Abstract
We previously demonstrated that 50% of (−)-epigallocatechin gallate (EGCG) was present in methylated form (4″-MeEGCG) in human prostate tissue, which is less bioactive. We therefore investigated whether quercetin, a natural inhibitor of catechol-O-methyl transferase (COMT), will inhibit EGCG methylation leading to enhanced antiproliferative activity of EGCG in prostate cancer cells. Incubation with both, quercetin and EGCG, for 2 hr increased the cellular concentrations of EGCG by 4 to 8-fold and 6 to 10-fold in androgen-independent PC-3 cells and androgen-dependent LNCaP cells, respectively. Concurrently, the percent of 4″-MeEGCG in the total EGCG was decreased from 39% to 15% in PC-3 cells and from 61% to 38% in LNCaP cells. Quercetin and EGCG in combination synergistically inhibited cell proliferation, caused cell cycle arrest and induced apoptosis in PC-3 cells. In LNCaP cells EGCG and quercetin exhibited a stronger antiproliferative activity leading to an additive effect. The synergistic effect of these two agents in PC-3 cells could be based on the fact that EGCG primarily inhibited COMT activity while quercetin reduced the amount of COMT protein. In summary, quercetin combined with EGCG in vitro demonstrated enhanced inhibition of cell proliferation by increasing the intracellular concentration of EGCG and decreasing EGCG methylation.
Keywords: Catechol-O-methyl transferase, experimental, green tea polyphenol, prostate cancer, quercetin
INTRODUCTION
Tea and tea polyphenols are promising chemopreventive and chemotherapeutic agents against a variety of tumors including prostate cancer (CaP) (1, 2). However, enhancing the tissue bioavailability of green tea polyphenols (GTPs) and inhibiting conversion into less active metabolites in vivo may enhance the health benefits of green tea against human cancers (1, 2). Green tea is abundant in monomeric GTPs including (−)-epigallocatechin (EGC), (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin (EC), and (−)-epicatechin-3-gallate (ECG), with EGCG being the most abundant and most biologically active component (1). However, GTPs are extensively transformed in vivo leading to enhanced excretion or reduced chemopreventive activity. The non-gallated GTPs such as EGC and EC undergo glucuronidation and sulfation while the gallated GTPs EGCG and ECG are mainly present in the free form (3). All GTPs with catechol groups are methylated by catechol-O-methyl transferase (COMT) leading to a decrease in urine excretion (4). Previously we found that around 50 percent of EGCG was present in methylated form (4″-O-methyl EGCG) in human prostate tissue obtained at prostatectomy after consumption of 6 cups (48 oz.) of green tea daily for 3–5 weeks (5). A similar degree of methylation of EGCG was found in mouse tissues including lung, kidney and the xenograft prostate tumors after green tea consumption (2). Methylation significantly decreases the anti-proliferative activity of EGCG in cultured LNCaP prostate cancer cells (5). Quercetin, a flavonoid found in onions, apples, red grapes and other fruits and vegetables, is known to inhibit the activity of COMT (6). Both EGCG and quercetin have been shown to inhibit proliferation and induce apoptosis in prostate cancer cells (7, 8). Both flavonoids have been demonstrated to inhibit the growth of CWR22 xenograft prostate tumor in severe combined immune deficient (SCID) mice and in athymic nude mice (9, 10).
The present study was designed to determine whether treatment with the combination of quercetin and EGCG will increase the cellular concentration of non-methylated EGCG, thereby enhancing the antiproliferative and pro-apoptotic effect of EGCG against CaP. The combined effects of EGCG and quercetin were examined in two prostate cancer cell lines, androgen-dependent LNCaP cells and androgen-independent PC-3 cells.
MATERIALS AND METHODS
Cell Line and Cell Culture
PC-3 and LNCaP prostate cancer cells were obtained from American Type Culture Collection (ATCC, Manassas, VA), and cultured in F-12K (ATCC) and RPMI 1640 medium with L-Glutamine (Mediatech Inc., Manassas, VA), respectively, supplemented with 10% (v:v) of fetal bovine serum (FBS) (USA Scientific, Ocala, FL), 100 IU/ml of penicillin and 100 µg/ml of streptomycin (Invitrogen Inc, Carlsbad, CA) at 37 °C in a 5% CO2 incubator.
