Abstract
cAMP-dependent protein kinase (PKA) plays a critical role in nervous system development by modulating sonic hedgehog and bone morphogenetic protein signaling. In the current studies, P19 embryonic carcinoma cells were neuronally differentiated by expression of the proneural basic helix-loop-helix transcription factor Ascl1. After expression of Ascl1, but prior to expression of neuronal markers such as microtubule associated protein 2 and neuronal β-tubulin, P19 cells demonstrated a large, transient increase in both mRNA and protein for the endogenous protein kinase inhibitor (PKI)β. PKIβ-targeted shRNA constructs both reduced the levels of PKIβ expression and blocked the neuronal differentiation of P19 cells. This inhibition of differentiation was rescued by transfection of a shRNA-resistant expression vector for the PKIβ protein, and this rescue required the PKA-specific inhibitory sequence of the PKIβprotein. PKIβ played a very specific role in the Ascl1-mediated differentiation process since other PKI isoforms were unable to rescue the deficit conferred by shRNA-mediated knockdown of PKIβ. Our results define a novel requirement for PKIβ and its inhibition of PKA during neuronal differentiation of P19 cells.
Keywords: cAMP, differentiation, shRNA
INTRODUCTION
During differentiation of the nervous system, pluripotent neural stem cells give rise to a wide variety of neuronal and glial cell types. This differentiation involves the dynamic interplay of extrinsic environmental signals, cell-cell interactions, and intrinsic transcriptional regulatory events. The bone morphogenic proteins (BMPs) interact with complementary regional signals such as fibroblast growth factors (FGFs), and sonic hedgehog (Shh) to regulate early stages of neural stem cell expansion, self-renewal, lineage restriction, and incipient lineage commitment. The ability of these trophic signals to act within neurodevelopmental niches requires precise expression of members of the basic helix-loop-helix (bHLH) transcription factor family (reviewed in Takahashi and Liu, 2006). bHLH factors regulate the fate of neural progenitor cells by controlling proliferation, cell cycle exit, neurite outgrowth, and synaptogenesis (Sun et al., 2001; Nguyen and Woo, 2003). Gain- and loss-of-function studies have shown that precise temporal and spatial expression of bHLH transcription factors is critical for proper development of the nervous system (Casarosa et al., 1999).
As a bHLH factor, mammalian achaete-schute homolog 1 (Ascl1 or Mash1) is essential for the survival of neural progenitor cells, and plays a central role in generating neuronal diversity by regulating subtype specification as well as differentiation (Bertrand et al., 2002). Ascl1 is one of the earliest markers expressed in a subset of neural progenitor cells (Parras et al., 2004), and in the embryonic ventral telencephalon is essential for the production of neuronal precursor cells (Casarosa et al., 1999; Nieto et al., 2001). In the dorsal telencephalon, Ascl1, in concert with other proneural bHLH proteins from the Neurogenin family, promotes the neuronal commitment of multipotent progenitors while inhibiting their astrocytic differentiation (Nieto et al., 2001).
Previous research utilizing P19 embryonic carcinoma cells has shown that these cells function as pluripotent stem cells. Once induced to differentiate into neurons by retinoic acid and aggregation, they exhibit biochemical and developmental processes similar to those that occur in early embryogenesis. Furthermore, they share several properties in common with embryonic stem cells isolated from mice and humans (Thomson and Marshall, 1998). Remarkably, transient transfection of NeuroD2, Ascl1, Neurog1 and related proneural bHLH proteins has shown that these key transcription factors are sufficient to convert uncommitted P19 cells into differentiated neurons (Farah et al., 2000). The consequences of Ascl1 expression in P19 cells are similar to those observed in vivo (Johnson et al., 1992): the differentiation of these transfected cells is preceded by elevated expression of the cyclin-dependent kinase inhibitor p27kip1 and cell cycle withdrawal. Furthermore, these differentiated neurons exhibit electrophysiological properties of neurons (Farah et al., 2000; Huang et al., 2010). However, little is known about the signaling cascades triggered downstream of Ascl1 that are involved in the differentiation and eventual function of these cells.
As a modulator of the sonic hedgehog and BMP pathways, cAMP-dependent protein kinase (PKA) is an essential integrator of signaling pathways (Tiecke et al., 2007; Ohta et al., 2008; Ghayor et al., 2009; Pan et al., 2009). During development, the cAMP/PKA pathway is critically involved in regulation of gene expression, cell growth, and cell differentiation. At low levels of cAMP, PKA exists as a tetrameric holoenzyme composed of two catalytic subunits and two regulatory subunits. Two genes encoding catalytic subunits of PKA have been identified in mammalians, designated Cα and Cβ (Lee et al., 1983; Uhler et al., 1986; Hedin et al., 1987). Four genes encoding the regulatory subunits of PKA are grouped into two categories: type I and type II. The type II regulatory subunits (RIIα and RIIβ) contain an autophosphorylation site (Hofmann et al., 1975; Rosen and Erlichman, 1975), whereas the type I subunits (RIα and RIβ) are not autophosphorylated. The regulatory subunits are modular, highly dynamic proteins that bind to two molecules of cAMP, which results in their dissociation from the catalytic subunits of PKA. These free catalytic subunits then go on to phosphorylate specific serine or threonine residues on PKA substrates, eliciting changes in their biological function (Corbin et al., 1988; Taylor et al., 1990). In addition, the regulatory subunits also serve to specifically target the PKA holoenzyme to the A-kinase anchoring proteins (AKAPs) within the cell (Banky et al., 1998; Newlon et al., 1999).
In addition to the regulatory and catalytic subunits, the protein kinase inhibitor (PKI) proteins are important physiological regulators of PKA (Dalton and Dewey, 2006). Three genes encoding different isoforms of PKIs (PKIα, PKIβ and PKIγ) have been characterized in mammals and these genes show conserved tissue-specific expression (Collins and Uhler, 1997; Zheng et al., 2000). PKIs were first identified as competitive inhibitors of the catalytic subunits and proposed to modulate the threshold for activation of PKA by cAMP (Ashby and Walsh, 1972). Later, PKIs were also shown to cause translocation of the catalytic subunit from the nucleus to the cytoplasm (Wen et al., 1994). PKIγ has been shown to be required for the termination of immediate early gene induction by PKA (Chen et al., 2005) and PKIα has been shown to suppress the Nodal-Pitx2 pathway in chick embryos (Kawakami and Nakanishi, 2001). In this study, we characterized PKA activation in P19 cells and demonstrated induction of all three isoforms of PKI during Ascl1-induced P19 neuronal differentiation. The magnitude of induction varied by isoform, and each PKI transcript also exhibited a distinct temporal pattern of expression. shRNA-mediated knockdown of each isoform showed that PKIβ—the most highly induced isoform in our model system—and its inhibition of PKA activity is necessary for Ascl1-induced neuronal differentiation in P19 cells.
EXPERIMENTAL METHODS
Materials
The following primary antibodies were used in these experiments: CREB, phosphorylated CREB, GAPDH, polyclonal Map2 (Cell Signaling Technology), Flag, monoclonal Map2 (Sigma-Aldrich), β-III-tubulin (Covance) and Ascl1 (BD Pharmingen). In addition, a polyclonal antibody was raised against peptides for PKIβ (64-KDQGQPKTPLNEGK-78) and synthesized from Invitrogen. Secondary horseradish peroxidase-conjugated antibodies were obtained from Cell Signaling Technology. A secondary Alexa Fluor conjugated antibody (goat anti-mouse Alexa Fluor 546) was purchased from Invitrogen. Lentiviral shRNA vectors were obtained from Open Biosystems; identification numbers along with hairpin sequences may be found in Supplementary Table 1. The hairpin shRNA sequences under the control of the human U6 promoter in the pLKO.1 vector were transfected directly into P19 cells without virus production.
Cell culture, transfection, and treatment
P19 cells were obtained from the American Type Culture Collection and cultured in Minimal Essential Medium Alpha (MEMα; Gibco) supplemented with 7.5% calf serum (CS; HyClone), 2.5% fetal bovine serum (FBS; HyClone), and 1% penicillin/streptomycin (Gibco). HEK-293T cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 10% FBS (HyClone). Cells were kept at a temperature of 37°C, a minimum relative humidity of 95%, and an atmosphere of 5% CO2 in air. Cells were maintained below 80% confluence and passaged by dissociating them into single cells using TrypLE Express (Gibco). Cells were transfected using the TransIT-LT1 transfection reagent (Mirus) following the manufacturer’s instructions as described (Huang et al., 2010). When necessary, the appropriate parental expression plasmid DNA was added to maintain a constant total amount of DNA. US2-Neo and US2-Cα DNAs were constructed from previously described plasmids (Huggenvik et al., 1991; Chung et al., 2006). US2-CαK72M encodes for a protein that renders PKA catalytically inactive by mutating a lysine residue near the N-terminus of the kinase in the protein kinase subdomain II to a methionine. This residue has frequently been mutated to eliminate the catalytic activity of protein kinases (Zoller et al., 1981; Huggenvick et al., 1991). 8-(4-Chlorophenylthio)adenosine-3’,5’-cyclic monophosphorothioate (8-CPT-cAMP; Sigma-Aldrich) was dissolved in DMSO to a concentration of 20 mM. 8-CPT-cAMP was pre-diluted in serum-free media to a working concentration of 200 µM and added to cells that had been serum-starved for a minimum of 2 hours prior to treatment.
