Abstract
Introduction
Pancreatic leak or fistula is the most frequent complication following left pancreatectomy. We performed a single-blinded, parallel-group, randomized controlled trial comparing stapled left pancreatectomy with stapled left pancreatectomy using mesh reinforcement of the staple line with either Seamguard® or Peristrips Dry®.
Methods
All patients undergoing left pancreatectomy at a large tertiary hospital were eligible for participation. Patients were randomized to either mesh reinforcement or no mesh reinforcement intraoperatively after being determined a candidate for resection. Patients were blinded to the result of their randomization for 6 weeks. Primary outcome measure was clinically significant leak as defined by the ISGPF pancreatic leak grading system.
Results
One hundred patients were randomized to either mesh (54) or no mesh (46) reinforcement of their pancreatic transection. There was one death in each group. ISGPF grade B and C leaks were seen in 1.9% (1/53) of patients undergoing resection with mesh reinforcement and 20% (11/45) of patients without mesh reinforcement (p=0.0007).
Conclusions
Mesh reinforcement of pancreatic transection line significantly reduces the incidence of significant pancreatic fistula in patients undergoing left pancreatectomy.
Trial Registration
Clinicaltrials.gov: NCT01359410
Introduction
Left pancreatectomy (resection of the pancreas left of the superior mesenteric vein) for benign and malignant disease of the pancreas is becoming an increasingly more common procedure performed worldwide, with most being performed for benign or malignant tumors of the pancreas (57%) and < 25% for chronic pancreatitis (1). Pancreatic occlusion failure (POF) or pancreatic leak is the leading cause of morbidity following left pancreatectomy, with a frequency of 5–64% (1–3) in retrospective studies. It is defined as the drainage of greater than 50 ml of amylase-rich fluid (> 3-fold elevation above the upper limit of normal in serum) after postoperative day 3, or pancreatic disruption identified radiographically (4). A variety of reinforcement measures have been applied in an effort to decrease POF, including ligation of the main pancreatic duct (5), biologic glues (6,7) and stapler devices (8,9), none of which have shown superiority.
Further complicating the picture had been the lack of a standard definition of what constituted a clinically significant POF. It wasn’t until 2005, when the International Study Group on Pancreatic Fistula Definition (ISGPF) published the consensus definition of what constitutes a pancreatic leak or fistula and a severity grading scale (10) that was subsequently validated (11), that a standard definition has been applied with widespread use.
With an increase in the use in laparoscopic techniques in safely performing left pancreatectomy (12, 13), both for benign and malignant pathology (14), staple devicesare becoming a more common method of transection. The recently published DISPACT trial demonstrated in a randomized, controlled fashion that stapled transection is as safe as hand-sewn anastomosis of the pancreatic remnant (8). Our group previously examined the role of reinforced stapler devices (15) and found a significant decrease in POF with the use of mesh reinforcement at the pancreatic transection line. This was confirmed by another group (16), though both trials suffered from small patient numbers and a non-randomized study design. The purpose of this study was to determine if mesh reinforcement decreases POF in stapled transection of the left pancreas.
Methods
This was a single institution, randomized controlled trial with a two group, parallel-group superiority design conducted in the United States. Eligible participants were all adults aged 18 or over with diseases of the pancreatic body or tail who were undergoing elective left pancreatectomy. All patients had to have an ECOG functional status of at least 2 and have an expected life expectancy of at least 100 days. Patients known to be pregnant or possess a contraindication to left pancreatic resection were excluded from the study. All patients were provided written informed consent for study participation. The study took place at Barnes-Jewish Hospital, a tertiary referral hospital in Saint Louis, MO, between the dates of 7/1/2007 and 11/30/2010. The study protocol was approved by the internal review board of the Washington University School of Medicine and registered on clinicaltrials.gov (NCT01359410).