Cellular Absorption of EGCG and Quercetin
PC-3 cells and LNCaP cells were allowed to grow to 50–60 percent confluency in 100 mm Petri dishes. Due to relatively low cellular uptake rate of EGCG and the detection limit of HPLC detection, a higher concentration of EGCG at 80µM was used for the cellular absorption experiments. PC-3 cells were incubated with fresh serum-complete medium containing 80µM EGCG (Sigma Chemicals, St Louis, MO), 10µM quercetin (Sigma Chemicals), 20µM quercetin, 80µM EGCG + 10µM quercetin, or 80µM EGCG + 20µM quercetin for 2, 24, or 48h. LNCaP cells were treated the same except that lower concentrations of quercetin (5µM and 10µM) were used in consistence with the proliferation assay described below. To minimize the effect of hydrogen peroxide (H2O2) that may be formed by autoxidation and/or dimerization of EGCG and quercetin in medium (11), 50 units/ml of catalase was added to the medium prior to EGCG and quercetin in all the mechanistic experiments in the present study. The procedures for cell harvest was described previously (5). Briefly, the medium was removed and the dishes were washed with 10 ml of PBS for 3 times. The dishes were placed on ice and cells were collected and homogenized in 100µl of 2% ascorbic acid in water. The homogenate was centrifuged at 10,000 rpm for 15 min and the supernatant was transferred and protein precipitated for detection by HPLC-CoulArray electrochemical detection system (ESA, Chelmsford, MA). Cytosolic EGCG and quercetin concentrations were normalized by cytosolic protein determined by the Bio-Rad protein assay according to the manufacturer’s protocol (Bio-Rad Laboratories, Hercules, CA). All the experiments were repeated three times.
Cell Proliferation Assay
PC-3 and LNCaP cells were seeded into 96-well plates at a density of 8×103 per well. PC-3 cells were treated with the following: vehicle control (DMSO), 40µM EGCG, 10µM quercetin, 20µM quercetin, 40µM EGCG + 10µM quercetin, or 40µM EGCG + 20µM quercetin for 24h and 48h. Lower concentrations of quercetin at 5µM or 10µM alone or in combination with 40µM EGCG were used for LNCaP treatment based on our preliminary results that LNCaP cells were more sensitive to EGCG and quercetin and the dose of quercetin at 20µM was too much to see a combined effect. Cell proliferation was determined with adenosine triphosphate (ATP) assay using the CellTiter-Glo® Luminescent Cell Viability Assay kit (Promega Corporation, Madison, WI) which has been shown in our previous studies to be more accurate than MTT assay in measurement of cell proliferation under the treatment of GTPs (12). Each concentration had five replications and the experiment was repeated three times.
Flow Cytometry Analysis of Cell Cycle and Apoptosis
When 50–60% confluent in 75ml flask, PC-3 cells were treated with vehicle control, 40µM EGCG, 10µM quercetin, 20µM quercetin, 40µM EGCG + 10µM quercetin, or 40µM EGCG + 20µM quercetin; LNCaP cells were treated with vehicle control, 40µM EGCG, 5µM quercetin, 10µM quercetin, 40µM EGCG + 5µM quercetin, or 40µM EGCG + 10µM quercetin for 24h and 48h. All the cells, including those attached to the bottom and floating in medium, were collected. After centrifugation at 1000 rpm for 5 min, the pellet containing 1×106 cells was resuspended in 500µl of hypotonic DNA staining buffer (0.1% sodium citrate, 0.3% (v:v) Triton-x 100, 0.01% Propidium iodide (PI), 0.002% ribonuclease A), and cell cycle distribution was detected using an FACScan Analytic Flow Cytometer (BD Biosciences, San Jose, CA). The apoptotic cells were determined by double staining with FITC-conjugated Annexin V and PI according to the manufacturer’s instruction (BD Biosciences) to identify phosphatidylserine exposure and membrane integrity using the FACScan Analytic Flow Cytometer within 30 min of staining, and a total of 10,000 events were acquired for FL1 versus FL2 dot plot analysis. Cells at early stage of apoptosis with FITC Annexin V positive and PI negative were used for the calculation of apoptosis percentage. The results were analyzed using ModFit LT 3.1 SP3 software (Verity Software House, Topsham, ME). The experiment was performed at least in duplicate and was repeated twice.
Determination of COMT Activity
PC-3 cells and LNCaP cells cultured in 60 mm Petri dishes were treated with EGCG and quercetin at the same concentrations as that for cell proliferation assays. After 2, 24, or 48h, the cells were harvested and COMT activity were measured followed the procedures described by Reenilä et al (13) with some modifications. Briefly, medium was removed and the dishes were washed with 5 ml of cold PBS for 3 times. The cells were collected and homogenized in 10mM Na2HPO4 buffer (pH 7.4) containing 0.5mM dithiothreitol. The homogenates were centrifuged at 900g for 10 min at 4°C and protein concentrations in the supernatant were measured by the Bio-Rad protein assay (Bio-Rad Laboratories). The supernatants were stored at −70°C until use. The COMT activity was evaluated based on the formation of the methyl metabolite vanillic acid (3–methoxy-4-hydroxybenzoic acid) from dihydroxybenzoic acid (DHBAc) catalyzed by COMT. Briefly, the cell preparation containing 100µg protein was incubated at 37°C with 0.2mM S-adenosyl-L-methionine iodide (AdoMet) (Sigma Chemicals), 5mM MgCl2and 200µM DHBAc, buffered with 100mM Na2HPO4 buffer (pH 7.4) in a total volume of 125µl. After 30 min, the reaction was terminated by adding 25µl of 4M perchloric acid. Protein was removed by centrifuge at 14,000 rpm for 15 min, and the supernatant was detected by HPLC-CoulArray detection system for vanillic acid which had a main peak at 500mV. The COMT enzyme activity was expressed as nmol vanillic acid formed/h/mg protein. The experiment was performed in triplicate.
