Abstract
Background/Aims
There is increased amyloid-β protein precursor (AβPP) expression and amyloid-β protein (Aβ) production in the brain shortly following cerebral ischemic stroke in rodent models. It has been postulated that this may seed amyloid deposition in the brain. On the other hand, it remains unclear how cerebral ischemia affects preexisting Aβ deposits in the brain. Here we determine the consequences of focal ischemic stroke on existing Aβ pathology in Tg-SwDI transgenic mice.
Methods
At 12 months of age, Tg-SwDI mice were subjected to photo-induced focal cerebral ischemia in one hemisphere. One, 7, or 21 days after lesioning, the amount of deposited Aβ in the ischemic and control hemispheres was measured using ELISA. Image analysis was used to visualize deposited Aβ and the presence of microglia/macrophages.
Results
After 7 days, and further after 21 days, there was a dramatic reduction in the amount of deposited Aβ and increased presence of microglia/macrophages in the ischemic hemisphere of the mice.
Conclusions
Focal cerebral ischemia leads to clearance of deposited Aβ in Tg-SwDI mice starting at 7 days with almost complete removal in the ischemic area by 21 days. The delayed clearance of Aβ following focal cerebral ischemia may involve the infiltration of activated neuroinflammatory cells.
Key Words: Amyloid-β protein, Alzheimer's disease, Ischemia, Clearance, Transgenic mice
Introduction
The progressive accumulation of amyloid-β protein (Aβ) in senile plaques and the cerebral vasculature is a prominent feature of Alzheimer's disease (AD) and several related disorders [1]. Aβ is derived from amyloid-β protein precursor (AβPP) through sequential proteolysis by β- and γ-secretase activities [1]. Situations that can increase expression and/or proteolytic processing of AβPP, including ischemic stroke, have been implicated in promoting Aβ deposition in the brain and contributing to the development of AD [2].
In animal models, there are numerous reports demonstrating that AβPP expression is increased after an ischemic challenge [3,4]. Although rats are the most frequently used animal models for ischemic studies, they generally do not develop cerebral Aβ deposits. Rather, human AβPP transgenic mice that develop age-dependent cerebral deposits of human Aβ have been useful models to demonstrate increased expression of AβPP, elevated levels of Aβ peptides, and deposition of Aβ peptides shortly following the introduced lesion [5]. On the other hand, how ischemic stroke influences preexisting cerebral Aβ deposits in older transgenic mice is less clear. Tg-SwDI mice are a unique transgenic model that expresses neuronal human cerebral amyloid angiopathy utant AβPP that produces Dutch (E22Q) and Iowa (D23N) double-mutant Aβ peptides in the brain [6]. Tg-SwDI mice were shown to develop early-onset cerebral microvascular amyloid and abundant diffuse parenchymal Aβ deposition [6,7]. In this study, we investigated how ischemic stroke affects preexisting Aβ deposits using the aged Tg-SwDI mouse.
Experimental Procedures
We used the photo-induced cerebral ischemic stroke model that introduces a permanent focal lesion in one brain hemisphere as described [8]. This procedure was performed in 12-month-old Tg-SwDI mice that present abundant cerebral Aβ deposition [7]. Groups of lesioned Tg-SwDI mice (n = 5–8 animals per group) were allowed to survive for 1, 7, or 21 days after which time they were sacrificed, perfused, and their brains collected. The levels of total Aβ peptides were measured by ELISA of guanidine extracts of the collected tissue samples as described [6,7]. In parallel studies, the harvested brains were fixed in 70% ethanol, followed by xylene treatment, and 10-μm-thick coronal tissue sections were prepared. Deposited Aβ in the brains of the mice was detected by immunolabeling with polyclonal antibody raised to human Aβ1–28 and Alexa Fluor 488-conjugated secondary antibody. Infiltrating microglia/macrophages in the brains were detected by immunolabeling with monoclonal antibody 5D4 to keratin sulfate and Alexa Fluor 594-conjugated secondary antibody. Total Aβ burden in the ischemic and contralateral control regions was quantified on the same set of systematically sampled Aβ-immunolabeled sections using NIH image J 1.32 software [7].
Results
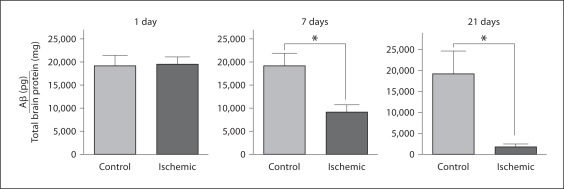
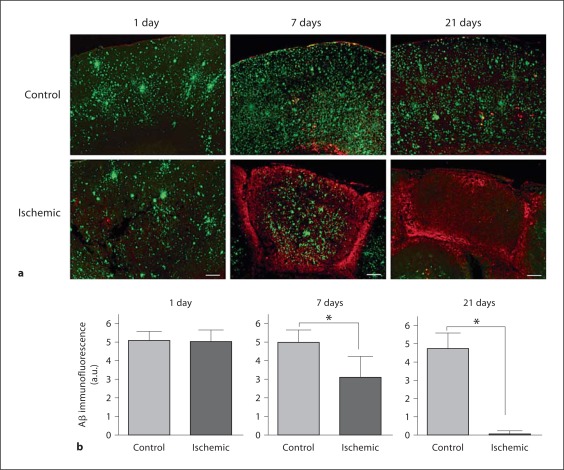
As shown in figure 1, 1 day following the ischemic lesion there was no detectable difference in the amount of Aβ in the ischemic or control hemispheres. However, at 7 days after the ischemic lesion, there was a highly significant (p < 0.01) nearly 50% decrease in the amount of extracted Aβ in the ischemic region. Moreover, 21 days following the ischemic lesion the amount of Aβ was reduced by nearly 90% in the ischemic region compared to the contralateral control hemisphere. These results obtained by quantitative ELISA were confirmed by image analysis of immunolabeled deposited Aβ in ischemic and contralateral control regions of brain tissue sections again showing a dramatic reduction in Aβ levels 7 and 21 days following the ischemic lesion (fig. 2). Concurrent with the loss of deposited Aβ, there was a marked increase in the presence of infiltrated microglia/macrophages in the ischemic tissue (fig. 2a).