Patients were randomized to receive either stapled transection of the pancreas alone or stapled transection with reinforcement using one of two commercially available mesh buttress devices (Seamguard®, W.L. Gore, Flagstaff, AZ, or Peristrips Dry®, Synovis, St. Paul, MN). Randomization was performed using a random number generator and occurred after the intraoperative determination that the patient (a) did not have a contraindication to left pancreatic resection and (b) that the pancreas was not too thick for application of the stapler (Echelon Laparoscopic Stapler with 4.8mm endoscopic linear stapler load, Ethicon, Cornelia, GA). Any patient with intraoperative exclusion had explicit documentation as to why he/she was excluded. The patient and outcome assessor were masked to the technique used. Every patient received one intra-abdominal drain left in the bed of the removed portion of the pancreas. Postoperatively, each patient had drain fluid amylase measured after the initiation of oral feeds and prior to removal of the drain, done at least 3 days postoperatively. POF was defined as drainage of over 50 ml/day of amylase rich fluid (drain fluid amylase above 3 times the normal serum level) or any radiographic evidence of a fluid collection in the pancreatic resection bed.
The primary endpoint was the development of a clinically significant postoperative pancreatic fistula at any time, identified as being a grade B or C fistula or any fistula that altered the patients’ management in any way. Determination of severity of pancreatic fistula was done using both the ISGPF and Zurich-based classification schemes (15) for pancreatic fistula/pancreatic occlusion failure (figure 1). Secondary endpoints included any fistula (including grade A fistulas), length of time a fistula exists (identified as postoperative days with a drain in place), additional procedures and non-pancreatic complications.
Figure 1.
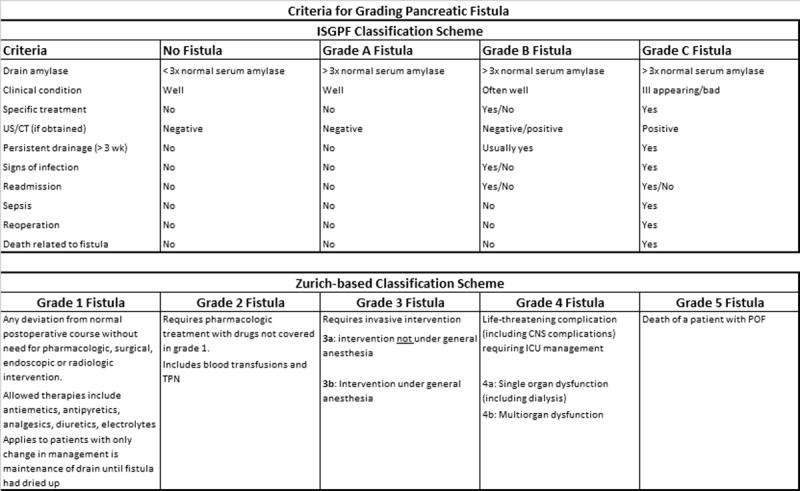
Criteria for Grading Pancreatic Fistula/Pancreatic Occlusion Failure
Statistical analysis
Demographic and clinical characteristics of the sample were summarized using descriptive statistics. The balance of these baseline factors between two arms were assessed using Chi-square test or Student t-test as appropriate. The between-group differences in pancreatic leak rates, as well as additional procedures and non-pancreatic complications, were compared by Fisher’s exact test or Chi-square test as appropriate. The distributions of time to drain removal were summarized by Kaplan-Meier product limit method and compared using log-rank test. A multivariate logistic regression was also fitted to identify demographic and clinical characteristics that could be independently predictive of POF.
The sample size for this study was determined based on the primary endpoint. We assumed clinically significant POF of <5% and 20% in the treatment and standard treatment arms respectively. A total of 140 patients (70 per arm) were required to detect such a difference with 80% power at a two-sided 0.05 significance level. Three pre-specified interim analyses were planed when approximately 25%, 50%, and 75% of the target recruitment was achieved. The Lan-DeMets spending function was used to ensure that such a “peek” did not bias final conclusions (17). Specifically, the significance levels for the interim analyses and the final test were specified as <0.00001, 0.003, 0.018 and 0.044 respectively. The trial was terminated after the 3rd interim analysis because of overwhelming difference in the efficacy data.
