Abstract
Rationale
Animal models of tobacco addiction rely on administration of nicotine alone or nicotine combined with isolated constituents. Models using tobacco extracts derived from tobacco products and containing a range of tobacco constituents might more accurately simulate tobacco exposure in humans.
Objective
To compare the effects of nicotine alone and an aqueous smokeless tobacco extract in several addiction-related animal behavioral models.
Methods
Nicotine alone and nicotine dose-equivalent concentrations of extract were compared in terms of their acute effects on intracranial self-stimulation (ICSS) thresholds, discriminative stimulus effects, and effects on locomotor activity.
Results
Similar levels of nicotine and minor alkaloids were achieved using either artificial saliva or saline for extraction, supporting the clinical relevance of the saline extracts used in these studies. Extract produced reinforcement-enhancing (ICSS threshold-decreasing) effects similar to those of nicotine alone at low to moderate nicotine doses, but reduced reinforcement-attenuating (ICSS threshold-increasing) effects at a high nicotine dose. In rats trained to discriminate nicotine alone from saline, intermediate extract doses did not substitute for the training dose as well as nicotine alone. Locomotor stimulant effects and nicotine distribution to brain were similar following administration of extract or nicotine alone.
Conclusions
The reinforcement-attenuating and discriminative stimulus effects of nicotine delivered in an extract of a commercial smokeless tobacco product differed from those of nicotine alone. Extracts of tobacco products may be useful for evaluating the abuse liability of those products and understanding the role of non-nicotine constituents in tobacco addiction.
Keywords: Nicotine, smokeless tobacco, non-nicotine tobacco constituents, extract, intracranial self-stimulation, nicotine discrimination, locomotor sensitization
Introduction
The primary role of nicotine in maintaining tobacco use is well established (Benowitz 2008; U.S. Department of Health and Human Services 1999) but increasing evidence suggests that other compounds in tobacco may also contribute to tobacco addiction. For example, acetaldehyde, monoamine oxidase (MAO) inhibitors, and minor tobacco alkaloids (e.g., nornicotine) can either mimic or enhance nicotine’s behavioral and neuropharmacological effects in animals (e.g., Bardo et al. 1999; Belluzzi et al. 2005; Dwoskin et al. 1999; Foddai et al. 2004; Guillem et al. 2005; Villegier et al. 2007), and a cocktail of nicotine and several minor alkaloids had greater reinforcing efficacy than nicotine alone (Clemens et al. 2009).
These studies strongly suggest that the effects of nicotine can be influenced by other tobacco constituents. However, they have all examined each constituent or class of constituents (i.e., minor alkaloids) in isolation from the many other chemicals present in tobacco or tobacco smoke. While this approach has been useful for identifying constituents that might contribute to the behavioral effects of tobacco exposure, it is not analogous to tobacco product exposure in humans per se. Tobacco or tobacco smoke contains thousands of chemicals and, for the vast majority, their effects and extent of absorption are not known. Some of these uncharacterized compounds may contribute, positively or negatively, to tobacco addiction. Moreover, it is the summation or interaction of these compounds that determines the actual effects of a tobacco product. This aggregate effect may not be adequately captured through the administration of just one or a limited panel of tobacco constituents alone. An additional limitation of isolated constituent studies is that the doses administered may not match the doses actually delivered during tobacco use.
Animal models using extracts derived from tobacco products and containing a comprehensive range of constituents might more closely simulate tobacco exposure in humans. This approach could help validate findings from studies which use isolated constituents by determining whether constituent effects are altered in the presence of the myriad of other constituents in tobacco or tobacco smoke, at relative concentrations similar to those occurring during tobacco exposure. Providing animals with exposures that more closely resemble those of humans using tobacco products may also improve the predictive validity of current animal models, expand our understanding of the mechanisms underlying tobacco addiction, and facilitate developing therapies for cessation of tobacco use. Given that administration of tobacco extracts could simulate actual tobacco product exposure per se, extract data may be useful to the Food and Drug Administration (FDA) for evaluating the relative abuse liability and toxicity of current tobacco products and novel products designed to meet specified performance standards.
Limited data suggest that use of tobacco or smoke extracts in preclinical models is feasible, and that extract effects may differ from those of nicotine alone. For example, tobacco or smoke extracts were more potent than nicotine alone in exerting certain neuropharmacological effects relevant to tobacco addiction (e.g., brain MAO inhibition, dopamine reuptake inhibition, 5HT neural inhibition) (Ambrose et al. 2007; Carr and Basham 1991; Carr et al. 1992; Touiki et al. 2007), consistent with the ability of isolated constituents to enhance nicotine’s effects on these systems (e.g., Crooks and Dwoskin 1997; Foddai et al. 2004; Villegier et al. 2007). In addition, nicotine alone and a nicotine dose-equivalent smoke extract inhibited extinction of an avoidance task equally (Driscoll and Battig 1970), but produced opposite effects on swimming endurance in rats (Battig 1970).
The primary goal of this study was to begin evaluating the effects of an aqueous smokeless tobacco extract in several animal models related to tobacco addiction. To validate the use of a saline vehicle for the extract, we compared levels of nicotine and minor alkaloids in extracts prepared using either saline or artificial saliva as the extraction solution. Nicotine alone and concentrations of extract containing an equivalent nicotine dose were subsequently compared in terms of their acute effects on intracranial self-stimulation (ICSS) thresholds. This assay is believed to measure nicotine’s reinforcement-enhancing (i.e., ICSS threshold-lowering) effects at low to moderate doses (Harrison et al. 2002; Huston-Lyons and Kornetsky 1992) and its reinforcement-attenuating (i.e., threshold-increasing) effects at high doses (Fowler et al. 2011; Spiller et al. 2009). The latter phenomenon may be related to nicotine’s acute aversive effects (see Fowler et al. 2011; Spiller et al. 2009). Nicotine’s reinforcement-enhancing and aversive effects may both contribute to the abuse liability of tobacco (Caggiula et al. 2009; Chaudhri et al. 2006; Donny et al. 2003; Laviolette and van der Kooy 2003; Sellings et al. 2008). We also compared the potential for extract and nicotine alone to produce similar discriminative stimulus (subjective) effects and induce locomotor sensitization (LMS), two additional behavioral measures that may have relevance to tobacco addiction (DiFranza and Wellman 2007; Smith and Stolerman 2009; Vezina et al. 2007; Wooters et al. 2009). Finally, given that certain non-nicotine constituents (e.g., MAO inhibitors) may influence nicotine pharmacokinetics (e.g, Zhang et al. 2001), we compared serum and brain nicotine levels following injection of nicotine alone or extract to examine whether differences in nicotine distribution may have contributed to any differences in behavioral effects between formulations.
