Abstract
Initiation of Adenovirus (Ad) DNA replication occurs by a protein-priming mechanism in which the viral precursor terminal protein (pTP) and DNA polymerase (pol) as well as two nuclear DNA-binding proteins from uninfected HeLa cells are required. Biochemical studies on the pTP and DNA polymerase proteins separately have been hampered due to their low abundance and their presence as a pTP-pol complex in Ad infected cells. We have constructed a genomic sequence containing the large open reading frame from the Ad5 pol gene to which 9 basepairs from a putative exon were ligated. When inserted behind a modified late promoter of vaccinia virus the resulting recombinant virus produced enzymatically active 140 kDa Ad DNA polymerase. The same strategy was applied to express the 80 kDa pTP gene in a functional form. Both proteins were overexpressed at least 30-fold compared to extracts from Adenovirus infected cells and, when combined, were fully active for initiation in an in vitro Adenovirus DNA replication system.
Full text
PDF




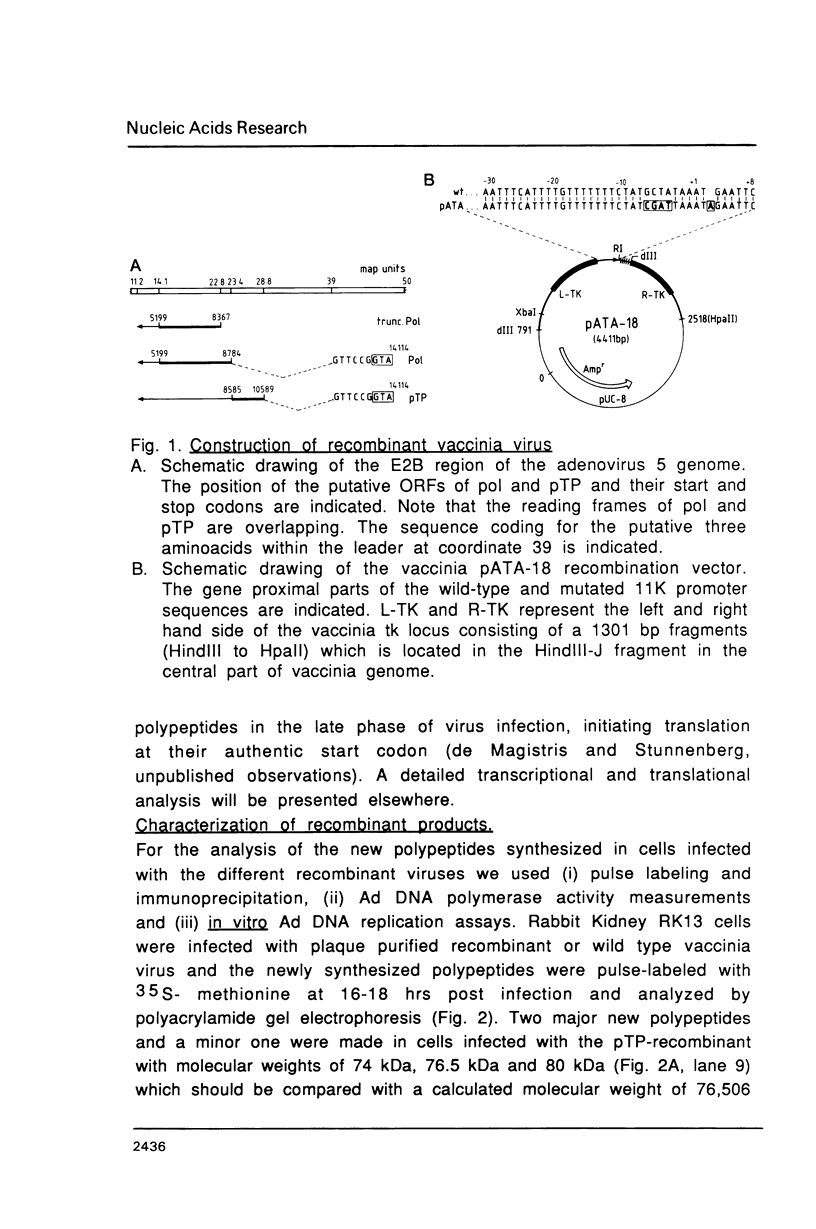
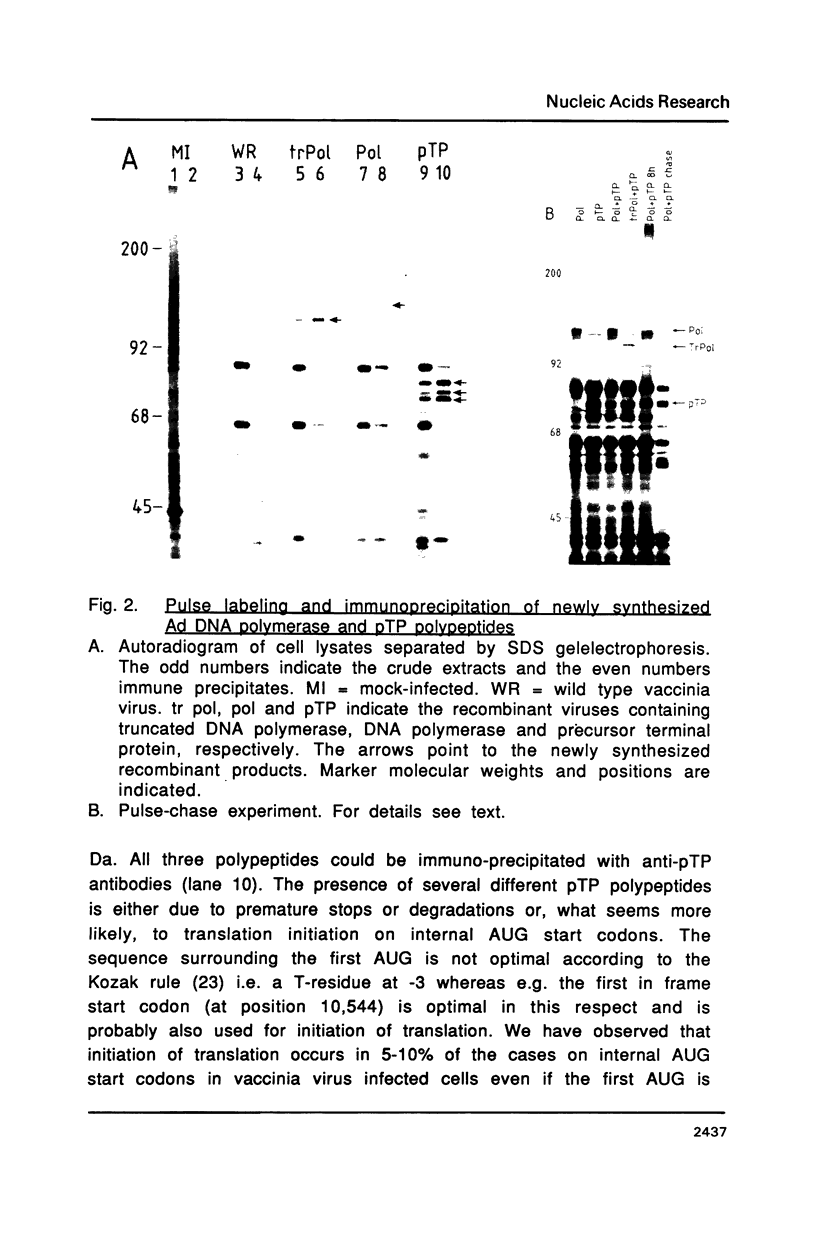
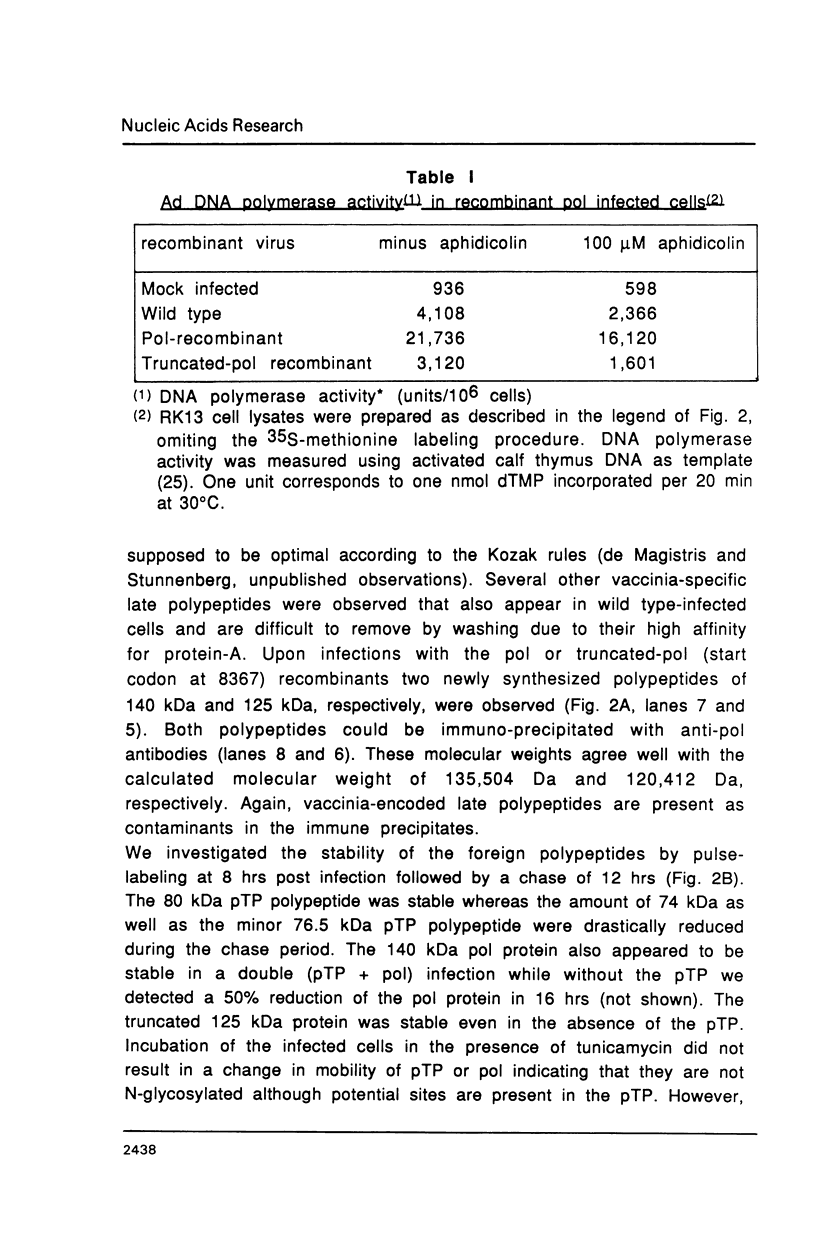
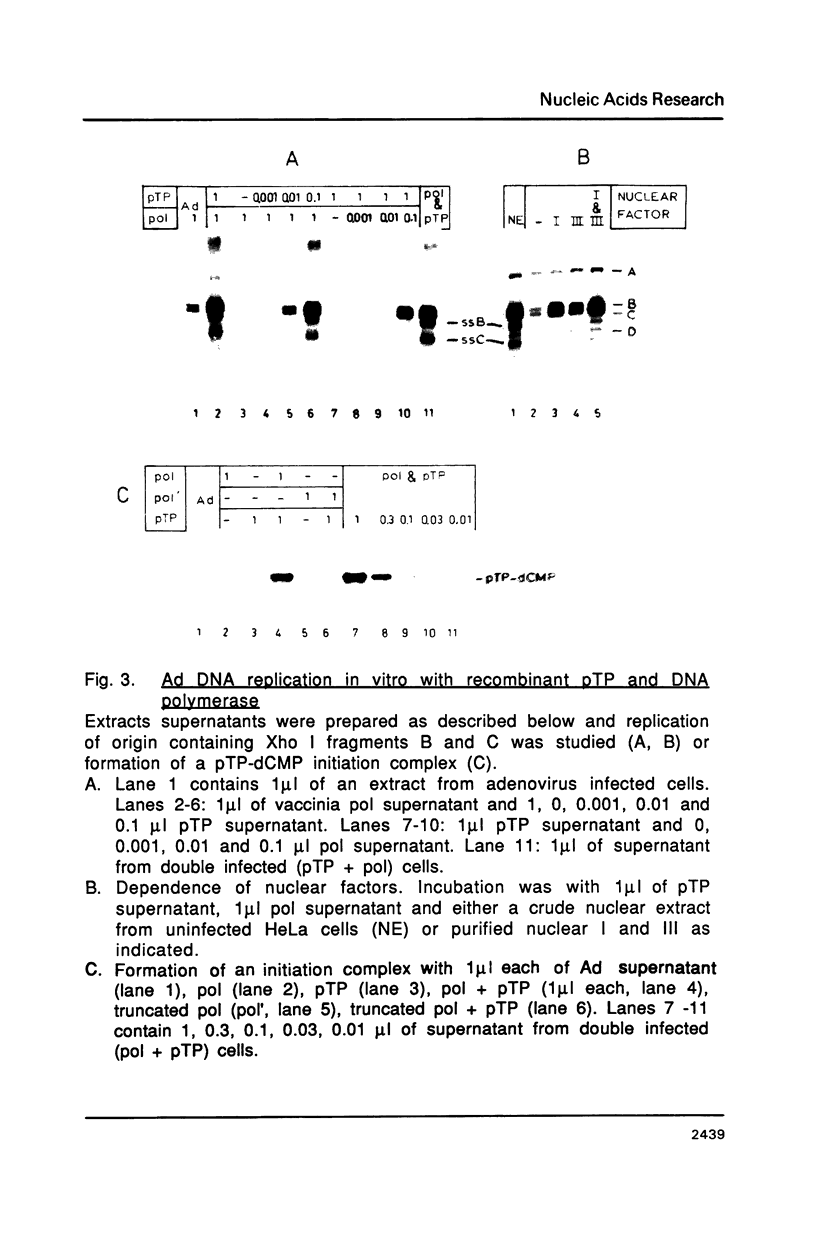





