 5/NR/WMD: −5.5 mmHg [−9.9, −1.1]. |
HbA1c: AT: 17/NR/WMD: −0.7% [−1.0, −0.4]; RT: 6/NR/WMD: −0.5% [−1.0, −0.1]; A + RT: 5/NR/WMD: −0.8% [−1.3, −0.2]. |
| Boulé et al84 | Type 2 DM | AT | VO2 max: 9/266/SES: 0.53 [0.18, 0.88] | HbA1c: 8/NR/WMD: −0.71 [−1.1, −0.32] |
| O’Brien et al91 | HIV – range of severity | AT |
VO2
max: 5/276/WMD: 2.6 mL/kg/min [1.2, 4.1]; Strength: ↑ in 5 of 6 studies |
Interval AT: CD4 cell counts: 2/45/WMD: 69.6 cells/mm3 [14.1, 125.1]; AT: Profile of moods: 2/65/WMD: −7.7 [−13.5, −1.9]. |
| Krogh et al93 | Depression | 9 AT; 3 RT; 1 A + RT | Depressive symptoms: 13/272/SES: −0.40 [−0.66, −0.14] | |
| Herring et al94 | Anxiety and chronic illness | AT, RT, balance | Anxiety symptoms: 38/NR/SES: 0.29 [0.23, 0.36] | |
| Mead et al96 | Depression | AT, RT, A + RT |
Depression symptoms AT: 17/640/SES: −0.63 [−0.95, −0.30]; RT: 2/69/SES: −1.34 [−2.07, −0.61]; A + RT: 4/198/SES: −1.47 [−2.56, −0.37] |
|
| Rethorst et al95 | Depression | AT, RT, AT + RT | Depression scores: 58/2982/ES: −0.80 [0.92, 0.67]. | |
| Lawlor and Hopker92 | Depression | AT |
Depressive symptoms: 9/461/SES: −1.1 [−1.5 to −0.6]; Beck depression: 9/461/WMD: −7.3 [−10.0, −4.6] |
Notes: WMD (weighted mean difference) is a calculation that provides an average mean difference of studies by weighting the means more highly when the n is larger and the variance is smaller. If the WMD is provided, the unit value for the measure is shown. SES (standardized effect size) is usually calculated by determining the difference between the pre-post values for the intervention and control groups and dividing this difference by the respective standard deviation of differences for the intervention group or the average SD of the differences for both groups.
Abbreviations: AT, aerobic training; BMD, bone mineral density; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; MD, mean difference; OR, odds ratio; QoL, quality of life; RR, rate ratio; RT, resistance training; SES, standardized effect size; 6 MWD, six-minute walking distance; SBP, systolic blood pressure; TUG: timed up-and-go; WMD, weighted mean difference.
Only statistically significant outcomes are reported in Table 4. When available, quantitative data for each metaanalysis was described including the number of studies, the number of participants, the standardized effect size or weighted mean difference, risk ratio, odds ratio, and 95% confidence intervals. If this information was not available, an attempt was made to determine the number of studies that reported a significantly positive change versus the total number of studies that measured the outcome. Nonsignificant findings or trends are not reported.
Results
Study selection
A search of the eight databases yielded 3276 citations and abstracts after duplicates were removed. After independent screening of the citations by two reviewers, 362 full-text articles were selected and reviewed for inclusion. Fifteen additional articles were identified from manual searching of reference lists. The main reasons for excluding articles were that they were not an SR, that participants did not have COPD/AECOPD or one of the other conditions of the search strategy, there was no exercise intervention, the exercise intervention was not clearly described or not differentiated from concurrent treatment, there was no comparison between an exercise group and a control group, the SR reported on similar data but had few articles, the article was not available in English, or the article was published before 2000. No SRs were identified that compared exercise to a control group in people with asthma or bronchiectasis. Fifty-eight SRs met the inclusion criteria.
Quality assessment
The number of RCTs in any of the SRs that related to our question ranged from 4 to 83. The average AMSTAR score was 7.5 out of a potential 11. The most common items not reported were a list of excluded articles (only 26% of SRs provided this list), an examination of publication bias (ie, funnel plot or Egger regression test, which 45% reported), a statement regarding conflict of interest (50% reported), a selection and abstraction of data performed by two reviewers (65% reported), and a search of the gray literature (59% reported). All other items were addressed by ≥71% of the SRs.
Characteristics of participants
The age of participants ranged between 18 years and the “very elderly”. SRs that described people with HIV or depression had the youngest participants. Gender varied from 100% male to 100% female. SRs that described older adults had the largest sample sizes.
Characteristics of interventions and outcomes in the different chronic conditions
Characteristics of the interventions are detailed in Table 3, while outcomes in the different chronic conditions are shown in Table 4.
AECOPD
One SR investigated the benefits of aerobic and resistance training in people with COPD (AMSTAR of 11).39 Aerobic and resistance training of the extremities improved walk distance performance and quality of life measures in addition to decreasing dyspnea, compared to the control group. Exercise also reduced admissions to hospital and mortality as demonstrated by meta-analyses of five and three RCTs, respectively.39 The SR included studies that initiated exercise immediately after or up to 6 weeks after treatment initiation for AECOPD as part of pulmonary rehabilitation on an out- or in-patient basis. Frequency of training ranged from three times a week to twice daily. The intensity of aerobic training was not reported.
