Abstract
Background
Induction of a humoral response against amyloid-β peptide may be beneficial for Alzheimer's disease (AD) patients and may alleviate the onset and progression of AD. DNA-based vaccination provides a unique alternative method of immunization for treatment and prevention of AD. Currently, the two major delivery methods used for enhancing DNA uptake and immune responses to DNA vaccines in humans are electroporation (EP) and gene gun (GG). Objective: The goal of this translational study was to evaluate the efficacy of an AD DNA epitope vaccine (DepVac) delivered intramuscularly by EP or intradermally by GG.
Methods
Humoral and cellular immune responses to immunization with DepVac were evaluated by ELISA and ELISPOT, respectively. Functional activity of the antibodies was also assessed.
Results
EP- and GG-mediated immunizations with DepVac induced similar anti-amyloid-β (Aβ) antibody and T cell responses. Anti-Aβ antibodies bound to amyloid plaques in AD brain tissue and to toxic forms of Aβ42 peptide.
Conclusion
Both delivery methods are effective at promoting potent antibodies specific for Aβ.
Key Words: Alzheimer's disease, DNA epitope vaccine, Electroporation, Gene gun, Immune responses
Introduction
Data from preclinical studies and clinical trials indicate that high titers of anti-amyloid-β (Aβ) antibodies may prevent the accumulation of toxic forms of Aβ peptide and can be beneficial to Alzheimer's disease (AD) patients [1,2,3,4,5]. However, in one clinical trial, immunization with an adjuvant Aβ peptide vaccine resulted in meningoencephalitis in 6% of subjects, likely due to the generation of autoreactive T cells [5,6,7]. Previously, we suggested that an epitope vaccine composed of a self-B cell epitope of Aβ peptide and a foreign T helper cell epitope would be safe in humans since it should reduce the risk of an adverse T-cell-mediated autoimmune response while inducing a strong antibody response to Aβ. Using this strategy, we have developed different epitope vaccines based on peptide, recombinant protein or DNA [8,9,10]. Our DNA-based epitope vaccine (DepVac), composed of three copies of a short Aβ B cell epitope fused with the foreign universal Th cell epitope, PADRE, and macrophage-derived chemokine, was immunogenic and safe in mice [8]. However, although DNA vaccines have been used to induce immune responses in small animal models for more than a decade, low immunogenicity in large animals and humans has limited their utility.
Accordingly, successful translation of a DNA vaccine to the clinical setting requires a suitable method for effective intracellular delivery. Electroporation (EP)- and gene gun (GG)-mediated administration are the most effective physical methods for delivering DNA plasmids in vivo and both are currently being tested in clinical trials [11,12]. GG is a needle-free ballistic device which delivers gold beads coated with plasmid DNA into the epidermal layer of skin and drives strong immune response to DNA vaccines in animals [8] and humans [12]. EP is a technique for intracellular delivery based on the brief application of electrical fields in the target region of tissue. EP delivery of DNA vaccines enhances immune responses by several orders of magnitude compared to conventional injection in animal models [13] and humans [11]. A previous study demonstrated superior T cell responses and tumor control with EP delivery compared to GG [14]. The goal of the current study was to evaluate the efficacy of antibody responses to DepVac delivered intramuscularly by EP or intradermally by GG. The results indicate that DepVac delivered by both methods induced potent titers of anti-Aβ antibodies, as well as strong cellular responses against the foreign Th epitope, PADRE, but not self-Aβ40.
Animals and Methods
Mice, DNA Immunizations, Detection of Humoral and Cellular Immune Responses and Functional Activity of Antibodies
C57BL/6 mice (5–6 weeks old; Jackson Laboratory, Calif., USA) were immunized on days 0, 14 and 28 with 10 μg/mouse DepVac [8] or noncoding control vector in a single anterior tibial muscle using the TDS-IM EP system (Ichor Medical Systems, Calif., USA) as described in Luxembourg et al. [15] or by intradermal delivery with three shots into the skin of the abdomen with GG bombardment (Bio-Rad, Calif., USA) [8,10]. Concentrations of anti-Aβ antibodies and their isotype profiles were measured in sera collected 12 days after each administration as described previously [8,10]. To detect binding of immune sera to different forms of Aβ42 peptide, a dot blot assay was run as described in Petrushina et al. [9]. To detect binding of immune sera to native amyloid (plaques), immunohistochemistry was performed on brain sections from a control or AD patient obtained from the University of California, Irvine, Calif., USA [8,9,10]. T cell proliferation was analyzed in splenocyte cultures restimulated in vitro with 10 μg/ml of PADRE peptide, Aβ40 peptide, or with an irrelevant peptide using a [3H]-thymidine incorporation assay; stimulation index was calculated as described earlier [10]. ELISPOT assay was used to determine the number of antigen-specific cells producing cytokines (IFN-γ and IL-4) as described in Petrushina et al. [9].