Differentiation of P19 cells
Tissue cultures plates were laminin coated using the procedure described in Huang et al. (2010). P19 cells were seeded at a density of 3.0 × 105 cells/ml onto uncoated tissue culture plates and allowed to recover for 24 h prior to transfection. After 6 h of transfection, cells were dissociated using TryplE Express (Gibco) and then passaged onto laminin coated tissue culture plates. Cells were treated with 7.5 µg/ml puromycin (InvivoGen) 12 h after transfection. After 24 h of transfection, the media was changed to stop puromycin selection. On day four, the media was changed to Neurobasal media (Gibco) supplemented with B27 (Gibco) and GlutaMAX (Invitrogen). Media was changed every 24 h thereafter.
Construction of PKI expression vectors
The sequence resulting in the 78 amino acid isoform of murine PKIβ was PCR amplified from a PKIβ 7.1 plasmid (previously described in Scarpetta and Uhler, 1993) using the primer pair shown in Supplementary Table 2. The resulting PCR fragment was subcloned into the pGEM-T Easy vector system (Promega). The DNA was EcoRI/XbaI digested and then subcloned into EcoRI/XbaI digested US2 vector downstream of the ubiquitin promoter. This plasmid was further modified such that base pair complementation was not possible with the shRNA we found to be most effective at knocking down PKIβ expression, but the final amino acid sequence of the protein remained the same. Silent mutations within the shRNA target sequence were introduced via PCR using the primer pairs found in Supplementary Table 2. Briefly, the first round of PCR generated a 5’ mutant fragment (using the PKIβmut 5’out and PKIβmut 3’in primers) and a 3’ mutant fragment (using the PKIβmut 5’in and PKIβmut 3’out primers) that had 24 overlapping nucleotides. These were then used as templates for the second round of PCR using the outer primer pairs (PKIβmut 5’out and PKIβmut 3’out) shown in Supplementary Table 2. The amplified fragment was EcoRI/XbaI digested and subcloned into EcoRI/XbaI digested US2 to create the US2-PKIβ expression vector.
In order to create the null mutant pUS2- PKIβ DNA construct, four amino acid residues (Phe18, Arg23, Arg26, Arg27) were mutated to alanine residues using the mutagenic oligonucleotide primers shown in Supplementary Table 2. Mutations were introduced via PCR as described for the US2-PKIβ expression vector. The full-length mutated PCR product was EcoRI/XbaI digested and subcloned into EcoRI/XbaI digested US2 to create the US2-PKIβnull expression vector.
Mammalian expression vectors for human (h)PKIα and murine (m)PKIγ were constructed from previously described plasmids (Scarpetta and Uhler, 1993; Collins and Uhler, 1997). The coding sequences for PKIα and PKIγ were PCR amplified using the primer pairs shown in Supplementary Table 2. The resulting PCR fragments were subcloned into the pGEM-T Easy vector system (Promega). The DNAs were EcoRI/XbaI digested and then subcloned into EcoRI/XbaI digested US2 vector downstream of the ubiquitin promoter to generate pUS2-hPKIα and pUS2-mPKIγ. All of the PKI constructs were sequenced to ensure that only the intended mutations were introduced. All oligonucleotides were synthesized by Invitrogen
RNA isolation and qRT-PCR analysis
Total RNA was purified from P19 cells with TRIzol reagent (Invitrogen). cDNA was synthesized from 2 µg of total RNA with SuperScript II Reverse Transcriptase and random hexamer primers (Invitrogen). Transcript levels were determined by quantitative real-time PCR (qRT-PCR) using the SYBR Green PCR Master Mix (Applied Biosystems) and the MyiQ single-color real-time PCR detection system (Bio-Rad). The specificity of the amplification was verified with a heat dissociation protocol (from 72°C to 98°C) after the final cycle of the PCR. Each amplification determination was done in triplicate. Expression levels were calculated using the delta-delta CT method, with GAPDH serving as the normalization control. The individual primer sequences used to amplify target genes can be found in Supplementary Table 2. A paired student’s t-test was performed to compare the two groups, with data presented as means ± standard deviation with the significance level set at p < 0.05.
Dual luciferase reporter assay
Firefly and Renilla luciferase assays used the Dual Luciferase Reporter Assay kit (Promega). To account for differences in transfection efficiencies, firefly luciferase activity was normalized to that of renilla luciferase generated from the US2-RL plasmid (Huang et al., 2010). Luciferase determinations were repeated a minimum of three times, and results were expressed as means ± standard deviation. The statistical significance of transactivation data was determined using a student’s paired t-test with the significance level set at p < 0.05.
Protein kinase activity assays
The PepTag assays were performed according to the manufacturer’s instructions (Promega). This assay utilizes the Leu-Arg-Arg-Ala-Ser-Leu-Gly (Kemptide) peptide substrate tagged with a fluorescent dye. Upon phosphorylation, the net charge of this peptide changes from +1 to −1, which subsequently alters its migration when run on an agarose gel. Briefly, lysed cell extract expressing PKIβ or functionally null PKIβ proteins was incubated with the tagged Kemptide substrate and activator buffers at 30°C, and the reaction was resolved on a 1% agarose gel. Active protein was detected by the phosphorylated Kemptide migration toward the anode. Quantitative assay of kinase activity was based on density measurements of the bands using ImageJ software (http://rsb.info.nih.gov/ij) from three independent experiments. The statistical significance of differences in kinase activity was determined using a student’s paired t-test with the significance level set at p < 0.05.
Basal kinase activity measurement required a modified procedure similar to a radiometric kinase assay used previously (Uhler and Abou-Chebl, 1992; Collins and Uhler, 1997). Cell pellets were resuspended in homogenization buffer (10 mM NaPO4 pH 7.0, 1 mM EDTA, 1 mM dithiothreitol, 250 mM sucrose), sonicated, and protein concentrations were adjusted to 2 mg/ml. Assays (25 µl volume) were performed for 30 min at 30°C and contained 100 µM ATP, 5 mM MgAc, 15 µM fluorescent Kemptide (Promega), 10 mM Tris pH 7.4, 250 µM 3-isobutyl-1-methylxanthine, 5 mM dithiothreitol, 2.5 mM NaF and 400 µg/ml of cell extract. At the end of the 30 min incubation, tubes were frozen on dry ice then heated to 100°C for 5 min. Following electrophoresis of the assay mixture to separate phosphorylated from unphosphorylated Kemptide as described (Promega), gel slices containing the phosphorylated and unphoshorylated substrate were excised, solubilized and quantitated in a fluorescence plate reader. Basal kinase activity is expressed in Units/mg with a Unit equivalent to 1 pmol of ATP transferred per minute.
SDS-PAGE and western blot analysis
Cells were washed twice with Dulbecco’s phosphate buffered saline (DPBS; Hyclone) and lysed in buffer containing 10 mM NaH2PO4·H2O, 1 mM EDTA, 1 mM DTT, 250 mM sucrose, 10 mM sodium fluoride, and complete EDTA-free protease inhibitors (Roche). Lysates were sonicated, and protein concentrations were determined by the bicinchonic acid protein assay (Bio-Rad). Equal amounts of total protein were denatured at 95°C in the presence of SDS, DTT, and β-mercaptoethanol. Samples were resolved on 16% Tris-HCl gels and transferred onto a 0.2 µm nitrocellulose membrane (Whatman). Membranes were blocked for 2 h in PBS supplemented with 5% non-fat dried milk, 2% polyvinylpyrrolidone (PVP-40), and 0.1% Triton X-100 and subsequently incubated in primary antibody diluted in PBS supplemented with 0.5% bovine serum albumin and 0.1% Triton X-100 overnight at 4°C. Membranes were washed three times for 10 min with TBST (50 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20), and then incubated with a 1:2,000 dilution of HRP-conjugated secondary antibody in TBST supplemented with 5% non-fat dried milk. Following the final set of three 10 min washes with TBST, the blots were developed using Lumi-Light Western Blotting Substrate (Roche) according to the manufacturer’s instructions. Quantitative assay of antigen expression was based on density measurements of protein bands using ImageJ software.
Rapid amplification of cDNA ends (RACE)
The 5’-cDNA ends were obtained with the SMART™ RACE cDNA Amplification Kit (Clontech Laboratories, Inc). For 5’-RACE, 1 µg total RNA from the 36 h time point (see Figure 4) was reverse transcribed with the 5’-RACE CDS Primer and SMART II A Oligonucleotide (provided). Three gene-specific primers for PKIβ were designed based on the sequence reported in the NCBI database and can be found in Supplementary Table 2. 5’-RACE PCR was performed with either PKIβ 3.1, 3.2, or 3.3 along with Universal Primer A Mix according to the SMART™ RACE cDNA Amplification Kit user manual. Negative controls containing only the UPM or only gene-specific primers were also performed. The amplified cDNA products were isolated, cloned into the pGEM-T Easy vector (Promega), and sequenced.
Figure 4. PKIβ expression during Ascl1-induced differentiation.
A. qRT-PCR of cells induced to differentiate via transient transfection of Ascl1 shows a 2500-fold increase in PKIβ expression at the peak 36 h time point relative to the 0 h time point. PKIβ mRNA levels are given as the mean ± S.D. normalized to GAPDH levels. B. Western blotting for expression of PKIβ under the same conditions in (A). Induced PKIβ protein was observed between 36 and 72 h post-transfection, and occurred after the induction of Ascl1 expression. Densitometric analysis was performed to quantify and compare protein levels with GAPDH controls, and the relative intensity values are shown underneath each corresponding blot.