This study used extracts prepared from smokeless tobacco rather than cigarette smoke for several reasons. First, the tobacco industry is introducing a variety of smokeless “modified risk tobacco products” (MRTPs) claimed to be safer or less addictive than conventional tobacco products, and methods for assessing the potential impact of MRTPs on individual and public health are needed (e.g., Hatsukami et al. 2007; Hatsukami et al. 2005; Hatsukami et al. 2010; Pederson and Nelson 2007; Zeller and Hatsukami 2009). Second, aqueous smokeless tobacco extracts are easily prepared, characterized, and administered in these models. Third, these extracts should provide a very close representation of tobacco constituent exposure in smokeless tobacco users, because saliva provides a similar aqueous extraction. A similarly suitable extract of tobacco smoke does not exist because smokers are exposed to both water-soluble and insoluble components in inhaled smoke, and water-insoluble components are difficult to administer to animals. Therefore, use of the simpler and more clinically-relevant smokeless tobacco extracts is a logical first step in developing animal models of tobacco product exposure.
Materials and Methods
Animals
Male Holtzman Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275–325 g at arrival were housed individually in a temperature- and humidity-controlled colony room with unlimited access to water. Rats in Experiments 2 and 3 were housed under a reversed 12-hr light/dark cycle and tested for ICSS/nicotine discrimination during the dark (active) phase. Rats in Experiment 4 were housed under a regular 12-hr light/dark cycle so that LMS testing would occur during the light (inactive) phase, as is standard in our laboratory and others (e.g., Green et al. 2003; Harris et al. 2010; Roiko et al. 2008). Beginning one week after arrival, rats in Experiments 2 and 3 were food-restricted to 18 g/day rat chow to facilitate operant performance and avoid detrimental effects of long-term ad libitum feeding on health. Rats in Experiment 4 were not food-restricted due to the relatively short duration of the protocol. As part of separate studies, all rats in Experiment 2 had previously received a 7-day continuous s.c. infusion of either saline or nicotine alone (3.2 mg/kg/day), some rats in Experiment 3a (see below) had received an i.v. infusion of control immunoglobin or the nicotine-specific monoclonal antibody Nic311 (see Keyler et al. 2005; Pentel et al. 2006), and all rats in Experiment 3b had received prior exposure to restraint stress. However, rats were treatment free and their performance had been maintained under their respective behavioral assay for 4–9 weeks before beginning the present experiments (32 ± 6 days, 41 ± 6 days, and 65 ± 19 days (mean ± SEM) for Experiments 2, 3a, and 3b, respectively). In addition, we used fully counterbalanced, within-subject designs in these experiments (see below), such that any effects of experimental history would equally influence the nicotine alone and extract conditions. All rats in Experiment 4 were experimentally naïve. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Drugs
Nicotine-alone solutions consisted of (−)-Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) dissolved in sterile saline. Aqueous tobacco extract was prepared from Kodiak smokeless tobacco product (purchased from stores in the Minneapolis area between June 2009 and December 2010) using a modification of a published method (Demady et al. 2003). Tobacco product was mixed with vehicle (saline or artificial saliva for Experiment 1; saline for Experiments 2–4) at a concentration of 450 mg/ml for 18 hours at room temperature using a tube tipper. The resulting solution was filtered through gauze, centrifuged at 5000 rpm for 20 min, and the supernate was vacuum-filtered through Whatman #3 paper followed by a 0.2 μm sterile filter. The nicotine concentration was determined, and extract was diluted to the appropriate nicotine concentration for each study. Therefore, the concentration of all constituents varied in proportion to the nicotine concentration, such that the ratio between nicotine and non-nicotine constituents was maintained at each nicotine dose. The pH of all solutions was adjusted to 7.4 using dilute NaOH. Nicotine doses are expressed as the base. All injections were administered s.c. in a volume of 1 ml/kg. Adjusting the pH of extract alters its free nicotine base concentrations and, as such, does not fully replicate this smokeless tobacco product in its marketed form. However, our intent was to control this aspect of nicotine delivery to exploit the potential contribution of non-nicotine constituents.
Experiment 1: Effects of extraction solution on nicotine and minor alkaloid levels
Artificial saliva preparation
Artificial saliva was prepared by dissolving NaCl (1.4 mg/ml), KCl (0.5 mg/ml), CaCl2 (0.1 mg/ml), MgCl2 (0.025 mg/ml), NaH2PO4 (0.15 mg/ml), urea (0.09 mg/ml), and glucose (0.2 mg/ml) in HLPC-grade deionized water (Chou and Que Hee 1994; Pappas et al. 2008). The pH was adjusted to 7.0 and human salivary α-amylase (2.5 units/ml), mucin (2.7 mg/ml), lysozyme chloride (0.7 units/ml), and acid phosphatase (0.004 units/ml) were added (all salts and proteins obtained from Sigma Chemical Co.). The solution was stirred for at least 2 hr and to homogeneity prior to use.
Alkaloid analyses
Nicotine levels in extracts prepared using either artificial saliva or saline were analyzed by gas chromatography-mass spectrometry (GC-MS) (Stepanov et al. 2005). Analysis of nornicotine, anatabine, and anabasine was carried out after their conversion to tertiary amine derivatives via reductive alkylation with propionaldehyde and sodium borohydride (Jacob et al. 1993). The propyl derivatives were analyzed by GC-MS/MS as described elsewhere (Stepanov et al. 2008).