COPD (stable)
Six SRs investigated the benefits of AT and RT in people with COPD, and the AMSTAR scores ranged between 5 and 10.40–45 AT and RT of the extremities improved maximal exercise capacity, walk distance performance, and quality of life measures in outpatient COPD compared to a control group as described by a high quality SR with an AMSTAR score of 10.42 Whether people with mild-to-moderate COPD with AECOPD benefit from exercise training is equivocal, as summarized by two SRs with AMSTAR scores of 5 and 8.40,44 Exercise training that focused on RT improved leg strength and cycling endurance as described by one review with an AMSTAR of 8.43 Unsupported and supported arm exercise showed improvement in half of the studies evaluated by one SR.41 Home exercise demonstrated improvement in exercise capacity in all studies that assessed this attribute and half of the studies that assessed quality of life.45 Dyspnea was significantly improved by aerobic and resistance trainings in two reports.42,44
Walking and cycling were the most common AT whereas free weights and Theraband™ were the most common resistance used during RT. Frequency of training ranged from twice weekly to twice daily. The intensity of aerobic training was either not described in the SRs or, in one report, was quantified as a percentage of walk test distance.45 RT intensity ranged from one-third of a (repetition maximum) RM to near maximal intensity for the number of repetitions. The time for each session varied between 30 and 120 minutes, or was defined in terms of repetitions and sets for RT.41,43 Durations of studies ranged between four weeks and 18 months. There was an obvious focus on AT and RT in the SRs, and no reports described the outcomes from balance or functional training in people with COPD.
Older adults
Overall, exercise training results in improved physical and functional performance for older adults, as demonstrated by seven SRs,46–52 although initial health status influenced both the ability to participate in certain programs as well as the outcomes of the training. Most SRs and many RCTs included a mix of RT, BT, and functional training rather than focusing on a single type of training. Thus, evidence for the most part is provided from multifaceted training rather than a singular approach. Many other aspects of the training varied widely, with a training frequency most often of two to three times a week, total duration that ranged from 2 to 104 weeks, and a variable intensity level (Table 3). SRs, which had AMSTAR scores ranging from 5 to 11, showed that mixed training improved balance47,49,52 and strength,47,51,52 reduced falls,48 and improved functional measures such as the 6-minute walk distance (6MWD).46,49,51,52 An SR that had a singular focus on RT with an AMSTAR of 11 showed widespread improvements in lower extremity strength, aerobic capacity, and several physical performance tests (Table 4).50 In addition, this SR provided evidence that RT reduced the relative risk of death and pain.50
Heart failure
Six meta-analyses53–58 investigated the benefits of AT and/or RT in patients with chronic heart failure. Most of these papers had AMSTAR scores ranging from 6 to 8, although one57 had a score of 3. Based on these papers, it can be concluded that AT and RT significantly improved numerous cardiopulmonary exercise outcomes, including increasing VO2 max, 6MWD, maximal workload, and anaerobic threshold. It also improved several cardiac system factors, including increasing the heart rate and cardiac output during maximal exercise, improving left ventricular ejection fraction, and the end-diastolic and end-systolic ventricular volume. Two meta-analyses also reported that exercise significantly improved quality of life in these patients.55,58
There were no meta-analyses that investigated the role of AT and/or RT on muscle strength or endurance. Three reviews without meta-analyses investigated the benefits of exercise on a large number of outcomes, including several that are specific to muscle performance.59–61 Exercise improved upper and lower extremity strength and muscle endurance, increased oxidative enzyme activity in the quadriceps muscle, and increased forearm blood flow. However, these results must be interpreted with caution as these reports were SRs without meta-analyses, and the AMSTAR scores were relatively low (2 to 6), with few details quantifying the magnitude of the effect.
The AT was relatively consistent across all the studies, in terms of frequency, mode, and intensity. Subjects exercised 2–7 days per week, with most trials within each SR providing the exercise intervention for 3 days per week. Cycle ergometry and treadmill walking were the most commonly used modalities. Most studies prescribed an AT intensity based on a maximal cardiopulmonary exercise test, with exercise intensities ranging from 50% to 80% of VO2 max. The time per session and the durations of the programs were very variable, ranging from 12 to 100 minutes and 1 to 14 months, respectively. Where described, the resistance training prescription tended to be based on 40% to 80% of one RM.
Ischemic heart disease
Seven SRs investigated the benefits of early mobilization, anaerobic training, and/or RT in ischemic heart disease patients.62–68 The AMSTAR scores ranged from 3 to 10, with two reviews having a score of 3,63,67 and one a score of 5.68 The remaining reviews had AMSTAR scores exceeding 8.
The study populations were predominantly male. There was variation in the specific type of intervention (both AT and RT), and differing endpoints were utilized to evaluate benefit and responses. Five reviews examined the effects of AT,62,63,65,66,68 three reported the effects of both AT and RT,62,66,68 one examined the sole effect of early in-hospital mobilization,64 and one examined the sole effect of RT.67 Some work focused on reporting disease-specific outcomes (ie, recurrent myocardial infarction, cholesterol and triglyceride levels, ejection fraction, and end-systolic and end-diastolic volumes),62,64,65,66 while others studied indices of overall activity and fitness (ie, walking distance, maximal aerobic capacity, exercise duration, and muscle strength)63,67,68 in these populations with ischemic heart disease. Quality of life and health care utilization were not studied or specifically reported, and significant variation was evident in the duration of follow-up.
Early in-hospital mobilization demonstrated nonsignificant trends towards decreased total mortality and nonfatal re-infarction.64 The results suggest that anaerobic and RT reduces the risk of total cardiac death, recurrent myocardial infarction, as well as cholesterol and triglyceride levels. Improvements were also demonstrated in peak oxygen uptake, exercise duration, exercise workload, and 6MWD. The findings suggest that benefits were greater when exercise programming was initiated soon after an ischemic event (as early as 1 week post-myocardial infarction), and when exercise program duration exceeded either 3 or 6 months (rather than <3 months).
Peripheral vascular disease
Exercise training significantly improved the walking distance and pain-free walking distance in patients with peripheral vascular disease (PVD) when compared to standard care as evidenced by two meta-analyses with relatively high AMSTAR scores of 9 and 7.69,70 Exercise training was generally supervised. The optimal intensity was not clear, although it was suggested that patients exercised until near maximal pain. The majority of the studies applied an exercise regimen of two to three times per week for 30–60 minutes, with these usually incorporating walking, leg exercises, or treadmill training. Some encouraged additional home exercise.