Analysis of COMT Protein Expression
PC-3 cells and LNCaP cells were treated with vehicle control, 40µM EGCG, 10µM quercetin, or 40µM EGCG + 10µM quercetin for 2h, 24h and 48h. The procedure for cell harvest and protein extraction was described before (5). Briefly, the medium was removed and cells were washed three times with cold PBS. The cells were lysed in cold lysis buffer for 5 min on ice and the crude lysate was passed through 26 ½ G needle and cleared by centrifugation. The protein concentration was measured by the Bio-Rad protein assay (Bio-Rad Laboratories).
For the Western blot analysis, 20 µg of protein was loaded and separated on a 10% Bis-Tris gel (Invitrogen Inc.). Proteins were electrotransferred to nitrocellulose membranes and blocked in Tris-buffered saline with 0.1% Tween 20 and 5% nonfat milk for 1 hour at room temperature. Membranes were incubated with rabbit anti-human COMT antibody (sc-25844) (Santa Cruz, CA) at a dilution of 1:1000 overnight at 4°C. Goat anti-rabbit IgG-Horseradish Peroxidase was used as the second antibody. Protein was visualized and analyzed using a ChemiDoc XRS chemiluminescence detection and imaging system (Bio-Rad Laboratories). β-actin protein was used as loading control.
Statistical Analysis
SPSS (Version 18.0, Chicago, IL) was used for statistical analyses. Data were expressed as mean ± standard deviation (SD). Comparison of means was performed by two independent samples t-test, or one-way analysis of variance with Tukey’s posttest. Synergistic action of a combination of EGCG and quercetin was present if the effect of the combination exceeded the additive effects of the individual components (14). Differences were considered significant if P<0.05.
RESULTS
Intracellular Concentration and Methylation of EGCG and Quercetin
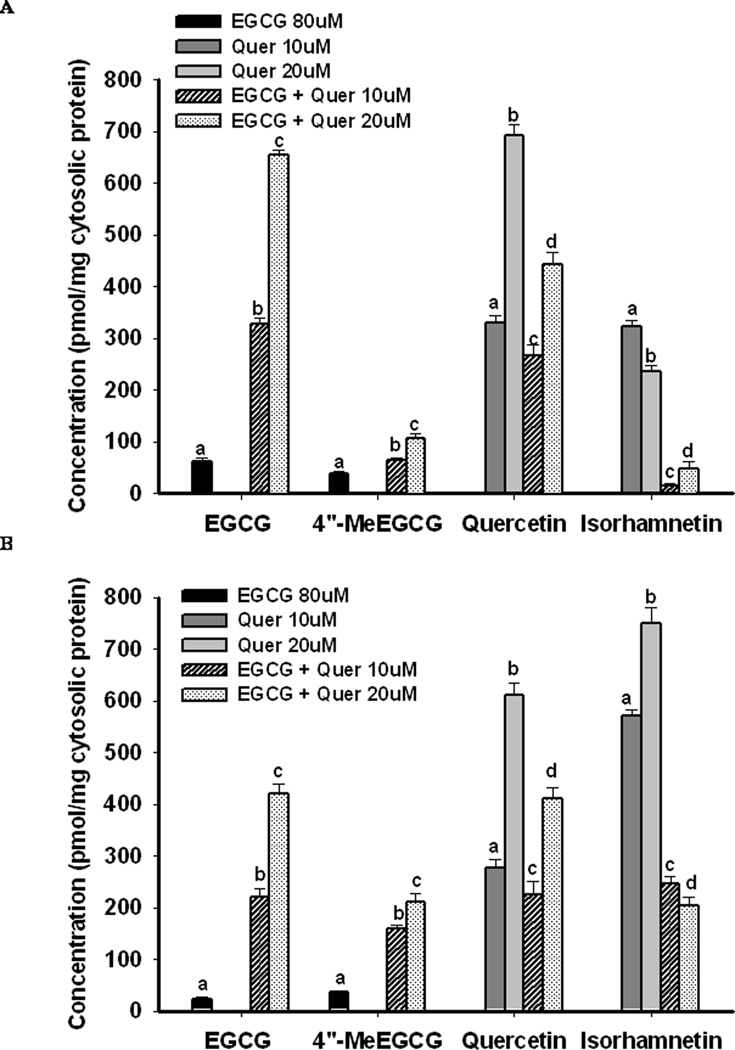
Both EGCG and its methylated metabolite 4″-MeEGCG were found in PC-3 and LNCaP cells when treated with 80µM of EGCG for 2h (Figure 1), 24 or 48h (supplementary data, Figure 1, PC-3 cells). The highest concentration of EGCG was found at 2h of treatment. Co-treatment with quercetin with 10µM and 20µM (5µM and 10µM for LNCaP cells) dramatically increased cellular absorption of EGCG by 4 to 8-fold and 6 to 10-fold compared to EGCG alone at 2h in PC-3 cells and LNCaP cells, respectively, in a dose-dependent manner. Concurrently, the percent of 4″-MeEGCG compared to total EGCG within cells was significantly decreased from 39% to 15% in PC-3 cells and from 61% to 38% in LNCaP cells at 2h post treatment (P<0.05). The increased cellular absorption and decreased methylation of EGCG was also observed at 24h and 48h in both cell lines when incubated with the combination of EGCG and quercetin (supplementary data, Figure 1, PC-3 cells). When incubated with quercetin alone, quercetin and its methyl metabolites 3′-O-methyl quercetin (isorhamnetin) and 4′-O-methyl quercetin (tamarixetin, data not shown) were found in both cell lines at significantly higher concentration compared to EGCG (Figure 1). In PC-3 the methylation rate of quercetin and EGCG was decreased compared to LNCaP cells. The co-treatment with EGCG decreased the cellular absorption of quercetin by 30–50% compared to the treatment with quercetin alone. The methylation of quercetin was significantly inhibited by the co-treatment as demonstrated by a decreased ratio of isorhamnetin to quercetin from 100% to 6% and 30% to 11% with low dose and high dose of quercetin, respectively in PC-3 cells, and from 210% to 110% and 120% to 50% in LNCaP cells (P<0.05) (Figure 1).