Immunocytochemistry
Cells were washed once in DPBS and then fixed in 4% formaldehyde in PBS for 20 min. Cells were washed three times in PBS, and then blocked for 1 h in PBS supplemented with 2% goat serum and 0.1% Triton X-100. Cells were probed with primary antibodies diluted in blocking solution for 2 h at 23°C. After washing in PBS, cells were incubated with AlexaFluor conjugated secondary antibodies for 1 h at 23°C, followed by three PBS washes. For nuclear counterstaining, the cells were incubated in 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen) for 10 min before being washed twice in PBS and imaged using an inverted Olympus IX70 fluorescence microscope. The percent of cells differentiated under each condition was calculated using the ratio of differentiated cells to the total number of cells (visualized by DAPI staining). Cells were qualified as differentiated if the processes were three times the length of the cell body. The statistical significance between groups was determined using a student’s paired t-test with the significance level set at p < 0.05.
RESULTS
Characterization of the PKA-CREB pathway in undifferentiated P19 cells
Initially, we sought to determine the role of PKA signaling in the neuronal differentiation of P19 cells. Previous studies to assess cAMP-inducible transcription with the F9 embryonic carcinoma cells demonstrated those cells to be refractory to cAMP, and the F9 cells only became cAMP-responsive following RA-induced differentiation (Strickland and Mahdavi, 1978; Strickland et al., 1980). P19 embryonic carcinoma cells have been widely used to study neuronal differentiation, but have not been examined previously for PKA function. To determine whether P19 cells exhibit PKA-CREB characteristics similar to F9 cells, we used a membrane-permeable analog of cAMP (8-CPT-cAMP) to carry out a PKA activation time course. P19 cells were treated for varying lengths of time with 8-CPT-cAMP and then subjected to western blot analysis. Similar levels of total CREB and PKA catalytic subunit (Cα) protein were observed at all time points (Figure 1A). pCREB immunoreactivity prior to 8-CPT-cAMP treatment was nearly undetectable, but increased after 15 min of exposure to 8-CPT-cAMP and remained elevated until 2 h, after which levels declined. The kinetics of CREB phosphorylation is typically transient in nature, peaking at approximately 30 min post-stimulation and subsequently diminishing to basal levels after 3–4 h following dephosphorylation of Ser133 by the protein phosphatases PP-1 and/or PP-2A (Hagiwara et al., 1992; Wadzinski et al., 1993).
Figure 1. Activity of the cAMP/PKA signaling pathway in P19 cells.
A. P19 cells were treated with 200 µM 8-CPT-cAMP for the indicated lengths of time. Untreated cells (0 min) were included as a negative control. Western blotting shows an induction of pCREB in response to cAMP treatment. B. P19 and HEKT cells were transfected with the indicated DNAs for 24 h. Western blot analysis showed an increase in pCREB levels in both P19 and HEKT cells that was abolished upon mutation of a critical lysine residue (K72M). C. Transcriptional activity of a CRE-luciferase reporter in response to Cα and cAMP. In both P19 and HEKT cell lines, co-transfection with Cα or cAMP treatment yielded an increase in the relative luciferase activity of the CRE-luciferase reporter.
We also examined the ability of P19 cells to respond to exogenous and constitutively active PKA catalytic subunit (Cα). Functional PKA signaling in HEKT cells has been extensively studied and this cell line served as a positive control for transfected exogenous Cα subunit activity (Roche et al., 1996; Chow and Wang, 1998; Papadopoulou et al., 2004). P19 and HEKT cells were transfected with expression vectors for wild-type Cα or a mutant form of Cα (K72M), which significantly reduces the catalytic activity of the kinase (Brown et al., 1990; Huggenvik et al., 1991). As expected, western blot analysis again shows comparable levels of CREB between HEKT and P19 cells (Figure 1B). In both cell lines, pCREB immunoreactivity increases when cells are transfected with the wild-type Cα expression vector. This increase is PKA activity-dependent, since no increase in pCREB immunoreactivity was seen in cells transfected with the mutant Cα (K72M) expression vector. An antibody against the catalytic subunit of PKA detected both the exogenous Cα and K72M, the latter of which has previously been reported to migrate faster than its wild-type counterpart due reduced autophosphorylation (Iyer et al., 2005).
Finally, we characterized the transcriptional response of P19 cells to both cAMP treatment and exogenous Cα by utilizing a CRE-containing reporter vector composed of the human chorionic gonadotropin (HCG) promoter driving expression of firefly luciferase. The promoter of the HCG gene has been extensively used for reporter analysis, and the proximal 180 bp of the promoter contains two adjacent CREs that mediate basal and cAMP-stimulated transcription (Delegeane et al., 1987; Jameson et al., 1989; Mellon et al., 1989; Pittman et al., 1994). P19 cells exhibited a 14-fold increase in CRE-luciferase activity upon transfection with a wild-type Cα expression vector, and a 17-fold increase in response to cAMP treatment (Figure 1C). HEKT cells exhibited a 38-fold increase in CRE-luciferase activity upon transfection with a wild-type Cα expression vector, and a 23-fold increase in response to cAMP treatment. This is in agreement with the results shown in Figure 1A, suggesting that PKA-regulated transcription via CREB is functional in undifferentiated P19 cells. In both cell lines, transfection with the K72M expression vector showed significantly less induction of CRE-luciferase activity. Together, these data show that P19 cells are a tractable system for studying cAMP-mediated effects, and suggest that PKA-CREB phosphorylation in P19 cells is similar to many other cell types such as HEKT cells but distinct from that reported for F9 embryonic carcinoma cells.
Characterization of PKA activity in differentiating P19 cells
Transfection of proneural bHLH proteins such as Ascl1 has previously been shown to convert P19 cells into a relatively homogenous population of electrophysiologically differentiated neurons (Farah et al., 2000; Vojtek et al., 2003; Huang et al., 2010). P19 cells were transiently transfected with either an empty plasmid expression vector (pUS2) or an expression vector for Ascl1 (pUS2-Ascl1). After 120 h following transfection, the majority of cells had adopted a neuronal morphology with round cell bodies and one or more long processes (Figure 2A). Neuron-specific class III β-tubulin (recognized by the TuJ1 monoclonal antibody) is widely accepted as a neuronal marker, and immunocytochemistry showed a high percentage (> 30%) of cells that had TuJ1-immunoreactive processes 5 days after transfection with Ascl1. In contrast, cells transfected with the parental pUS2 expression vector maintained the morphology of undifferentiated P19 cells but did show some detectable perinuclear immunoreactivity with TuJ1. This basal level of TuJ1 immunoreactivity was not due to the Neurobasal media change, as limited expression of TuJ1 is observed in untransfected P19 cells as well (data not shown). Western blot analysis showed that Ascl1 protein was transiently induced, with expression that preceded mature neuronal marker expression (Figure 2B). Neuronal markers such as microtubule-associated protein 2 (Map2) and β-III-tubulin were induced following expression of Ascl1, with levels detectable as early as 36 h after transfection and remaining elevated throughout the time course.
Figure 2. PKA activity changes during neuronal differentiation of P19 cells.
A. TuJ1 staining (red) of P19 cells transiently transfected with US2 or US2-Ascl1 for 120 h. Nuclei were stained with DAPI and appear blue. In the absence of Ascl1, no TuJ1-positive processes were observed. In the presence of Ascl1, TuJ1-positive cells were evident that had a distinct neuronal morphology. Scale bar = 100 µm. B. Western blot for changes in protein expression during Ascl1-induced neuronal differentiation. C. Kinase assays optimized to detect basal levels of PKA activity over the time course of differentiation show that PKA undergoes a transient, 2.7-fold decrease in activity during Ascl1-induced neuronal differentiation.
To detect potential changes in PKA activity as P19 cells differentiate into neurons, a kinase assay was optimized to detect basal levels of PKA activity over the time course of differentiation. Basal kinase activity was measured at approximately 200 U/mg at 0 h, and a decrease in PKA activity was observed beginning as early as 24 h after transfection with Ascl1, undergoing a maximal, 2.7-fold reduction in kinase activity at 36 h post-transfection (Figure 2C). Levels of PKA activity then gradually recovered over the remaining duration of the time course. Total PKA kinase activity at all time points was approximately 2,000 U/mg (data not shown). The fluctuations in PKA activity were not due to a reduction in PKA C subunit protein as levels of catalytic subunit remain comparable across all time points (Figure 2B).
Regulation of PKI expression during neuronal differentiation
Microarray hybridization studies of genes induced after transient transfection of P19 cells with Ascl1 showed a significant, but transient, increase in expression of all three PKI isoforms (data not shown, D. Turner unpublished data). Since the PKI proteins are important physiological regulators of PKA-mediated phosphorylation events, we pursued the possibility that PKIs were responsible for the reduction in PKA activity observed during the Ascl1-induced differentiation process. To confirm the microarray findings, we verified the microarray-based changes in PKI gene expression using qRT-PCR (Figure 3A). Because the spliced isoforms of the PKIα and PKIγ genes are less complex than those of the PKIβ gene, we first verified induction of the PKIα and PKIγ genes. In concordance with the microarray data, PKIα and PKIγ were induced after transient expression of Ascl1: maximal (18-fold) induction of PKIα was seen at 120 h, while maximal (6-fold) PKIγ induction was observed at 36 and 48 h. To determine whether either of these isoforms is required for neuronal differentiation, shRNA vectors targeting the specific PKI isoforms in P19 cells were employed. The efficacy of five different shRNA constructs, each targeting a different region of the PKIα and PKIγ transcripts, were evaluated via qRT-PCR analysis of cells transfected with the shRNA vector. Four shRNA vectors produced a statistically significant reduction in levels of PKIα transcript and two shRNA vectors were successful at reducing expression of PKIγ (Figure 3B). Despite these reductions in mRNA transcript levels, co-transfection with these shRNAs did not result in significant effect on neuronal differentiation since these cells differentiated normally as determined by immunostaining (Figure 3C).