Hypertension
Exercise training (mainly aerobic exercise) significantly lowered the systolic (SBP) and diastolic blood pressure (DBP) in people with hypertension, as supported by four meta-analyses.71–74 Two of the SRs73,74 had AMSTAR scores of 8 of 11 while two others had scores of 7 and 4.71,72 VO2 max increased significantly and resting heart rate (HR) decreased after exercise training.72 Exercise training also demonstrated significant improvement in cardiovascular risk factors as shown by an increase in high density lipoprotein cholesterol, and decreases in glucose, insulin levels, and the homeostatic model assessment index (a measure of insulin resistance), despite the inclusion of normotensives in the analysis.72 Reductions were greater for SBP in African– American individuals and for DBP in Asian individuals, but only a small number of trials were conducted in people of different ethnic backgrounds.74
A variety of study designs were included in the meta-analyses, but the majority of the studies included training programs that involved mainly aerobic exercise (walking, jogging, cycling), usually three to five times per week, for 30 to 60 minutes, at moderate intensity. According to two SRs there is no relationship between blood pressure (BP) response and training characteristics (intensity, frequency), except for a lesser BP reduction associated with a longer total trial duration, probably due to loss of compliance in long trials.72,74
Because aerobic exercise also showed positive effects in normotensives, these data suggest that aerobic exercise is an important strategy not only in treating high blood pressure, but could potentially be used as prophylaxis.74
Obesity
Data on the effect of physical activity on fitness outcomes in this population were limited. In post-menopausal obese women, an exercise-based program improved VO2 max as shown in one RCT reported by one SR.75 A Cochrane review with an AMSTAR score of 11 did not report the effect of physical activity on VO2 max but found a decrease in DBP, reduced triglycerides and glucose, and increased HDL levels.76 The majority of trials in the two SRs consisted of AT, usually two to three times per week, at 60%–80% of VO2 max or HRmax, with different durations utilized.
Osteoarthritis
Results from the five SRs examining the effects of exercise on patients with knee or hip OA consistently show beneficial results for fitness levels and functional performance, though emphasis was primarily on disease-specific outcomes such as joint pain or physical dysfunction.77–81 AMSTAR scores were between 7 and 9, and most studies failed to report publication bias or conflicts of interest.
Isotonic RT targeting the quadriceps or hamstrings muscle groups was the most common exercise intervention, though AT and range of motion exercises were also included. Control interventions primarily included patient education or usual care with no additional treatment. Most exercise interventions lasted 3–6 months, though some were as short as 4 weeks and as long as 24 months. Some studies required daily exercise, while others included only two or three sessions per week. Individual sessions were typically 45–60 minutes in length and complied with usual dosage characteristics for exercise in healthy populations (ie, three sets of ten repetitions for resistance training, and 50% to 80% of maximum heart rate for aerobic interventions).
Pain and physical function were consistently improved following exercise, but no particular type of exercise was found to be superior. Studies examining RT found improvements in strength compared to control interventions,77,81 while studies examining the effects of AT found improvements in aerobic capacity, walking speed, and walking distance.77–80 All studies found exercise to be a safe and feasible intervention with minimal side effects for patients with OA.
Osteoporosis
Most of the SRs identified in this study investigated the benefits of exercise in the prevention of osteoporosis or osteopenia in healthy, pre- or post-menopausal women. Two SRs investigated the impact of exercise in women diagnosed with osteoporosis or osteopenia, and these had AMSTAR scores of 782 and 683, indicating moderate quality overall. One of these was an SR without a meta-analysis that evaluated the benefits of AT, RT, BT, and gait on numerous exercise- and disease-related outcomes. It was reported that exercise resulted in improvements in balance measures such as the timed-up-and-go, upper extremity strength, and walking velocity, as well a reduction in fall incidence and fall-related fractures.82 The other paper included a meta-analysis investigating the benefits of RT with or without balance exercise, and reported that exercise improved several quality-of-life domains including physical function, pain, and vitality.83
Type 2 diabetes mellitus
Seven meta-analyses investigated the benefits of RT and or AT in people with type 2 diabetes with AMSTAR scores that ranged from 6 to 11.84–90 Aerobic exercise improved VO2 max as evidenced by two SRs with AMSTAR scores of 6 and 11.84,89 Two SRs with AMSTAR scores of 6 also demonstrated reductions in SBP and DBP after AT, RT, or a combination of AT and RT.85,88 Strength measures improved after RT, as described by one SR with an AMSTAR of 7.86 Glycosylated hemoglobin (HbA1c), a measure of blood sugar levels, was the most consistent outcome reported in all of the included SRs, with a reduction in this seen in all reviews regardless of the type of exercise training.84–90
The exercise interventions described were quite varied across the SRs. AT consisted of stationary cycling, outdoor cycling, walking, jogging, swimming, rowing, or skiing. RT was performed using a Theraband, free weights, or weight machines. AT intensity ranged 50%–85% of VO2 max, 35%–85% of HRmax, or 40%–80% of HRR. RT intensity ranged 50%–85% of one RM. The exercise sessions were between 30 and 120 minutes, and the duration of the program ranged from 5 to 52 weeks. The SRs primarily focused on the benefits of AT, RT, or a combination of these interventions. However, no studies focused on balance or functional training, or reported functional or quality-of-life outcomes.
HIV/AIDS
Interval and continuous AT improved VO2 max in patients living with HIV, as evidenced by a Cochrane review with an AMSTAR score of 10.91 This SR also described improved strength in five of the six studies that reported this measure. Additional benefits from exercise training include no change in the viral load, an increase in CD4 cell counts after interval AT, and improved Profile of Moods scores after AT. A summary of the interventions applied is shown in Table 3.