Results
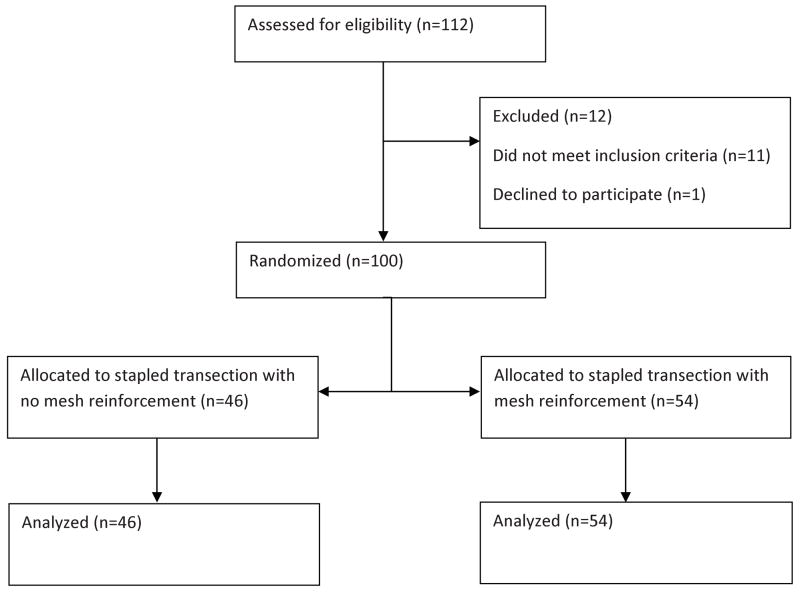
From July 2007-November 2010, 112 patients met eligibility for the study and were taken to the operating room for planned left pancreatectomy (figure 2). One patient declined to be in the trial and 11 patients were found to be ineligible for the trial. Of the patients excluded, 2 were found to have adrenal masses not invading the pancreas and no pancreatectomy was required. One patient was found to be unresectable at time of operation due to metastases and 1 patient required a total pancreatectomy. Four required pancreatic division very close to the head (right side of the portal vein) and stapler application was either challenging or would have made margin assessment difficult or inadequate. Three patients had an exceptionally thick pancreas in the planned transection area (>3 cm) which the surgeon believed precluded the safe application of the stapler. This left 100 patients in the intention-to-treat population who were randomized to either mesh reinforcement of the staple line (54) or no mesh reinforcement of the stapled pancreatic transection (46). Follow up was obtained on all patients for 100 days or until the final drain was removed, whichever came last. The trial ended early after finding superiority in the mesh reinforcement group on the third planned interim analysis.
Figure 2.
Randomization Flowchart
The study groups were well-balanced for all patient and procedure characteristics with the exception of body mass index, which was lower in the mesh reinforcement group than in the no reinforcement group (p=0.02, see table 1). Most patients in each group underwent simultaneous splenectomy (43/46) in no mesh group, 50/54) in mesh group). There were 8 intraoperative complications that occurred and are summarized in table 2.
Table 1.
