Abstract
Background
The vascular deposition of amyloid known as cerebral amyloid angiopathy (CAA) – an age-associated condition and a common finding in Alzheimer's disease – compromises cerebral blood flow, causing macro/microhemorrhages and/or cognitive impairment. Very little is known about the mechanisms causing CAA-related degeneration of cerebral vascular cells. The Dutch E22Q familial amyloid-β (Aβ) variant is primarily associated with CAA, and manifests clinically with severe cerebral hemorrhages. Objective: We aimed to determine the molecular mechanisms causing apoptosis of cerebral endothelial cells in the presence of wild-type Aβ40 or its vasculotropic E22Q variant.
Methods
We challenged human brain microvascular endothelial cells with both Aβ variants, and studied the apoptotic pathways triggered by these peptides.
Results
Caspase-mediated apoptotic pathways were elicited by both peptides within time frames correlating with their aggregation properties and formation of oligomeric/protofibrillar assemblies. Our data revealed a primary activation of caspase-8 (typically triggered by death receptors) with secondary engagement of caspase-9, with cytochrome C and apoptosis-inducing factor release from the mitochondria, suggesting the independent or synergistic engagement of extrinsic and intrinsic apoptotic mechanisms. Conclusion: Our data demonstrate the induction of caspase-8- and caspase-9-dependent mitochondrial-mediated apoptotic pathways by Aβ oligomers/protofibrils in vascular cells, likely implicating a primary activation of death receptors.
Key Words: Cerebral amyloid angiopathy, Alzheimer's disease, Endothelial cells, Apoptosis, Aggregation, Amyloid, Caspases, Mitochondria
Introduction
The deposition of amyloid in and around cerebral vessels is an often underestimated lesion that causes vascular damage, micro/macrohemorrhages and dysfunction of the entire neurovascular unit [1,2]. The main component of vascular deposits found in Alzheimer's disease (AD) brains is the 40-amino-acid-long amyloid-β (Aβ40) peptide. Mutations at positions 21–23 or 34 generate Aβ variants that associate with cerebral amyloid angiopathy (CAA), hemorrhagic stroke and dementia in early-onset familial forms of AD. The aggressive E22Q-Aβ40 mutant causes cerebral hemorrhage in a disorder known as hereditary cerebral hemorrhage with amyloidosis Dutch type [3], characterized by recurrent strokes and vascular dementia in the absence of neurofibrillar pathology, with fatal cerebral bleeding resulting from the massive amyloid deposition in leptomeningeal and cortical vessels. Studies on the molecular pathogenesis of CAA and AD are currently centered on the structural changes affecting the respective amyloid subunits, with the transition to β-sheet-rich aggregated species being considered today as a key player in the disease process [4]. Insoluble amyloid species accumulate at the vascular walls, causing degeneration of the smooth muscle cell and endothelial cell (EC) layers while the presence of CAA and concomitant microhemorrhages in small capillary vessels lacking the smooth muscle cell layer emphasizes the relevance of studying the mechanism of Aβ-dependent cell death in brain ECs. Apoptosis has been reported as a major contributor to the cell death mechanisms associated with AD, with increasing data from ours and other groups demonstrating the importance of intermediate Aβ aggregates, in particular oligomers and protofibrils, for the mechanism of cytotoxicity [5,6]. However, the identification of the events triggering this apoptotic cascade is still a hotly debated issue. In the studies described herein, we establish a direct relationship between the aggregation state of the peptides deposited in sporadic and familial CAA cases, and their effect on EC death, with particular emphasis on the molecular events triggering the apoptotic machinery.
Methods
Peptides
Synthetic homologues of wild-type (WT) Aβ40 and the E22Q variant were synthesized using N-tert-butyloxycarbonyl chemistry by James I. Elliott at Yale University and purified by reverse-phase high-performance liquid chromatography on a Vydac C4 column (Western Analytical, Murrieta, Calif., USA) [7]. Peptides were dissolved to 1 mM in hexafluoroisopropanol (Sigma). After a 1-hour incubation and lyophilization to remove hexafluoroisopropanol, peptides were thoroughly dissolved to 5 mM in dimethyl sulfoxide, followed by the addition of deionized water and 2× PBS, pH 7.4, to a final concentration of 1 mg/ml in PBS. Peptides were either incubated at 37°C for the aggregation studies or diluted into culture media at the required concentrations for the cell culture experiments.
Electron Microscopy
Peptides were placed onto carbon-coated 400-mesh Cu/Rh grids (Ted Pella, Inc.) and stained with 1% uranyl acetate in distilled water (Polysciences, Inc.). Stained grids were examined in a Philips CM-12 transmission electron microscope and photographed with a Gatan (4k × 4k) digital camera at NYU School of Medicine Image Core Facility.