Figure 1.
Cellular uptake and metabolism of EGCG and quercetin under different treatments for 2h. PC-3 (A) and LNCaP (B) cells were treated with the indicated concentrations of EGCG and quercetin alone or in combination. Cellular contents were detected 2h after treatment. The bars grouped by compounds with different superscript letters represent significant difference between treatments (P<0.05). Error bars represent standard deviation.
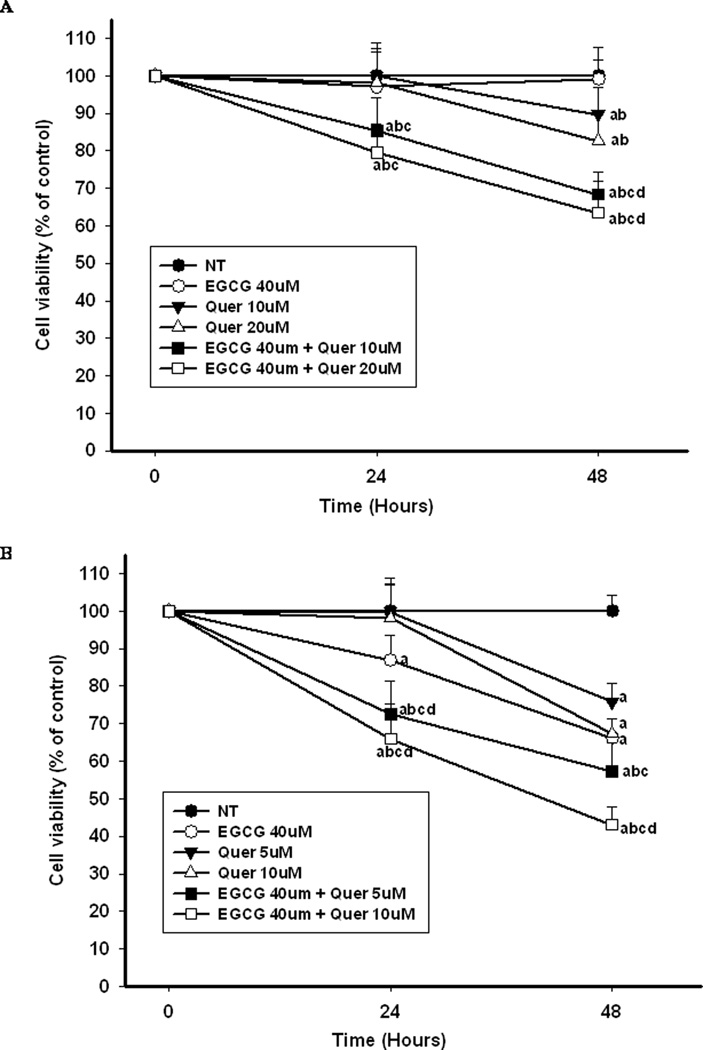
Inhibition of Cell Proliferation
To test the additive/synergistic effect of co-treatment with EGCG and quercetin we selected concentrations below the IC50 for treatment. Therefore when PC-3 cells were treated with EGCG or quercetin alone, no inhibitory effect or very small inhibitory effect on cell proliferation was observed during 48 hr compared to the control (Figure 2A). However, co-treatment of quercetin with EGCG synergistically enhanced the inhibitory effect on the proliferation of PC-3 cells in a dose-dependent manner. Compared to the sum inhibition rate of EGCG and quercetin alone, the combination of EGCG with 10µM or 20µM of quercetin increased the inhibition of PC-3 cell proliferation by 15% and 20%, or 21% and 19%, respectively at 24h and 48h. In LNCaP cells EGCG and quercetin treatment exhibited a stronger antiproliferative effect than in PC-3 cells (Figure 2B). Therefore a lower concentration of quercetin was used for the co-treatment. EGCG alone at 40µM inhibited the proliferation of LNCaP cells by 15% and 30% compared to the control at 24h and 48h, respectively (P<0.05). Quercetin alone at the same concentration (10µM) showed a 3-fold stronger inhibitory effect in LNCaP cells than in PC-3 cells at 48h. The combination of EGCG and quercetin significantly enhanced the inhibition in an additive manner leading to 60% inhibition of LNCaP cell proliferation with 40µM EGCG + 10µM quercetin at 48h as compared to the control (Figure 2B).
