Abstract
Neuronal ceroid lipofuscinoses (NCLs) comprise a heterogeneous group of metabolic storage diseases that present with the accumulation of autofluorescent lipopigment, neurodegeneration and premature death. Nine genes have been thus far identified as the cause of different types of NCL, with ages at onset ranging from around birth to adult, although the underlying etiology of the disease still remains elusive. We present a family with typical NCL pathology in which we performed exome sequencing and identified a single homozygous mutation in ATP13A2 that fully segregates with disease within the family. Mutations in ATP13A2 are a known cause of Kufor–Rakeb syndrome (KRS), a rare parkinsonian phenotype with juvenile onset. These data show that NCL and KRS may share etiological features and implicate the lysosomal pathway in Parkinson's disease.
INTRODUCTION
The neuronal ceroid lipofuscinoses (NCLs) are a heterogeneous group of inherited progressive degenerative diseases that affect the brain and sometimes the retina. They are characterized by lysosomal accumulation of autofluorescent lipopigment that display typical ultrastructural patterns (1). There are now nine genes known to contain pathogenic mutations causing NCL: CLN1 (PPT1 [MIM 256730]), CLN2 (TPP1 [MIM 204500]), CLN3 (MIM 204200), CLN4 (DNAJC5 (MIM 611203)), CLN5 (MIM 256731), CLN6 (MIM 601780), CLN7 (MFSD8 [MIM 610951]), CLN8 (MIM 600143), CLN10 (CTSD [MIM 610127]) that cause onset at varying ages from birth to adulthood. Despite these recent extraordinary advances in the identification of causative genes, the etiology of the disease remains elusive.
Recently, two reports (2,3) in the same dog model for a late-onset NCL suggested that mutations in ATP13A2 might be the cause of the disease in that animal. ATP13A2 belongs to the P-type superfamily of ATPases that transport inorganic cations and other substrates across cell membranes. It has previously been shown in humans that loss-of-function mutations in ATP13A2 cause Kufor–Rakeb syndrome (KRS), a very rare form of autosomal-recessive hereditary parkinsonism with dementia and juvenile onset (4). The relationship between the disease presented by the dog model of NCL and KRS is not evident, as the clinical features of both diseases do not appear to overlap significantly. KRS typically presents with rigidity, bradykinesia, spasticity, supranuclear upgaze paresis and dementia. NCL disease varies according to the underlying gene defect and severity of mutation, but typically includes seizures, a progressive intellectual and motor deterioration, and in children but usually not adult onset cases, visual failure. In addition, NCL shows autofluorescent storage material with typical ultrastructural patterns and in most juvenile onset cases vacuolated lymphocytes (5). Brain magnetic resonance imaging shows global atrophy for both syndromes, while iron deposition is typically seen only in KRS.
Here, we present a large NCL family from Belgium (Fig. 1), in which, although extensively studied in the past (6–8), no mutation underlying the disease had been identified. The clinical history of affected siblings is similar. All affected showed difficulties in learning from around the ages of 8 years, with vacuolated lymphocytes and ultrastructural pathology that resembled that for NCL. The index case (II-1) had an unsteady gait, myoclonus and mood disturbance from age 11 to 13, progressing to clear extrapyramidal involvement with akinesia and rigidity, and dysarthric speech over the next 5 years. At this point, the patient was found to be responsive to L-dopa, but although considered, a diagnosis of young-onset Parkinson's disease was not made. Interestingly, following L-dopa administration, the patients developed dyskinesias something that parallels what has been reported in KRS (9,10). At age 25, myoclonus was severe, speech was unintelligible, there was loss of muscle strength, amyotrophy and the patient was wheelchair bound. Neurological exam showed evidence of pyramidal involvement, cerebellar ataxia and bulbar syndrome (dysphonia, dysphagia and dysarthria). There was no apparent retinal involvement, although the patient was reported to have slow vertical ocular movements, which is also interesting given the known occurrence of supranuclear gaze palsy with upgaze restriction in KRS, and the patient eventually died at age 36 of pulmonary emboli. Muscle biopsy showed numerous subsarcolemmal autofluorescence bodies with a fingerprint appearance in electron microscopy and suggestion of neurogenic muscular atrophy. Post-mortem pathological examination showed abundant neuronal and glial lipofuscinosis involving the cortex, basal nuclei, cerebellum, but sparing the white matter, with whorled lamellar inclusions typical of NCL in electron microscopy. Lipofuscin deposits were confirmed in the retina.
