Abstract
Purpose
To describe a combination of treatment modalities used for the successful eradication of Fusarium endophthalmitis.
Design
Interventional case series.
Participants
Three consecutive patients with keratitis-associated Fusarium endophthalmitis.
Methods
After failure of traditional management options, a combination of intravitreal and long-term, high-dose systemic voriconazole, topical antifungal medications, and surgical intervention, with penetrating keratoplasty, lensectomy, and endoscopic-guided pars plana vitrectomy, was administered to each patient.
Results
All three cases achieved full resolution of the infection, with a final Snellen visual acuity score of 20/50 to 20/70.
Conclusions
An aggressive combination of therapeutic modalities, including the removal of subiris abscesses, might be needed for the successful resolution of Fusarium endophthalmitis.
Keywords: endophthalmitis, fungal, fusarium, keratitis, keratoplasty, voriconazole
Introduction
Fusarium is a saprotrophic mold, with worldwide distribution, that is responsible for destructive mycotic keratitis. In portions of the Southern United States, keratomycosis accounts for 6% of all microbial keratitis cases, and Fusarium species comprise 62% of fungal isolates.1 Although cases are often associated with trauma,1,2 a 2006 international outbreak of Fusarium keratitis, secondary to ReNu with MoistureLoc contact lens cleaning solution (Bausch and Lomb, Rochester, NY), produced at least 130 confirmed cases in the United States alone.3 Two cases of contact lens-induced keratitis, progressing to endophthalmitis, were reported due to this outbreak.4
Fusarium is visually destructive, due to its high rates of resistance against many antifungal mediations,5 relentless infiltration into ocular tissues,6 mycotoxicity, 7,8 and intravascular invasion with occlusion.9 It can perforate the cornea and produce an infiltrative endophthalmitis in over 6% of keratitis cases, which leaves at least 60% of eyes with finger-counting or worse visual acuity, and 30% of eyes with phthisis, or requiring enucleation.6,10 This manuscript will describe the clinical course and successful resolution of three consecutive cases of Fusarium endophthalmitis secondary to keratitis. Two of the cases were the result of soft contact lens wearing; the final case resulted from traumatic injury to the cornea. All three cases illustrate a uniform combination of treatments, involving the removal of subiris abscesses, to sterilize the eye and achieve a Snellen visual acuity of 20/50 to 20/70.
Methods
Review of patient records was approved by the Institutional Review Board of the University of Michigan, and conforms to all privacy rules set forth by the Health Insurance Portability and Accountability Act.
Case 1
A 32-year-old healthy female contact lens wearer was referred to the Cornea Service at our academic ophthalmology institution with a 10-day history of increasing redness, pain, and blurring of vision in the right eye. She had previously started treatment with topical ofloxacin (0.3%) and prednisolone acetate (1%). At presentation, the examination revealed a Snellen visual acuity of 20/20 with a medium-sized superficial corneal ulcer and indistinct infiltrate at the margins. Corneal cultures were obtained, and prednisolone acetate treatment was immediately discontinued. Three days after presentation, an emergent penetrating keratoplasty was required for a corneal perforation. The cultures revealed unspeciated Fusarium. The patient began treatment with frequent topical natamycin (5%), amphotericin B deoxycholate (0.15%), as well as oral ketoconazole (200 mg per day). Prednisolone acetate (1%) was also prescribed to prevent graft rejection. Two weeks later, the patient was referred to the Retina Service because of increased corneal graft infiltration and anterior segment inflammation with a new hypopyon. Due to presumed intraocular invasion of Fusarium, 5 μg of amphotericin B deoxycholate was injected into the vitreous, and oral voriconazole (VFEND®, Pfizer, New York, NY) (200 mg