Figure 2.
Cell proliferation under different treatments during 48 hr. PC-3 cells (A) and LNCaP cells (B) were treated with the indicated concentrations of EGCG and quercetin alone or in combination for 24h and 48h. Cell proliferation was measured by ATP assay. The superscript letters represent significant difference between groups (P<0.05): a compared to vehicle control (NT); b compared to 40µM of EGCG treatment; c compared to low dose of quercetin treatment; d compared to high dose of quercetin treatment. Error bars represent standard deviation.
Impact on Cell Cycle and Apoptosis
EGCG and quercetin alone or in combination induced both cell cycle arrest and apoptosis in LNCaP cells and PC-3 cells (Tables 1 and 2) with a stronger effect observed at 48h than at 24h (data not shown). Consistent with the higher anti-proliferative effects of EGCG and quercetin demonstrated in LNCaP cells, stronger effects on the arrest of cell cycle and induction of apoptosis were also observed in LNCaP cells compared to PC-3 cells. Both EGCG and quercetin alone induced S-phase and G2/M phase cell cycle arrest in the two cell lines. Co-treatment of EGCG and quercetin significantly enhanced the arrest of cell cycle at S-phase and G2 phase and synergistically increased apoptosis in both of the cell lines with a 2–3 folds stronger effect in LNCaP cells than in PC-3 cells.
Table 1.
Cell cycle distribution and apoptosis of PC-3 cells under different treatments
| Treatment (µmol/L) |
Cell cycle distribution (%) |
Apoptosis (%) |
||
|---|---|---|---|---|
| G1/G0 | S | G2/M | ||
| NT | 77.5 ± 0.3a | 14.7 ± 0.1a | 7.8 ± 0.4a | 3.5 ± 0.1a |
| EGCG 40 | 74.6 ± 0.1b | 15.7 ± 0.3b | 9.7 ± 0.4b | 5.0 ± 0.3b |
| Quer 10 | 72.3 ± 0.3c | 15.9 ± 0.2b | 11.8 ± 0.3c | 4.4 ± 0.1c |
| Quer 20 | 67.6 ± 0.4d | 17.3 ± 0.4c | 15.1 ± 0.4d | 5.3 ± 0.1b |
| EGCG 40 + Quer 10 | 60.6 ± 0.2e | 19.3 ± 0.2d | 20.1 ± 0.2e | 7.9 ± 0.3d |
| EGCG 40 + Quer 20 | 50.0 ± 0.9f | 23.4 ± 0.2e | 26.6 ± 0.9f | 9.5 ± 0.3e |
PC-3 cells were treated with the indicated concentrations of EGCG and quercetin alone or in combination. Cell cycle distribution and cell apoptosis were detected at 48h post treatment by flow cytometry. Data are presented as mean ± SD. Groups with different superscript letters in each column represent significant difference between groups (P<0.05).
Table 2.
Cell cycle distribution and apoptosis of LNCaP cells under different treatments
| Treatment (µmol/L) |
Cell cycle distribution (%) |
Apoptosis (%) |
||
|---|---|---|---|---|
| G1/G0 | S | G2/M | ||
| Control (NT) | 75.7 ± 0.8a | 15.6 ± 0.4a | 8.7 ± 0.3a | 3.2 ± 0.1a |
| EGCG 40 | 65.0 ± 0.7b | 18.8 ± 0.1b | 16.2 ± 0.6b | 8.3 ± 0.2b |
| Quer 5 | 68.4 ± 0.5c | 17.1 ± 0.7c | 14.5 ± 0.5c | 6.6 ± 0.1c |
| Quer 10 | 60.5 ± 0.3d | 20.0 ± 0.2b | 19.5 ± 0.5d | 7.5 ± 0.1d |
| EGCG 40 + Quer 5 | 52.4 ± 0.4e | 20.9 ± 0.6b | 26.7 ± 0.4e | 12.1 ± 0.3e |
| EGCG 40 + Quer 10 | 42.8 ± 0.3f | 23.5 ± 0.7d | 33.7 ± 0.7f | 17.6 ± 0.1f |
LNCaP cells were treated with the indicated concentrations of EGCG and quercetin alone or in combination. Cell cycle distribution and cell apoptosis were detected at 48h post treatment by flow cytometry. Data are presented as mean ± SD. Groups with different superscript letters in each column represent significant difference between groups (P<0.05).