Figure 3. PKIα and PKIγ are not required for Ascl1-induced neuronal differentiation.
A. qRT-PCR analysis of PKIα and PKIγ gene expression over a time course of Ascl1-induced neuronal differentiation shows that P19 cells undergo a transient increase in PKIα and PKIγ mRNA expression. Results are shown as the mean ± S.D. normalized to GAPDH levels. B. qRT-PCR analysis of shRNA constructs for efficacy of knockdown for each gene. Negative controls (NC) are cells transfected with the empty US2 vector. Positive controls (PC) are cells transfected with Ascl1. *p < 0.05. C. Representative images showing that P19 cells differentiate normally even when PKIα or PKIγ are knocked down. TuJ1 staining is shown in red, and DAPI-stained nuclei appear blue. Scale bar = 100 µm.
PKIβ was the most highly induced isoform in our microarray hybridization analyses, and qRT-PCR confirmed an increase in the relative expression of PKIβ transcript, showing a 2,500-fold increase after the overexpression of Ascl1 at 36 h (Figure 4A). This increase in PKIβ transcript levels corresponds to the time point at which a decrease in PKA activity was observed (see Figure 2C). Western blot analysis using a PKIβ antibody showed that the expression of the PKIβ protein also transiently increases during the differentiation process: the PKIβ protein is first detectable at 36 h, which corresponds to the peak mRNA expression in the qRT-PCR results, and then declines. The PKIβ immunoreactivity on the western blots appears at the expected molecular weight of 15.5 kDa (Scarpetta and Uhler, 1993), and the peak induction of PKIβ protein occurs after that of the Ascl1 protein, with Ascl1 expression peaking at 24 h and becoming undetectable by 120 h. This expression pattern is in accordance with previous data showing that Ascl1 is transiently expressed in proliferating neural precursors: the protein appears before overt neuronal differentiation and disappears as markers of the mature neuronal phenotype are expressed (Lo et al., 1991; Casarosa et al., 1999). The relative immunoreactivity of PKIβ or Ascl1 protein to GAPDH protein is shown underneath each blot and indicates at least a 6-fold increase in PKIβ immunoreactivity. These combined results show that both PKIβ mRNA and protein levels increase during Ascl1-induced neuronal differentiation of P19 cells
PKIβ is necessary for Ascl1-induced neuronal differentiation
As done for PKIα and PKIγ, specific shRNA vectors were used to knockdown PKIβ and determine whether PKIβ expression is required for the neuronal differentiation of P19 cells. Five different shRNAs were assayed for efficacy of knockdown of the PKIβ gene at 36 h, the time point where PKIβ mRNA expression peaks (Figure 5A). As compared to the positive control (PC; cells transfected with Ascl1), all five shRNAs significantly reduced PKIβ mRNA transcript levels (p < 0.05). However, shRNA1 did not decrease PKIβ expression as much as shRNA4 (which showed the greatest fold-reduction in PKIβ expression). To determine whether knocking down PKIβ results in changes in neuronal differentiation, we co-transfected P19 cells with Ascl1 in the presence of the shRNA1 or shRNA4 construct, and then used immunocytochemistry to assay for differences in neuronal differentiation 120 h post-transfection.
Figure 5. PKIβ is necessary for Ascl1-induced P19 neuronal differentiation.
A. qRT-PCR analysis of the efficacy of shRNAs targeted to the PKIβ gene. Negative controls (NC) are cells transfected with the empty US2 vector. Positive controls (PC) are cells transfected with Ascl1. Out of five different shRNA vectors tested, shRNA1 was the least effective, while shRNA4 was the most effective. Results are shown as the mean ± S.D. normalized to GAPDH levels. *p < 0.05, **p < 0.01. B. Immunostaining for expression of TuJ1 (red) shows that co-transfection with PKIβ shRNA4 results in fewer morphologically differentiated cells than either the positive control (PC) or the cells co-transfected with shRNA1. Nuclei were visualized with DAPI staining and appear blue. Scale bar = 100 µm. C. Quantitation of the percentage of cells differentiated in (B). Co-transfection with shRNA4 resulted in a significant reduction in the percentage of TuJ1-positive cells. Percentages are expressed as the mean ± S.D. **p < 0.01. D. Western blotting using antibodies against PKIβ and Map2 show that shRNA4 is the most effective at reducing both protein levels.
Cells transfected with shRNA1 showed fewer TuJ1-positive cells than the positive control (Figure 5B), but the difference in percentage of cells differentiated was not statistically significant (p = 0.09, Figure 5C). However, transfection with shRNA4 significantly (p < 0.01) reduced the percentage of differentiated cells. Western blot analysis of cell lysates using an antibody against Map2 supported our immunocytochemistry findings: a substantial increase in Map2 expression is observed in cells transfected with Ascl1, a slight decrease in Map2 expression is observed in cells co-transfected with shRNA1, and a dramatic decrease is seen in cells co-transfected with shRNA4 (Figure 5D). The levels of Map2 protein in cells transfected with shRNA4 are comparable to those of the negative control. These results show that not only is the shRNA4 construct the most effective at knocking down PKIβ mRNA and protein expression, but it also perturbs Ascl1-induced neuronal differentiation in P19 cells.
To examine transcriptional changes in response to shRNA-mediated knockdown of PKIβ expression, qRT-PCR analyses were carried out (Figure 6A). Transfection with PKIβ shRNA4 blocked induction of the PKIβ mRNA transcript without affecting PKIα expression, and resulted in an apparent, but statistically insignificant reduction of PKIγ transcript levels. This reduction is unlikely to be due to a direct effect of the PKIβ shRNA, since it targets a sequence not conserved in PKIγ. Furthermore, reducing expression of PKIβ did not affect the induction of Ascl1 transcript or early downstream targets: recent research has shown that Gadd45γ is a direct transcriptional target of Ascl1, and is also one of the earliest and most induced genes in the Ascl1-mediated differentiation process (Huang et al., 2010). qRT-PCR analysis showed that the induction of Gadd45γ transcript was comparable in cells where PKIβ expression had been knocked down. Therefore, early events in Ascl1-induced differentiation appear to be intact in the cells transfected with PKIβ shRNA4. However, neuronal markers such as Map2 were significantly reduced in cells where PKIβ expression had been knocked down compared to the controls with an overall 5-fold reduction in expression.
Figure 6. Gene expression changes in response to knockdown of PKIβ.
A. qRT-PCR analysis of mRNA transcript levels in Ascl1-transfected P19 cells in the presence (open circles) or absence (filled circles) of PKIβ shRNA4. PKIβ was the most highly induced of the three PKI isoforms, and co-transfection with PKIβ effectively reduced mRNA transcript levels without compensatory increases in PKIα or PKIγ expression. Reduced PKIβ expression did not affect the induction of Ascl1 transcript or the expression of a direct transcriptional target, Gadd45γ. Neuronal markers such as Map2 (Mtap2) had reduced expression levels in the presence of PKIβ shRNA. Results are shown as the mean ± S.D. normalized to GAPDH levels. B. Western blot analysis using antibodies against neuronal-specific β-III-tubulin shows that protein expression is induced at early time points under both conditions, but β-III-tubulin expression is significantly reduced at later time points in cells that received PKIβ shRNA.
Western blot analysis also verified that early gene expression changes appeared to be largely unaffected in cells where PKIβ expression was reduced (Figure 6B). The neuronal marker β-III-tubulin was still strongly induced between 24 to 48 h in PKIβ shRNA-transfected cells, but at later time points (72 to 120 h) there was no detectable expression of β-III-tubulin protein. In Ascl1-transfected cells, β-III-tubulin protein expression was detectable as early as 24 h and remained elevated throughout the time course. Ascl1 protein was also induced in both time courses, although its expression fell more quickly in PKIβ shRNA-transfected cells with protein expression becoming undetectable past 48 h, whereas in the controls Ascl1 remained detectable up to 72 h post-transfection. To our knowledge, there is no direct evidence linking PKIβ expression or PKA activity to Ascl1 stability or degradation.
Characterization of PKIβ in P19 cells
Since the PKIβ transcript is known to undergo significant alternative splicing (Scarpetta and Uhler, 1993; Kumar and Walsh, 2002), RACE amplification was used to characterize the PKIβ transcript in differentiating P19 cells. Using three different reverse primers (Supplementary Table 2) one major transcript was successfully amplified, which was subsequently isolated, cloned, and sequenced. RACE amplification products and a representative sequence from one amplified DNA clone is shown in Supplementary Figure 1. The predicted exon organization of the mouse PKIβ gene includes exons 1, 5, 6, 7, 9 and 10. RACE amplification results show that in P19 cells, exon 7 is absent (Figure 7A; open boxes represent non-coding regions, while the closed box represents the coding region). Exon 7 does not contain any elements crucial to the inherent activity of PKI, but it does include the region that makes it a highly potent inhibitor of cGMP-dependent protein kinase (PKG) (Kumar and Walsh, 2002). The predicted size of the PKIβ protein is 15.5 kDa, in accordance with our western blot results (Figure 4 and Figure 5).
