Abstract
We determined the DNA sequence of seventeen sigma elements and flanking regions in order to investigate the extent of the association between the yeast repetitive element, sigma, and tRNA genes. Fifteen of seventeen sigma elements analyzed begin at position -19 to -16 with respect to the 5' end of a tRNA-coding sequence. This region is close to the initiation point of tRNA gene transcription and contains a sequence which is modestly conserved for a number of tRNA genes. Two pairs of identical sigma elements occur as the long terminal repeats of a sequence which, together with flanking sigma elements, has the structural properties of a retrotransposon; this element has been named Ty3 (manuscript submitted). Hybridization analysis of yeast chromosomal DNA separated by orthogonal field alternation gel electrophoresis (OFAGE) showed that Ty3 and isolated sigma elements are distributed over many chromosomes in the yeast genome.
Full text
PDF








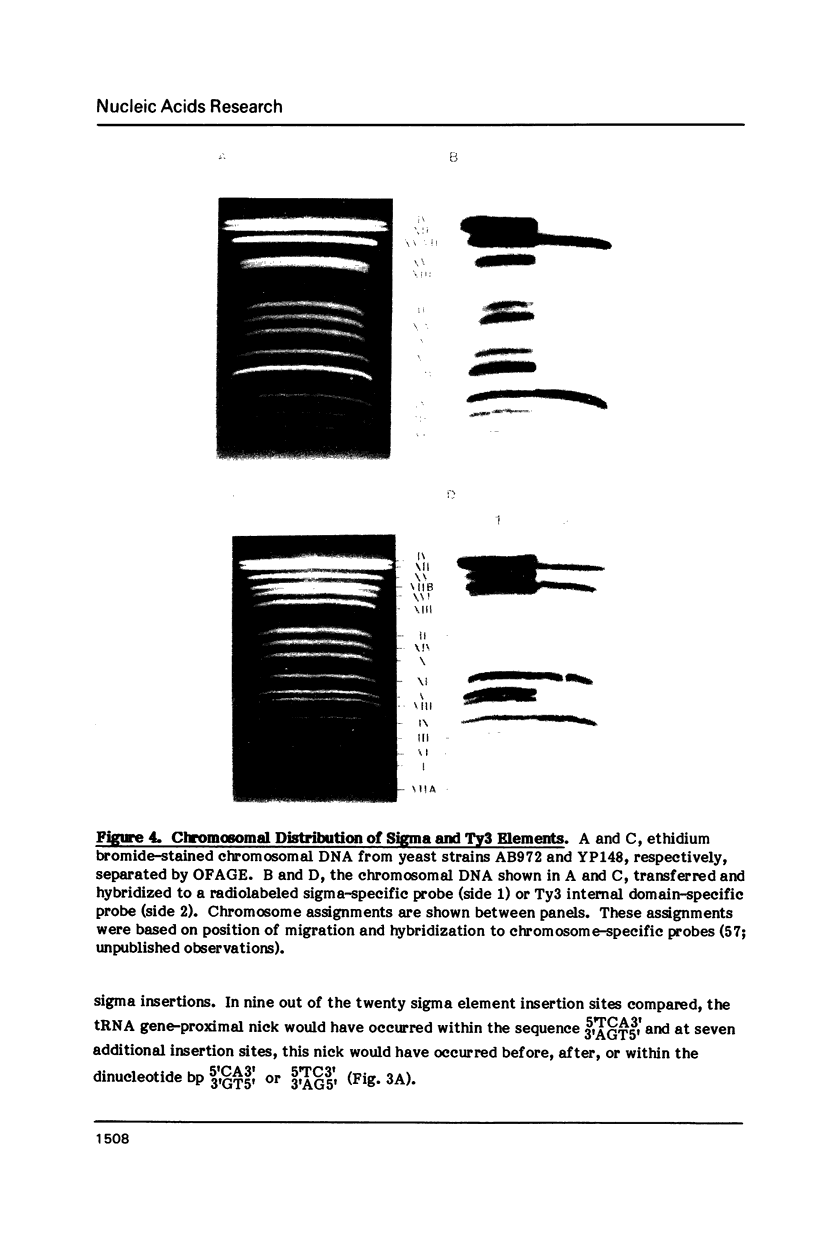







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstutz H., Munz P., Heyer W. D., Leupoid U., Kohli J. Concerted evolution of tRNA genes: intergenic conversion among three unlinked serine tRNA genes in S. pombe. Cell. 1985 Apr;40(4):879–886. doi: 10.1016/0092-8674(85)90347-2. [DOI] [PubMed] [Google Scholar]
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Baker R. E., Eigel A., Vögtel D., Feldmann H. Nucleotide sequences of yeast genes for tRNA(2), tRNA(2) and tRNA(1): homology blocks occur in the vicinity of different tRNA genes. EMBO J. 1982;1(3):291–295. doi: 10.1002/j.1460-2075.1982.tb01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Ebel J. P., Shershneva L. P., Krutilina A. I., Venkstern T. V., Bayev A. A., Dirheirmer G. The corrected nucleotide sequence of valine tRNA from baker's yeast. Biochimie. 1974;56(9):1211–1213. doi: 10.1016/s0300-9084(74)80013-1. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Friedman L., Sherman F. Correspondence of yeast UAA suppressors to cloned tRNASerUCA genes. J Mol Biol. 1981 Aug 15;150(3):375–387. doi: 10.1016/0022-2836(81)90553-2. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Sandmeyer S. B., Olson M. V. Consistent association between sigma elements and tRNA genes in yeast. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3292–3296. doi: 10.1073/pnas.80.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Camier S., Gabrielsen O., Baker R., Sentenac A. A split binding site for transcription factor tau on the tRNA3Glu gene. EMBO J. 1985 Feb;4(2):491–500. doi: 10.1002/j.1460-2075.1985.tb03655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleff D. T., Fink G. R. Genetic events associated with an insertion mutation in yeast. Cell. 1980 Aug;21(1):227–237. doi: 10.1016/0092-8674(80)90130-0. [DOI] [PubMed] [Google Scholar]