Experiment 2: Effects of nicotine alone and extract on baseline ICSS thresholds
Intracranial self-stimulation
Surgery, apparatus, and training procedure used here are described in detail elsewhere (Harris et al. 2010; Harris et al. 2011; Roiko et al. 2009). Briefly, animals were anesthetized with i.m. ketamine (75 mg/kg)/xylazine (7.5 mg/kg) and implanted with a bipolar stainless steel electrode in the medial forebrain bundle at the level of the lateral hypothalamus. Rats were later trained to respond for electrical brain stimulation using a modified version of the Kornetsky and Esposito (1979) discrete-trial current-threshold procedure (see Markou and Koob 1992). Each session was approximately 45 min and provided two dependent variables: ICSS thresholds (a measure of brain reinforcement function) and response latencies (a measure of non-specific (e.g., motor) effects).
Protocol
Animals (N = 8) were tested in daily ICSS sessions conducted Mon-Fri until thresholds were stable (i.e., less than 10% coefficient of variation over a 5-day period and no apparent trend). To habituate animals to the injection procedure, s.c. saline was administered 10 min prior to ICSS testing for at least 1 session and until thresholds were stable. Effects of 10-min pretreatment with nicotine alone (half of the animals) or extract (the other half) were subsequently determined at nicotine doses of 0, 0.06, 0.125, 0.25, 0.50, and 0.75 mg/kg. These doses bracket the range of nicotine doses that reduce or increase ICSS thresholds when administered acutely (Bauco and Wise 1994; Harrison et al. 2002; Huston-Lyons and Kornetsky 1992; Spiller et al. 2009). Because initial data indicated reduced potential for extract to elevate ICSS thresholds (see below), effects of a higher dose of extract (1.25 mg/kg) were evaluated in two rats. This dose of nicotine alone was not tested because doses ≥ 1.0 mg/kg produce severe non-specific effects (e.g., seizures, paralysis) that interfere with behavioral testing (unpublished data). Injections typically occurred on Tues and Fri, provided that thresholds were within baseline range on intervening days, and doses were administered in a counterbalanced order. Following completion of dose-response testing, animals were tested for ICSS under drug-free conditions for at least 2 weeks and until thresholds were stable. All rats then underwent the same procedure as described above, with the exception that formulation (i.e. nicotine alone versus extract) was crossed-over within each subject.
Experiment 3a: Effects of nicotine alone and extract on nicotine discrimination (0.4 mg/kg nicotine training dose)
Nicotine discrimination
Apparatus and training procedure used here have been described in detail elsewhere (LeSage et al. 2009). Briefly, animals (N = 16) were trained to discriminate nicotine alone (0.4 mg/kg) from saline using a 2-lever discrimination procedure. Lever pressing was reinforced under a terminal variable interval 15 sec schedule using 45-mg food pellets. Discrimination was assessed twice weekly (Tues and Fri) during 2-min extinction test sessions. Discrimination was considered stable when a) >80% responding occurred on the injection-appropriate lever during two consecutive saline and nicotine test sessions, b) >95% injection-appropriate responding occurred on six consecutive training sessions, and c) response rates (total responses/session) were stable (no trend across these four test sessions and six training sessions).
Protocol
Test sessions occurred as above, subject to stable discrimination performance on intervening training days. During these sessions, either nicotine alone (half of the animals) or extract (the other half) was substituted for the training dose at nicotine doses of 0.0, 0.05, 0.1, 0.2, and 0.4 mg/kg (counterbalanced order). This dose range is commonly used to generate nicotine generalization dose-effect functions (e.g., LeSage et al. 2009; Philibin et al. 2005; Stolerman and White 1996). Following completion of dose-response testing, baseline discrimination training was conducted for at least 2 weeks and until discrimination was stable. All rats then underwent the same procedure as described above, with the exception that formulation (i.e., nicotine alone versus extract) was crossed-over within each subject.
Experiment 3b: Effects of nicotine alone and extract on nicotine discrimination (0.1 mg/kg nicotine training dose)
Training dose can influence nicotine’s discriminative stimulus properties (Murray and Bevins 2007b; Smith and Stolerman 2009; Stolerman et al. 1984). To examine whether results observed in Experiment 3a generalized to a lower nicotine training dose, rats (N = 5) were tested using the same apparatus, training procedure, and protocol as described above with the exception that 1) the nicotine alone training dose was 0.1 mg/kg, and 2) nicotine alone/extract doses used for generalization testing were 0.0, 0.01, 0.03, 0.05, 0.1, and 0.2 mg/kg.
Experiment 4a: Effects of nicotine alone and extract on locomotor activity
Protocol
On each of two consecutive habituation days, all rats (N = 24) were tested for locomotor activity in open field activity chambers (described in Roiko et al. 2008) for 30 min (pre-test), injected with s.c. saline, and then immediately tested for activity for another 30 min (post-test). Total distance traveled during the post-test on the second day was used to match animals into groups (see below) with similar baseline activity levels.
Sensitization testing began two days after completion of habituation. On each day, rats in the saline group (negative control, n = 8) continued to be treated as during habituation (i.e., 30 min pre-test, s.c. saline injection, 30 min post-test). The remaining animals were treated identically with the exception that they received either nicotine alone or tobacco extract (n = 8/group) at a nicotine dose of 0.4 mg/kg prior to the post-test. Nicotine alone at this dose effectively induces LMS (e.g., Clarke and Kumar 1983; Zaniewska et al. 2008; Zubaran et al. 2000). Rats were treated in this manner Mon-Fri for two consecutive weeks (10 test days total). Drug administration and activity testing were then suspended for 10 days, after which all rats were tested as described above to assess the persistence of sensitization (challenge test).
Experiment 4b: Serum and brain nicotine levels following s.c. injection of nicotine alone or extract
Protocol
This experiment was conducted in a subset of rats from Experiment 4a (N = 16), at least 1 week after the challenge test. Rats were anesthetized with i.m. droperidol (2 mg/kg)/fentanyl (0.04 mg/kg) and subsequently injected s.c. with either nicotine alone or extract (0.4 mg/kg; n = 8 / formulation). Ten min later, rats were decapitated and trunk blood and brain were collected. Samples were collected near the time at which peak serum and brain nicotine levels should occur following s.c. nicotine (e.g., Ghosheh et al. 1999; Pratt et al. 1983). Timing of sample collection also coincided with the timing of behavioral testing in Experiments 2–4.
Nicotine assay
Serum and brain nicotine levels were measured using GC with nitrogen-phosphorous detection (Jacob et al. 1981). Brain nicotine levels were corrected for brain blood content (Hieda et al. 1999).