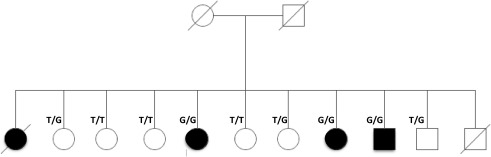
Figure 1.
Pedigree of the family studied. Pedigree showing complete segregation of p.M810R mutation in ATP13A2 in the available family members. T is the reference allele, while G represents the mutation.
The additional three affected individuals in this kindred showed a combination of progressive spinocerebellar ataxia, bulbar syndrome, extrapyramidal and pyramidal involvement, and intellectual deterioration (Table 1) [for additional clinical details and fludeoxyglucose-positron emission tomography findings see (7,8)].
Table 1.
Summary of the clinical descriptions of the affected individuals
| Patients | II-1 | II-5 | II-8 | II-9 |
|---|---|---|---|---|
| Gender | Female | Female | Male | Female |
| Age at onset | 13 | 14 | 13 | 16 |
| Age at examination | 35 | 31 | 26 | 28 |
| Rigidity | +++ | +++ | + | + |
| Akinesia | +++ | ++ | +++ | ++ |
| Rest tremor | ++ | – | – | N/A |
| Dysarthria/dysphasia | ++ | + | ++ | + |
| Uncoordinated movements | + | – | + | + |
| Behavioural instability | ++ | – | + | – |
| Intellectual impairment | ++ | + | + | + |
| Response to levodopa | +++ | +++ | ++ | + |
Adapted from (8).
RESULTS
In this work, we identified a unique homozygous mutation in ATP13A2 in a family with apparent autosomal-recessive juvenile onset NCL, using exome sequencing. We have, in addition, tested other available NCL families with onset in the juvenile or adult age range in which the genetic basis is unknown for mutations in ATP13A2 (n = 14), but have not found further mutations. Nonetheless, the exome data, together with previous reports in the dog model and in silico analysis of the mutation, clearly show causality of this variant in autosomal–recessive NCL.
From the cleaned set of results, generated from the exome sequencing of three individuals, we excluded all the variants that were not present in both affected siblings, and all the variants shared by the unaffected individual. From this list, we excluded all the synonymous variants, which led us to a final number of 25 variants (Supplementary Material, Table S1). Of these, only one was shared in the homozygous state in the affected individuals, c.T2429G in exon 22 of ATP13A2. This predicts the missense change p.Met810Arg. We also considered the hypothesis of compound heterozygous mutations being the underlying cause of disease. However, we did not identify compound heterozygous mutations that segregated with the disease in these three individuals.
Having all the living members of this kindred available at time of collection meant that we were able to verify segregation of the mutation in the family. We performed Sanger sequencing for exon 22 (RefSeq ID: NM_001141974) in all available samples and results showed complete segregation in the family (Fig. 1).
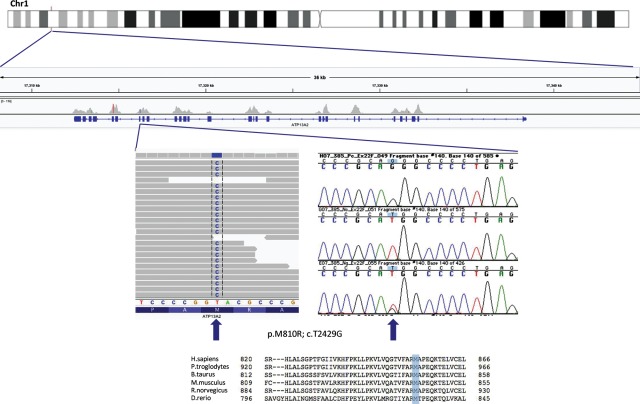
No mutations have been reported in residue 810 in other individuals with KRS or the dog model of NCL thus far; additionally, this is a highly conserved residue in ATP13A2 (Fig. 2), mutation of this residue is absent from the most recent single nucleotide polymorphism database (dbSNP) (Build 135, which includes the latest 1000 Genomes data as well as other large-scale population exome sequencing studies, comprising a number in excess of 3000 individuals with European ancestry and 1000 individuals with African ancestry) and both SIFT and PolyPhen2 attribute this variant with a very high probability of being deleterious (SIFT score is 0 while PolyPhen score is 1.000).