Demographics
| No Stapleline Reinforcement n=46 |
Mesh Reinforcement n=54 |
Total n=100 |
p-value | |
|---|---|---|---|---|
| Sex | 0.11 | |||
| Male | 25 | 20 | 45 | |
| Female | 21 | 34 | 55 | |
| Age (years) | 58.6 +/−13.4 | 57.5 +/−15.6 | 58.0 +/−14.7 | 0.70 |
| Race | 0.80 | |||
| White | 38 | 43 | 81 | |
| Black | 8 | 11 | 19 | |
| BMI (kg/m2) | 30.97 +/−7.28 | 27.91 +/−5.78 | 29.3 +/−6.7 | 0.02 |
| Indication for operation | ||||
| Adenocarcinoma | 10 | 8 | 18 | |
| Neuroendocrine tumor | 14 | 12 | 26 | |
| Metastatic tumor to pancreas | 3 | 3 | 6 | |
| Benign pancreatic tumor* | 13 | 24 | 37 | |
| Chronic pancreatitis | 4 | 3 | 7 | |
| Normal pancreas** | 2 | 4 | 6 | |
| Smoking history | 0.54 | |||
| Nonsmoker | 26 | 34 | 60 | |
| Smoker | 20 | 20 | 40 | |
| ASA | 0.38 | |||
| 2 | 19 | 26 | 45 | |
| 3 | 27 | 26 | 53 | |
| 4 | 0 | 2 | 2 | |
| Additional organ resection (excluding spleen) | 0.82 | |||
| Yes | 13 | 14 | 27 | |
| No | 33 | 40 | 73 | |
| Laparoscopic operation | 1.0 | |||
| Yes | 22 | 25 | 47 | |
| No | 24 | 29 | 53 | |
| Conversion of laparoscopic operation | 0.97 | |||
| Yes | 6 | 7 | 13 | |
| No | 22 | 25 | 47 | |
| Planned open operation | 18 | 22 | 40 | |
| Operative variables | ||||
| Operative time (minutes) | 208.46 +/−80.2 | 208.59 +/−59.7 | 208.5 +/−69.5 | 0.99 |
| Blood loss (ml) | 508 +/−613 | 487 +/−527 | 496 +/−565 | 0.85 |
| Blood transfusion | 5 (10.9%) | 7 (13%) | 12 (12%) | 0.75 |
| Intraoperative Complication | 2 | 6 | 8 | 0.28 |
Values expressed as mean +/− ]standard deviation.
BMI = body mass index. ASA = American Society of Anesthesiologists’ risk class
Benign pancreatic tumor includes intraductal papillary mucinous neoplasms, mucinous cystic neoplasms and serous cystadenomas
Normal pancreas indicates operation was performed for pathology elsewhere and pancreas was removed to ensure negative margins.
Table 2.
Intraoperative Complications Incurred by Patients
| Complication | Group (No Mesh vs Mesh) | Postoperative POF |
|---|---|---|
| Staple misfire on splenic artery requring revision | No Mesh | No |
| Bleeding at staple line | No Mesh | Yes |
| Hepatic artery injury | Mesh | No |
| Umbilical tape included in staple line requring revision | Mesh | Yes |
| Pulmonary embolus | Mesh | No* |
| Splenic avulsion | Mesh | No |
| Umbilical tape included in staple line requring revision | Mesh | No |
| Deserosalization of stomach | Mesh | Yes |
This patient expired prior to the development of POF
The postoperative hospital course for each group was also similar (table 3). Three patients required reoperation within 30 days, two in the no mesh reinforcement group (one splenic artery bleed in the post-anesthesia unit requiring emergent reoperation and one breakdown of a small bowel anastomosis postoperative day 5) and one in the mesh reinforcement group (gastroduodenal artery bleed in the post-anesthesia unit requiring emergent reoperation). Forty-seven patients (47.9%) were diagnosed with any POF, and 12 (12.2%) had a clinically significant POF (ISGPF grade B or C, Zurich grade 2–5). The grading of POF is illustrated in table 4. There was a statistically significant difference in the clinically significant POF seen between the mesh reinforcement group (1.9%) compared to the no mesh reinforcement group (24%, p =0.0007) and was true for both classification schemes. Two patients (both in no mesh reinforcement group) initially diagnosed with grade A/1 POF were upgraded to a clinically significant POF due to the need for drain exchange because of a non-functioning intraoperatively-placed drain, resulting in clinical symptoms.
Table 3.