Figure 7. Inhibitory activity of exogenous PKIβ expression vectors.
A. Schematic of PKIβ exon organization based on 5’ RACE sequencing. Open boxes represent non-coding regions, and the closed box represents the coding region. Exon sizes are indicated above the exon boxes. Target sequences for two representative PKIβ shRNA constructs are underlined. B. Amino acid sequence of murine PKIβ. The numbering of the sequence begins with the known initiator methionine, and is placed on the left of the diagram. Amino acid residues known to be important in high affinity binding of PKIα for the C subunit of PKA are indicated with an asterisk on the top line (Phe18, Arg23, 26, 27). All four of these residues were mutated to alanines to determine their importance for PKIβ function. C. PKA enzyme activity, as determined by kinase assays using a fluorescent PKA substrate peptide (f-kemptide), is inhibited by exogenous PKIβ. A representative UV-illuminated agarose gel of the products of kinase reactions run with f-kemptide and transfected cell homogenates is shown. PKA activity phosphorylates kemptide, which changes its net charge from +1 to −1. This allows the phosphorylated and nonphosphorylated forms of the substrate to be rapidly separated on an agarose gel. Densitometric analysis quantitated a 7-fold reduction of PKA activity in the presence of exogenous PKIβ. This inhibition is dependent on residues critical to binding of PKA, as the functionally inactive PKIβ protein (PKIβnull) failed to inhibit PKA activity. *p < 0.05.
With the identification of exon 1 by RACE amplification, we also constructed a promoter-reporter construct driving firefly luciferase to examine whether Ascl1 regulated transcriptional activity of the PKIβ promoter. This reporter consisted of a 1.2 kb DNA sequence 5’ of the transcriptional start site for exon 1 and contained two E-box (CANNTG) sequences. However, transfection of this reporter showed little firefly luciferase activity and no regulation by co-transfected Ascl1 (data not shown) although transfection of a previously characterized promoter-reporter construct for Gadd45γ was highly regulated (Huang et al., 2010).
Rescue of PKIβ during neuronal differentiation
If the changes in neuronal differentiation observed in Figures 5 and 6 are dependent on PKIβ protein expression, the effect of shRNAs for PKIβ should be rescued by introducing exogenous PKIβ protein. However, the shRNA4 construct targets a sequence within the PKIβ coding region (Figure 7A). Therefore, we created a PKIβ coding variant where the nucleotide sequence was altered to impede binding of shRNA4, but still produced the wild-type protein (Figure 7B). PKIs are competitive inhibitors of the catalytic subunit of PKA, and contain an inhibitor sequence Arg-Arg-Asn-Ala that serves to prevent phosphorylation (Van Patten et al., 1991). Studies on the PKIα isoform have shown that the inhibitor sequence is important for PKA inhibition, as substitutions of Arg18 and Arg19 significantly reduced PKI potency (Scott et al., 1986). Other extra-inhibitory sequence residues in PKIα are also important for the high potency inhibition of PKA: two residues outside the pseudosubstrate sequence that contribute significantly to PKI interactions with the catalytic subunit of PKA are Arg15 and Phe10 (Glass et al., 1989; Baude et al., 1994). The substitution of both residues leads to dramatic decreases in the efficacy of PKIα. The amino acids important for full inhibitory potency are conserved between PKIα and PKIβ, so we determined whether the conserved residues important in PKIα function are also critical for PKIβ inhibition of PKA. We introduced mutations in the corresponding four conserved amino acids (Phe18, Arg23, Arg26, and Arg27) in the PKIβ coding variant sequence (Figure 7B, indicated with asterisks). All four residues were mutated to alanines. A PKA kinase activity assay was used to determine whether these proteins were functionally active (PKIβ) or inactive (PKIβnull). Based on densitometry and the coupled kinase assay, exogenous PKIβ reduced kinase activity 7-fold, while the functionally inactive PKIβnull mutant failed to inhibit the activity of PKA (Figure 7C).
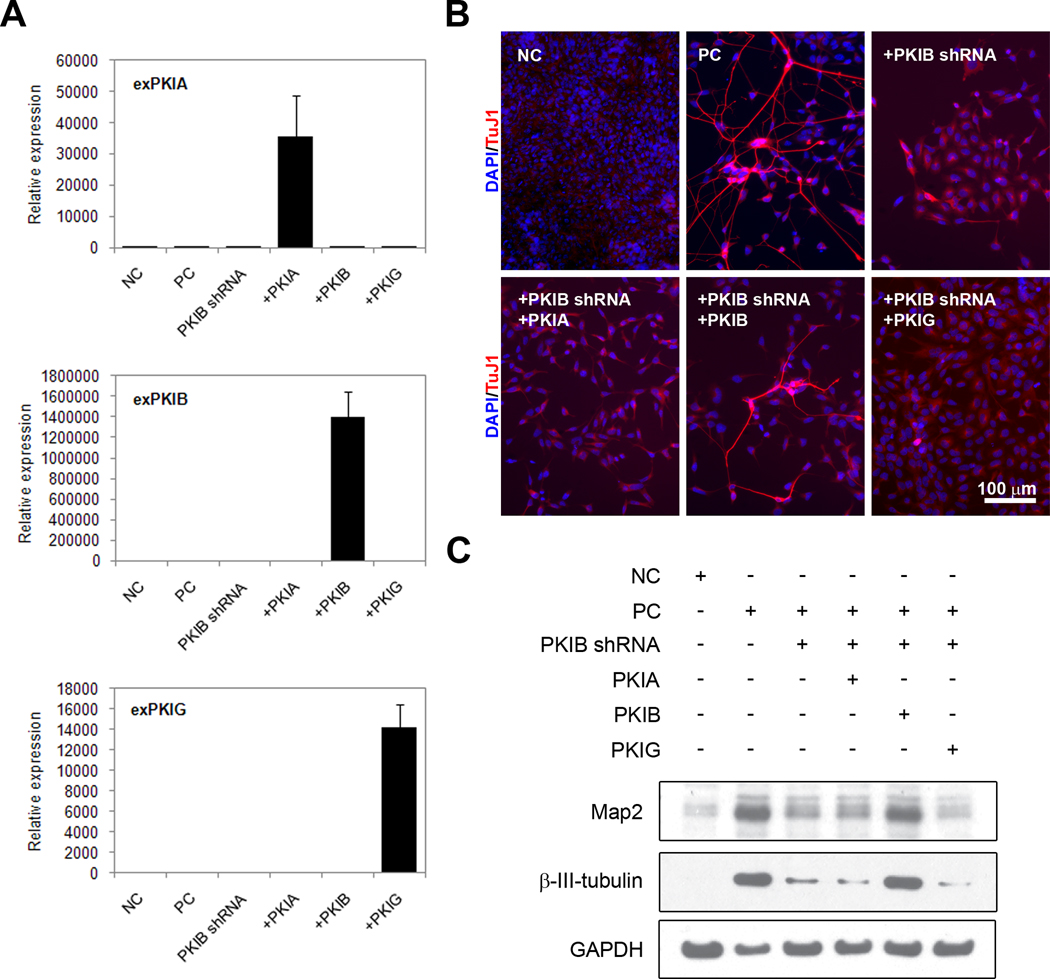
Transfection of P19 cells with the exogenous wild-type PKIβ construct restores the ability of P19 cells to differentiate in response to Ascl1 in the presence of shRNA4: immunocytochemistry shows increased number of TuJ1-positive cells with extended neurites relative to cells transfected with shRNA4 alone (Figure 8A). When P19 cells were co-transfected with the functionally inactive PKIβnull construct, a rescue of neuronal differentiation was not observed as evidenced by the lack of TuJ1-positive projections. Quantitation of the percentage of cells differentiated under each condition showed that 68% of the cells differentiated 120 h post-transfection with Ascl1 (Figure 8B). Co-transfection with shRNA4 reduced the percentage of differentiated cells to 1.6% of the total population. When exogenous PKIβ was introduced, differentiation was restored with 53% of the cells expressing TuJ1. This rescue requires the residues previously shown to be important for PKIα inhibition of PKA because the functionally inactive PKIβnull mutant did not rescue the differentiation, as evidenced by significantly fewer differentiated cells compared to both Ascl1-transfected cells and cells co-transfected with Ascl1, shRNA4, and exogenous PKIβ. Western blot analysis also showed increased Map2 (Figure 8B) and TuJ1 (data not shown) protein levels in cells co-transfected with the exogenous PKIβ. However, when cells were co-transfected with the functionally inactive PKIβnull expression vector, no significant difference in Map2 or TuJ1 protein levels were observed between this condition and cells transfected with shRNA4. Together, these data suggest that residues critical for PKIβ inhibition of PKA are also critical for PKIβ-mediated neuronal differentiation downstream of Ascl1.
Figure 8. Exogenous PKIβ rescues neuronal differentiation, and requires binding to PKA.
A. As previously shown (see Figure 5B), transient co-transfection of P19 cells with PKIβ shRNA4 resulted in fewer cells differentiating. Immunostaining for TuJ1 (red) shows that introduction of exogenous PKIβ rescued the phenotype, producing more TuJ1-positive cells that adopt a neuronal morphology. A functionally inactive PKIβ did not rescue the phenotype, as evidenced by decreased TuJ1-immunoreactivity and a lack of TuJ1-positive processes. Nuclei were visualized with DAPI staining and appear blue. Scale bar = 100 µm. B. Quantitation of the percentage of cells differentiated from (A). To be considered differentiated, cells had to be TuJ1-positive and also have processes three times the length of the cell body. Using these parameters, cells from three independent fields per condition were counted and expressed as the mean ± S.D. *p < 0.05. Western blotting for Map2 protein showed changes in expression that support the immunostaining results shown in (A).