Figure 2.
Identification of a homozygous mutation in ATP13A2. Figure showing the region on chromosome 1 where ATP13A2 is located, with the coverage and variant calling obtained from exome sequencing at the p.M810R locus for one affected sibling. Also shown are Sanger sequencing traces for one affected and two unaffected samples from the family and the protein sequence comparison displaying high conservation between species. The top panel on the chromatogram shows the affected individual (homozygous G at the position), while the two bottom panels show unaffected individuals (one homozygous wild-type and one heterozygous).
DISCUSSION
The vast majority of mutations in ATP13A2 associated with KRS are truncating mutations arising by deletion, duplication, insertion or splice site mutations (11), although there are two reports of missense variants causing KRS (9,12). It is not yet clear if there is a relationship between mutation type and disease severity. The mechanism through which mutations in ATP13A2 cause KRS is not fully understood, although it has been previously shown that the wild-type protein is located in the perimeter lysosomal membrane of transiently transfected cells, while the unstable truncated mutants are retained in the endoplasmic reticulum and degraded by the proteasome (4). We have previously proposed a lysosomal pathway for parkinsonism syndromes, centred around ceramide metabolism, where we have hypothesized that ATP13A2 mutations could be involved (13). Briefly, following the discovery that mutations in GBA, a gene known to cause the lysosomal storage disease Gaucher's disease, are also a risk factor for Parkinson's disease (PD), we hypothesized that ceramide metabolism could play an important role in the pathogenesis of PD. The link we proposed between ATP13A2 mutations and ceramide metabolism, although not a direct one, was based on the fact that ATP13A2 is a lysosomal transport protein thought to be responsible for the maintenance of lysosomal pH, where ceramide is metabolized. The hypothesis was based on increased intracellular levels of ceramide, derived from reduced activity of several proteins in the lysosomal pathway. Among them were GBA, ATP13A2, PANK2 and PLA2G6.
The implications of the present results are 2-fold: first they indicate that KRS and NCL, although separate clinical entities, share etiology components; secondly, we provide further support that a lysosomal pathway underlies the pathobiology of another parkinsonism syndrome, in addition to the previously confirmed GBA (14), PANK2 (15) and PLA2G6 (16)-linked diseases. Following the identification of risk conferring variability at GBA, which was conclusively demonstrated to be the strongest risk factor known for PD, identifying mutations in ATP13A2 in confirmed NCL cases undoubtedly corroborate the presence of parallels between these two types of disorders. It is also interesting to note that parkinsonism has previously been reported in NCL disease families (17–21). Parkinson-like movement disorder is always part of juvenile CLN3 disease (1). In fact, the frequency of parkinsonian signs in NCL disease and the partial phenotypic overlap between Kufor–Rakeb and NCL led Farias and colleagues to predict that Kufor–Rakeb is an NCL (3). Currently, there is no definitive pathological evidence of this as no Kufor–Rakeb brain tissue is available for the neuropathology study. However, biopsy analysis of a sural nerve from one KRS patient revealed cytoplasmic inclusions in Schwann cells and smooth muscle cells (22), which may be interpreted as reminiscent of NCL inclusions. Furthermore, the brains of NCL patients caused by Cathepsin D deficiency (CLN10 disease) show intense α-synuclein staining (23). Given the parallels between NCL and Parkinsonian disorders, it was surprising that the Tibetan terriers with NCL disease caused by ATP13A2 mutation did not present with signs of parkinsonism (3). From these data, it is tempting to propose that at least some cases of NCL disease and KRS are two different clinical entities belonging to the same ethiological disease spectrum, where ATP13A2 specifically and lysosomal pathways, in general, play pivotal roles in the neurodegenerative processes.
We propose that, in accordance with a new NCL classification scheme (1), the gene ATP13A2 should be linked to the symbol CLN12.
In summary, we present conclusive evidence that mutations in the parkinsonism gene ATP13A2 underlie a form of juvenile NCL, suggesting not only that these two diseases share pathobiological features, but also that KRS is indeed linked to the lysosomal pathway, a feature that is becoming increasingly evident for a variety of parkinsonian phenotypes.
