Abstract
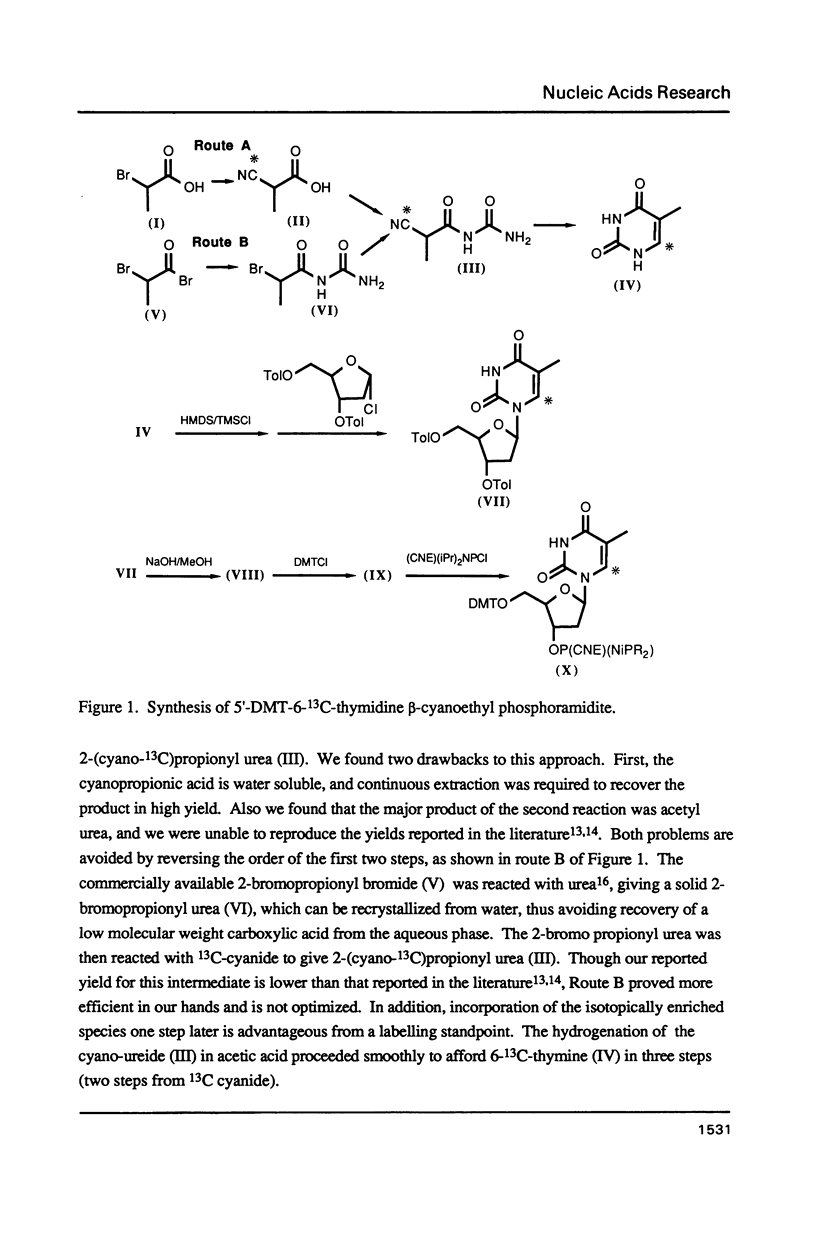
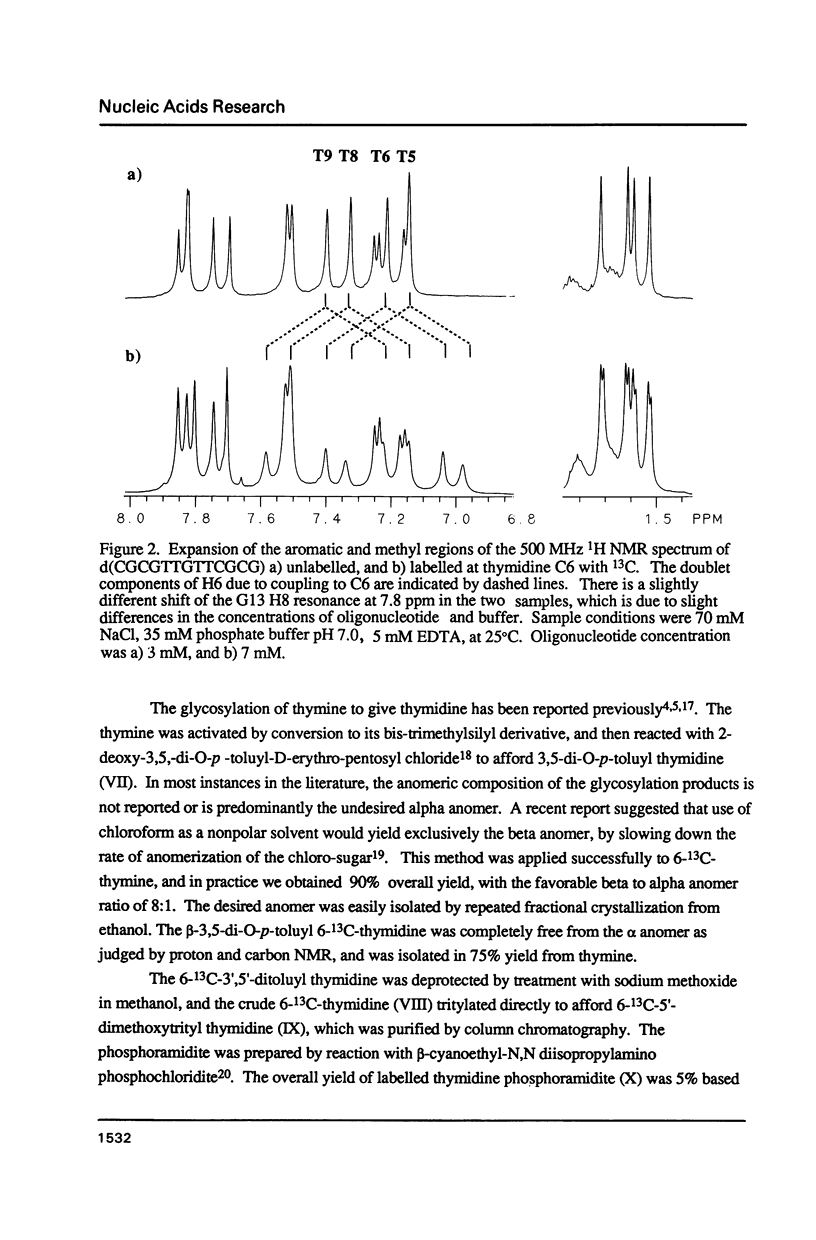
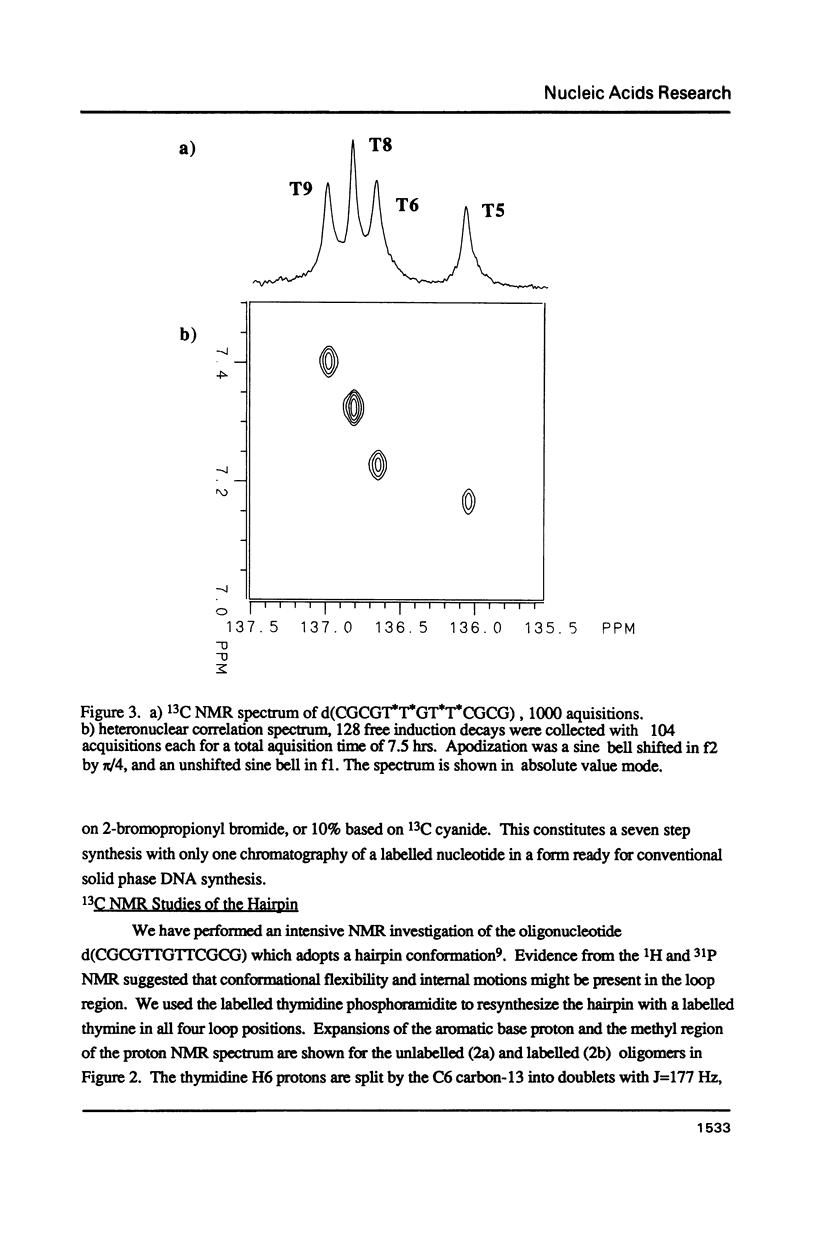
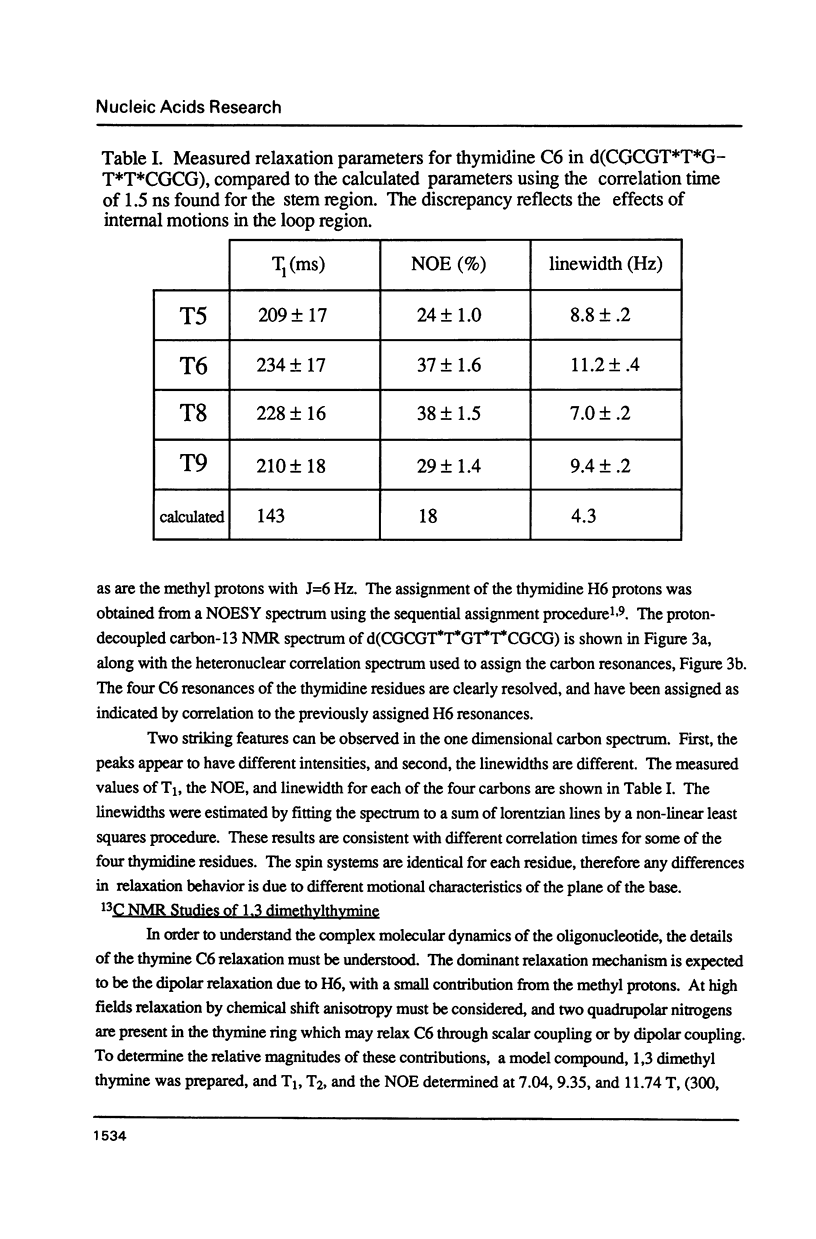
A thymidine phosphoramidite labelled at C6 with 13C has been synthesized, and incorporated into a synthetic oligonucleotide, d(CGCGT*T*GT*T*CGCG), which adopts a hairpin conformation. NMR relaxation measurements indicate that internal motion may be present in the loop region of the oligonucleotide. The relaxation behavior of a the C6 carbon in a model compound, N,N-1,3 dimethylthymine is examined in detail as a function of magnetic field strength to determine relative contributions of various mechanisms to the relaxation. The relaxation behaviour of the labelled carbons in the oligonucleotide is discussed in relation to these measurements.