Statistical analyses
Intracranial self-stimulation thresholds (in μA) and response latencies (sec) were expressed as percentage of baseline (i.e., mean during last 5 sessions prior to each dose-response determination). Nicotine discrimination was measured as the percentage of responding on the nicotine-appropriate lever (%NLR) and overall response rate (responses/second) during the 2-min extinction test sessions. Locomotor activity was measured as total horizontal distance traveled (in cm) over each 30-min post-test. The following secondary outcomes were also measured: vertical counts (rearing) and stereotypy counts (i.e., non-ambulatory horizontal activity) over each 30-min post-test; distance traveled within-session (i.e., separated into 5-min blocks) during post-tests on the first and final days of sensitization, and during the challenge test. In general, data were analyzed using one- or two-way ANOVA followed by Bonferroni or Dunnett post hoc tests as appropriate. Paired or independent sample t-tests were also used for some comparisons. See results for specific experiments for further details.
Results
Experiment 1: Extraction solution
Extracts prepared using either saline or artificial saliva had similar levels of nicotine and minor alkaloids (Table 1), as well as a similar relative percentage of total minor alkaloids (i.e., nornicotine + anabasine + anatabine) to total alkaloids (i.e., including nicotine; 1.72% versus 1.71%). Therefore, a saline extraction solution was used in Experiments 2–4.
Table 1.
Nicotine and minor alkaloid levels (ug/ml) in extracts prepared using saline or artificial saliva as the extraction vehicle.
| Alkaloid Levels (ug/ml)
|
||||
|---|---|---|---|---|
| Nicotine | Nornicotine | Anabasine | Anatabine | |
| Saline | 3110 | 28.7 | 14.8 | 10.8 |
| Artificial Saliva | 3110 | 27.2 | 15.9 | 11.0 |
Experiment 2: ICSS thresholds
Baseline measures
The nicotine alone and extract dose-response determinations did not differ in terms of baseline thresholds (Mean ± SEM = 82.8 ± 9.2 μA versus 83.4 ± 9.6 μA) or response latencies (2.5 ± 0.2 sec versus 2.5 ± 0.1 sec).
Thresholds
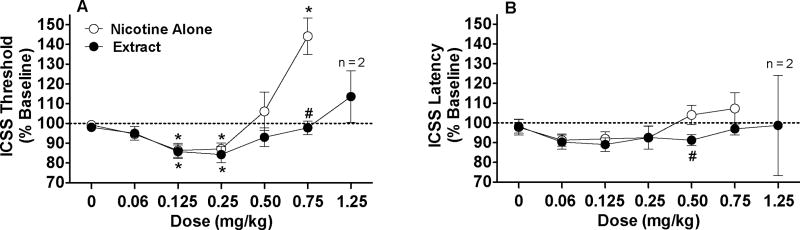
Extract produced threshold-decreasing effects similar to nicotine alone at low to moderate nicotine doses, but reduced threshold-increasing effects at the highest nicotine dose. There were significant main effects of formulation (F(1,7)=8.0, p <0.05) and dose (F(5, 35)=17.0, p <0.0001), and a significant formulation x dose interaction (F(5,35)=11.3, p <0.0001). Thresholds were lower for extract compared to nicotine alone at the 0.75 mg/kg dose (p <0.01) (Fig 1A). This effect did not appear to be influenced by experimental history (see Animals), as thresholds following nicotine alone versus extract (0.75 mg/kg) were 147.2 ± 17.4% versus 100.4 ± 0.34% in rats with a history of continuous saline infusion (n = 5) and 142.3 ± 12.0% versus 96.2 ± 5.6% in rats with a history of continuous nicotine infusion (n = 3). Within-formulation comparisons on data for all rats indicated a significant effect of dose for both the nicotine alone (F(5,35) =3.4, p < 0.0001) and extract (F(5,35) =2.9, p < 0.01) conditions. Thresholds were reduced at the 0.125 and 0.25 mg/kg doses for both formulations (p < 0.01 or 0.05), but were elevated at the 0.75 mg/kg dose for only the nicotine alone condition (p < 0.01; Fig 1A). Extract at 1.25 mg/kg only modestly elevated thresholds in the two animals receiving this dose (Fig 1A).
Figure 1.
ICSS thresholds (A) and response latencies (B) (expressed as percent of baseline, mean ± SEM) following s.c. injection of nicotine alone or extract (0 – 0.75 mg/kg). Threshold and latency data from the two rats administered s.c. extract 1.25 mg/kg are also shown. * Significantly different from saline (0 mg/kg), p < 0.05 or 0.01. # Significantly different from nicotine alone at that dose, p < 0.05 or 0.01.
Response latencies
There was no significant effect of formulation on response latency, but there was a significant main effect of dose (F(5,35)=3.8, p <0.01) and a formulation x dose interaction (F(5,35)=2.8, p <0.05). Latencies significantly differed between formulations at the 0.5 mg/kg dose (p < 0.05), but not at other doses (Fig 1B). Within-formulation comparison indicated a significant effect of dose for the nicotine alone condition (F(5,35)=5.4, p < 0.0001), although latencies did not differ significantly from saline at any dose. There was no effect of dose for the extract condition. Extract at 1.25 mg/kg did not consistently affect latencies in the two rats administered this dose (Fig 1B).