MATERIALS AND METHODS
Exome sequencing was performed on three individuals from this family: two affected (II-5, II-9) and an unaffected sibling (II-4). The approach used assumed a recessive mode of inheritance where shared variants could be determined in the affected cases and subsequently filtered out in the unaffected, while maintaining the number of sequencing runs to a minimum. Genomic DNA was prepared according to Illumina's TruSeq Sample Preparation v3 (Illumina, CA, USA) and capture was performed with Illumina's TruSeq Exome Enrichment according to the manufacturer's instructions. Sequencing was performed in Illumina's HiSeq2000 using 100 bp paired-end reads.
Following quality-control procedures, samples yielded between 7.1 and 8.5 Gb of high quality, aligned data. This amount of data represented mean target coverage between 55.9–65.6×, percentages of targets covered at greater than or equal to 10× of 88.9–90.6% and less than 1% of targets not being covered at least once. Sequence alignment and variant calling were performed against the reference human genome (UCSC hg19) using bwa (24) and the Genome Analysis Toolkit (25). PCR duplicates were removed prior to variant calling using the Picard software (http://picard.sourceforge.net/index.shtml). Based on the hypothesis that the mutation underlying this rare familial disease was not present in the general population, we excluded all SNPs identified in the 1000 Genomes project (www.1000genomes.org/) or in dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/, Build 132). We thank the Rare NCL Gene Consortium for encouraging collaborative research on variant NCL cases, and the families and physicians for providing samples.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
FUNDING
This work was supported in part by the Wellcome Trust/MRC Joint Call in Neurodegeneration award (WT089698), the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 281234, the Medical Research Council (core support at LMCB) and the Batten Disease Support and Research Association, USA. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Supplementary Material
REFERENCES
- 1.Mole S.E., Williams R.E., Goebel H.H. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford: Oxford University Press; 2011. [Google Scholar]
- 2.Wohlke A., Philipp U., Bock P., Beineke A., Lichtner P., Meitinger T., Distl O. A one base pair deletion in the canine ATP13A2 gene causes exon skipping and late-onset neuronal ceroid lipofuscinosis in the Tibetan terrier. PLoS Genet. 2011;7:e1002304. doi: 10.1371/journal.pgen.1002304. doi:10.1371/journal.pgen.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farias F.H., Zeng R., Johnson G.S., Wininger F.A., Taylor J.F., Schnabel R.D., McKay S.D., Sanders D.N., Lohi H., Seppala E.H., et al. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol. Dis. 2011;42:468–474. doi: 10.1016/j.nbd.2011.02.009. doi:10.1016/j.nbd.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez A., Heimbach A., Grundemann J., Stiller B., Hampshire D., Cid L.P., Goebel I., Mubaidin A.F., Wriekat A.L., Roeper J., et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. doi:10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 5.Anderson G., Smith V.V., Malone M., Sebire N.J. Blood film examination for vacuolated lymphocytes in the diagnosis of metabolic disorders; retrospective experience of more than 2,500 cases from a single centre. J. Clin. Pathol. 2005;58:1305–1310. doi: 10.1136/jcp.2005.027045. doi:10.1136/jcp.2005.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlier G., Dubru J.M. Familial juvenile Parkinsonism. Acta Paediatr. Belg. 1979;32:123–127. [PubMed] [Google Scholar]
- 7.Tome F.M., Brunet P., Fardeau M., Hentati F., Reix J. Familial disorder of the central and peripheral nervous systems with particular cytoplasmic lamellated inclusions in peripheral nerves, muscle satellite cells, and blood capillaries. Acta Neuropathol. 1985;68:209–217. doi: 10.1007/BF00690197. doi:10.1007/BF00690197. [DOI] [PubMed] [Google Scholar]