- Cigan A. M., Donahue T. F. The methionine initiator tRNA genes of yeast. Gene. 1986;41(2-3):343–348. doi: 10.1016/0378-1119(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Eigel A., Feldmann H. Ty1 and delta elements occur adjacent to several tRNA genes in yeast. EMBO J. 1982;1(10):1245–1250. doi: 10.1002/j.1460-2075.1982.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigel A., Olah J., Feldmann H. Structural comparison of two yeast tRNA Glu 3 genes. Nucleic Acids Res. 1981 Jun 25;9(12):2961–2970. doi: 10.1093/nar/9.12.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke D. R., Gegenheimer P., Abelson J. Nucleolytic processing of a tRNAArg-tRNAAsp dimeric precursor by a homologous component from Saccharomyces cerevisiae. J Biol Chem. 1985 Jan 25;260(2):1271–1279. [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Olah J., Friedenreich H. Sequence of a yeast DNA fragment containing a chromosomal replicator and a tRNA Glu 3 gene. Nucleic Acids Res. 1981 Jun 25;9(12):2949–2959. doi: 10.1093/nar/9.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafner J., Philippsen P. The yeast transposon Ty1 generates duplications of target DNA on insertion. Nature. 1980 Jul 24;286(5771):414–418. doi: 10.1038/286414a0. [DOI] [PubMed] [Google Scholar]
- Gafner J., Robertis E. M., Philippsen P. Delta sequences in the 5' non-coding region of yeast tRNA genes. EMBO J. 1983;2(4):583–591. doi: 10.1002/j.1460-2075.1983.tb01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Gangloff J., Keith G., Ebel J. P., Dirheimer G. The primary structure of aspartate transfer ribonucleic acid from brewer's yeast. II. Partial digestions with pancreatic ribonuclease and T 1 ribonuclease and derivation of complete sequence. Biochim Biophys Acta. 1972 Jan 31;259(2):210–222. [PubMed] [Google Scholar]
- Genbauffe F. S., Chisholm G. E., Cooper T. G. Tau, sigma, and delta. A family of repeated elements in yeast. J Biol Chem. 1984 Aug 25;259(16):10518–10525. [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness N. J., Atfield G. The nucleotide sequence of cysteine transfer ribonucleic acid from baker's yeast. Identification of the products from partial degradation of the molecule and derivation of the complete sequence. Biochem J. 1976 Feb 1;153(2):447–454. doi: 10.1042/bj1530447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Kingsman A. J., Gimlich R. L., Clarke L., Chinault A. C., Carbon J. Sequence variation in dispersed repetitive sequences in Saccharomyces cerevisiae. J Mol Biol. 1981 Feb 5;145(4):619–632. doi: 10.1016/0022-2836(81)90306-5. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Hicks J. B. A position-effect control for gene transposition: state of expression of yeast mating-type genes affects their ability to switch. Cell. 1981 Aug;25(2):517–524. doi: 10.1016/0092-8674(81)90070-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Irie T., Yoshida M., Takeishi K., Ukita T. The primary structure of yeast glutamic acid tRNA specific to the GAA codon. Biochim Biophys Acta. 1974 Oct 11;366(2):168–181. doi: 10.1016/0005-2787(74)90331-1. [DOI] [PubMed] [Google Scholar]