Experiment 3a: Nicotine discrimination (0.4 mg/kg training dose)
%NLR
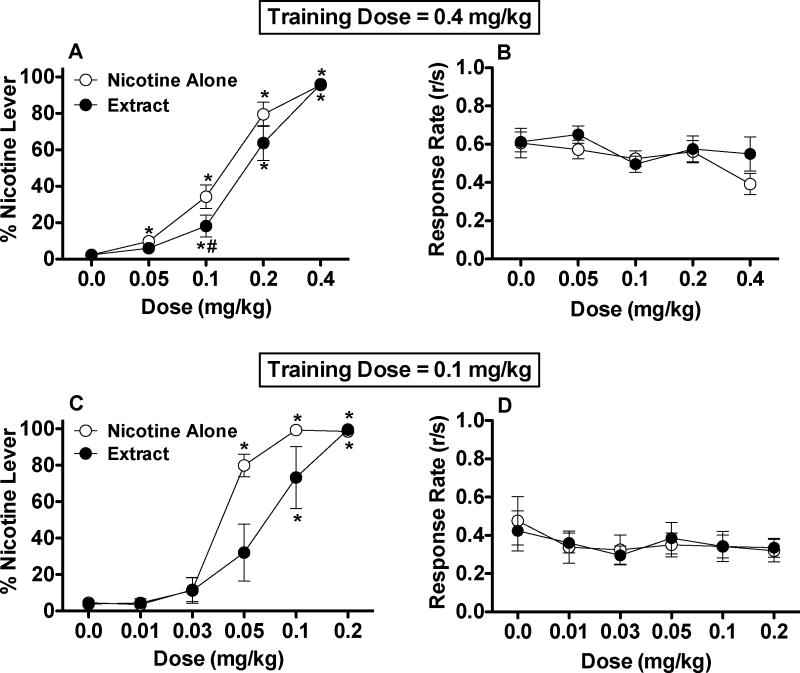
A small but significant attenuation of nicotine discrimination was observed for tobacco extract compared to nicotine alone (Fig 2A). There were significant main effects of formulation (F(1,15)=9.0, p <0.01) and dose (F(4,60)=134.9, p <0.0001), and a formulation x dose interaction (F(4,60)=2.6, p <0.05). Nicotine-appropriate responding was lower for extract compared to nicotine alone at the 0.1 mg/kg dose (p < 0.05). This effect did not appear to be influenced by experimental history (see Animals), as %NLR following nicotine alone versus extract (0.1 mg/kg) was 23.5 ± 8.2% versus 12.0 ± 5.3% in naïve rats (n = 6), 35.4 ± 10.5% versus 19.2 ± 14.3% in rats previously infused with control immunoglobulin (n = 6), and 30.8 ± 15.5% versus 15.0 ± 9.7% in rats previously infused with nicotine-specific antibody (n = 4). Within-formulation comparisons on data for all rats indicated an effect of dose for both the nicotine alone (F(4,60)=110.3, p < 0.0001) and extract (F(4,60)=74.2, p < 0.001) conditions. Nicotine-appropriate responding was elevated compared to saline at doses of 0.05 mg/kg nicotine alone and higher (p < 0.05) and doses of 0.1 mg/kg extract or higher (p < 0.05 or 0.01). Together, these findings show that the %NLR dose-response curve for extract was shifted to the right compared to that for nicotine alone.
Figure 2.
Percent of total responses on the nicotine-appropriate lever (mean ± SEM) and overall response rate (total responses/sec) following s.c. injection of nicotine alone or extract in Experiment 3a (0.4 mg/kg nicotine training dose, A and B) and Experiment 3b (0.1 mg/kg nicotine training dose, C and D). * Significantly different from saline (0 mg/kg), p < 0.05 or 0.01. # Significantly different from nicotine alone at that dose, p < 0.05. For clarity, significant main effects of formulation (extract versus nicotine alone) in Fig 2A and 2C are not shown.
Response rates
There was a significant main effect of dose on overall response rate (F(4,60)=3.2, p < 0.05), reflecting a modest reduction in response rates for both formulations at the 0.4 mg/kg dose (Fig 2B), but no effect of formulation or dose x formulation interaction was observed.
Experiment 3b: Nicotine discrimination (0.1 mg/kg nicotine training dose)
%NLR
As with the higher nicotine training dose in Experiment 3a, nicotine discrimination was significantly attenuated when extract was administered to rats trained with a 0.1 mg/kg dose of nicotine alone (Fig 2C). There were significant main effects of formulation (F(1,4)=8.0, p <0.05) and dose (F(5,20)=54.4, p <0.0001), and a formulation x dose interaction (F(5,20)=4.2, p <0.01). Nicotine-appropriate responding tended to be lower for extract compared to nicotine alone at the 0.05 mg/kg dose, but this difference was not statistically significant (t(4) = 3.3, p = .06). Within-formulation comparisons indicated a significant effect of dose for both the nicotine alone (F(5,20)=135.1, p < 0.0001) and extract (F(5,20)=16.0, p < 0.0001) conditions. Nicotine-appropriate responding was elevated compared to saline at doses of 0.05 mg/kg nicotine alone and higher (p < 0.01) and doses of 0.1 mg/kg extract and higher (p < 0.05 or 0.01), again suggesting that the %NLR dose-response curve for extract was shifted to the right compared to nicotine alone.
Response rates
There were no significant effects of dose, formulation, or interaction on overall response rates (Fig 2D).
Experiment 4a: Locomotor activity
Habituation
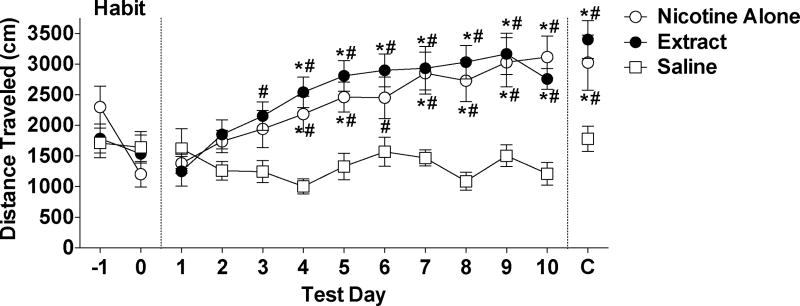
There was a significant main effect of test day (F(1,21) =4.6, p<0.05) on distance traveled, reflecting a decrease in activity across sessions for all groups, but no significant effect of group or group x day interaction (see Fig 3).
Figure 3.
Total distance traveled (Mean ± SEM) during each 30 min post-test during habituation, sensitization, and challenge phases (see text). * Significantly different from the s.c. saline group (negative control) on that test day, p < 0.05 or 0.01. # Significantly different from test day 1 for that group, p < 0.05 or 0.01.