- 8.De Volder A.G., Cirelli S., de Barsy T., Brucher J.M., Bol A., Michel C., Goffinet A.M. Neuronal ceroid-lipofuscinosis: preferential metabolic alterations in thalamus and posterior association cortex demonstrated by PET. J. Neurol. Neurosurg. Psychiatry. 1990;53:1063–1067. doi: 10.1136/jnnp.53.12.1063. doi:10.1136/jnnp.53.12.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Fonzo A., Chien H.F., Socal M., Giraudo S., Tassorelli C., Iliceto G., Fabbrini G., Marconi R., Fincati E., Abbruzzese G., et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. doi:10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- 10.Crosiers D., Ceulemans B., Meeus B., Nuytemans K., Pals P., Van Broeckhoven C., Cras P., Theuns J. Juvenile dystonia-parkinsonism and dementia caused by a novel ATP13A2 frameshift mutation. Parkinsonism Relat. Disord. 2011;17:135–138. doi: 10.1016/j.parkreldis.2010.10.011. doi:10.1016/j.parkreldis.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Vilarino-Guell C., Soto A.I., Lincoln S.J., Ben Yahmed S., Kefi M., Heckman M.G., Hulihan M.M., Chai H., Diehl N.N., Amouri R., et al. ATP13A2 variability in Parkinson disease. Hum. Mutat. 2009;30:406–410. doi: 10.1002/humu.20877. doi:10.1002/humu.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning Y.P., Kanai K., Tomiyama H., Li Y., Funayama M., Yoshino H., Sato S., Asahina M., Kuwabara S., Takeda A., et al. PARK9-linked parkinsonism in eastern Asia: mutation detection in ATP13A2 and clinical phenotype. Neurology. 2008;70:1491–1493. doi: 10.1212/01.wnl.0000310427.72236.68. doi:10.1212/01.wnl.0000310427.72236.68. [DOI] [PubMed] [Google Scholar]
- 13.Bras J., Singleton A., Cookson M.R., Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. doi:10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R., Bar-Shira A., Berg D., Bras J., Brice A., et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. doi:10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. doi:10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 16.Lei X., Zhang S., Bohrer A., Bao S., Song H., Ramanadham S. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–10185. doi: 10.1021/bi700017z. doi:10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burneo J.G., Arnold T., Palmer C.A., Kuzniecky R.I., Oh S.J., Faught E. Adult-onset neuronal ceroid lipofuscinosis (Kufs disease) with autosomal dominant inheritance in Alabama. Epilepsia. 2003;44:841–846. doi: 10.1046/j.1528-1157.2003.39802.x. doi:10.1046/j.1528-1157.2003.39802.x. [DOI] [PubMed] [Google Scholar]
- 18.Berkovic S.F., Carpenter S., Andermann F., Andermann E., Wolfe L.S. Kufs’ disease: a critical reappraisal. Brain. 1988;111:27–62. doi: 10.1093/brain/111.1.27. doi:10.1093/brain/111.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Nijssen P.C., Brusse E., Leyten A.C., Martin J.J., Teepen J.L., Roos R.A. Autosomal dominant adult neuronal ceroid lipofuscinosis: parkinsonism due to both striatal and nigral dysfunction. Mov. Disord. 2002;17:482–487. doi: 10.1002/mds.10104. doi:10.1002/mds.10104. [DOI] [PubMed] [Google Scholar]
- 20.Taschner P.E., de Vos N., Thompson A.D., Callen D.F., Doggett N., Mole S.E., Dooley T.P., Barth P.G., Breuning M.H. Chromosome 16 microdeletion in a patient with juvenile neuronal ceroid lipofuscinosis (Batten disease) Am. J. Hum. Genet. 1995;56:663–668. [PMC free article] [PubMed] [Google Scholar]
- 21.Lavrov A.Y., Ilyna E.S., Zakharova E.Y., Boukina A.M., Tishkanina S.V. The first three Russian cases of classical, late-infantile, neuronal ceroid lipofuscinosis. Eur. J. Paediatr. Neurol. 2002;6:161–164. doi: 10.1053/ejpn.2002.0584. doi:10.1053/ejpn.2002.0584. [DOI] [PubMed] [Google Scholar]
- 22.Paisan-Ruiz C., Guevara R., Federoff M., Hanagasi H., Sina F., Elahi E., Schneider S.A., Schwingenschuh P., Bajaj N., Emre M., et al. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov. Disord. 2010;25:1791–1800. doi: 10.1002/mds.23221. doi:10.1002/mds.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen V., Lindfors M., Ng J., Paetau A., Swinton E., Kolodziej P., Boston H., Saftig P., Woulfe J., Feany M.B., et al. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol. Brain. 2009;2:5. doi: 10.1186/1756-6606-2-5. doi:10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. doi:10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. doi:10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.