Postoperative Course Following Left Pancreatectomy
| No Mesh Reinforcement | Mesh Reinforcement | p-value | |
|---|---|---|---|
| Length of Stay (days) | 8.37 +/−6.8 | 7.28 +/−4.6 | 0.35 |
| Transfer to ICU | 0.75 | ||
| Yes | 6 | 5 | |
| No | 39 | 48 | |
| Discharged with drain | 0.84 | ||
| Yes | 25 | 28 | |
| No | 20 | 25 | |
| Wound infection | 0.24 | ||
| Yes | 5 | 2 | |
| No | 40 | 52 | |
| Reoperation in 30 days | 0.59 | ||
| Yes | 2 | 1 | |
| No | 43 | 53 | |
| Drain replaced | 0.001 | ||
| Yes | 11 | 1 | |
| No | 35 | 53 | |
| Days with drain* | 17 (7–40) | 9 (6–19) | 0.009 |
Median (95% confidence interval)
Table 4.
Grading Pancreatic Occlusion Failure by Definition
| No Mesh Reinforcement | Mesh Reinforcement | p-value | |
|---|---|---|---|
| ISGPF | |||
| A | 15 | 20 | 0.001 |
| B-C | 11 | 1 | |
| Zurich | |||
| 1 | 15 | 20 | |
| 2 | 0 | 0 | 0.001 |
| 3a | 8 | 1 | |
| 3b | 2 | 0 | |
| 4a | 0 | 0 | |
| 4b | 1 | 0 | |
| 5 | 0 | 0 | |
Forty-nine non-pancreatic adverse events occurred in 28 patients (29.6%) requiring 7 additional procedures. Twenty-eight occurred in patients without mesh reinforcement and 21 were in patients with mesh reinforcement. Table 5 includes a detailed list of other complications. There was no evidence of increased complications secondary to POF or increased POF due to higher severity complications. Of note, no patients who were discharged with a drain in place developed infections at the site of their drain.
Table 5.
Adverse Events
| No Mesh Reinforcement (46) | Mesh Reinforcement (54) | ||||
|---|---|---|---|---|---|
| Adverse events | Complication Grade* | POF | No POF | POF | No POF |
| Cerebrovascular accident | 4a | 1 | |||
| Myocardial inarction | 4a | 1 | |||
| Atrial fibrillation | 2 | 2 | |||
| Pulmonary embolus | 2 | 1 | 2 | ||
| Pneumonia | 2 | 2 | 3 | 1 | |
| Pleural effusion | 3a | 1 | |||
| Postoperative bleeding requring transfusion | 2 | 1 | 1 | ||
| Postoperative bleeding requring reoperation | 3b | 1 | 1 | ||
| Urinary retention | 1 | 1 | |||
| Acute renal failure (not requring dialysis) | 1 | 1 | |||
| Urinary tract infection | 2 | 1 | 2 | 1 | |
| New onset diabetes | 2 | 1 | 1 | ||
| Gastrointestinal bleed | 3a | 1 | |||
| Gastrointestinal anastomosis leak | 3b | 1 | 1 | ||
| Prolonged ileus | 2 | 2 | 2 | 1 | |
| Other intraabdominal abscess | 3a | 2 | 1 | ||
| Anaphylactic reaction to medication | 2 | 1 | |||
| Pancreatitis | 2 | 1 | |||
| Vascular pseudoaneurysm | 3a | 2 | |||
| IV infiltration with skin necrosis | 2 | 1 | |||
| Wound infection | 1 | 5 | 2 | ||
| Total | 20 | 8 | 10 | 11 | |
Clavien classification, Ann Surg 2004;240:205–13.
The time to drain removal was considered an important secondary outcome, as it identifies the length of time the patient has POF and also is the source of potential secondary patient morbidity (drain site infections, accidental drain removal requiring replacement). Patients in the mesh group were found to have a significantly shorter time period to drain removal when compared to patients in the no mesh reinforcement group (median drain time [95% CI] 9 [6–19] days vs 17 [7–40] days, mesh vs no mesh, p=0.009). However, most of this difference was related to the increased number of clinically significant leaks, which required prolonged drainage when compared to non-significant leaks/no leaks. There was no statistical decrease in the median number of days with a drain in place in patients with grade A POF (40 days [95% CI: 19–52 days] no mesh reinforcement vs 21 days [95% CI: 19–39 days] mesh reinforcement, p=0.30).