two times per day) was substituted for ketoconazole. The eye continued to worsen clinically over 4 days, with the development of vitreous opacities on B-scan ultrasound. An emergent 20-gauge pars plana vitrectomy, with cultures and lensectomy, was performed with a repeat intraocular injection of amphotericin B deoxycholate (5 μg) at the conclusion of surgery. The cultures reconfirmed unspeciated Fusarium, and were sent to another institution for sensitivity analysis. Initial postoperative improvement was quickly supplanted by increasing intraocular inflammation, and the eye received a third pars plana injection of amphotericin B deoxycholate (5 μg), followed by two injections of voriconazole (100 μg). However, intraocular inflammation continued to worsen. A second vitrectomy was performed, using endoscopic guidance because of significant corneal graft edema. The camera revealed large subiris and ciliary body abscesses, which were removed with the vitrectomy cutter (see Video http://youtu.be/SLvTMCQB8X0). A third intravitreal voriconazole injection (100 μg) was given at the conclusion of the surgery. Shortly afterwards, culture sensitivity results revealed resistance to amphotericin B deoxycholate and ketoconazole. In addition, the minimum inhibitory concentration (MIC) of voriconazole was >8 μg/mL, which suggested poor sensitivity. Because of this high MIC and the difficulty eradicating the infection previously, a fourth intravitreal voriconazole (100 μg) injection was given shortly after surgery, in addition to increasing the dose of oral voriconazole to 400 mg twice-daily. The eye showed slow clinical improvement. The high-dose oral voriconazole and topical natamycin were discontinued after 3 months. A repeat penetrating keratoplasty was later required due to graft failure. The Snellen best-corrected visual acuity measured 20/70 after 5 years of follow-up.
Case 2
A 48-year-old healthy female contact lens wearer was referred to the Cornea Service at our institution with a 7-day history of increased redness, pain, and blurring of vision in the right eye. She had previously started treatment with a topical combination of tobramycin (0.3%) and dexamethasone (0.1%). At presentation, examination revealed a Snellen visual acuity of 20/400 with a central corneal ulcer and underlying infiltrate. A corneal smear revealed filamentous elements. Dexamethasone and tobramycin were discontinued, and topical natamycin (5%), amphotericin B deoxycholate (0.15%), and oral voriconazole (200 mg per day) were initiated. The cultures revealed unspeciated Fusarium. Two weeks later, the patient was referred to the Retina Service because of increased corneal infiltration and anterior segment inflammation with a new hypopyon (Figure 1A). Voriconazole (100 μg) was injected through the pars plana, and the oral voriconazole was increased to 400 mg twice-daily. However, new vitreous opacities on B-scan ultrasonography and worsening anterior segment inflammation precipitated a penetrating keratoplasty, 20-gauge pars plana vitrectomy with cultures, lensectomy, and endoscopic exploration 2 days later. Subiris and ciliary body abscesses were visualized and excised during surgery. The surgical cultures failed to yield organisms. However, histology of the extracted corneal button revealed persistent fungal elements. The intraocular inflammation slowly improved. However, an infiltrate at the graft-host interface was suspicious for recurrent infection, and topical voriconazole (1%) was added to the topical natamycin (5%), amphotericin B deoxycholate (0.15%), and prednisolone acetate (1%) regimen. Despite this, the corneal infiltrate continued to worsen and required a second penetrating keratoplasty. The corneal button was negative for fungal elements. Four months postoperatively, the eye had no signs of recurrent infection (Figure 1B), and the oral voriconazole was discontinued along with the topical antifungal medications. The Snellen best-corrected visual acuity was 20/50 at 1.5 years of follow-up.
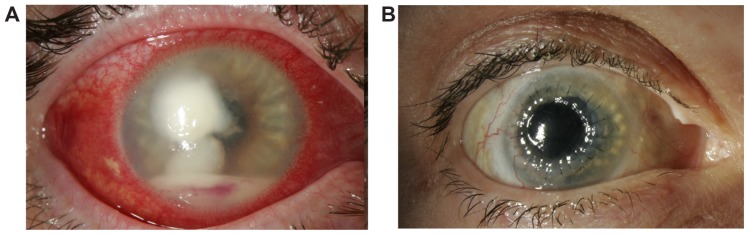
Figure 1.