Compensatory up-regulation of other PKI isoforms has been reported previously (Belyamani et al., 2001). Since PKIβ is the most highly induced PKI isoform, it is possible that it is required for differentiation simply because of its abundance relative to PKIα and PKIγ. In order to determine whether PKIα and PKIγ isoforms are able to rescue the neuronal differentiation of cells where PKIβ has been knocked down, exogenous PKIα or PKIγ were introduced into P19 cells that were also co-transfected with Ascl1 and PKIβ shRNA4. To verify the overexpression of these DNA vectors, qRT-PCR was performed using primers designed to specifically amplify the exogenous mRNA transcript (Supplementary Table 2), and significant induction of exogenous PKI transcripts were observed in the appropriate conditions (Figure 9A). A no reverse transcriptase control was included that validated these increases as being independent of DNA contamination (data not shown). As previously observed, transfection with exogenous, wild-type PKIβ partially restored the ability of P19 cells to differentiate in response to Ascl1. However, when PKIα and PKIγ were overexpressed, no rescue of neuronal differentiation was observed, as evidenced by a lack of TuJ1-positive cell bodies with significant processes (Figure 9B). Western blot analysis of cell lysates using antibodies against the neuronal markers Map2 and β-III-tubulin supported the immunocytochemistry findings, demonstrating a rescue in expression of both proteins only in cells transfected with PKIβ (Figure 9C). These findings suggest a role for PKIβ in Ascl1-mediated neuronal differentiation distinct from that of PKIα or PKIγ.
Figure 9. Other PKI isoforms are unable to compensate for loss of PKIβ.
A. qRT-PCR analysis for expression of exogenous PKI transcripts. B. As previously shown (see Figure 5B and Figure 8A), immunostaining for expression of TuJ1 (red) demonstrates that PKIβ is necessary for Ascl1-mediated neuronal differentiation of P19 cells, and that exogenous PKIβ is able to rescue the phenotype. Neither exogenous PKIα nor PKIγ are able to rescue the phenotype conferred by loss of PKIβ, thereby suggesting a unique functional role for the PKIβ isoform during neuronal differentiation. Nuclei were visualized with DAPI staining and appear blue. Scale bar = 100 µm. C. Western blotting using antibodies against the neuronal markers Map2 and β-III-tubulin showed changes in expression that support the immunostaining results shown in (B).
Constitutively active PKA prevents Ascl1-induced neuronal differentiation
In order to determine whether the modulation of PKA activity by PKIβ is critical for the Ascl1-induced differentiation, P19 cells were co-transfected with expression vectors for Ascl1 and the constitutively active Cα subunit (Figure 10A). Although an overall reduction in the density of cells was apparent after 120 h of transfection, cells that received Cα had a distinct lack of projections and TuJ1-immunoreactive cells. Western blot analysis (Figure 10B) corroborated the immunostaining results, with protein expression changes similar to those that occur upon knockdown of PKIβ (see Figure 6B): the neuronal proteins Map2 and β-III-tubulin are induced between 24 to 48 h in Cα-transfected cells, but at later time points (72 to 120 h) expression significantly declines. Ascl1 protein and its direct transcriptional target, Gadd45γ, were induced in both time courses, suggesting that early events in Ascl1-induced differentiation appear to be intact in the cells transfected with Cα, but that cells with high PKA activity fail to express markers indicative of a differentiated neuronal phenotype.
Figure 10. Constitutively active PKA prevents Ascl1-induced neuronal differentiation.
A. After 120 h of transfection with either US2 (NC), US2-Ascl1 (PC), or co-transfection with US2-Ascl1 and US2-Cα (PKA Cα), immunostaining for expression of TuJ1 (red) demonstrates that overexpression of PKA perturbs Ascl1-induced neuronal differentiation. Nuclei were visualized with DAPI staining and appear blue. Scale bar = 100 µm. B. Western blot analysis using antibodies against the neuronal-specific proteins Map2 and β-III-tubulin show that protein expression is induced at early time points under both conditions, but expression is significantly reduced at later time points in cells that received Cα. The induction of Ascl1 protein and the expression of a direct transcriptional target of Ascl1, Gadd45γ, are largely unaffected.
DISCUSSION
PKA is critical for phenotypic specification and transition in the adult and developing nervous system, but its role in neuronal differentiation remains controversial with contradictory roles emerging depending on cell type. In vitro, PKA activity inhibits neuronal differentiation in SH-SY5Y human neuroblastoma cells by blocking the initial steps of neurite elongation (Canals et al., 2005), and in NG108-15 cells, PKA activity also appears to inhibit neuritogenesis and neurite outgrowth rate (Tojima et al., 2003). In vivo, PKA has been shown to effectively inhibit the progression of retinal neurogenesis in zebrafish via effects on cell cycle exit (Masai et al., 2005). Conflicting research shows that in SH-SY5Y cells, PKA activity is necessary for the initial steps of cAMP-induced neurite elongation (Sanchez et al., 2004). Similarly, in hippocampal HiB5 cells, treatment with a cAMP analog results in a dramatic increase in neurite outgrowth (Kim et al., 2002). The importance of downstream effects of PKA activity were highlighted in a study where a dominant-negative inhibitor of CREB was shown to be effective in attenuating NGF-mediated differentiation of PC12 cells (Ahn et al., 1998). Despite these incongruities, these data suggest that the level of active PKA expressed in a neuronal cell can have profound effects on neurite formation, which in turn can alter the excitability of a cell and its ability to generate and transfer electrical signals within the nervous system. Examination of the PKA-CREB signaling pathway in P19 cells showed that 8-CPT-cAMP was capable of activating the PKA pathway, as evidenced by increased levels of pCREB. Exogenous Cα also produced activation of a CRE-reporter (Figure 1). During Ascl1-induced neuronal differentiation, a kinase assay demonstrated that P19 cells undergo a significant, but transient decrease in PKA activity early in the differentiation process (Figure 2).
Measurement of basal kinase activity during Ascl1-induced neuronal differentiation demonstrated a significant reduction in basal kinase activity. Since PKI proteins have the ability to inhibit basal kinase activity, we characterized the expression of these proteins. In our studies, following overexpression of Ascl1, microarray hybridization showed that P19 cells undergo a transient increase in all three isoforms of PKI, each displaying a unique temporal pattern of expression. We verified these results using qRT-PCR and found that the PKIβ transcript was the most highly induced, exhibiting a 2500-fold increase in expression (compared to an 18-fold and 6-fold expression for PKIα and PKIγ, respectively) that corresponded to the 2.7-fold decrease in PKA activity. We confirmed that the induction of PKIβ mRNA expression was accompanied by a significant increase in PKIβ protein (Figure 4). Since PKIs are specific inhibitory regulators of PKA, we hypothesized that PKIs could be responsible for the observed inhibition of PKA activity during Ascl1-mediated differentiation. shRNA constructs targeting each isoform were evaluated for their ability to knockdown expression of all three PKI genes, and although we successfully identified a number of effective shRNAs for each isoform, only those targeting the PKIβ gene prevented neuronal differentiation (compare Figure 3 with Figure 5). qRT-PCR analysis determined that the shRNA-mediated reduction in PKIβ mRNA and protein did not affect early events of Ascl1-mediated differentiation (e.g. Ascl1 and Gadd45γ induction), and also did not significantly affect the expression of other PKI isoforms (Figure 6). The alternative splice variant of PKIβ induced in P19 cells is a specific inhibitor of PKA (Figure 7) and the shRNA blockage of neuronal differentiation was partially rescued by overexpressing PKIβ protein. We found that this rescue of neuronal differentiation was dependent on four amino acid residues critical for binding of PKIβ to the catalytic subunit of PKA (Figure 7 and 8). Since compensatory up-regulation of other PKI isoforms has been reported previously (Belyamani et al., 2001), we also conducted experiments testing whether exogenous PKIα or PKIγ expression could rescue the phenotype conferred by antisense knockdown of PKIβ. Our results suggest a requirement for PKIβ and its association with PKA during the neuronal differentiation of P19 cells. Importantly, the observation that neither PKIα nor PKIγ were able to rescue the block to neuronal differentiation caused by PKIβ shRNAs demonstrates a unique role for PKIβ (Figure 9). Finally, very few culture systems have been described in which PKI gene transcription is regulated, making this P19 system a significant new model to study the physiological regulation of PKIs.
The three PKI protein isoforms—PKIα, PKIβ, and PKIγ—are produced from three evolutionarily conserved genes that have widespread but distinctive tissue distributions (Collins and Uhler, 1997; Zheng et al., 2000). Mice deficient in PKIα exhibited defects in skeletal muscle, but show no gross defects in development or fertility (Gangolli et al., 2000). PKIβ-deficient mice exhibited a partial loss of PKI activity in testis, but remained fertile with normal testis development and function (Gangolli et al., 2001). However, detailed studies of neuronal development in the PKIβ deficient mice have not been carried out. Remarkably, PKIα/β double-knockout mice were also viable and fertile with no obvious physiological defects (Belyamani et al., 2001). Mice deficient in PKIγ have not been described to date, and it is possible that PKIγ compensates to some extent for loss of PKIα and PKIβ in the mice deficient for the latter PKI isoforms. More recently, studies of osteosarcoma cells and fibroblasts have demonstrated that PKIγ is necessary for the efficient termination of PKA signaling in the nucleus (Chen et al., 2005).