Depression
The five SRs relating to depression were moderate-to-high quality (AMSTAR 8–11).92–96 Exercise programs (AT, RT, or a combination of these) for people diagnosed with major or minor depression were found to result in improved scores on depression and mood, particularly in the short term.92,93 Medium- to long-term effects were less clear.92,93 As a group of studies, the interventions were not well described. Most examined both AT, RT, and mixed AT plus RT, except one92 that focused on AT. The intensity of AT, if described, was moderate to high.93,95 Each session was 30 to 60 minutes in length, with these performed one to five times per week, and the duration of the programs was between 10 days and 52 weeks.
Discussion
This synthesis examined 58 systematic reviews of exercise training in people with AECOPD, COPD, or eleven chronic conditions commonly associated with COPD. Overall, this review provides Level 1A evidence97 that exercise training improves generic or disease-specific measures of fitness in several disease populations. Markers of endurance (aerobic capacity, 6MWD) were shown to improve in all conditions except depression, whereas strength was shown to improve in most conditions with the exception of PVD, hypertension, obesity, and depression. In addition, several studies in different disease populations also reported improvements in quality of life, function, control, or prevention outcomes. Depression was the only condition where SRs exclusively focused on disease-specific outcomes (measures of depression and anxiety).92–96 Exercise training also decreases the risk of mortality in older adults,50 and in those with COPD39 or ischemic heart disease.66
Overwhelmingly, the most common types of training provided to people with all conditions reviewed were AT and RT. Balance and functional training were less commonly applied SRs of exercise training. However, these interventions had widespread application in most SRs that examined older adults,46–49,51,52 and in one SR that examined OA patients79 and one that examined osteoporosis patients.82
The quality of the SRs for most conditions was moderate to excellent (>65%) as evaluated by AMSTAR scores. However, SRs that examined training outcomes in heart failure, ischemic heart disease, hypertension, and osteoporosis scored moderately poorly on the AMSTAR (≥51% but ≤61%). The listing of articles that were excluded from in the review was the most common item omitted from most SRs, which may have been due to page constraints. Even with this item excluded, the AMSTAR scores remained relatively low for heart failure (56%) whereas scores for the other conditions were ≥66%. In addition to the SR design and strength of AMSTAR scores, the number of participants and RCTs for each condition, including COPD, were large. These numbers further strengthen the foundation of evidence and recommendations for exercise training as an intervention that produces positive outcomes for COPD and commonly associated conditions.98
Although COPD patients often have one or more comorbidities, no information is available, on prescription parameters of exercise training for patients with multimorbidity. Training parameters from studies examining conditions that are common comorbidities might be applicable, however, consideration should be give to similarities of patient demographics and details of training, especially intensity. Given that the most common conditions associated with COPD are obesity (prevalence of 54%),26 osteoporosis (60%–70%)23 and osteoarthritis (increasing prevalence to 40% at age 75)27 (see Table 1), data from SRs describing these conditions may have the greatest relevance. The pertinence of these data is further strengthened by the similarity in age of participants from SRs that examined exercise training in people who were obese, had osteoporosis or osteoarthritis to those affected by COPD. In contrast, subjects were somewhat younger in some of the RCTs that examined obesity. Gender was mixed in all of these conditions, except in the case of the SRs on osteoporosis in which predominantly women were studied.82,83
The modalities of AT and RT for obesity, osteoporosis, and osteoarthritis were similar to those typically applied for COPD patients, although exercise training for these conditions utilized more diverse types of training. The intensity of AT appeared to be much higher for obese individuals and those with arthritis compared to that prescribed for COPD patients; intensity was commonly based on aerobic or HR parameters for the former compared to walk distance or dyspnea measures used in COPD patients. This raises the notion that a broader spectrum approach to exercise training in order to improve fitness in patients with COPD and comorbidities such as obesity, osteoporosis, and osteoarthritis may be effective. However, exercise training of the more complex COPD patient (with multiple comorbidities) may result in smaller gains of disease-specific outcomes due to the inability to achieve comparable AT and RT intensities (compared to those achieved in patients with a single condition). These issues merit further study.
Because obesity is a major cause of morbidity and a significant risk factor for other comorbidities such as heart disease and diabetes,26,75,76 SRs addressing exercise fitness outcomes other than body composition are needed in order to determine the overall impact of exercise training in this particular group. Although altering body composition may be the primary outcome for people who are obese, understanding the training and physiologic determinants to achieve that goal are essential; thus fitness outcomes such as aerobic capacity and strength measures should be reported in order to better evaluate the type and intensity of training that results in improvement. Moreover, it has also recently been demonstrated that some obese COPD patients have better fitness outcomes than non-obese COPD patients.99 Determining how physical activity interventions can attenuate obesity, reduce cardiovascular risk factors, and improve overall fitness in people with COPD needs further attention.
Depression is a common comorbidity in COPD with estimates of prevalence ranging between 10% and 80%.25 SRs that examined the effect of exercise on depression had participants with an age range similar to those with COPD, and the interventions were similar to many of the other conditions in this review. A major contrast to the other SRs was that only disease-specific outcomes and no generic fitness measures were reported. All five SRs demonstrated significant decreases in depression or anxiety scores.92–96 These benefits were reported regardless of whether AT, RT, or a combination of AT and RT were applied. Of interest is the finding that the benefits decreased in response to longer training programs. More RCTs are needed to examine clinical sub-groups (minor, moderate, major depression), to compare different forms of exercise, and to clarify the appropriate dose/intensity of physical exercise in addition to the mechanism of improvement. Designing a training program that might result in gains related to self-efficacy would be very different from a regimen that requires improved aerobic fitness or an endorphin response to induce a therapeutic benefit. A better understanding of the type of depression most commonly experienced by COPD will also provide further insight into if and how exercise training can reduce the clinical severity of these two conditions.