Case 2. (A) The patient’s clinical condition, following 3 weeks of treatment with topical and oral antibiotics, with severe corneal infiltrate and hypopyon. (B) The patient’s eye 4 months after initial presentation, with a clear corneal graft and a quiet eye.
Notes: The clinical result represents a combined approach of aggressive medical treatment with topical, oral, and intravitreal antibiotics, in conjunction with endoscopic pars plana vitrectomy, involving lensectomy and removal of sub-iris abscesses, as well as two penetrating keratoplasties.
Case 3
A 54-year-old healthy male was referred to the Cornea Service at our institution 4 days after he was struck in the left eye by a tree branch. He had previously started treatment with a combination of topical tobramycin (0.3%) and dexamethasone (0.1%) in addition to ciprofloxacin (0.3%). However the ocular redness persisted, and visual acuity worsened. At presentation, examination revealed a Snellen visual acuity of 20/30 with multifocal corneal infiltrates. Corneal cultures were obtained, and the previously-prescribed drops were changed to topical natamycin (5%), neomycin sulfate (0.5%), and moxifloxacin (0.5%) empirically. Two days later, visual acuity had dropped to hand motion with enlargement of the corneal ulcer and development of anterior segment infiltration. However there was no evidence of vitritis on B-scan ultrasound. An aqueous culture was obtained. An aqueous culture was obtained, and an empiric injection of voriconazole (100 ug) was given for presumed fungal invasion. Topical amphotericin B deoxycholate (0.15%) and systemic voriconazole (200 mg twice-daily) were added to the regimen. The aqueous culture revealed unspeciated Fusarium. Because the corneal and anterior chamber inflammation failed to improve and a follow-up B-scan ultrasound demonstrated new vitreous opacities, voriconazole (100 μg) was again injected into the vitreous cavity and the systemic voriconazole dose was increased to 400 mg twice-daily. However, the inflammation continued to worsen, which prompted surgical intervention with a penetrating keratoplasty, 20-gauge pars plana vitrectomy with lensectomy, and endoscopic exploration. Multiple subiris and ciliary body abscesses were removed using endoscopic visualization. Postoperative management included: topical amphotericin B deoxycholate (0.15%), natamycin (5%) and cyclosporine (1%), oral voriconazole (400 mg twice-daily), and two additional voriconazole (100 μg) injections through the pars plana. The corneal and intraocular inflammation slowly resolved, and the medication regimen was maintained for 3 months. Culture sensitivities, reported nearly a month after the surgery, revealed minimum inhibitory concentrations of 4 μg/mL for amphotericin B and natamycin and >8 μg/mL for voriconazole. The best-corrected Snellen visual acuity was 20/60 after 5 years of follow-up.
Results
Each of the three consecutive cases was characterized by culture-positive Fusarium keratitis with subsequent anterior chamber invasion and vitreous involvement. The infection failed to respond to several treatments, including a variety of topical, intravitreal, and systemic antifungal medications. The endophthalmitis eventually cleared with a multimodal approach, including medical treatment with topical natamycin and amphotericin B deoxycholate, multiple intraocular voriconazole (VFEND®, Pfizer, New York, NY) injections, and high-dose, long-duration oral voriconazole (400 mg twice-daily). Surgical management included penetrating keratoplasty, endoscopic- guided pars plana vitrectomy with subiris abscess removal, and lensectomy. In each case, clearance of the infection occurred after endoscopic removal of subiris and ciliary body abscesses. Both cases in which the sensitivity of Fusarium to voriconazole was measured demonstrated an MIC > 8 μg/mL. Despite this poor voriconazole sensitivity, the combined approach resulted in sterilization of the eye, and a final Snellen visual acuity score of 20/50 to 20/70.