Progression from a clinically insignificant POF to aclinically significant POF was observed in two patients, both in the no mesh reinforcement treatment arm. Both patients experienced clogging of their drains leading to the development of clinical symptoms and the need for radiographically-guided catheter exchange.
Subgroup analysis was performed to identify if either mesh (Seamguard® or Peristrips Dry®) had decreased occurrence of POF. While the study was not powered to detect this difference, there does not appear to be a difference in the rates of POF based on the type of mesh used, as 10 of 23 patients (43.5%) who were reinforced with Seamguard® and 11 of 30 (36.7%) patients who were reinforced with Peristrips Dry® developed POF (p=0.78), and only one patient in either group developed a clinically significant POF.
The effect of the indication for operation (type of tumor, pancreatitis) was also analyzed (table 6). The rate of POF in patients with adenocarcinoma was significantly decreased in patients with mesh reinforced transection (1/8, 12.5%) when compared to no mesh reinforcement (7/10, 70%), but the study was not designed or powered to make those conclusions.
Table 6.
Pathologic Indication for Left Pancreatectomy
| No Mesh Reinforcement | Mesh Reinforcement | ||||
|---|---|---|---|---|---|
| Tumor Type | No POF | POF | No POF | POF | p-value |
| Adenocarcinoma | 3 | 7 | 7 | 1 | 0.05 |
| Neuroendocrine tumor | 4 | 10 | 8 | 4 | 0.11 |
| Metastatic tumor to pancreas | 2 | 1 | 2 | 1 | 1.0 |
| Benign pancreatic tumor | 7 | 6 | 11 | 13 | 1.0 |
| Chronic pancreatitis | 2 | 2 | 2 | 1 | 1.0 |
| Normal pancreas | 2 | 0 | 3 | 1 | 1.0 |
An attempt was made to identify any preoperative risk factors that could predict if the patient would develop POF. Using multivariate analysis, the only variable that predicted a decrease in rate of POF, both clinically significant (p=0.01) as well as any POF (p=0.04), was if the patient received mesh reinforcement or not. Other variables, including BMI, age, ethnicity, gender, smoking, additional organs resected, intraoperative complications and if the operation was performed laparoscopically did not predict POF (figure 3). There was also no correlation between operative time (201.8 +/−58.4 minutes for no POF vs 216.4 +/−81 minutes for POF, p=0.31; 231.7+/−122.1 minutes for clinically significant POF, p=0.28)) or blood loss (517.8 +/−499.88 ml for no POF vs 476.1 +/−639.4 for POF, p=0.72) and the development of POF.
Figure 3.
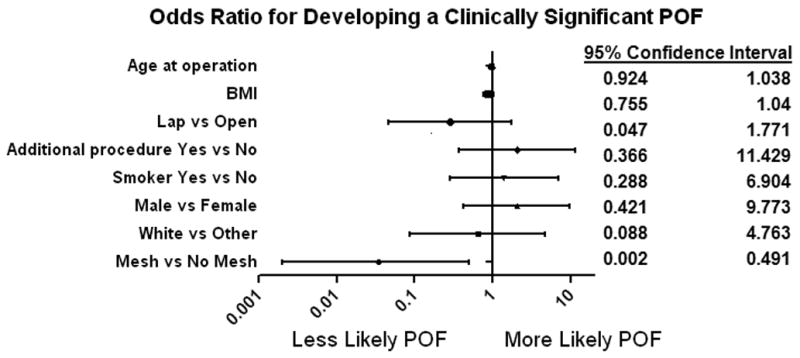
Odds Ratio for Developing Pancreatic Occlusion Failure by Risk Category
Discussion
As patients of both sexes, all ages and with a wide array of pancreatic diseases were included, the results indicate that reinforcing the stapled transection line when performing left pancreatectomy significantly decreases the rate of clinically significant pancreatic occlusion failure. Furthermore, we found a significant difference in the time it took to remove drainage catheters in the patients who had mesh placed when compared with those patients without drains. While we did not have any drain-site infections in our patients, the potential does exist, making earlier safe removal beneficial. Furthermore, the longer the drain remains in place, the more clinic visits the patient must make for drain checks, increasing inconvenience for the patient, and the higher the risk for development of clinically significant leak (18).