Impact on COMT Activity and Protein Expression
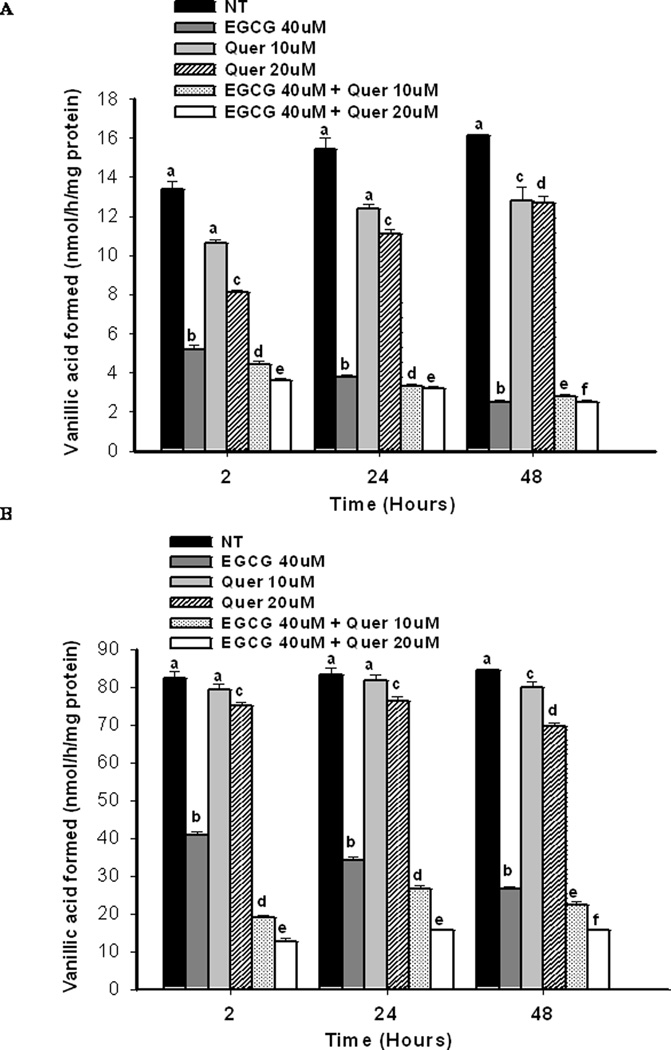
Compared to quercetin, treatment with 40 µM of EGCG exhibited a stronger inhibition of COMT activity by 61%, 75%, 85% compared to the control at 2h, 24h, and 48h, respectively in PC-3 cells (Figure 3A), and 50%, 59%, 72% in LNCaP cells (Figure 3B). Co-treatment of EGCG and 10µM of quercetin significantly increased the inhibition of COMT activity in LNCaP cells in a dose-dependent manner with an inhibition of 85%, 81%, 82% compared to the control at 2h, 24h, and 48h (Figure 3B). However, in PC-3 cells an increased inhibition of COMT activity by the co-treatment was only observed at 2h post treatment (Figure 3A). No significant effects on COMT protein expression were observed at 2h in either PC-3 or LNCaP cells (supplementary data, Figure 2A and B) However the COMT protein expression for LNCaP cells showed a trend to decrease at the two hour point when treated with the EGCG-quercetin combination. EGCG at 40µM showed little inhibitory effect on COMT protein expression while quercetin at 10µM demonstrated a small, but significant, inhibition as compared to the control by 10% in PC-3 cells at 48h and 22% and 20% in LNCaP cells at 24h and 48h, respectively (P<0.05) (Figure 4A and B). Co-treatment of EGCG and quercetin enhanced the inhibition of COMT protein expression to 12% and 24% in PC-3 cells, 33% and 30% in LNCaP cells at 24 and 48h, respectively.
Figure 3.
Impact on COMT activity by different treatments. PC-3 cells (A) and LNCaP cells (B) were treated with the indicated concentrations of EGCG and quercetin alone or in combination for the desired time points. COMT activity was evaluated based on the formation of the methyl metabolite vanillic acid (3-methoxy-4-hydroxybenzoic acid) from dihydroxybenzoic acid (DHBAc) catalyzed by COMT. Groups with different superscript letters at each time point represent significant difference between groups (P<0.05). Error bars represent standard deviation.
Figure 4.
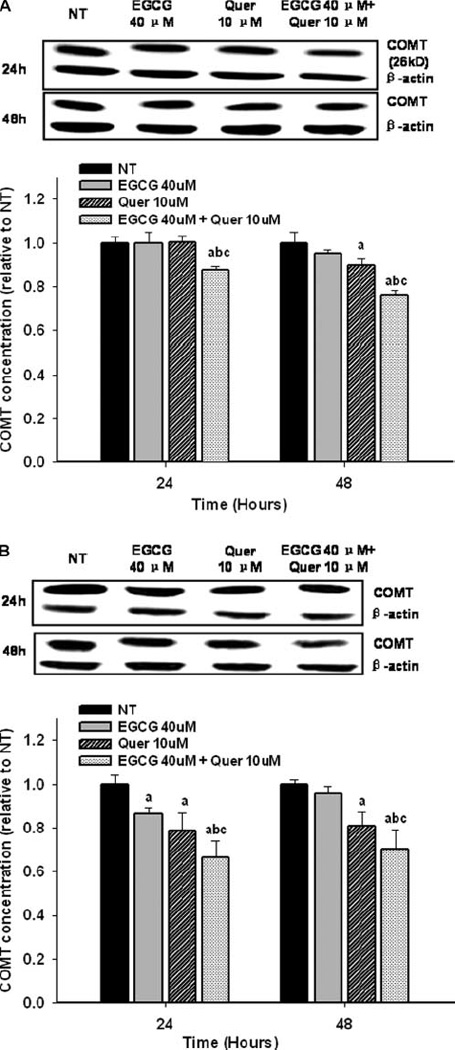
Impact on COMT protein expression by different treatments. PC-3 cells (A) and LNCaP cells (B) were treated with the indicated concentrations of EGCG and quercetin alone or in combination for 24h and 48h. COMT protein expression was evaluated by Western blot. The superscript letters represent significant difference between groups (P<0.05): a compared to vehicle control (NT); b compared to 40 µM of EGCG treatment; c compared to 10 µM of quercetin treatment. Error bars represent standard deviation.