Cell Cultures
Immortalized human brain microvascular endothelial cells hCMEC/D3 [8] were cultured in complete EBM-2 medium (Lonza) containing growth supplements and 2.5% fetal bovine serum. Peptide treatments were performed in EBM-2 with 1% fetal bovine serum.
Cell Death ELISA
The extent of apoptosis caused by the different Aβ peptides was assessed by quantitation of nucleosome formation with the Cell Death ELISAplus kit (Roche Applied Science).
Immunocytochemistry
ECs were plated on glass chamber slides (Thermo Fisher Scientific), precoated with type I collagen. After 1 day in culture, cells were treated with the peptides, and subsequently fixed with 4% paraformaldehyde. Slides were separately incubated with monoclonal anti-cytochrome C (CytC) antibody (BD Biosciences), and polyclonal anti-apoptosis-inducing factor (AIF) antibody (Santa Cruz) followed, respectively, by Alexa Fluor 488-conjugated anti-mouse or anti-rabbit IgG (Invitrogen). Fluorescence was visualized with a Nikon Eclipse E 800 Deconvolution Microscope using Image-Pro Plus software (Media Cybernetics Inc., Bethesda, Md., USA) for image acquisition and processing and Autodeblur (Media Cybernetics) for 3-dimensional deconvolution.
Caspase-3/7, -8, and -9 Activity Assays
Caspase-3/7 activity was measured in cells treated with the Aβ variants by Apo-ONE fluorescence assay (Promega) while caspase-8 and -9 activation was evaluated by luminescent assays (Caspase-Glo 8 and Caspase-Glo 9, Promega). Fluorescence/luminescence were evaluated in a Synergy HT Multi-Mode Microplate Reader (Biotek).
Results
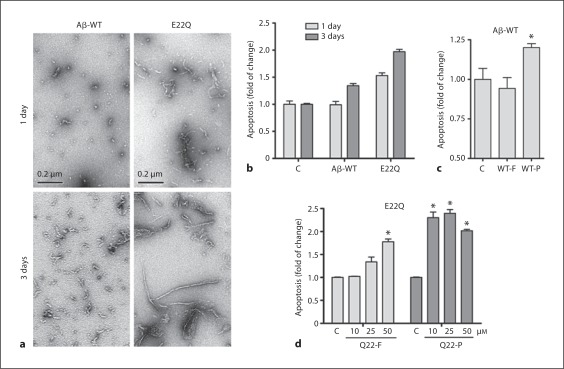
The aggregation status of Aβ-WT and E22Q peptides was monitored by transmission electron microscopy (fig. 1a). After 1 day of incubation of Aβ-WT at physiological salt concentrations, a few small globular species representing oligomeric conformations were observed, while E22Q presented abundant protofibrillar structures (up to 200 nM in length) in combination with numerous oligomeric assemblies. After 3-day aggregation, the conformations present in the Aβ-WT preparation were similar to the ones exhibited by E22Q at 1 day, whereas this variant displayed long fibrillar components together with the protofibrillar elements. To determine the proapoptotic effect of these peptides on ECs, we analyzed nucleosome formation by cell death ELISA (Roche). Apoptosis reached a 1.5-fold increase in cells treated for 1 day with E22Q peptide, while at the same time point Aβ-WT had no effect (fig. 1b). Levels of nucleosome formation after a 3-day treatment with Aβ-WT were similar to those elicited by Aβ-E22Q after 1 day – correlating to the comparable aggregation species present in the two preparations at the respective time points – whereas ECs treated with E22Q peptide for 3 days presented a 2-fold increase in apoptosis compared to untreated controls (fig. 1b). To further establish whether intermediate aggregation species were responsible for the peptide toxicity, we measured nucleosome formation in cells treated for 1 day with Aβ-WT, either freshly solubilized or preaggregated for 2 days to allow the formation of oligomers/protofibrils. The level of apoptosis, while being comparable to the untreated controls in cells challenged with fresh peptide, was significantly elevated in cells treated with preaggregated peptide (fig. 1c). For the aggressive Aβ-E22Q, we evaluated apoptosis induction after a 20-hour challenge with 10, 25 or 50 μM fresh and preaggregated peptide. The 2-day preaggregation rendered the peptide more toxic for ECs, being the amount of nucleosomes in cells treated with each concentration of preaggregated E22Q higher in all cases than after treatment with fresh peptide (fig. 1d). To better understand the mechanism of Aβ-induced apoptosis in ECs, we performed immunocytochemistry experiments to analyze the possible release of the mitochondrial proteins CytC and AIF. Figure 2a shows that in control conditions CytC (top panels) and AIF (bottom panels) presented the punctuate distribution characteristic of mitochondrial localization. CytC and AIF were released into the cytoplasm after a 1- or 2-day challenge with E22Q, respectively, or after a 3-day treatment with both peptides, indicating that the two Aβ variants induced similar apoptotic pathways, albeit within different time frames. The activation of different members of the caspase family was also investigated. Figure 2b illustrates the activation of the effector caspase-3/7 in ECs challenged for 6 h or 3 days with the Aβ peptides, compared to untreated controls at the same time points. E22Q induced caspase-3/7 activation after only a 6-hour treatment, while after 3 days both Aβ-WT and E22Q induced an almost 2-fold increase in caspase-3/7 activity. Activation of the initiator caspases-8 and -9, indicating the participation of extrinsic or intrinsic apoptotic pathways, respectively, was evaluated in ECs treated with fresh AβE22Q (fig. 2c, top panel), or Aβ-WT preaggregated for 3 days (fig. 2c, bottom panel). In both cases, after a 4-hour treatment, caspase-8 activity was higher than that of caspase-9, whereas after a 24-hour peptide incubation the activity of both caspases was upregulated to similar levels. These results suggest the participation of extrinsic, death-receptor-mediated apoptotic pathways, initially involving activation of caspase-8, which, after cleavage of Bid and translocation of its truncated form to the mitochondria, induce the release of CytC and subsequent activation of caspase-9.