Exercise training for older adults showed the greatest contrasts to interventions applied to COPD and other associated conditions in this review. This is especially important given that many individuals with COPD who present for health care interventions are 65 or older. Major foci appeared to be on balance and functional training rather than predetermined intensities of AT or RT.46–49,51,52 In addition, frequently reported outcomes by the SRs on the elderly included balance/falls, and physical performance measures that were functional (ie, timed up-and-go, stair climbing) as opposed to more physiologic measures of strength and endurance. Exercise training for older adults may provide an excellent model to further broaden exercise prescription for COPD patients because, as with the elderly,48,49 people with COPD have a high relative risk of falls.100 In addition, the physical activity level of COPD patients is often comparable to much older, healthier adults. Thus, a greater focus on balance and functional training may not only reduce falls in COPD patients but might also result in greater gains in daily physical activity outside of pulmonary rehabilitation.101
Limitations
Although this synthesis of systematic reviews examined several conditions commonly associated with COPD, the participants within each of the SRs most often had a homogeneous presentation of the condition of interest. Only SRs of the elderly appeared to include participants with diverse and multiple morbidities. For this reason, it might be challenging to apply modalities in a sufficient dose in order to induce both generic and disease-specific benefits that address a complex patient with COPD and multimorbidities. The more functional approach of physical activity training, even in the frail elderly, did result in functional gains, and this approach may also prove to be beneficial to the AECOPD patients with multimorbidities.
Many SRs included lengthy training programs but did not compare the benefits of shorter versus longer programs, with the exception of SRs that evaluated those with hypertension72,74 and depression.92,93 These SRs indicated a slightly diminished response with longer training programs, which may have resulted in a blunting of the physiologic benefits of exercise, decreased adherence to the exercise training program, or a continued onslaught of inciting factors that could no longer be countered by the benefits of exercise training.
Conclusions
In summary, this systematic review showed evidence of significant benefits from exercise training in AECOPD, COPD, and many conditions that are common comorbidities associated with COPD. Exercise training, primarily consisting of AT and/or RT, leads to meaningful generic and disease-specific benefits in these conditions. Meta-analyses of endurance (aerobic or exercise capacity, 6MWD) were shown to improve in most conditions whereas meta-analyses of strength were shown to improve in five of the 13 conditions (that is, COPD, older adults, heart failure, ischemic heart disease, and diabetes). Additional research is required to determine the added benefit of RT plus AT in several conditions that are common comorbidities associated with COPD.
A broader approach to exercise and activity prescription in pulmonary rehabilitation for AECOPD may induce therapeutic benefits to ameliorate clinical sequelae associated with COPD and comorbidities. For example, BT and functional training, similar to that applied in older adults, could potentially be very effective in improving functional outcomes including fall risk, and may have a greater impact on daily physical activity than recently demonstrated.101
Appendix 1 Search strategy for Medline Ovid
Most of the numbered items are entered as keywords or phrases in the search box on the main page of Medline OvidSP. However, some line items at the end of each concept (eg, line 7) are commands to combine previous items in a particular fashion for the main concept. Asterisks indicate truncated terms in Medline. As well, abbreviations (ie, adj3) can be used to link words together.
Similar search terms were used in all databases. However, the preferred term could differ and the user needed to determine if the alternate term provided in a database was suitable. In addition, the manner of truncation, labeling for title or abstract, and combining of terms differed amongst databases.
Concept: COPD
lung diseases, obstructive/or exp pulmonary disease, chronic obstructive/or bronchitis, chronic/
exp Pulmonary Emphysema/
(obstruct* adj3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)).ti,ab.
((chronic airflow or chronic airway*) adj2 (disease* or disorder* or obstruct* or limitation*)).ti,ab.
(COPD or COAD or chronic bronchi* or emphysema* or hyperlucent lung*).ti,ab.
(chronic adj3 obstructive).ti,ab.
or/1–6
Concept: comorbidities
8. bronchial diseases/or asthma/or bronchial hyperreactivity/or bronchial spasm/or bronchiectasis/or exp bronchitis/or bronchopneumonia/
9. (bronchial disease* or asthma* or bronchial asthma* or bronchial hyperreactivit* or bronchospasm*).ti,ab.
10. (bronchiectas* or bronchitis or bronchopneumonia* or bronchial pneumonia*).ti,ab.
11. exp Pneumonia/
12. (pneumonia* or pneumoniti* or pulmonary inflammation* or lung inflammation* or lobar pneumonia*). ti,ab.
13. Influenza, Human/
14. ((influenza adj3 human*) or human flu or influenza* or grippe).ti,ab.
15. respiratory insufficiency/
16. (respiratory insufficiency or respiratory failure or respiratory depression).ti,ab.
17. heart diseases/or arrhythmias, cardiac/or heart failure/or myocardial ischemia/or myocardial infarction/or pulmonary heart disease/
18. (arrythmia or arrhythmia or cardiac arrhythmia* or cardiac dysrhythmia*).ti,ab.
19. (heart failure or cardiac failure or myocardial failure or heart decompensation or myocardial infarction).ti,ab.
20. (pulmonary heart disease* or cor pulmonale).ti,ab.
21. Hypertension/
22. (hypertension or high blood pressure).ti,ab.
23. Diabetes Mellitus/
24. (diabetes mellitus or diabetes).ti,ab.
25. Obesity/or Overweight/
26. (obesity or overweight).ti,ab.
27. Sleep Apnea, Obstructive/
28. (sleep apnea or obstructive sleep apnea or obstructive sleep apnea syndrome).ti,ab.
29. Depression/
30. (depression* or depressive symptoms or emotional depression*).ti,ab.
31. Anxiety/
32. (anxiet* or nervousness or anxious).ti,ab.
33. Anemia/
34. (anemia* or anaemia).ti,ab.
35. Osteoporosis/
36. (osteoporos#s or senile osteoporos#s or bone-loss*). ti,ab.