In clinical practice, we find it difficult to clearly separate grade B and C pancreatic fistulae using the criteria outlined by the ISGPF consensus definition, leading to the grouping of clinically significant versus not significant. Because of this lack of clarity, we also graded the complications using the classification system proposed by Strasberg and colleagues based on the University of Zurich’s grading of surgical complications. While not yet validated, we believe it allows for a more objective categorization of the severity of the complication, as the definitions are based on an escalating provision of care and does not contain any categories with vagaries (“Yes/No” or “usually yes”) or subjectivity (often well appearing vs ill appearing). Both groups identify the same set of patients as without clinically significant leak (those with an elevated drain amylase but no clinical symptoms). The natural course for these patients is unknown, as almost all of these patients seem to recover with drainage alone.
There were several limitations to our study. The study was all performed at one hospital, where 96% of the resections were performed by 3 hepatobiliary surgeons, all of whom use essentially the same operative technique. Furthermore, we were unable to identify any complications directly due to the mesh materials. However, it is possible these complications are very rare and that our study did not include enough patients to potentially identify these potential harms. We also did not consistently record the consistency of the pancreatic gland at the site of transection. Conventional wisdom states that patients with chronic inflammation have firmer pancreases and are at a lower risk for developing POF than patients with otherwise normal glands (tumors). This has been used in previous studies (9) to define those patients with a “high risk” (soft) pancreas or “low risk” (firm) pancreas in terms of developing POF. In our patients, there was no statistical difference in the distribution of predisposition to high risk of POF versus low risk groups. There was a difference in the baseline BMI of patients in the two groups, with patients receiving mesh reinforcement having a significantly lower mean BMI (30.97 vs 27.91, p=0.02). However, BMI did not appear to be a significant predictor of the development of pancreatic leak (table 7), so we feel that this difference in the two groups is clinically insignificant. We did observe a difference in POF in patients undergoing resection for adenocarcinoma between groups, though the study is underpowered to conclude this.
Table 7.
Multivariate Analysis of Risk Factors for the Development of POF
| Any POF | Clinically Significant POF | |
|---|---|---|
| Mesh reinforcement | 0.04 | 0.01 |
| Ethnicity | 0.68 | 0.67 |
| Age | 0.15 | 0.49 |
| Gender | 0.97 | 0.38 |
| Smoker | 0.96 | 0.67 |
| BMI | 0.41 | 0.14 |
| Additional resection | 0.61 | 0.42 |
| Laparoscopic operation | 0.15 | 0.18 |
| Intraoperative complication | 0.22 | 0.97 |
When examining our patients who did not have stapled transection of the pancreas, we identified two situations in which participating surgeons decided that it was not safe or technically possible to apply the stapler. The pancreas that was deemed “too thick” in 3 patients although there were other similarly thick pancreases which were stapled included in the trial. In 4 patients the tumor that was very proximal (typically located to the right of the superior mesenteric vein), making it technically difficult to adequately apply the stapler while assuring an adequate tumor margin. While there was no true defined maximum pancreatic thickness that prevented the use of the stapler, the authors believe that clinicians who want to start using this technique should use caution and check that fracture did not occur if the stapler is applied to a pancreas with an estimated thickness over 3cm or 2cm in firm pancreatic tissue. A slow controlled closure of the stapler is mandatory to allow time for the tissue to compress without fracturing.