DISCUSSION
This study demonstrated that quercetin increased the cellular uptake of EGCG and inhibited methylation of EGCG in prostate cancer cells leading to enhanced biological activity in inhibition of proliferation and induction of apoptosis by the combined treatment compared to either substance alone. Green tea, in which EGCG is the major active chemical, is a promising anti-cancer agent with respect to its bioactivity and safety (1). In vitro cell culture studies have shown that GTPs target multiple signaling pathways in anti-carcinogenesis such as NF-κB pathway, mitogen-activated protein kinase pathway, epidermal growth factor receptor mediated pathway, and the insulin-like growth factor mediated pathway (1, 15). However, the low bioavailability and extensive biotransformation of GTPs in vivo limits the health beneficial effect of green tea in humans (16).
A single nucleotide polymorphism in the gene encoding for COMT at position 158 has been shown to reduce the enzymatic activity by 40% (17). The importance of catechol O-methylation in the evaluation of the effect of green tea on cancer risk has been suggested by an epidemiological study in Asian-American women showing that breast cancer risk was significantly reduced only among tea drinkers possessing at least one low-activity COMT allele (18). This finding was further supported by evidence from our laboratory and other investigators demonstrating that the most bioactive component of green tea, EGCG, was largely methylated in vivo and the methylation significantly decreased its anticancer activity (5, 19). Thus, through the inhibition of COMT the formation of less active methyl metabolites may be reduced and the anticancer potency of GTPs and quercetin may be enhanced. Our results presented here demonstrated that EGCG exhibited a stronger inhibitory effect on COMT activity compared to quercetin. However, quercetin was able to inhibit COMT protein expression while EGCG showed little effect. The co-treatment of EGCG and quercetin enhanced the inhibition of COMT in both activity and protein levels resulting in significantly decreased methylation of EGCG and quercetin in the prostate cancer cells compared to EGCG or quercetin alone. Recently, Landis-Piwowar et al. reported an increase in proteasome inhibition and apoptosis induction in breast cancer cells by EGCG treatment when COMT activity was decreased (20), which is in support of the important role of COMT in tea intervention.
Our data also demonstrated a dramatic increase in cellular content of EGCG in both prostate cancer cell lines by co-incubation of EGCG with quercetin. Quercetin is a potent inhibitor of multidrug-resistance proteins (MRPs) including MRP-1 (21) which has been found involved in the efflux of GTPs from the cells (22, 23). We speculate that the increased cellular content of EGCG observed in this study may have partly resulted from the inhibition of MRP-1 by quercetin. In addition, the co-treatment led to a decrease in quercetin absorption in both cell lines, possibly due to a competition of transport into the cells with EGCG (24, 25). However, despite the decrease in quercetin content, co-treatment was associated with an increase in anti-proliferation, inhibition of cell cycle and stimulation of apoptosis.
The antiproliferative effect of EGCG and quercetin was stronger in LNCaP cells than PC-3 cells, suggesting that different pathways or mechanisms may be affected in the two cell lines in response to EGCG or quercetin treatment. LNCaP and PC-3 cells differ in p53 status, a tumor suppressor protein regulating cell cycle and apoptosis (26). In p53 wild-type LNCaP cells, the induction of apoptosis and cell cycle arrest by EGCG and quercetin may be primarily via a p53-dependent pathway (26). In p53 null PC-3 cells apoptosis may be mainly stimulated through p53-independent activation of p21 pathway (26–28). The loss of p53 expression may contribute to the decreased sensitivity of PC-3 cells to EGCG and quercetin treatment as indicated by the presented data (26, 27). The observed increase in antiproliferative and cell cycle inhibitory effects by the combination of EGCG and quercetin on androgen independent PC-3 cells supports our hypothesis that the combined supplementation may be effective in the treatment of later stage CaP. This is also supported by a recent study by Tang et al. in which quercetin and EGCG exhibited a synergistic effect to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition (29). In our study the combination of EGCG and quercetin significantly increased the percentage of cells in S-phase and G2/M phase, which may indicate an enhanced cell cycle arrest at S phase and G2/M phase and induction of apoptosis at G1/G0 phase. Depending on culture conditions other investigators demonstrated that both EGCG and quercetin induced S-phase and G2/M phase cell cycle arrest in PC-3 cells and LNCaP cells (28, 30, 31). However, G0/G1 phase arrest by EGCG was also observed by other investigators in LNCaP and DU145 cells when cells were starved for 36h to arrest them in G0 phase before EGCG treatment (7). In our study we added catalase to the culture medium prior to the addition of EGCG or quercetin to minimize the artificial effect on cell proliferation and apoptosis by the reactive oxygen species such as H2O2which can be formed by autoxidation and/or dimerization of EGCG and quercetin in medium (11). Therefore, the effects observed in this study are expected to better reflect the physiological situation in vivo.