Sensitization
Nicotine alone and extract elicited a similar degree of LMS. There were significant main effects of group (F(2,189)=10.0, p < 0.0001) and test day (F(9,189) = 16.6, p < 0.0001) on distance traveled during sensitization, and a significant group x day interaction (F(18,189) = 5.8, p < 0.0001). Activity in both the nicotine alone and extract groups was higher than in the saline group beginning on test day 4 and continuing throughout nearly all test days (ps < 0.05 or 0.01; Fig 3). The nicotine alone and extract groups did not differ from one another on any test day. Within-group comparisons indicated a significant effect of session for the nicotine alone group (F(9,63)=15.4, p < 0.0001) and the extract group (F(9,63)=10.3, p < 0.0001), with activity increased compared to test day 1 beginning on either test day 3 (extract group) or test day 4 (nicotine alone group). There was a marginally significant effect of session for the saline group (F(9,63)=8.9, p = 0.056), reflecting a modest reduction in activity across test days (Fig 3).
Challenge
Persistence of LMS was similar for both formulations. There were significant differences in distance traveled between groups on the challenge day (F(2,21)= 6.3, p<0.01), with the nicotine alone and extract groups differing from the saline group (p < 0.01 and 0.05, respectively), but not from each other. In addition, activity on the challenge day was higher compared to test day 1 for the nicotine alone group (t(7)=4.7 p<0.01) and the extract group (t(7)=7.2, p<0.001), but not for the saline group.
Secondary outcomes
Analyses of all secondary outcomes (e.g., vertical and stereotypy counts, see statistical analyses) revealed a similar pattern of results as observed for distance traveled (data not shown).
Experiment 4b: Serum and brain nicotine levels
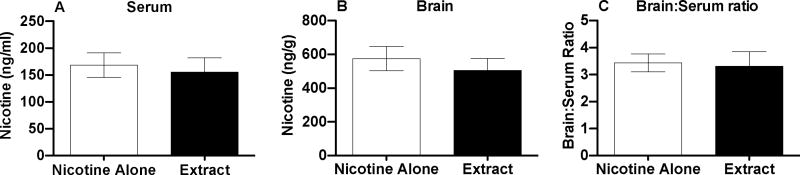
Nicotine alone and extract (0.4 mg/kg, s.c.) produced similar serum (p=0.31) and brain (t(14)=1.9, p=0.074) nicotine levels (Fig 4A and 4B), and similar brain:serum nicotine concentration ratios (p=0.59) (Fig 4C).
Figure 4.
Serum (A) and brain (B) nicotine levels and brain:serum nicotine concentration ratios (C) (Mean ± SD) following 10-min pretreatment with s.c. nicotine alone or extract (0.4 mg/kg).
Discussion
This study found that the reinforcement-attenuating (or perhaps aversive) and discriminative stimulus effects of nicotine delivered in an aqueous smokeless tobacco extract differed from those of nicotine alone. In contrast, extract and nicotine alone produced similar reinforcement-enhancing and locomotor stimulant effects. Nicotine serum and brain levels following injection of nicotine alone and extract did not differ significantly, suggesting that behavioral data were not likely influenced by between-formulation differences in nicotine pharmacokinetics.
Reduced aversive effects of nicotine may increase the likelihood or rate of tobacco use (Fowler et al. 2011; Laviolette and van der Kooy 2003; Rodriguez and Audrain-McGovern 2004; Sellings et al. 2008; Urban 2010; Wilmouth and Spear 2004). The lack of elevation in ICSS thresholds at high extract doses may reflect an attenuation of nicotine’s acute aversive effects by other constituents in extract, although this interpretation should be validated in more traditional assays of aversive drug effects (e.g., conditioned taste aversion) using experimentally naïve subjects. As such, our findings suggest a novel mechanism by which non-nicotine constituents may contribute to tobacco addiction. Isolation and characterization of constituents in extract mediating this effect (e.g., via fractionation assays, Chan et al. 1999; Hecht et al. 1981; Johnson et al. 2009) represents an important area for future work. Monoamine oxidase inhibitors (e.g., harman or norharman) are potential candidates because limited data suggest that MAO inhibition may influence nicotine’s acute aversive effects (Agatsuma et al. 2006). Comparing effects of nicotine alone and extract on neuropharmacological systems implicated in nicotine’s acute aversive effects (e.g., dopamine in the nucleus accumbens core, alpha5 nicotinic receptor activation in the medial habenula, Fowler et al. 2011; Sellings et al. 2008) would also help to elucidate further this potential mechanism.
The mechanism underlying the reduced discriminative-stimulus effects of nicotine in extract is unclear. Some non-nicotine constituents might antagonize nicotine’s effects without producing any added discriminative effects of their own (e.g., a nicotinic receptor antagonist). To our knowledge, no constituents with such activity have yet been identified. Alternatively, some non-nicotine constituents may themselves produce discriminative effects that, in combination with those of nicotine, result in a compound discriminative stimulus that perceptually differs from nicotine alone (Murray and Bevins 2007a; Stolerman et al. 1987). Some studies have demonstrated that isolated non-nicotine constituents can produce discriminative-stimulus effects of their own (Goldberg et al. 1989; MacInnes and Handley 2002; Pratt et al. 1983; Stolerman et al. 1984; York 1981). However, in the case of minor alkaloids that were measured in the extract (i.e., nornicotine, anabasine, and anatabine), the doses administered in the present study were lower than those needed to produce discriminative-stimulus effects (e.g., Goldberg et al. 1989). In the case of other constituents that were not measured in the extract (e.g., beta-carbolines), the doses that were likely administered in the present study were also almost certainly lower than those needed to produce discriminative stimulus effects (e.g., MacInnes and Handley 2002). Finally, future studies are needed to determine whether the long history of nicotine discrimination training in the rats prior to the present experiment may have influenced the effect of non-nicotine constituents.
In contrast to the nicotine discrimination and ICSS assays, there were no differences between extract and nicotine alone in the locomotor behavior assay. This may be attributable to the nicotine dose that was studied, as differences between extract and nicotine alone were not apparent at similar doses in the discrimination and ICSS assays. However, other methodological factors unique to the locomotor study (e.g., use of a normal light cycle, the lack of food deprivation, use of naïve animals) may also account for these findings.