Statistical Analysis
Prism 3.03 software (GraphPad Software, Calif., USA) was applied in the statistical analysis of the data. Statistically significant differences were examined using a t test and p < 0.05 was considered significant.
Results and Discussion
DepVac Induces Strong and Therapeutically Potent Anti-Aβ Antibodies in Mice Immunized with TDS-IM and GG
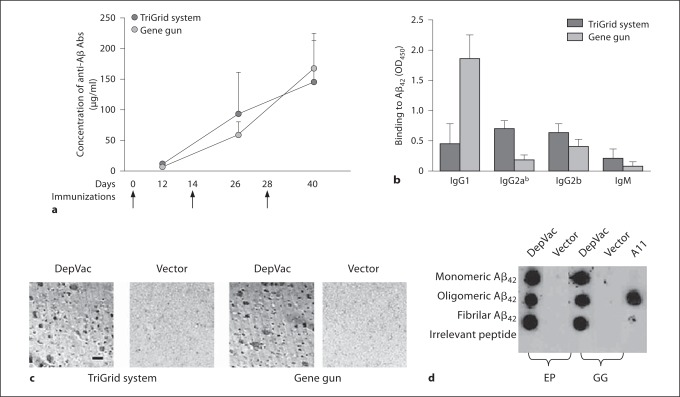
In order to select a suitable device for delivering DepVac to humans, we evaluated the efficacy of DepVac after EP-mediated intramuscular delivery and compared it to that with intradermal delivery by GG. Experimental and control animals received three immunizations and the kinetics of anti-Aβ antibody responses were analyzed in individual sera collected from mice after each injection (fig. 1a). The results demonstrated that both methods are effective in inducing strong humoral immune responses. The level of anti-Aβ antibodies after EP versus GG delivery was equivalent; after the third immunization, concentrations of antibodies were 145.1 ± 69.2 and 167.9 ± 57.3 μg/ml, respectively. Of note, intradermal and intramuscular immunizations of mice with DepVac without using any delivery device generated low titers of anti-Aβ antibodies: 0.84 ± 1.4 and 21.3 ± 17.5 μg/ml, respectively. No anti-Aβ response was seen in mice immunized with noncoding vector. Interestingly, EP-mediated vaccination induced predominantly IgG2ab isotypes of antibodies (IgG1/IgG2ab ratio was 0.61), whereas the antibodies produced after GG vaccination were mostly of the IgG1 isotype (IgG1/IgG2ab ratio was 11.87) (fig. 1b). These data were in accordance with other reports [8,9,16,17], which indicates that these techniques may differ in activation of Th1 and Th2 pathways. One possible explanation could be connected with differential delivery of DNA. In case of muscle immunization, CpG motifs on plasmid backbone may interact with receptors in APC located in a target tissue and induce proinflammatory cytokines and Th1 type of immune response [18], while GG inoculation delivers DNA directly into the nucleus bypassing CpG receptors. Other explanations for these differences are also possible.
Fig. 1.
DepVac delivered by EP or GG induces equivalent anti-Aβ responses (n = 8 per group). Anti-Aβ antibody concentrations were calculated by ELISA using a calibration curve generated with anti-Aβ 6E10 monoclonal antibody (Signet, Dedham, Mass., USA) (a). DepVac delivered by EP induces antibody predominantly of IgG2ab isotype, whereas GG delivery induces predominantly IgG1 isotype (sera were used at dilution 1:500) (b). Immune, but not control sera bind to amyloid plaques in the brain section of the cortical region from an AD case (magnification × 10, scale bar 100 μm) (c), and to all species of Aβ42 peptide in dot blot (positive control A11 antibody binds to oligomers only) (d).
To demonstrate the functional activity of the anti-Aβ antibodies, we analyzed binding of antisera to Aβ plaques in brain tissues from an AD case. The immune sera from the EP and GG delivery groups bound to β-amyloid plaques and this binding was specific since it was blocked by preabsorption of antisera with Aβ42 peptide (data not shown) and was not observed with control sera (fig. 1c). Currently, it is thought that oligomers are the most toxic forms of Aβ responsible for disrupting neuronal functions and inducing cognitive decline in AD [1]. Therefore, we analyzed binding of immune and control sera to various forms of amyloid and demonstrated that only immune sera bound to monomeric, oligomeric and fibrillar Aβ42 peptide (fig. 1d).