Discussion
Fusarium endophthalmitis can result from penetrating trauma,6 keratitis,10 and intraocular surgery11 in immunocompetent individuals. It can also occur endogenously in immunocompromised patients.9,12 The first two individuals in this case series developed endophthalmitis from Fusarium keratitis secondary to soft contact lens wear; the third patient acquired endophthalmitis from trauma-induced Fusarium keratitis.
Eradicating Fusarium endophthalmitis is notoriously difficult. Persistence of the infection despite standard treatments10 or delayed recurrence of infection can occur for multiple reasons.13 First, Fusarium has high rates of resistance to some antifungal medications – which was the situation in at least two of these cases.5 Poor susceptibility to voriconazole is less common, but there are at least two other case reports that document resistance of Fusarium to voriconazole.14,15 Also, these cases raise the possibility that refractory cases might be secondary to undetected abscesses, which provide a nidus for persistent infection. Removal of the abscesses hidden beneath the iris was the event that, ultimately, initiated clinical improvement in each of these cases. Finally, topical corticosteroids were being used at presentation in all the cases. In the first two cases, corticosteroids were used postoperatively to prevent graft rejection. Studies suggest that topical corticosteroids might worsen Fusarium infection by inhibiting the antifungal phagocytic action of immune cells.16–18 Cyclosporine, which has both antifungal and antirejection properties, has demonstrated effectiveness when used topically on therapeutic keratoplasties related to mycotic keratitis and may be a viable alternative to corticosteroids when a keratoplasty is required, as was the approach in Case 3.16,19
The older treatment modalities for ocular Fusarium infections are convoluted. Medical options have included: topical natamycin6 amphotericin B deoxycholate,6 and clotrimazole; 6 subconjunctival amphotericin B deoxycholate6 and miconazole;6 intracameral amphotericin B deoxycholate;6 intravitreal amphotericin B deoxycholate;6 systemic miconazole6 thiobendazole,6 fluconazole,10 ketoconazole,10 amphotericin B deoxycholate,20 amphotericin B lipid complex, 21 and flucytosine.20 Surgical approaches have included: penetrating keratoplasty,10 lensectomy,10 iridectomy,10 and pars plana vitrectomy.6,10 Case reports11,20 have described some successes with various combinations of these treatments. However, larger case series6,10 describe a predominantly dismal outcome. A number of these traditional medical and surgical modalities were incorporated without success in Case 1. Therefore, we switched to oral and intraocular voriconazole, in addition to novel surgical management. This new approach salvaged the eye, and was then applied to the two subsequent cases with relatively successful outcomes.
Voriconazole, with broad-spectrum antifungal properties, high oral bioavailability, and rapid systemic absorption, was approved by the US Food and Drug Administration in 2002, for invasive Aspergillus, Fusarium, and Scedosporium infections. Although it is off-label for ocular indications, several small descriptive reports have suggested that it is safe and effective when directed against Fusarium infections in human eyes.4,22–25 The MIC of voriconazole towards ocular Fusarium isolates usually ranges between 2–8 μg/mL, with few cases of resistance.5 By contrast, Fusarium exhibits relatively high resistance rates to fluconazole, itraconazole, miconazole, ketoconazole, posaconazole, flucytosine, and amphotericin B deoxycholate.5,11 Voriconazole also demonstrates time-dependent activity, which suggests that maximizing the duration of exposure might optimize fungistatic activity.26 We attempted to achieve an intraocular concentration of voriconazole consistently in excess of the typical MIC by utilizing both systemic and intravitreal routes.
Systemically administered voriconazole in a non-inflamed human eye achieves an intravitreal concentration of 0.81–1.12 μg/mL.23,27 We felt that an elevated dose of 400 mg, two times per day, might better maximize long-term intraocular concentrations. In conjunction with the Infectious Disease Service, we monitored liver function tests weekly and were cognizant of potential adverse interactions with other systemic medications. Tu et al described two cases of Fusarium endophthalmitis in which the patients were unable to tolerate oral voriconazole due to systemic toxicity. However, another review suggested that the majority of patients taking oral voriconazole for this indication tolerated the therapy long enough to achieve clearance of the infection.15,28 All of our patients were able to tolerate the higher dose for 3–4 months.