Behaviorally active non-nicotine tobacco constituents identified to date enhance nicotine’s behavioral effects, especially its reinforcing effects (e.g., Belluzzi et al. 2005; Clemens et al. 2009; Dwoskin et al. 1999; Guillem et al. 2005). To our knowledge, this is the first study to report that non-nicotine constituents do not affect nicotine’s reinforcement-enhancing (ICSS threshold-lowering) effects and actually reduce some of nicotine’s other behavioral effects. These unexpected findings may be due to the complexity of the neurochemical interactions that a tobacco extract might produce. The interactions between nicotine and non-nicotine constituents have previously been demonstrated in isolation from the thousands of other chemicals in tobacco. Our findings suggest that there may be unidentified constituents that could oppose the effects of nicotine and/or other behaviorally active constituents. Alternatively, the levels of non-nicotine constituents in extract may have simply been lower than the artificially high levels that are sometimes used when these constituents are studied in isolation (e.g., Dwoskin et al. 1999; Guillem et al. 2005; Villegier et al. 2007). In addition, the effects of non-nicotine constituents have most often been demonstrated in nicotine self-administration models, and different mechanisms mediating drug self-administration may account for discrepancies with other assays (Smith and Stolerman 2009; Wise 2002). Regardless of the explanation, these data suggest that examining the relationship between nicotine and non-nicotine constituents in the context of the complex chemical milieu to which tobacco users are exposed may provide unique insights into the behavioral and neurochemical mechanisms of tobacco addiction.
It is unlikely that the present findings were influenced by non-specific toxic or motoric effects of extract. A similar extract was orally administered to rats for 4 weeks with no obvious adverse effects (Skott et al. 2006). Similarly, no general signs of toxicity (e.g., reduced food intake and/or weight loss) were observed in rats exposed to extract in this study. Extract and nicotine alone also produced similar effects on response latency and rate measures in the ICSS and nicotine discrimination assays, respectively, and similar locomotor effects in the LMS assay. Nonetheless, it will be important to carefully consider this issue in future studies.
Nicotine alone and extract produced similar peak serum and brain:serum nicotine concentration ratios, consistent with previous reports that non-nicotine tobacco constituents have little or no effect on nicotine disposition in humans or animals (Benowitz et al. 2004; Cao et al. 2007). Nonetheless, measurement of serum and brain nicotine levels at additional time points and characterization of other nicotine pharmacokinetic parameters would be useful to confirm that nicotine pharmacokinetics are not altered when nicotine is administered in an extract. There was a nonsignificant trend for extract to produce lower brain nicotine levels, but this 10% difference is not likely to account for the > 40% difference in ICSS threshold values between the 0.75 mg/kg dose of extract and nicotine alone in Experiment 2. In support of this, an even higher extract dose (1.25 mg/kg) administered to two rats produced little change in thresholds.
Experiment 1 was conducted for preliminary verification that saline extraction would be reasonably representative of a salivary extraction, which is the mode of exposure in humans. We chose to profile the minor alkaloids because of their demonstrated influence on nicotine’s behavioral effects (e.g., Clemens et al. 2009; Dwoskin et al. 1999). Although there was good correspondence in the alkaloid profiles between the saline and artificial saliva extracts, it is possible that the extraction profiles may differ for other important constituents (e.g., MAO inhibitors, acetaldehyde). Further validation of the saline extraction procedures will require much more extensive chemical profiling.
Further development of the present and other models of tobacco extract exposure is particularly important in light of the Family Smoking Prevention and Tobacco Control Act enacted in 2009. This law mandates that the FDA set performance standards for current tobacco products, including reduced nicotine yields or levels of other constituents, if deemed appropriate for protection of public health (Hatsukami et al. 2010; Zeller and Hatsukami 2009). The FDA is also required to examine new products to determine if they are substantially equivalent to current products, and if not, whether they pose an increased risk to individual or public health. Animal models using the present and other tobacco extracts may be useful for identifying constituents that need to be regulated and examining the relative abuse liability of MRTPs and other novel smokeless tobacco products. Employment of animal models of tobacco extract exposure in conjunction with models using isolated constituents will be important to this process. For example, studies of isolated compounds can identify chemicals that may need to be regulated, while studies of extracts may indicate whether existing levels of those chemicals within a broader context of constituents might have an impact on product abuse liability and need to be reduced.
Acknowledgments
Supported by the Minneapolis Medical Research Foundation (MMRF) Translational Addiction Research Program (Harris PI), an MMRF Career Development Award (#7572, LeSage PI), and DA026444 (NIDA, LeSage PI). The authors would like to thank Dr. Steven Hecht for providing the alkaloid assay, and Drs. Hecht and Dorothy Hatsukami for helpful discussion during the early stages of this work. We would also like to thank Mylissa Staley, David Shelley, Christina Mattson, Daniel Tran, and Aleksandar Knezevich for technical assistance.
Footnotes
No conflicts of interest.