37. Osteoarthritis/
38. (osteoarthritis or osteoarthritides or osteoarthros#s or degenerative arthritis).ti,ab.
39. osteopenia.mp.
40. exp aged/or frail elderly/
41. (aged or elderly or frail elderly or frail elder* or frail older adult*).ti,ab.
42. or/8–41
43. 7 or 42
Concept: hospitalized patients
44. exp Inpatients/
45. Hospitalization/
46. (inpatient* or in-patient* or hospitalized or hospitalised or hospitalization*).ti,ab.
47. or/44–46
48. 43 and 47
Concept: exercise
49. exp exercise/or exp walking/
50. exp Exercise Therapy/
51. Physical Fitness/
52. Exercise Movement Techniques/
53. (exercise* or physical exercise* or exercise therap* or walk* or ambulat* or physical fitness or physical activity). ti,ab.
54. (exercise* adj5 train*).mp.
55. (strength* adj5 train*).mp.
56. exp “Physical Therapy (Specialty)”/
57. (physiother* or physical therap*).mp.
58. Rehabilitation/
59. rehabilitat*.mp.
60. or/49–59
61. 48 and 60
62. animals/not humans/
63. 61 not 62
64. limit 63 to English
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. Programmes and projects: Chronic Respiratory Diseases: Burden of COPD Web page. Geneva, Switzerland: World Health Organization; 2011. [Accessed October 5, 2011]. Available from: http://www.who.int/respiratory/copd/burden/en/index.html. [Google Scholar]
- 2.de Melo MN, Ernst P, Suissa S. Rates and patterns of chronic obstructive pulmonary disease exacerbations. Can Respir J. 2004;11(8):559–564. doi: 10.1155/2004/813058. [DOI] [PubMed] [Google Scholar]
- 3.Vestbo J. Clinical assessment, staging, and epidemiology of chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc. 2006;3(3):252–256. doi: 10.1513/pats.200510-107SF. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JM, Haddon JM, Bradley-Kennedy C, Kuramoto L, Ford GT RUSIC Study Group. Resource use study in COPD (RUSIC): a prospective study to quantify the effects of COPD exacerbations on health care resource use among COPD patients. Can Respir J. 2007;14(3):145–152. doi: 10.1155/2007/921914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittmann N, Kuramoto L, Seung SJ, Haddon JM, Bradley-Kennedy C, Fitzgerald JM. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102(3):413–421. doi: 10.1016/j.rmed.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Tu JV. Are elderly patients with obstructive airway disease being prematurely discharged? Am J Respir Crit Care Med. 2000;161(5):1513–1517. doi: 10.1164/ajrccm.161.5.9907031. [DOI] [PubMed] [Google Scholar]
- 7.Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58(9):752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16(10):1033–1038. doi: 10.1016/0277-9536(82)90175-7. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296–1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. [DOI] [PubMed] [Google Scholar]
- 10.Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Jr, Tinetii ME. A controlled trial of a nursing-centered intervention in hospitalized elderly medical patients: the Yale Geriatric Care Program. J Am Geriatr Soc. 1993;41(12):1353–1360. doi: 10.1111/j.1532-5415.1993.tb06487.x. [DOI] [PubMed] [Google Scholar]
- 11.Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645–652. [PubMed] [Google Scholar]
- 12.Mahoney JE, Sager MA, Jalaluddin M. New walking dependence associated with hospitalization for acute medical illness: incidence and significance. J Gerontol A Biol Sci Med Sci. 1998;53A(4):M307–M312. doi: 10.1093/gerona/53a.4.m307. [DOI] [PubMed] [Google Scholar]
- 13.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129(3):536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Facing the Facts: The Impact of Chronic Disease in Canada. Geneva, Switzerland: World Health Organization; 2011. [Accessed November 21, 2011]. Available from: http://www.who.int/chp/chronic_disease_report/media/CANADA.pdf. [Google Scholar]
- 15.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 16.World Diabetes Foundation. Diabetes Facts. Web page. Gentofte, Denmark: World Diabetes Foundation; [Accessed October 6, 2011]. [updated May 5, 2011]. Available from: http://www.worlddiabetesfoundation.org/composite-35.htm. [Google Scholar]
- 17.Young AS, Klap R, Shoai R, Wells KB. Persistent depression and anxiety in the United States: prevalence and quality of care. Psychiatr Serv. 2008;59(12):1391–1398. doi: 10.1176/appi.ps.59.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu K, Chen Z, Lipscome LL Canadian Hypertension Education Program Outcomes Research Taskforce. Prevalence and incidence of hypertension from 1995–2005 a population-based study. CMAJ. 2008;178(11):1429–1435. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 21.International Osteoporosis Foundation. Facts and Statistics about Osteoporosis and its Impact. [Accessed February 29, 2012]. Available from: http://www.iofbonehealth.org/facts-and-statistics.html#factsheet-category-23.