Repeated intraocular injections of voriconazole were added to maximize intraocular concentration. In keeping with previous reports discussing the safe dosing of intravitreal voriconazole, we injected 100 μg through the pars plana to achieve a presumed vitreous cavity concentration of approximately 20–25 μg/mL.4,22,23,29–33 Injections were administered as frequently as every 48 hours, since voriconazole has a relatively short half-life. In the rabbit vitreous, the half-life measured 2.5 hours. However, clearance may be more rapid in rabbits than in humans.34 The clearance rate most likely accelerated further following vitrectomy.35
In our patients, voriconazole was injected into the vitreous cavity instead of into the anterior chamber to maximize the duration of action and minimize anterior segment complications in these previously phakic eyes. However, a recent report described successful eradication of Fusarium in the anterior chamber when doses of voriconazole (100 μg) were injected intracamerally on a daily basis for up to 8 days.24 Alternatively, topical voriconazole (1%) has good corneal penetration and was shown to achieve a concentration of at least 6.5 μg/mL in the anterior chamber.4,22,36 If initiated when the infection is limited to the anterior segment, these approaches may avoid the morbidity of more invasive procedures.
Once the infection reaches the vitreous, surgery is often needed to augment therapy.6 Pflugfelder et al reported that four of six patients (67%) with Fusarium endophthalmitis required penetrating keratoplasty, 83% required vitrectomy, 100% required lensectomy, 67% required iridectomy, and 67% were left with no light perception vision.6 Similarly, Dursun et al reported that nine of ten patients (90%) required penetrating keratoplasty, 40% required vitrectomy, 50% required lensectomy, 10% required iridectomy, and 30% were left with no light perception vision.10 The cases described here all required penetrating keratoplasty, pars plana vitrectomy, and lensectomy. With endoscopic visualization, we identified and removed subiris and ciliary body abscesses during the vitrectomy, which were not apparent on clinical examination. This approach may have reduced the infectious load and allowed for improved access of voriconazole to any residual Fusarium. In addition, it may have prevented more drastic surgical interventions, such as a sector or complete iridectomy. As far as we know, this is the first report to describe subiris abscesses and removal in the management of Fusarium endophthalmitis.
In summary, we achieved successful resolution of three consecutive cases of Fusarium endophthalmitis with functional visual outcomes. An aggressive therapeutic approach, including a combination of intravitreal and long-term, high-dose systemic voriconazole, topical natamycin and amphotericin B deoxycholate, penetrating keratoplasty, lensectomy, and endoscopic guided pars plana vitrectomy resulted in the eradication of the Fusarium infections. This approach was developed after more traditional treatment modalities failed. Although this report is retrospective, uncontrolled, and describes a number of variables utilized in the treatment of Fusarium endophthalmitis, it is clear that the removal of subiris and ciliary body abscesses seemed to be the unifying event that initiated clinical improvement in each case. Thus, this combination of treatments, including the removal of abscesses, might decrease the morbidity of Fusarium endophthalmitis and allow for improved visual outcomes.
Acknowledgments
This work was supported by a Postdoctoral Clinical/Translational Science Fellowship Award from the American Diabetes Association-Merck.
Portions of this manuscript were presented as a poster at the American Academy of Ophthalmology Annual Meeting, November, 2006, Las Vegas, NV.
Footnotes
Disclosure
The authors have no financial interest in any of the products discussed in this paper.