- Kuntzel B., Weissenbach J., Dirheimer G. Structure primaire des tRNA Arg/III de levure de bière. II. Hydrolyses partielles des tRNA Arg/III par les ribonucléases pancréatique et T1 et éstablissement de leur structure primaire complète. Biochimie. 1974;56(8):1069–1087. doi: 10.1016/s0300-9084(74)80096-9. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Hall B. D. Mutations at the Saccharomyces cerevisiae SUP4 tRNA(Tyr) locus: isolation, genetic fine-structure mapping, and correlation with physical structure. Mol Cell Biol. 1982 Dec;2(12):1501–1513. doi: 10.1128/mcb.2.12.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nelböck P., Stucka R., Feldmann H. Different patterns of transposable elements in the vicinity of tRNA genes in yeast: a possible clue to transcriptional modulation. Biol Chem Hoppe Seyler. 1985 Nov;366(11):1041–1051. doi: 10.1515/bchm3.1985.366.2.1041. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Page G. S., Sentenac A., Piper P. W., Worthington M., Weiss R. B., Hall B. D. Only one of two closely related yeast suppressor tRNA genes contains an intervening sequence. Nature. 1981 Jun 11;291(5815):464–469. doi: 10.1038/291464a0. [DOI] [PubMed] [Google Scholar]
- Page G. S., Hall B. D. Characterization of the yeast tRNA Ser genomic organization and DNA sequence. Nucleic Acids Res. 1981 Feb 25;9(4):921–934. doi: 10.1093/nar/9.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penswick J. R., Martin R., Dirheimer G. Evidence supporting a revised sequence for yeast alanine tRNA. FEBS Lett. 1975 Jan 15;50(1):28–31. doi: 10.1016/0014-5793(75)81033-7. [DOI] [PubMed] [Google Scholar]
- Petes T. D. Yeast ribosomal DNA genes are located on chromosome XII. Proc Natl Acad Sci U S A. 1979 Jan;76(1):410–414. doi: 10.1073/pnas.76.1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixa G., Dirheimer G., Keith G. Sequence of tRNA Ile IAU from brewer's yeast. Biochem Biophys Res Commun. 1984 Mar 30;119(3):905–912. doi: 10.1016/0006-291x(84)90859-3. [DOI] [PubMed] [Google Scholar]
- Raymond K. C., Raymond G. J., Johnson J. D. In vivo modulation of yeast tRNA gene expression by 5'-flanking sequences. EMBO J. 1985 Oct;4(10):2649–2656. doi: 10.1002/j.1460-2075.1985.tb03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Gagnon G. C. Patterns of proviral insertion and deletion in avian leukosis virus-induced lymphomas. J Virol. 1986 Jan;57(1):28–36. doi: 10.1128/jvi.57.1.28-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Fink G. R. DNA rearrangements associated with a transposable element in yeast. Cell. 1980 Aug;21(1):239–249. doi: 10.1016/0092-8674(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S. B., Olson M. V. Insertion of a repetitive element at the same position in the 5'-flanking regions of two dissimilar yeast tRNA genes. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7674–7678. doi: 10.1073/pnas.79.24.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O., Mao J., Ogden R., Beckmann J., Sakano H., Abelson J., Söll D. Dimeric tRNA precursors in yeast. Nature. 1980 Oct 23;287(5784):750–752. doi: 10.1038/287750a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Saffran W., Welsh J., Haas R., Goldenberg M., Cantor C. R. New techniques for purifying large DNAs and studying their properties and packaging. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):189–195. doi: 10.1101/sqb.1983.047.01.024. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Teh H. S., Ley A. N., D'Obrenan P. The nucleotide sequences and coding properties of the major and minor lysine transfer ribonucleic acids from the haploid yeast Saccharomyces cerevisiae S288C. J Biol Chem. 1973 Jun 25;248(12):4475–4485. [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Venegas A., Rutter W. J. Yeast tRNA3Leu gene transcribed and spliced in a HeLa cell extract. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5963–5967. doi: 10.1073/pnas.78.10.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman D. J., Better M., Geiduschek E. P. Electron-microscopic examination of the binding of a large RNA polymerase III transcription factor to a tRNA gene. J Mol Biol. 1985 Sep 20;185(2):451–455. doi: 10.1016/0022-2836(85)90417-6. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Venegas A., Weinberg F., Bishop R., Rutter W. J. Structure of yeast phenylalanine-tRNA genes: an intervening DNA segment within the region coding for the tRNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):190–194. doi: 10.1073/pnas.75.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arsdell S. W., Stetler G. L., Thorner J. The yeast repeated element sigma contains a hormone-inducible promoter. Mol Cell Biol. 1987 Feb;7(2):749–759. doi: 10.1128/mcb.7.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas A., Gonzalez E., Bull P., Valenzuela P. Isolation and structure of a yeast initiator tRNAmet gene. Nucleic Acids Res. 1982 Feb 11;10(3):1093–1096. doi: 10.1093/nar/10.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaya S., Steffen D. L., Robinson H. L. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J Virol. 1986 Nov;60(2):683–692. doi: 10.1128/jvi.60.2.683-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Warmington J. R., Anwar R., Newlon C. S., Waring R. B., Davies R. W., Indge K. J., Oliver S. G. A 'hot-spot' for Ty transposition on the left arm of yeast chromosome III. Nucleic Acids Res. 1986 Apr 25;14(8):3475–3485. doi: 10.1093/nar/14.8.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Whitehouse H. L. Duplex breaks in DNA as recombination initiators. Nature. 1983 Dec 15;306(5944):645–646. doi: 10.1038/306645a0. [DOI] [PubMed] [Google Scholar]
- Williamson V. M. Transposable elements in yeast. Int Rev Cytol. 1983;83:1–25. doi: 10.1016/s0074-7696(08)61684-8. [DOI] [PubMed] [Google Scholar]
- Willis I., Hottinger H., Pearson D., Chisholm V., Leupold U., Söll D. Mutations affecting excision of the intron from a eukaryotic dimeric tRNA precursor. EMBO J. 1984 Jul;3(7):1573–1580. doi: 10.1002/j.1460-2075.1984.tb02013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zachau H. G., Dütting D., Feldmann H. The structures of two serine transfer ribonucleic acids. Hoppe Seylers Z Physiol Chem. 1966;347(4):212–235. doi: 10.1515/bchm2.1966.347.1.212. [DOI] [PubMed] [Google Scholar]
- del Rey F. J., Donahue T. F., Fink G. R. sigma, a repetitive element found adjacent to tRNA genes of yeast. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4138–4142. doi: 10.1073/pnas.79.13.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey F., Donahue T. F., Fink G. R. The histidine tRNA genes of yeast. J Biol Chem. 1983 Jul 10;258(13):8175–8182. [PubMed] [Google Scholar]