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Kovacs S. A., Smith C., Kopper R. A., Schmidt P. G. Complete nuclear magnetic resonance signal assignments and initial structural studies of [13C]methyl-enriched yeast transfer ribonucleic acid. Biochemistry. 1983 Mar 15;22(6):1402–1408. doi: 10.1021/bi00275a013. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Zanatta N., Holak T. A., Levy G. C., van Boom J. H., Wang A. H. Conformation and dynamics of short DNA duplexes: (dC-dG)3 and (dC-dG)4. J Biomol Struct Dyn. 1984 Jun;1(6):1373–1386. doi: 10.1080/07391102.1984.10507526. [DOI] [PubMed] [Google Scholar]
- Hilliard P. R., Jr, Smith R. M., Rill R. L. Natural abundance carbon-13 nuclear magnetic resonance studies of histone and DNA dynamics in nucleosome cores. J Biol Chem. 1986 May 5;261(13):5992–5998. [PubMed] [Google Scholar]
- Hubbard A. J., Jones A. S., Walker R. T. An investigation by 1H NMR spectroscopy into the factors determining the beta:alpha ratio of the product in 2'-deoxynucleoside synthesis. Nucleic Acids Res. 1984 Sep 11;12(17):6827–6837. doi: 10.1093/nar/12.17.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. C., Hilliard P. R., Jr, Levy L. F., Rill R. L., Inners R. Carbon 13 spin-lattice relaxation, linewidth, and nuclear Overhauser enhancement measurements of nucleosome length DNA. J Biol Chem. 1981 Oct 10;256(19):9986–9989. [PubMed] [Google Scholar]
- Olsen J. I., Schweizer M. P., Walkiw I. J., Hamill W. D., Jr, Horton W. J., Grant D. M. Carbon-13 NMR relaxation studies of pre-melt structural dynamics in [4-13C-uracil] labeled E. coli transfer RNAIVal. Nucleic Acids Res. 1982 Jul 24;10(14):4449–4464. doi: 10.1093/nar/10.14.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P. G., Playl T., Agris P. F. Internal dynamics of transfer ribonucleic acid determined by nuclear magnetic resonance of carbon-13-enriched ribose carbon 1. Biochemistry. 1983 Mar 15;22(6):1408–1415. doi: 10.1021/bi00275a014. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Schmidt P. G., Petsch J., Agris P. F. Nuclear magnetic resonance signal assignments of purified [13C]methyl-enriched yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1985 Mar 12;24(6):1434–1440. doi: 10.1021/bi00327a023. [DOI] [PubMed] [Google Scholar]