Studies indicate that multiple forms of PKIβ exist, related by covalent modification and alternate translational initiation (Van Patten et al., 1991; Van Patten et al., 1997; Zheng et al., 2000; Kumar and Walsh, 2002). PKIβ was first isolated from rat testis as a 70 amino acid protein, but the genomic sequence suggested that an alternate form might exist, arising as a consequence of alternate translational initiation. This species, now termed PKIβ78, is equipotent with PKIβ70, and also occurs in vivo. Six additional species of PKIβ are also evident in tissues: two of these represent the phospho forms of PKIβ78 and PKIβ70, while the other four represent phospho and dephospho forms of two higher molecular mass PKIβ species. These latter forms are currently termed PKIβ109 and PKIβY, and their molecular identities have yet to be fully determined (Kumar et al., 1997). Our data indicate that the form expressed in P19 cells corresponds to the 78 amino acid isoform of PKIβ expressed in the brain (Kumar et al., 1997). Furthermore, the gene organization of PKIβ elucidated from our RACE studies indicates that the P19 cell PKIβ78 is a specific inhibitor of PKA. Other isoforms of PKIβ exist that are dual-specificity inhibitors of both PKA and PKG, but the sequences required for PKG inhibition are located in exon 7, a region that is absent in the cDNA of PKIβ in P19 cells. Human PKIB shares a 70% homology to mouse PKIβ, most notably within the sequences for the pseudosubstrate site and nuclear export signal. In humans, PKIB is the predominant isoform expressed in the brain, and the PKIB cDNA also encodes a peptide of 78 amino acids (Zheng et al., 2000). Because of the sequence homology between human and mouse PKIβ and similar patterns of tissue specific expression, it is possible that PKIβ may play a role in human as well.
Although the tissue-specific expression of mammalian PKI genes has been well characterized in past studies, tissue-specific functions of the PKIs have not been described in detail previously. The findings reported here suggest that PKIβ can be highly regulated by bHLH proteins such as Ascl1 and that PKIβ has an isoform-specific role in the neuronal differentiation of P19 cells. More detailed studies in the P19 cells and in PKIβ-deficient animals should provide greater clarity into the properties of PKIβ that are important for neuronal differentiation.
Supplementary Material
A. PKIβ gene organization was determined using the Smart™ RACE cDNA amplification kit, and a representative gel of the amplified products is shown. B. Representative PKIβ nucleotide sequence from an isolated cDNA clone. The beginning of each exon of the PKIβ gene is underlined.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the helpful discussions of Ms. Tanya Marrocco-Redmond, Mr. John Whang and Ms. Ginger Kubish. This work utilized Molecular Biology Core of the Michigan Diabetes Research and Training Center, funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was supported by NIH/NINDS R01NS051472 (MDU).
Footnotes
DISCLOSURE/ CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banky P, Huang LJ, Taylor SS. Dimerization/docking domain of the type Ialpha regulatory subunit of cAMP-dependent protein kinase. Requirements for dimerization and docking are distinct but overlapping. J Biol Chem. 1998;273:35048–35055. doi: 10.1074/jbc.273.52.35048. [DOI] [PubMed] [Google Scholar]
- Baude EJ, Dignam SS, Reimann EM, Uhler MD. Evidence for the importance of hydrophobic residues in the interactions between the cAMP-dependent protein kinase catalytic subunit and the protein kinase inhibitors. J Biol Chem. 1994;269:18128–18133. [PubMed] [Google Scholar]
- Belyamani M, Gangolli EA, Idzerda RL. Reproductive function in protein kinase inhibitor-deficient mice. Mol Cell Biol. 2001;21:3959–3963. doi: 10.1128/MCB.21.12.3959-3963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Blume C, Benz PM, Walter U, Ha J, Kemp BE, Renne T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem. 2007;282:4601–4612. doi: 10.1074/jbc.M608866200. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Mattei P, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol. 1986;114:453–462. doi: 10.1016/0012-1606(86)90209-5. [DOI] [PubMed] [Google Scholar]
- Brown NA, Stofko RE, Uhler MD. Induction of alkaline phosphatase in mouse L cells by overexpression of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1990;265:13181–13189. [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Cadd G, McKnight GS. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989;3:71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Canals M, Angulo E, Casado V, Canela EI, Mallol J, Vinals F, Staines W, Tinner B, Hillion J, Agnati L, Fuxe K, Ferre S, Lluis C, Franco R. Molecular mechanisms involved in the adenosine A and A receptor-induced neuronal differentiation in neuroblastoma cells and striatal primary cultures. J Neurochem. 2005;92:337–348. doi: 10.1111/j.1471-4159.2004.02856.x. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Chen S, Ji M, Paris M, Hullinger RL, Andrisani OM. The cAMP pathway regulates both transcription and activity of the paired homeobox transcription factor Phox2a required for development of neural crest-derived and central nervous system-derived catecholaminergic neurons. J Biol Chem. 2005;280:41025–41036. doi: 10.1074/jbc.M503537200. [DOI] [PubMed] [Google Scholar]
- Chow YW, Wang HL. Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J Neurochem. 1998;70:2606–2612. doi: 10.1046/j.1471-4159.1998.70062606.x. [DOI] [PubMed] [Google Scholar]
- Chung KH, Hart CC, Al-Bassam S, Avery A, Taylor J, Patel PD, Vojtek AB, Turner DL. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 2006;34:e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SP, Uhler MD. Characterization of PKIγ, a novel isoform of the protein kinase inhibitor of cAMP-dependent protein kinase. J Biol Chem. 1997;272:18169–18178. doi: 10.1074/jbc.272.29.18169. [DOI] [PubMed] [Google Scholar]
- Collins SP, Uhler MD. Cyclic AMP- and cyclic GMP-dependent protein kinases differ in their regulation of cyclic AMP response element-dependent gene transcription. J Biol Chem. 1999;274:8391–8404. doi: 10.1074/jbc.274.13.8391. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Cobb CE, Beebe SJ, Granner DK, Koch SR, Gettys TW, Blackmore PF, Francis SH, Wells JN. Mechanism and function of cAMP- and cGMP-dependent protein kinases. Adv Second Messenger Phosphoprotein Res. 1988;21:75–86. [PubMed] [Google Scholar]
- Delegeane AM, Ferland LH, Mellon PL. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987;7:3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdouets C, Matesic G, Molina CA, Foulkes NS, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Gangolli EA, Belyamani M, Muchinsky S, Narula A, Burton KA, McKnight GS, Uhler MD, Idzerda RL. Deficient gene expression in protein kinase inhibitor alpha Null mutant mice. Mol Cell Biol. 2000;20:3442–3448. doi: 10.1128/mcb.20.10.3442-3448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- Ghayor C, Ehrbar M, San Miguel B, Gratz KW, Weber FE. cAMP enhances BMP2-signaling through PKA and MKP1-dependent mechanisms. Biochem Biophys Res Commun. 2009;381:247–252. doi: 10.1016/j.bbrc.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RM, Ji-Buechler Y, Taylor SS. Identification of electrostatic interaction sites between the regulatory and catalytic subunits of cyclic AMP-dependent protein kinase. Protein Sci. 1997;6:1825–1834. doi: 10.1002/pro.5560060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DB, Lundquist LJ, Katz BM, Walsh DA. Protein kinase inhibitor-(6–22)-amide peptide analogs with standard and nonstandard amino acid substitutions for phenylalanine 10. Inhibition of cAMP-dependent protein kinase. J Biol Chem. 1989;264:14579–14584. [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Haffner C, Jarchau T, Reinhard M, Hoppe J, Lohmann SM, Walter U. Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. Embo J. 1995;14:19–27. doi: 10.1002/j.1460-2075.1995.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hedin L, McKnight GS, Lifka J, Durica JM, Richards JS. Tissue distribution and hormonal regulation of messenger ribonucleic acid for regulatory and catalytic subunits of adenosine 3',5'-monophosphate-dependent protein kinases during ovarian follicular development and luteinization in the rat. Endocrinology. 1987;120:1928–1935. doi: 10.1210/endo-120-5-1928. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Beavo JA, Bechtel PJ, Krebs EG. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975;250:7795–7801. [PubMed] [Google Scholar]
- Huang HS, Kubish GM, Redmond TM, Turner DL, Thompson RC, Murphy GG, Uhler MD. Direct transcriptional induction of Gadd45gamma by Ascl1 during neuronal differentiation. Mol Cell Neurosci. 44:282–296. doi: 10.1016/j.mcn.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchon D, Ozon R, Fischer EH, Demaille JG. The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation. A 4-step scheme for meiotic maturation. Mol Cell Endocrinol. 1981;22:211–222. doi: 10.1016/0303-7207(81)90092-7. [DOI] [PubMed] [Google Scholar]
- Huggenvik JI, Collard MW, Stofko RE, Seasholtz AF, Uhler MD. Regulation of the human enkephalin promoter by two isoforms of the catalytic subunit of cyclic adenosine 3',5'-monophosphate-dependent protein kinase. Mol Endocrinol. 1991;5:921–930. doi: 10.1210/mend-5-7-921. [DOI] [PubMed] [Google Scholar]