- 22.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 23.Kopec JA, Rahman MM, Berthelot JM, et al. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J Rheumatol. 2007;34(2):386–393. [PubMed] [Google Scholar]
- 24.Rutten FH, Cramer MJ, Lammers JW, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8(7):706–711. doi: 10.1016/j.ejheart.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 26.Eisner MD, Blanc PD, Sidney S, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson GT, Claverley PM, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a Revolution in COPD Health Study. Chest. 2009;136(6):1456–1465. doi: 10.1378/chest.08-3016. [DOI] [PubMed] [Google Scholar]
- 28.Smidt N, de Vet HC, Bouter LM, et al. Effectiveness of exercise therapy: a best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71–85. doi: 10.1016/s0004-9514(05)70036-2. [DOI] [PubMed] [Google Scholar]
- 29.Kujala UM. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med. 2009;43(8):550–555. doi: 10.1136/bjsm.2009.059808. [DOI] [PubMed] [Google Scholar]
- 30.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 31.Canadian Society for Exercise Physiology. Canadian Physical Activity Guidelines: Clinical Practice Guideline Development Report. Ottawa, Canada: Canadian Society for Exercise Physiology; 2011. [Accessed October 14, 2011]. Available from: http://www.csep.ca/CMFiles/Guidelines/CPAGuideline_Report_JAN2011.pdf. [Google Scholar]
- 32.Lemmens V, Oenema A, Knut IK, Brug J. Effectiveness of smoking cessation interventions among adults: a systematic review of reviews. Eur J Cancer Prev. 2008;17(6):535–544. doi: 10.1097/CEJ.0b013e3282f75e48. [DOI] [PubMed] [Google Scholar]
- 33.Ernst E. A systematic review of systematic reviews of homeopathy. Br J Clin Pharmacol. 2002;54(6):577–582. doi: 10.1046/j.1365-2125.2002.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujala UM. Evidence for exercise therapy in the treatment of chronic disease based on at least three randomized controlled trials – summary of published systematic reviews. Scand J Med Sci Sports. 2004;14(6):339–345. doi: 10.1111/j.1600-0838.2004.413.x. [DOI] [PubMed] [Google Scholar]
- 35.Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11:119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Phys Ther. 2005;85(11):1208–1223. [PubMed] [Google Scholar]
- 37.Higgins JPT, Green S, editors. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [Accessed February 29, 2012]. [updated March 2011] Available from: http://www.cochranehandbook.org/
- 38.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Puhan MA, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;1:CD005305. doi: 10.1002/14651858.CD005305.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Chavannes N, Vollenberg JJ, van Schayck CP, Wouters EF. Effects of physical activity in mild to moderate COPD: a systematic review. Br J Gen Pract. 2002;52(480):574–578. [PMC free article] [PubMed] [Google Scholar]
- 41.Janaudis-Ferreira T, Hill K, Goldstein R, Wadell K, Brooks D. Arm exercise training in patients with chronic obstructive pulmonary disease: as systematic review. J Cardiopulm Rehabil Prev. 2009;29(5):277–283. doi: 10.1097/HCR.0b013e3181b4c8d0. [DOI] [PubMed] [Google Scholar]
- 42.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 43.O’Shea SD, Taylor NF, Paratz JD. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: a systematic review. Chest. 2009;136(5):1269–1283. doi: 10.1378/chest.09-0029. [DOI] [PubMed] [Google Scholar]
- 44.Salman GF, Mosier MC, Beasley BW, Calkins DR. Rehabilitation for patients with chronic obstructive pulmonary disease: meta-analysis of randomized controlled trials. J Gen Intern Med. 2003;18(3):213–221. doi: 10.1046/j.1525-1497.2003.20221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16(2):134–143. doi: 10.1097/MCP.0b013e32833642f2. [DOI] [PubMed] [Google Scholar]
- 46.Chin A, Paw MJ, van Uffelen JG, Riphagen I, van Mechelen W. The functional effects of physical exercise training in frail older people: a systematic review. Sports Med. 2008;38(9):781–793. doi: 10.2165/00007256-200838090-00006. [DOI] [PubMed] [Google Scholar]
- 47.Forster A, Lambley R, Young JB. Is physical rehabilitation for older people in long-term care effective? Findings from a systematic review. Age Ageing. 2010;39(2):169–175. doi: 10.1093/ageing/afp247. [DOI] [PubMed] [Google Scholar]
- 48.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;4:CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- 49.Howe TE, Rochester L, Jackson A, Banks PM, Blair VA. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2008;4:CD004963. doi: 10.1002/14651858.CD004963.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rydwik E, Frändin K, Akner G. Effects of physical training on physical performance in institutionalised elderly patients (70+) with multiple diagnoses. Age Ageing. 2004;33(1):13–23. doi: 10.1093/ageing/afh001. [DOI] [PubMed] [Google Scholar]
- 52.Weening-Dijksterhuis E, de Greef MH, Scherder EJ, Slaets JP, van der Schans CP. Frail institutionalized older persons: a comprehensive review on physical exercise, physical fitness, activities of daily living, and quality-of-life. Am J Phys Med Rehabil. 2011;90(2):156–168. doi: 10.1097/PHM.0b013e3181f703ef. [DOI] [PubMed] [Google Scholar]
- 53.Chien CL, Lee CM, Wu YW, Chen TA, Wu YT. Home-based exercise increases exercise capacity but not quality of life in people with chronic heart failure: a systematic review. Aust J Physiother. 2008;54(2):87–93. doi: 10.1016/s0004-9514(08)70041-2. [DOI] [PubMed] [Google Scholar]
- 54.Hwang CL, Chien CL, Wu YT. Resistance training increases 6-minute walk distance in people with chronic heart failure: a systematic review. J Physiother. 2010;56(2):87–96. doi: 10.1016/s1836-9553(10)70038-2. [DOI] [PubMed] [Google Scholar]
- 55.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12(7):706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49(24):2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 57.Hwang R, Marwick T. Efficacy of home-based exercise programmes for people with chronic heart failure: a meta-analysis. Eur J Cardiovasc Prev Rehabil. 2009;16(5):527–535. doi: 10.1097/HJR.0b013e32832e097f. [DOI] [PubMed] [Google Scholar]
- 58.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Benton MJ. Safety and efficacy of resistance training in patients with chronic heart failure: research-based evidence. Prog Cardiovasc Nurs. 2005;20(1):17–23. doi: 10.1111/j.0889-7204.2005.03888.x. [DOI] [PubMed] [Google Scholar]
- 60.Cahalin LP, Ferreira DC, Yamada S, Canavan PK. Review of the effects of resistance training in patients with chronic heart failure: potential effects upon the muscle hypothesis. Cardiopulm Phys Ther J. 2006;17(1):15–28. [Google Scholar]