Fig. 1.
ELISA measurement of total Aβ levels in Tg-SwDI mice following focal ischemic stroke. Level of total Aβ peptides in ischemic and contralateral control tissues 1, 7, and 21 days following ischemia. Data presented are the mean 8 SD of 5–8 mice per group per time point. * p < 0.01.
Fig. 2.
a Immunolabeling of Aβ deposits and microglia/macrophages in ischemic and contralateral control brain tissues. Scale bars = 200 μm. b Quantitative image analysis of Aβ deposition in Tg-SwDI mice following focal ischemic stroke. Level of Aβ deposition in ischemic and contralateral control tissues at 1, 7, and 21 days following ischemia. Data presented are the mean ± SD of 5–8 mice per group per time point. * p < 0.01.
Discussion
The objective of the present study was to determine the effect of permanent focal ischemic stroke on preexisting Aβ deposits in Tg-SwDI mice, a transgenic model of early-onset and extensive Aβ deposition in the brain. In our studies, we found that shortly following ischemic stroke (1 day) there was no discernible effect on the amount of preexisting Aβ deposits in the ischemic lesion. In other experiments using younger Tg-SwDI mice without preexisting Aβ deposits, 1 day after induction of a focal ischemic stroke we found an elevation in AβPP expression and small increases in the amount of Aβ peptides in the ischemic lesion (data not shown). Although this same small effect likely occurs after 1 day in the 12-month-old Tg-SwDI mice used in the present study, the additional amount of accumulated Aβ would be negligible compared to the extensive amount of preexisting Aβ deposits observed at this age. However, 7 and 21 days following induction of the ischemic lesion, a dramatic reduction in the amount of preexisting Aβ deposits was observed supported by ELISA measurement of Aβ peptide levels extracted from the collected tissues and quantitative image analysis of deposited Aβ in brain tissue sections (fig. 1 and 2, respectively).
Although previous studies have demonstrated an increase in AβPP expression and Aβ production shortly following ischemic stroke in rodent models, our present results indicate that during later stages of the ischemic lesion there is a marked clearance of preexisting Aβ deposits. This suggests that in older animals with deposits any initial increase in local Aβ levels would be overcome by enhanced clearance mechanisms. The robust clearance of preexisting Aβ deposits following an ischemic lesion might result from increased infiltration and activation of neuroinflammatory cells such as microglia/macrophages (fig. 2a). This response may lead to enhanced cellular uptake and/or production of extracellular Aβ-degrading enzymes that can clear the deposited Aβ peptides.
Acknowledgements
This work was supported by National Institutes of Health grant NS52533. Antibody reagents for the Aβ ELISAs were generously provided by Lilly Research Laboratories.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Jendroska K, Poewe W, Daniel SE, Pluess J, Iwessen-Schmidt H, Paulsen J, Barthel S, Schelosky L, Cervos-Navarro J, DeArmond SJ. Ischemic stress induces deposition of amyloid β immunoreactivity in human brain. Acta Neuropathol. 1995;90:461–466. doi: 10.1007/BF00294806. [DOI] [PubMed] [Google Scholar]
- 3.Pierce JE, Trojanowski JQ, Graham DI, Smith DH, McIntosh TK. Immunohistochemical characterization of alterations in the distribution of amyloid precursor proteins and beta-amyloid peptide after experimental brain injury in the rat. J Neurosci. 1996;16:1083–1090. doi: 10.1523/JNEUROSCI.16-03-01083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Groen T, Puurunen K, Maki H-M, Sivenius J, Jolkkonen J. Transformation of diffuse β-amyloid precursor protein and β-amyloid deposits to plaques in the thalamus following transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- 5.Sadowski M, Pankiewicz J, Scholtzovva H, Li YS, Quartermain D, Duff K, Wisniewski T. Links between the pathology of Alzheimer's disease and vascular dementia. Neurochem Res. 2004;29:1257–1266. doi: 10.1023/b:nere.0000023612.66691.e6. [DOI] [PubMed] [Google Scholar]
- 6.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 7.Miao J, Xu F, Davis J, Otte-Holler I, Verbeek MM, Van Nostrand WE. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol. 2005;167:505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J-K, Park M-S, Kim Y-S, Moon K-S, Joo S-P, Kim T-S, Kim S-H. Photochemically induced cerebral ischemia in a mouse model. Surg Neurol. 2007;67:620–625. doi: 10.1016/j.surneu.2006.08.077. [DOI] [PubMed] [Google Scholar]