References
- 1.Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101(6):1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- 2.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113(1):109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296(8):953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg KD, Flynn HW, Jr, Alfonso EC, Miller D. Fusarium endophthalmitis following keratitis associated with contact lenses. Ophthalmic Surg Lasers Imaging. 2006;37(4):310–313. doi: 10.3928/15428877-20060701-08. [DOI] [PubMed] [Google Scholar]
- 5.Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137(5):820–825. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 6.Pflugfelder SC, Flynn HW, Jr, Zwickey TA, et al. Exogenous fungal endophthalmitis. Ophthalmology. 1988;95(1):19–30. doi: 10.1016/s0161-6420(88)33229-x. [DOI] [PubMed] [Google Scholar]
- 7.Gopinathan U, Ramakrishna T, Willcox M, et al. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp Eye Res. 2001;72(4):433–442. doi: 10.1006/exer.2000.0971. [DOI] [PubMed] [Google Scholar]
- 8.Jones BR, Jones DB, Lim AS, Bron AJ, Morgan G, Clayton YM. Corneal and intra-ocular infection due to Fusarium solani. Trans Ophthalmol Soc UK. 1970;89:757–779. [PubMed] [Google Scholar]
- 9.Patel AS, Hemady RK, Rodrigues M, Rajagopalan S, Elman MJ. Endogenous Fusarium endophthalmitis in a patient with acute lymphocytic leukemia. Am J Ophthalmol. 1994;117(3):363–368. doi: 10.1016/s0002-9394(14)73147-2. [DOI] [PubMed] [Google Scholar]
- 10.Dursun D, Fernandez V, Miller D, Alfonso EC. Advanced fusarium keratitis progressing to endophthalmitis. Cornea. 2003;22(4):300–303. doi: 10.1097/00003226-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer C, Alio J, Rodriguez A, Andreu M, Colom F. Endophthalmitis caused by Fusarium proliferatum. J Clin Microbiol. 2005;43(10):5372–5375. doi: 10.1128/JCM.43.10.5372-5375.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezai KA, Eliott D, Plous O, Vazquez JA, Abrams GW. Disseminated Fusarium infection presenting as bilateral endogenous endophthalmitis in a patient with acute myeloid leukemia. Arch Ophthalmol. 2005;123(5):702–703. doi: 10.1001/archopht.123.5.702. [DOI] [PubMed] [Google Scholar]
- 13.Weissgold DJ, Orlin SE, Sulewski ME, Frayer WC, Eagle RC., Jr Delayed-onset fungal keratitis after endophthalmitis. Ophthalmology. 1998;105(2):258–262. doi: 10.1016/s0161-6420(98)92938-4. [DOI] [PubMed] [Google Scholar]
- 14.Proenca-Pina J, Ssi Yan Kai I, Bourcier T, Fabre M, Offret H, Labetoulle M. Fusarium keratitis and endophthalmitis associated with lens contact wear. Int Ophthalmol. 2010;30(1):103–107. doi: 10.1007/s10792-008-9290-7. [DOI] [PubMed] [Google Scholar]
- 15.Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592) Am J Ophthalmol. 2007;143(2):222–227. doi: 10.1016/j.ajo.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 16.Bell NP, Karp CL, Alfonso EC, Schiffman J, Miller D. Effects of methylprednisolone and cyclosporine A on fungal growth in vitro. Cornea. 1999;18(3):306–313. doi: 10.1097/00003226-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 17.O’Day DM, Ray WA, Head WS, Robinson RD, Williams TE. Influence of corticosteroid on experimentally induced keratomycosis. Arch Ophthalmol. 1991;109(11):1601–1604. doi: 10.1001/archopht.1991.01080110139051. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Au Eong KG, Chan WK, Tseng PS. Fusarium keratitis following the use of topical antibiotic-corticosteroid therapy in traumatised eyes. Ann Acad Med Singapore. 1996;25(6):862–865. [PubMed] [Google Scholar]