- 61.Spruit MA, Eterman RM, Hellwig VA, Janssen PP, Wouters EF, Uszko-Lencer NH. Effects of moderate-to-high intensity resistance training in patients with chronic heart failure. Heart. 2009;95(17):1399–1408. doi: 10.1136/hrt.2008.159582. [DOI] [PubMed] [Google Scholar]
- 62.Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143(9):659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 63.Cornish AK, Broadbent S, Cheema BS. Interval training for patients with coronary artery disease: a systematic review. Eur J Appl Physiol. 2011;111(4):579–589. doi: 10.1007/s00421-010-1682-5. [DOI] [PubMed] [Google Scholar]
- 64.Cortes OL, Villar JC, Devereaux PJ, DiCenso A. Early mobilisation for patients following acute myocardiac infarction: a systematic review and meta-analysis of experimental studies. Int J Nurs Stud. 2009;46(11):1496–1504. doi: 10.1016/j.ijnurstu.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Haykowsky M, Scott J, Esch B, et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials. 2011;12:92. doi: 10.1186/1745-6215-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2009;1:CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira JL, Galvão CM, Rocha SM. Resistance exercises for health promotion in coronary patients: evidence of benefits and risks. Int J Evid Based Healthc. 2008;6(4):431–439. doi: 10.1111/j.1744-1609.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 68.Valkeinen H, Aaltonen S, Kujala UM. Effects of exercise training on oxygen uptake in coronary heart disease: a systematic review and meta-analysis. Scand J Med Sci Sports. 2010;20(4):545–555. doi: 10.1111/j.1600-0838.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 69.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;4:CD000990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg. 2007;34(1):1–9. doi: 10.1016/j.ejvs.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 71.Kelley GA, Kelley KA, Tran ZV. Aerobic exercise and resting blood pressure: a meta-analytic review of randomized, controlled trials. Prev Cardiol. 2001;4(2):73–80. doi: 10.1111/j.1520-037x.2001.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46(4):667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 73.Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24(2):215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 74.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136(7):493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 75.Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39(2):176–184. doi: 10.1093/ageing/afp251. [DOI] [PubMed] [Google Scholar]
- 76.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ottawa Panel. Evidence-based clinical practice guidelines for therapeutic exercises and manual therapy in the management of osteoarthritis. Phys Ther. 2005;85(9):907–971. [PubMed] [Google Scholar]
- 78.Brosseau L, Pelland L, Wells G, et al. Efficacy of aerobic exercises for osteoarthritis (part II): a meta-analysis. Phys Ther Rev. 2004;9(3):125–145. [Google Scholar]
- 79.Devos-Comby L, Cronan T, Roesch SC. Do exercise and self-management interventions benefit patients with osteoarthritis of the knee? A metaanalytic review. J Rheumatol. 2006;33(4):744–756. [PubMed] [Google Scholar]
- 80.Lange AK, Vanwanseele B, Fiatarone Singh MA. Strength training for treatment of osteoarthritis of the knee: a systematic review. Arthritis Rheum. 2008;59(10):1488–1494. doi: 10.1002/art.24118. [DOI] [PubMed] [Google Scholar]
- 81.Pelland L, Brosseau L, Wells G, et al. Efficacy of strengthening exercises for osteoarthritis (part I): a meta-analysis. Phys Ther Rev. 2004;9(2):77–108. [Google Scholar]
- 82.de Kam D, Smulders E, Weerdesteyn V, Smits-Engelsman BC. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: a systematic review of randomized controlled trials. Osteoporos Int. 2009;20(12):2111–2125. doi: 10.1007/s00198-009-0938-6. [DOI] [PubMed] [Google Scholar]
- 83.Li WC, Chen YC, Yang RS, Tsauo JY. Effects of exercise programmes on quality of life in osteoporotic and osteopenic postmenopausal women: a systematic review and meta-analysis. Clin Rehabil. 2009;23(10):888–896. doi: 10.1177/0269215509339002. [DOI] [PubMed] [Google Scholar]
- 84.Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003;46(8):1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- 85.Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34(5):1228–1237. doi: 10.2337/dc10-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Aust J Physiother. 2009;55(4):237–246. doi: 10.1016/s0004-9514(09)70003-0. [DOI] [PubMed] [Google Scholar]
- 87.Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: a meta-analysis of randomized-controlled trials. Public Health. 2007;121(9):643–655. doi: 10.1016/j.puhe.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29(11):2518–2527. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 89.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009;3:CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1C levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 91.O’Brien K, Nixon S, Tynan AM, Glazier RH. Aerobic exercise interventions for people living with HIV/AIDS: implications for practice, education, and research. Physiother Can. 2006;58(2):114–129. [Google Scholar]
- 92.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krogh J, Nordentoft M, Sterne JA, Lawlor DA. The effect of exercise in clinically depressed adults: systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2011;72(4):529–538. doi: 10.4088/JCP.08r04913blu. [DOI] [PubMed] [Google Scholar]
- 94.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170(4):321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 95.Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- 96.Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev. 2008;4:CD004366. doi: 10.1002/14651858.CD004366.pub3. [DOI] [PubMed] [Google Scholar]
- 97.Centre for Evidence Based Medicine. Levels of Evidence. EBM Tools. [Accessed February 29, 2012]. Available at http://www.cebm.net/index.aspx?o=1025.
- 98.Marciniuk DD, Brooks D, Butcher S, et al. Optimizing pulmonary rehabilitation in COPD – practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J. 2010;17(4):159–168. doi: 10.1155/2010/425975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O’Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180(10):964–971. doi: 10.1164/rccm.200904-0530OC. [DOI] [PubMed] [Google Scholar]
- 100.Roig M, Eng JJ, MacIntyre DL, et al. Falls in people with chronic obstructive pulmonary disease: an observational cohort study. Resp Med. 2011;105(3):461–469. doi: 10.1016/j.rmed.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134(2):273–280. doi: 10.1378/chest.07-2655. [DOI] [PubMed] [Google Scholar]