References
- Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I, Hiroi N. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum Mol Genet. 2006;15:2721–31. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- Ambrose V, Miller JH, Dickson SJ, Hampton S, Truman P, Lea RA, Fowles J. Tobacco particulate matter is more potent than nicotine at upregulating nicotinic receptors on SH-SY5Y cells. Nicotine Tob Res. 2007;9:793–9. doi: 10.1080/14622200701485117. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–6. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Battig K. Differential effects of nicotine and tobacco smoke alkaloids on swimming endurance in the rat. Psychopharmacologia. 1970;18:300–4. doi: 10.1007/BF00412675. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther. 1994;271:294–301. [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–15. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025–35. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Carr LA, Basham JK. Effects of tobacco smoke constituents on MPTP-induced toxicity and monoamine oxidase activity in the mouse brain. Life Sci. 1991;48:1173–7. doi: 10.1016/0024-3205(91)90455-k. [DOI] [PubMed] [Google Scholar]
- Carr LA, Basham JK, York BK, Rowell PP. Inhibition of uptake of 1-methyl-4-phenylpyridinium ion and dopamine in striatal synaptosomes by tobacco smoke components. Eur J Pharmacol. 1992;215:285–7. doi: 10.1016/0014-2999(92)90040-b. [DOI] [PubMed] [Google Scholar]
- Chan WS, Chowdhry S, Chang T, Kew RR. Initial characterization of the complement activating compounds in extracts of smokeless tobacco. Immunobiology. 1999;201:64–73. doi: 10.1016/S0171-2985(99)80047-3. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chou CC, Que Hee SS. Bioassay-driven analysis of chewing tobacco extracts. Environment Tox and Chem. 1994;13:1177–1186. [Google Scholar]
- Clarke PBS, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009:1–12. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–53. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Demady DR, Lowe ER, Everett AC, Billecke SS, Kamada Y, Dunbar AY, Osawa Y. Metabolism-based inactivation of neuronal nitric-oxide synthase by components of cigarette and cigarette smoke. Drug Metab Dispos. 2003;31:932–7. doi: 10.1124/dmd.31.7.932. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. doi: 10.1080/14622200601078277. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Battig K. The effect of nicotine and total alkaloids extracted from cigarette smoke on avoidance behavior in rats under extinction procedure. Psychopharmacologia. 1970;18:305–13. doi: 10.1007/BF00412676. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berl) 1999;145:442–51. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–6. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2′-(14)C]nicotine. Drug Metab Dispos. 1999;27:1448–55. [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 2003;170:235–41. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol Biochem Behav. 2010;96:217–27. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2273-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. Am J Prev Med. 2007;33:S368–78. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Giovino GA, Eissenberg T, Clark PI, Lawrence D, Leischow S. Methods to assess potential reduced exposure products. Nicotine Tob Res. 2005;7:827–44. doi: 10.1080/14622200500266015. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19:e1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Carmella S, Mori H, Hoffmann D. A study of tobacco carcinogenesis. XX. Role of catechol as a major cocarcinogen in the weakly acidic fraction of smoke condensate. J Natl Cancer Inst. 1981;66:163–9. [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, Pentel PR. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 1999;143:150–7. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–9. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Liang G, Shulgin AT, Benowitz NL. Gas chromatographic-mass spectrometric method for determination of anabasine, anatabine and other tobacco alkaloids in urine of smokers and smokeless tobacco users. J Chromatogr. 1993;619:49–61. doi: 10.1016/0378-4347(93)80445-a. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol Biomarkers Prev. 2009;18:3263–304. doi: 10.1158/1055-9965.EPI-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, Lesage MG, St Peter JV, Stewart S, Fuller S, Le CT, Pentel PR. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: Dose- and affinity-response relationships. Drug Metab Disposition. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–6. [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003;8:50–9. 9. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–7. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes N, Handley SL. Characterization of the discriminable stimulus produced by 2-BFI: effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine and ibogaine. Br J Pharmacol. 2002;135:1227–34. doi: 10.1038/sj.bjp.0704579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–9. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007a;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol. 2007b;18:707–16. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- Pappas RS, Stanfill SB, Watson CH, Ashley DL. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J Anal Toxicol. 2008;32:281–91. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- Pederson LL, Nelson DE. Literature review and summary of perceptions, attitudes, beliefs, and marketing of potentially reduced exposure products: communication implications. Nicotine Tob Res. 2007;9:525–34. doi: 10.1080/14622200701239548. [DOI] [PubMed] [Google Scholar]
- Pentel PR, Dufek MB, Roiko SA, Lesage MG, Keyler DE. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. J Pharmacol Exp Ther. 2006;317:660–6. doi: 10.1124/jpet.105.097873. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Vann RE, Varvel SA, Covington HE, 3rd, Rosecrans JA, James JR, Robinson SE. Differential behavioral responses to nicotine in Lewis and Fischer-344 rats. Pharmacol Biochem Behav. 2005;80:87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology (Berl) 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- Rodriguez D, Audrain-McGovern J. Construct validity analysis of the early smoking experience questionnaire for adolescents. Addict Behav. 2004;29:1053–7. doi: 10.1016/j.addbeh.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of Immunotherapy against nicotine in rats. J Pharmacol Exp Ther. 2008;325:985–93. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol Biochem Behav. 2009;93:105–11. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Baharnouri G, McQuade LE, Clarke PB. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur J Neurosci. 2008;28:342–52. doi: 10.1111/j.1460-9568.2008.06341.x. [DOI] [PubMed] [Google Scholar]
- Skott M, Andreassen TT, Ulrich-Vinther M, Chen X, Keyler DE, LeSage MG, Pentel PR, Bechtold JE, Soballe K. Tobacco extract but not nicotine impairs the mechanical strength of fracture healing in rats. J Orthop Res. 2006;24:1472–9. doi: 10.1002/jor.20187. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009:295–333. doi: 10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–6. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Hecht SS, Ramakrishnan S, Gupta PC. Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Int J Cancer. 2005;116:16–9. doi: 10.1002/ijc.20966. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob Res. 2008;10:1773–82. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–9. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Rauch RJ, Norris EA. Discriminative stimulus effects of a nicotine-midazolam mixture in rats. Psychopharmacology (Berl) 1987;93:250–6. doi: 10.1007/BF00179943. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, White JA. Impact of training history on discrimination of a drug mixture by rats. Behav Pharmacol. 1996;7:483–494. [PubMed] [Google Scholar]
- Touiki K, Rat P, Molimard R, Chait A, de Beaurepaire R. Effects of tobacco and cigarette smoke extracts on serotonergic raphe neurons in the rat. Neuroreport. 2007;18:925–9. doi: 10.1097/WNR.0b013e32811d6d21. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Nicotine addiction: Health consequences of smoking. DHHS; 1999. [Google Scholar]
- Urban R. Early smoking experience in adolescents. Addict Behav. 2010;35:612–5. doi: 10.1016/j.addbeh.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine- induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1625–38. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–25. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bevins RA, Bardo MT. Neuropharmacology of the interoceptive stimulus properties of nicotine. Curr Drug Abuse Rev. 2009;2:243–55. doi: 10.2174/1874473710902030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL. The ethanol stimulus in rats with differing ethanol preferences. Psychopharmacology (Berl) 1981;74:339–43. doi: 10.1007/BF00432743. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Stefanski R, Przegalinski E, Filip M. Effect of varenicline on the acute and repeated locomotor responses to nicotine in rats. Synapse. 2008;62:935–9. doi: 10.1002/syn.20564. [DOI] [PubMed] [Google Scholar]
- Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–32. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kilicarslan T, Tyndale RF, Sellers EM. Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab Dispos. 2001;29:897–902. [PubMed] [Google Scholar]
- Zubaran C, Shoaib M, Stolerman IP. The development and expression of locomotor sensitization to nicotine in the presence of ibogaine. Behav Pharmacol. 2000;11:431–6. doi: 10.1097/00008877-200008000-00009. [DOI] [PubMed] [Google Scholar]