Fig. 1.
Correlation between Aβ peptide aggregation and toxicity for endothelial cells. a Electron microscopy studies showing the structural species present after 1 day and 3 days of incubation under physiological conditions in Aβ40-WT and E22Q preparations. b Apoptosis induced by Aβ variants. ECs were treated with 50 μM Aβ-WT or E22Q for 1 or 3 days. c Apoptosis levels in ECs treated for 24 h with 50 μM Aβ-WT, either freshly solubilized (WT-F) or preaggregated for 2 days (WT-P). * p ≤ 0.05. d Apoptosis in ECs treated for 20 h with 10, 25, or 50 μM E22Q, either freshly solubilized (Q22-F) or preaggregated for 2 days (Q22-P). * p ≤ 0.01. Apoptosis in b–d – evaluated as nucleosome formation assessed by cell death ELISA – is represented as fold of change compared to untreated controls.
Fig. 2.
Release of mitochondrial proteins and caspase activation in Aβ-treated endothelial cells. a Immunocytochemistry visualizing CytC and AIF in ECs treated with 50 μM Aβ-WT and E22Q (1- and 3-day treatment for CytC; 2- and 3-day treatment for AIF). Punctuate pattern denotes mitochondrial localization under control conditions, while decrease in mitochondrial staining and cytoplasmic localization is evident after 1-day E22Q challenge and 3-day Aβ-WT treatment. b Caspase-3/7 activation was measured in EC after a 6-hour and 3-day treatment with Aβ-WT and E22Q. c Top panel: caspase-8 (light gray bars) and caspase-9 (dark gray bars) activity was measured in ECs treated for 4 and 24 h with freshly solubilized Aβ-E22Q. Bottom panel: caspase-8 (light gray bars) and caspase-9 (dark gray bars) activity was measured in ECs treated for either 4 or 24 h with Aβ-WT preaggregated for 3 days. Caspase activity is represented as fold of change compared to untreated controls at the same time point.
Conclusions
The Aβ40-E22Q genetic variant exhibits enhanced aggregation kinetics, forming protofibrillar and fibrillar assemblies more rapidly than the WT Aβ40 counterpart. The early stages of apoptosis in cerebral microvascular ECs precede fibril formation, correlating with the presence of intermediate-sized oligomeric and protofibrillar assemblies. Aβ40 variants, albeit requiring different time frames for maximal effect, are strong inducers of the apoptotic mitochondrial pathway, triggering CytC and AIF release from the mitochondria and activation of the effector caspases-3/7. The participation of both caspase-8 and -9, with a primary activation of caspase-8, indicates involvement of extrinsic death-receptor-mediated pathways in Aβ-induced brain vascular cell apoptosis.
Acknowledgements
This work was supported by NIH grants AG30539, NS051715, AG008051 ADC Pilot, the Alzheimer's Association, and the American Heart Association.
References
- 1.Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlokovic BV. The blood brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Levy E, Carman MD, Fernandez Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 4.Walsh DM, Selkoe DJ. A beta oligomers – A decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 5.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid betaprotein oligomers. Proc Natl Acad Sci USA. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fossati S, Cam J, Meyerson J, Mezhericher E, Romero IA, Couraud PO, Weksler BB, Ghiso J, Rostagno A. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-beta variants in cells composing the cerebral vessel walls. FASEB J. 2010;24:229–241. doi: 10.1096/fj.09-139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miravalle L, Tokuda T, Chiarle R, Giaccone G, Bugiani O, Tagliavini F, Frangione B, Ghiso J. Substitutions at codon 22 of Alzheimer's Aβ peptide induce diverse conformational changes and apoptotic effects in human cerebral endothelial cells. J Biol Chem. 2000;275:27110–27116. doi: 10.1074/jbc.M003154200. [DOI] [PubMed] [Google Scholar]
- 8.Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]