EP- and GG-Mediated Immunizations with DepVac Induce Strong Cellular Immune Responses
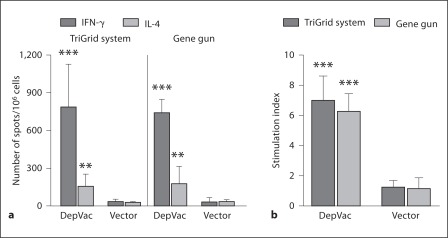
To determine if enhancement of antibody responses after DepVac immunization by TDS-IM and GG is tied to higher T helper cell activation, we analyzed T cell responses in vaccinated mice. ELISPOT assay demonstrated that EP and GG delivery methods induced activation of similar numbers of IFN-γ and IL-4 producing cells after restimulation of immune splenocytes with PADRE peptide, which were significantly higher compared with control groups (p ≤ 0.001 and p ≤ 0.01, respectively) (fig. 2a). We also evaluated antigen-specific T cell activation by measuring T cell proliferation in splenocyte cultures restimulated with PADRE or irrelevant peptide. Data generated in this proliferation assay (fig. 2b) were in accordance with ELISPOT data presented in figure 2a. Of note, as we expected from our previous studies [8,9], anti-Aβ T cell responses were not detected in immune splenocyte cultures (data not shown).
Fig. 2.
DepVac delivered by TDS-IM or GG induces similar levels of cellular responses measured by ELISPOT assay (a), and [3H]-thymidine incorporation assay (b). Activation of splenocytes with the Th epitope PADRE was significantly higher in DepVac-immunized mice compared with vector-immunized groups (** p ≤ 0.01; *** p ≤ 0.001, n = 8 per group).
In conclusion, this study demonstrated that EP and GG delivery of DepVac were equally effective in generating anti-Aβ humoral and anti-PADRE cellular immune responses, although the antibody isotype responses were significantly different. With GG delivery, a much smaller dose range can be evaluated than with intramuscular EP since only a limited amount of DNA can be loaded on the gold beads; thus, multisite administrations may be required in humans to achieve target responses. Therefore, based on the data presented here plus the larger potential dosing range provided by intramuscular EP delivery, we initiated preclinical testing of DepVac in rabbits and nonhuman primates using EP. If safe and effective in these species, the vaccine will be evaluated in a human clinical trial.
Acknowledgements
This work was supported by funding from NIH (AG-20241, NS-50895, NS-065518 and NS-057395) and Alzheimer's Association (IIRG-0728314). H.D. and N.M. were supported by NIA training grant AG000096. AD case tissues were provided by UCI ADRC (grant P50-AG16573).
References
- 1.Agadjanyan M, Cribbs D. Active and passive Abeta-immunotherapy: preclinical and clinical studies and future directions. Part 1. CNS Neurol Disord Drug Targets. 2009;8:1–6. doi: 10.2174/187152709787601849. [DOI] [PubMed] [Google Scholar]
- 2.Town T. Inflammation, immunity, and Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:129–131. doi: 10.2174/187152710791012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcock DM. The usefulness and challenges of transgenic mouse models in the study of Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:386–394. doi: 10.2174/187152710791556168. [DOI] [PubMed] [Google Scholar]
- 4.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 6.Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer's disease. Neuromolecular Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- 7.Boche D, Nicoll JA. The role of the immune system in clearance of Abeta from the brain. Brain Pathol. 2008;18:267–278. doi: 10.1111/j.1750-3639.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, et al. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine – A novel immunotherapeutic strategy. PLos ONE. 2008;3:e21–e24. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, et al. Alzheimer's disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Aβ species in amyloid precursor protein transgenic mice. J Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, et al. DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther. 2010;17:261–271. doi: 10.1038/gt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Drunen Littel-van den Hurk S. Hannaman D. Electroporation for DNA immunization: clinical application. Expert Rev Vaccines. 2010;9:503–517. doi: 10.1586/erv.10.42. [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Evans K, McElwaine-Johnn H, Sharpe M, Oxford J, et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine. 2009;27:2506–2512. doi: 10.1016/j.vaccine.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 13.Livingston BD, Little SF, Luxembourg A, Ellefsen B, Hannaman D. Comparative performance of a licensed anthrax vaccine versus electroporation based delivery of a PA encoding DNA vaccine in rhesus macaques. Vaccine. 2010;28:1056–1061. doi: 10.1016/j.vaccine.2009.10.111. [DOI] [PubMed] [Google Scholar]
- 14.Best SR, Peng S, Juang CM, Hung CF, Hannaman D, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27:5450–5459. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luxembourg A, Hannaman D, Nolan E, Ellefsen B, Nakamura G, et al. Potentiation of an anthrax DNA vaccine with electroporation. Vaccine. 2008;26:5216–5222. doi: 10.1016/j.vaccine.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 16.Town T, Tan J, Sansone N, Obregon D, Klein T, et al. Characterization of murine immunoglobulin G antibodies against human amyloid-β1–42. Neurosci Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- 17.Town T, Vendrame M, Patel A, Poetter D, DelleDonne A, et al. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer's beta-amyloid(1–42) J Neuroimmunol. 2002;132:49–59. doi: 10.1016/s0165-5728(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 18.Moreno S, Timon M. DNA vaccination: an immunological perspective. Immunologia. 2004;123:41–55. [Google Scholar]