- Iyer GH, Garrod S, Woods VL, Jr, Taylor SS. Catalytic independent functions of a protein kinase as revealed by a kinase-dead mutant: study of the Lys72His mutant of cAMP-dependent kinase. J Mol Biol. 2005;351:1110–1122. doi: 10.1016/j.jmb.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Jameson JL, Albanese C, Habener JF. Distinct adjacent protein-binding domains in the glycoprotein hormone alpha gene interact independently with a cAMP-responsive enhancer. J Biol Chem. 1989;264:16190–16196. [PubMed] [Google Scholar]
- Johnson JE, Zimmerman K, Saito T, Anderson DJ. Induction and repression of mammalian achaete-scute homologue (MASH) gene expression during neuronal differentiation of P19 embryonal carcinoma cells. Development. 1992;114:75–87. doi: 10.1242/dev.114.1.75. [DOI] [PubMed] [Google Scholar]
- Kim G, Choe Y, Park J, Cho S, Kim K. Activation of protein kinase A induces neuronal differentiation of HiB5 hippocampal progenitor cells. Brain Res Mol Brain Res. 2002;109:134–145. doi: 10.1016/s0169-328x(02)00550-8. [DOI] [PubMed] [Google Scholar]
- Kumar P, Van Patten SM, Walsh DA. Multiplicity of the beta form of the cAMP-dependent protein kinase inhibitor protein generated by post-translational modification and alternate translational initiation. J Biol Chem. 1997;272:20011–20020. doi: 10.1074/jbc.272.32.20011. [DOI] [PubMed] [Google Scholar]
- Kumar P, Walsh DA. A dual-specificity isoform of the protein kinase inhibitor PKI produced by alternate gene splicing. Biochem J. 2002;362:533–537. doi: 10.1042/0264-6021:3620533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275:36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- Lee DC, Carmichael DF, Krebs EG, McKnight GS. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1983;80:3608–3612. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977;252:1712–1718. [PubMed] [Google Scholar]
- Masai I, Yamaguchi M, Tonou-Fujimori N, Komori A, Okamoto H. The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation. Development. 2005;132:1539–1553. doi: 10.1242/dev.01714. [DOI] [PubMed] [Google Scholar]
- Matyakhina L, Lenherr SM, Stratakis CA. Protein kinase A and chromosomal stability. Ann N Y Acad Sci. 2002;968:148–157. doi: 10.1111/j.1749-6632.2002.tb04333.x. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Mellon PL, Clegg CH, Correll LA, McKnight GS. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989;86:4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon MG, Roy M, Morikis D, Hausken ZE, Coghlan V, Scott JD, Jennings PA. The molecular basis for protein kinase A anchoring revealed by solution NMR. Nat Struct Biol. 1999;6:222–227. doi: 10.1038/6663. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Nakagawa K, Imai Y, Katagiri T, Koike T, Takaoka K. Cyclic AMP enhances Smad-mediated BMP signaling through PKA-CREB pathway. J Bone Miner Metab. 2008;26:478–484. doi: 10.1007/s00774-008-0850-8. [DOI] [PubMed] [Google Scholar]
- Oyen O, Eskild W, Beebe SJ, Hansson V, Jahnsen T. Biphasic response to 3',5'-cyclic adenosine monophosphate (cAMP) at the messenger ribonucleic acid level for a regulatory subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1988;2:1070–1076. doi: 10.1210/mend-2-11-1070. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol. 2009;326:177–189. doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol Endocrinol. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman RH, Clay CM, Farmerie TA, Nilson JH. Functional analysis of the placenta-specific enhancer of the human glycoprotein hormone alpha subunit gene. Emergence of a new element. J Biol Chem. 1994;269:19360–19368. [PubMed] [Google Scholar]
- Plet A, Evain D, Anderson WB. Effect of retinoic acid treatment of F9 embryonal carcinoma cells on the activity and distribution of cyclic AMP-dependent protein kinase. J Biol Chem. 1982;257:889–893. [PubMed] [Google Scholar]
- Reinhard M, Halbrugge M, Scheer U, Wiegand C, Jockusch BM, Walter U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. Embo J. 1992;11:2063–2070. doi: 10.1002/j.1460-2075.1992.tb05264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Roger PP, Reuse S, Maenhaut C, Dumont JE. Multiple facets of the modulation of growth by cAMP. Vitam Horm. 1995;51:59–191. doi: 10.1016/s0083-6729(08)61038-9. [DOI] [PubMed] [Google Scholar]
- Rosen OM, Erlichman J. Reversible autophosphorylation of a cyclic 3':5'-AMP-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1975;250:7788–7794. [PubMed] [Google Scholar]
- Sanchez S, Jimenez C, Carrera AC, Diaz-Nido J, Avila J, Wandosell F. A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem Int. 2004;44:231–242. doi: 10.1016/s0197-0186(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Scarpetta MA, Uhler MD. Evidence for two additional isoforms of the endogenous protein kinase inhibitor of cAMP-dependent protein kinase in mouse. J Biol Chem. 1993;268:10927–10931. [PubMed] [Google Scholar]
- Scott JD, Glaccum MB, Fischer EH, Krebs EG. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986;83:1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Glaccum MB, Zoller MJ, Uhler MD, Helfman DM, McKnight GS, Krebs EG. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci U S A. 1987;84:5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MH, Mavila N, Wang WH, Vega Alvarez S, Hall MC, Andrisani OM. Time-dependent activation of Phox2a by the cyclic AMP pathway modulates onset and duration of p27Kip1 transcription. Mol Cell Biol. 2009;29:4878–4890. doi: 10.1128/MCB.01928-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S, Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978;15:393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21:347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Sun T, Echelard Y, Lu R, Yuk DI, Kaing S, Stiles CD, Rowitch DH. Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Curr Biol. 2001;11:1413–1420. doi: 10.1016/s0960-9822(01)00441-9. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Liu FC. Genetic patterning of the mammalian telencephalon by morphogenetic molecules and transcription factors. Birth Defects Res C Embryo Today. 2006;78:256–266. doi: 10.1002/bdrc.20077. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, Anand GS. Dynamics of signaling by PKA. Biochim Biophys Acta. 2005;1754:25–37. doi: 10.1016/j.bbapap.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Thompson EB, Medh RD, Zhou F, Ayala-Torres S, Ansari N, Zhang W, Johnson BH. Glucocorticoids, oxysterols, and cAMP with glucocorticoids each cause apoptosis of CEM cells and suppress c-myc. J Steroid Biochem Mol Biol. 1999;69:453–461. doi: 10.1016/s0960-0760(99)00063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Marshall VS. Primate embryonic stem cells. Curr Top Dev Biol. 1998;38:133–165. doi: 10.1016/s0070-2153(08)60246-x. [DOI] [PubMed] [Google Scholar]
- Tiecke E, Turner R, Sanz-Ezquerro JJ, Warner A, Tickle C. Manipulations of PKA in chick limb development reveal roles in digit patterning including a positive role in Sonic Hedgehog signaling. Dev Biol. 2007;305:312–324. doi: 10.1016/j.ydbio.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Tojima T, Kobayashi S, Ito E. Dual role of cyclic AMP-dependent protein kinase in neuritogenesis and synaptogenesis during neuronal differentiation. J Neurosci Res. 2003;74:829–837. doi: 10.1002/jnr.10754. [DOI] [PubMed] [Google Scholar]
- Uhler MD, Carmichael DF, Lee DC, Chrivia JC, Krebs EG, McKnight GS. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986;83:1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler MD, Abou-Chebl A. Cellular concentrations of protein kinase A modulate prostaglandin and cAMP induction of alkaline phosphatase. J Biol Chem. 1992;267:8658–8665. [PubMed] [Google Scholar]
- Van Patten SM, Ng DC, Th'ng JP, Angelos KL, Smith AJ, Walsh DA. Molecular cloning of a rat testis form of the inhibitor protein of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991;88:5383–5387. doi: 10.1073/pnas.88.12.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Patten SM, Donaldson LF, McGuinness MP, Kumar P, Alizadeh A, Griswold MD, Walsh DA. Specific testicular cellular localization and hormonal regulation of the PKIalpha and PKIbeta isoforms of the inhibitor protein of the cAMP-dependent protein kinase. J Biol Chem. 1997;272:20021–20029. doi: 10.1074/jbc.272.32.20021. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Taylor J, DeRuiter SL, Yu JY, Figueroa C, Kwok RP, Turner DL. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol. 2003;23:4417–4427. doi: 10.1128/MCB.23.13.4417-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski BE, Wheat WH, Jaspers S, Peruski LF, Jr, Lickteig RL, Johnson GL, Klemm DJ. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol. 1993;13:2822–2834. doi: 10.1128/mcb.13.5.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Lee H, Huang MW, Scholz PM, Weiss HR. Opposing functional effects of cyclic GMP and cyclic AMP may act through protein phosphorylation in rabbit cardiac myocytes. J Auton Pharmacol. 2000;20:111–121. doi: 10.1046/j.1365-2680.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- Zheng L, Yu L, Tu Q, Zhang M, He H, Chen W, Gao J, Yu J, Wu Q, Zhao S. Cloning and mapping of human PKIB and PKIG, and comparison of tissue expression patterns of three members of the protein kinase inhibitor family, including PKIA. Biochem J. 2000;349:403–407. doi: 10.1042/0264-6021:3490403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller MJ, Nelson NC, Taylor SS. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981;256:10837–10842. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. PKIβ gene organization was determined using the Smart™ RACE cDNA amplification kit, and a representative gel of the amplified products is shown. B. Representative PKIβ nucleotide sequence from an isolated cDNA clone. The beginning of each exon of the PKIβ gene is underlined.