- 19.Perry HD, Doshi SJ, Donnenfeld ED, Bai GS. Topical cyclosporin A in the management of therapeutic keratoplasty for mycotic keratitis. Cornea. 2002;21(2):161–163. doi: 10.1097/00003226-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Rowsey JJ, Acers TE, Smith DL, Mohr JA, Newsom DL, Rodriguez J. Fusarium oxysporum endophthalmitis. Arch Ophthalmol. 1979;97(1):103–105. doi: 10.1001/archopht.1979.01020010043010. [DOI] [PubMed] [Google Scholar]
- 21.Goldblum D, Frueh BE, Zimmerli S, Böhnke M. Treatment of postkeratitis fusarium endophthalmitis with amphotericin B lipid complex. Cornea. 2000;19(6):853–856. doi: 10.1097/00003226-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Reis A, Sundmacher R, Tintelnot K, Agostini H, Jensen HE, Althaus C. Successful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazole. Br J Ophthalmol. 2000;84(8):932–933. doi: 10.1136/bjo.84.8.928d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005;139(1):135–140. doi: 10.1016/j.ajo.2004.08.077. [DOI] [PubMed] [Google Scholar]
- 24.Shen YC, Wang CY, Tsai HY, Lee HN. Intracameral voriconazole injection in the treatment of fungal endophthalmitis resulting from keratitis. Am J Ophthalmol. 2010;149(6):916–921. doi: 10.1016/j.ajo.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Durand ML, Kim IK, D’Amico DJ, et al. Successful treatment of Fusarium endophthalmitis with voriconazole and Aspergillus endophthalmitis with voriconazole plus caspofungin. Am J Ophthalmol. 2005;140(3):552–554. doi: 10.1016/j.ajo.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Klepser ME, Malone D, Lewis RE, Ernst EJ, Pfaller MA. Evaluation of voriconazole pharmacodynamics using time-kill methodology. Antimicrob Agents Chemother. 2000;44:1917–1920. doi: 10.1128/aac.44.7.1917-1920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122(1):42–47. doi: 10.1001/archopht.122.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol. 2008 Jul;92(7):871–878. doi: 10.1136/bjo.2007.136515. [DOI] [PubMed] [Google Scholar]
- 29.Scott IU, Cruz-Villegas V, Flynn HW, Jr, Miller D. Delayed-onset, bleb-associated endophthalmitis caused by Lecythophora mutabilis. Am J Ophthalmol. 2004;137(3):583–585. doi: 10.1016/j.ajo.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Nehemy MB, Vasconcelos-Santos DV, Torqueti-Costa L, Magalhaes EP. Chronic endophthalmitis due to verticillum species after cataract surgery treated (or managed) with pars plana vitrectomy and oral and intravitreal voriconazole. Retina. 2006;26(2):225–227. doi: 10.1097/00006982-200602000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Sen P, Gopal L, Sen PR. Intravitreal voriconazole for drug-resistant fungal endophthalmitis: case series. Retina. 2006;26(8):935–939. doi: 10.1097/01.iae.0000250011.68532.a2. [DOI] [PubMed] [Google Scholar]
- 32.Kramer M, Kramer MR, Blau H, Bishara J, Axer-Siegel R, Weinberger D. Intravitreal voriconazole for the treatment of endogenous Aspergillus endophthalmitis. Ophthalmology. 2006;113(7):1184–1186. doi: 10.1016/j.ophtha.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 33.Gao H, Pennesi ME, Shah K, et al. Intravitreal voriconazole: an electroretinographic and histopathologic study. Arch Ophthalmol. 2004;122(11):130. doi: 10.1001/archopht.122.11.1687. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y-C, Wang M-Y, Wang CY, et al. Clearance of intravitreal voriconazole. Invest Ophthalmol Vis Sci. 2007;48:2238–2241. doi: 10.1167/iovs.06-1362. [DOI] [PubMed] [Google Scholar]
- 35.Doft BH, Weiskopf J, Nilsson-Ehle I, Winquard LB., Jr Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology. 1985;92:1601–1605. doi: 10.1016/s0161-6420(85)33838-1. [DOI] [PubMed] [Google Scholar]
- 36.Vemulakonda GA, Hariprasad SM, Mieler WF, Prince RA, Shah GK, VanGelder RN. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol. 2008;126(1):18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]