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Tucker A. D., Philipson L. Primary structural relationships may reflect similar DNA replication strategies. Virology. 1986 Mar;149(2):208–216. doi: 10.1016/0042-6822(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker B. M., van Ormondt H. The nucleotide sequence of fragment HindIII-C of human adenovirus type 5 DNA (map positions 17.1-31.7). Gene. 1984 Jan;27(1):115–120. doi: 10.1016/0378-1119(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D. Physical mapping of vaccinia virus temperature-sensitive mutations. Virology. 1983 Dec;131(2):385–393. doi: 10.1016/0042-6822(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Jones E. V., Moss B. Homology between DNA polymerases of poxviruses, herpesviruses, and adenoviruses: nucleotide sequence of the vaccinia virus DNA polymerase gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3659–3663. doi: 10.1073/pnas.83.11.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Freimuth P. I., Ginsberg H. S. Codon insertion mutants of the adenovirus terminal protein. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7816–7820. doi: 10.1073/pnas.83.20.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friefeld B. R., Korn R., de Jong P. J., Sninsky J. J., Horwitz M. S. The 140-kDa adenovirus DNA polymerase is recognized by antibodies to Escherichia coli-synthesized determinants predicted from an open reading frame on the adenovirus genome. Proc Natl Acad Sci U S A. 1985 May;82(9):2652–2656. doi: 10.1073/pnas.82.9.