In summary, quercetin enhanced the effect of EGCG against prostate cancer cells via increasing the cellular uptake and decreasing methylation of EGCG. Given the safety of these two phytochemicals, the combination is promising to be a novel regimen to enhance the chemoprevention and chemotherapy in CaP. In addition, based on the ubiquitous distribution of COMT in different organs, the combination may benefit patients of other types of cancer. Further studies in a xenograft model are underway to confirm these findings in vivo.
Supplementary Material
Acknowledgments
Funding: This work was supported by the NIH Grant RO1 CA116242
Footnotes
Conference Presentation: Presented at the American Association for Cancer Research (AACR) Translational Cancer Medicine 2010 annual meeting held in San Francisco, CA.
REFERENCES
- 1.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henning SM, Wang P, Heber D. Chemopreventive effects of tea in prostate cancer: Green tea versus black tea. Mol Nutr Food Res. 2011;55:905–920. doi: 10.1002/mnfr.201000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henning SM, Choo JJ, Heber D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J Nutr. 2008;138:1529S–1534S. doi: 10.1093/jn/138.8.1529S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, et al. Genetic Association Between the COMT Genotype and Urinary Levels of Tea Polyphenols and Their Metabolites among Daily Green Tea Drinkers. Int J Mol Epidemiol Genet. 2010;1:114–123. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila Pa) 2010;3:985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab Dispos. 2004;32:497–504. doi: 10.1124/dmd.32.5.497. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 8.Aalinkeel R, Bindukumar B, Reynolds JL, Sykes DE, Mahajan SD, et al. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate. 2008;68:1773–1789. doi: 10.1002/pros.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma ZS, Huynh TH, Ng CP, Do PT, Nguyen TH, et al. Reduction of CWR22 prostate tumor xenograft growth by combined tamoxifen-quercetin treatment is associated with inhibition of angiogenesis and cellular proliferation. Int J Oncol. 2004;24:1297–1304. [PubMed] [Google Scholar]
- 10.Siddiqui IA, Zaman N, Aziz MH, Reagan-Shaw SR, Sarfaraz S, et al. Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–839. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 11.Yang GY, Liao J, Li C, Chung J, Yurkow EJ, et al. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–2039. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One. 2010;5:e10202. doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reenila I, Tuomainen P, Mannisto PT. Improved assay of reaction products to quantitate catechol-O-methyltransferase activity by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1995;663:137–142. doi: 10.1016/0378-4347(94)00433-6. [DOI] [PubMed] [Google Scholar]
- 14.Bharadwaj R, Vidya A, Dewan B, Pal A. An in vitro study to evaluate the synergistic activity of norfloxacin and metronidazole. Indian J Pharmacol. 2003;35:220–226. [Google Scholar]
- 15.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 16.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–S151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 17.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;61:6716–6722. [PubMed] [Google Scholar]
- 18.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63:7526–7529. [PubMed] [Google Scholar]
- 19.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, et al. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007;213:252–260. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 20.Landis-Piwowar K, Chen D, Chan TH, Dou QP. Inhibition of catechol-Omicron-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (−)-EGCG. Oncol Rep. 2010;24:563–569. doi: 10.3892/or_00000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Zanden JJ, van der Woude H, Vaessen J, Usta M, Wortelboer HM, et al. The effect of quercetin phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochem Pharmacol. 2007;74:345–351. doi: 10.1016/j.bcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Hong J, Lu H, Meng X, Ryu JH, Hara Y, et al. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 23.Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos. 2011;39:920–926. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nait Chabane M, Al Ahmad A, Peluso J, Muller CD, Ubeaud G. Quercetin and naringenin transport across human intestinal Caco-2 cells. J Pharm Pharmacol. 2009;61:1473–1483. doi: 10.1211/jpp/61.11.0006. [DOI] [PubMed] [Google Scholar]
- 26.Hastak K, Agarwal MK, Mukhtar H, Agarwal ML. Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J. 2005;19:789–791. doi: 10.1096/fj.04-2226fje. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 28.Vijayababu MR, Kanagaraj P, Arunkumar A, Ilangovan R, Aruldhas MM, et al. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J Cancer Res Clin Oncol. 2005;131:765–771. doi: 10.1007/s00432-005-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang SN, Singh C, Nall D, Meeker D, Shankar S, et al. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenouda NS, Zhou C, Browning JD, Ansell PJ, Sakla MS, et al. Phytoestrogens in common herbs regulate prostate cancer cell growth in vitro. Nutr Cancer. 2004;49:200–208. doi: 10.1207/s15327914nc4902_12. [DOI] [PubMed] [Google Scholar]
- 31.Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr Cancer. 2000;38:116–122. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.