Clinically significant postoperative POF remains a potentially morbid complication. Recently, the DISPACT trial demonstrated equivalency in stapler versus hand-sewn closure of left pancreatectomy, with similar rates of clinically significant POF as our patient population that did not receive mesh reinforcement. With the shift towards minimally invasive left pancreatectomy, the use of stapled transection will become a more prominent fixture in the surgeon’s arsenal. Reinforcing the staple line with some form of mesh buttress material appears to lessen the risk of clinically significant POF without any identified additional morbidity directly related to the mesh itself. We advocate the use of mesh reinforcement of stapled pancreatic transection for left pancreatectomy and a low threshold for discharging patients home with their drains in place. These efforts should decrease the rate of clinically significant POF inpatients undergoing left pancreatectomy.
Acknowledgments
Funding: This trial was funded by the Department of Surgery at Washington University School of Medicine. Statistical support was provided by the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842. Salary support was provided for three authors (NAH, MRP, FMJ) by the Washington University Surgical Oncology Training Grant (NIH-5T32CA00962122). Other surgeons providing technical assistance include J. Christopher Eagon, MD, Christopher Anderson, MD, and Maria Doyle, MD.
References
- 1.Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–700. doi: 10.1097/00000658-199905000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez JR, Germes SS, Pandharipande PV, Gazelle GS, Thayer SP, Warshaw AL, et al. Implications and cost of pancreatic leak following distal pancreatic resection. Arch Surg. 2006;141:361–366. doi: 10.1001/archsurg.141.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knaebel HP, Diener MK, Wenter MN, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–46. doi: 10.1002/bjs.5000. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–92. doi: 10.1097/00000658-199510000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PWT. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg. 2003;90:190–196. doi: 10.1002/bjs.4032. [DOI] [PubMed] [Google Scholar]
- 6.Fisher WE, Chai C, Hodges SE, Wu MF, Hilsenbeck SG, Brunicardi FC. Effect of BioGlue® on the incidence of pancreatic fistula following pancreas resection. J Gastrointest Surg. 2008;12:882–890. doi: 10.1007/s11605-008-0479-x. [DOI] [PubMed] [Google Scholar]
- 7.Suc B, Msika S, Fingerhut A, Fourtanier G, Hay JM, Holmieres F, et al. Temporary fibrin glue occlusion of the main pancreatic duct in the prevention of intra-abdominal complications after pancreatic resection: prospective randomized trial. Ann Surg. 2003;237:57–65. doi: 10.1097/00000658-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrone CR, Warshaw AL, Rattner DW, Berger D, Zheng H, Rawal B, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 2008;12:1691–1698. doi: 10.1007/s11605-008-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, et al. Effectiveness of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT trial): a randomized, controlled multicentre trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)60237-7. in press. [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho CS, Kooby DA, Schmidt CM, Nakeeb A, Bentrem DJ, Merchant NB, et al. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg. 2011;253:975–980. doi: 10.1097/SLA.0b013e3182128869. [DOI] [PubMed] [Google Scholar]
- 13.Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Arch Surg. 248:438–446. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 14.Kooby DA, Hawkins WG, Schmidt CM, Weber SM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779–785. doi: 10.1016/j.jamcollsurg.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Johnston FM, Cavataio A, Strasberg SM, Hamilton NA, Simon PO, Trinkaus K, et al. The effect of mesh reinforcement of a stapled transection line on the rate of pancreatic occlusion failure after distal pancreatectomy: review of a single institution’s experience. HPB (Oxford) 2009;11:25–31. doi: 10.1111/j.1477-2574.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M, Hayashi MS, Nguyen NT, Nguyen TD, McCloud S, Imagawa DK. Use of Seamguard to prevent pancreatic leak following distal pancreatectomy. Arch Surg. 2009;144:894–899. doi: 10.1001/archsurg.2009.39. [DOI] [PubMed] [Google Scholar]
- 17.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 18.Diener MK, Mehr KT, Wente MN, Kieser M, Buchler MW, Seiler CM. Risk-benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbecks Arch Surg. 2011;396:41–52. doi: 10.1007/s00423-010-0716-0. [DOI] [PubMed] [Google Scholar]