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Hassin D., Korn R., Horwitz M. S. A major internal initiation site for the in vitro translation of the adenovirus DNA polymerase. Virology. 1986 Nov;155(1):214–224. doi: 10.1016/0042-6822(86)90181-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Ariga H. Multiple rounds of adenovirus DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1476–1480. doi: 10.1073/pnas.78.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwant M. M., van der Vliet P. C. Differential effect of aphidicolin on adenovirus DNA synthesis and cellular DNA synthesis. Nucleic Acids Res. 1980 Sep 11;8(17):3993–4007. doi: 10.1093/nar/8.17.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum J. O., Field J., Hurwitz J. The adenovirus DNA binding protein and adenovirus DNA polymerase interact to catalyze elongation of primed DNA templates. J Biol Chem. 1986 Aug 5;261(22):10218–10227. [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. W., Williams J. Cellular transformation by adenovirus type 5 is influenced by the viral DNA polymerase. J Virol. 1987 Nov;61(11):3630–3634. doi: 10.1128/jvi.61.11.3630-3634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucienniczak A., Schroeder E., Zettlmeissl G., Streeck R. E. Nucleotide sequence of a cluster of early and late genes in a conserved segment of the vaccinia virus genome. Nucleic Acids Res. 1985 Feb 11;13(3):985–998. doi: 10.1093/nar/13.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., Kusters H. G., Gmelig Meyling F. H., van der Vliet P. C. Inhibition of adenovirus DNA replication in vitro by autoimmune sera. Eur J Biochem. 1986 Jan 15;154(2):363–370. doi: 10.1111/j.1432-1033.1986.tb09406.x. [DOI] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Rekosh D., Lindenbaum J., Brewster J., Mertz L. M., Hurwitz J., Prestine L. Expression in Escherichia coli of a fusion protein product containing a region of the adenovirus DNA polymerase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2354–2358. doi: 10.1073/pnas.82.8.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri Y., Sanford T., Aguirre P., Padmanabhan R. Immunological analysis of 140-kDa adenovirus-encoded DNA polymerase in adenovirus type 2-infected HeLa cells using antibodies raised against the protein expressed in Escherichia coli. Virology. 1987 Oct;160(2):389–399. doi: 10.1016/0042-6822(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L. M., Hong J. S., Wei Y. F., Engler J. A. Nucleotide sequence of the genes encoded in early region 2b of human adenovirus type 12. Gene. 1986;46(2-3):187–195. doi: 10.1016/0378-1119(86)90403-8. [DOI] [PubMed] [Google Scholar]
- Shu L. M., Horwitz M. S., Engler J. A. Expression of enzymatically active adenovirus DNA polymerase from cloned DNA requires sequences upstream of the main open reading frame. Virology. 1987 Dec;161(2):520–526. doi: 10.1016/0042-6822(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. The mechanism of adenovirus DNA replication and the characterization of replication proteins. Curr Top Microbiol Immunol. 1984;109:53–73. doi: 10.1007/978-3-642-69460-8_2. [DOI] [PubMed] [Google Scholar]
- Tsernoglou D., Tucker A. D., Van der Vliet P. C. Crystallization of a fragment of the adenovirus DNA binding protein. J Mol Biol. 1984 Jan 15;172(2):237–239. doi: 10.1016/s0022-2836(84)80042-x. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries E., Bloemers S. M., van der Vliet P. C. Incorporation of 5-bromodeoxycytidine in the adenovirus 2 replication origin interferes with nuclear factor 1 binding. Nucleic Acids Res. 1987 Sep 25;15(18):7223–7234. doi: 10.1093/nar/15.18.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen B. G., van der Vliet P. C. Temperature-sensitive initiation and elongation of adenovirus DNA replication in vitro with nuclear extracts from H5ts36-, H5ts149-, and H5ts125-infected HeLa cells. J Virol. 1983 May;46(2):642–648. doi: 10.1128/jvi.46.2.642-648.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]





