Abstract
Exposure to acute stressful experience can enhance the later ability to acquire new memories about associations between stimuli. This enhanced learning is observed during classical eyeblink conditioning of both hippocampal-dependent and -independent learning. It can be induced within minutes of the stressful event and persists for days. Here we examined the role of the major stress hormones glucocorticoids in the enhancement of learning after stress. In the first two experiments, it was determined that adrenalectomy (ADX), with and without replacement of basal levels of corticosterone, prevented the stress-induced enhancement of trace conditioning, a task that is dependent on the hippocampus for acquisition. In a third experiment, demedullation, which removes the adrenal medulla but leaves the adrenal cortex and corticosterone levels intact, did not affect the enhancement of learning after stress. In a fourth experiment, ADX prevented the stress-induced enhancement of delay conditioning, a hippocampal-independent task. In a final experiment, it was determined that one injection of stress levels of corticosterone enhanced new learning within minutes but not new learning 24 h later. Together these results suggest that endogenous glucocorticoids are necessary and sufficient for transiently enhancing acquisition of new associative memories and necessary but insufficient for persistently enhancing their acquisition after exposure to an acute stressful experience.
Introduction
Stressful experience can positively affect the subsequent acquisition of associative memories. Indeed, exposure to a variety of acute stressors including intermittent tailshocks, water deprivation, and swim stress enhances classical conditioning procedures such as the eyeblink response, in which a tone stimulus is paired with subsequent stimulation of the eyelid (Berry and Swain, 1989; Shors et al., 1992). During this associative learning task, the animal must blink in response to eyelid stimulation and eventually “learns” to blink in response to the tone. The enhancement of conditioning after stressful experience occurs with other conditioned responses such as those of heart rate (Wilson et al., 1975). Moreover, the enhancement is not restricted to simple procedural tasks such as delay conditioning, in which the stimuli overlap in time, but also occurs during trace conditioning, a more difficult task in which stimuli are separated by a temporal gap (Beylin et al., 1998). This task is dependent on the hippocampal formation for acquisition (Beylin et al., 2001; Solomon et al., 1986). Acute stress also overcomes deficits in performance that occur after exposure to explicitly unpaired stimuli (Beylin et al., 1998). The stress-induced enhancement of associative learning is rapidly induced (within minutes of the stressor) and persistently expressed (Servatius and Shors, 1994; Shors, 2001). Not only does performance continue to increase over days of training, but new learning is also enhanced several days after stressor cessation. The enhancement is sensitive to psychological cues such as those associated with the context; rats that are stressed and returned for training in the same context respond more than those trained in a different context (Shors and Servatius, 1997). [Although not addressed in this report, the enhancing effect of stress on learning is unique to males and does not occur in females (Wood and Shors, 1998; Wood et al., 2001).]
When exposed to such stressors as those that enhance learning, the organism responds by activating a complex series of physiological and behavioral responses that often involve the hypothalamic–pituitary–adrenal axis and autonomic nervous system. The release of glucocorticoids from the adrenal cortex and epinephrine from the adrenal medulla constitute an important part of the stress response which presumably enhances the organism’s ability to deal with stress (Munich et al., 1984). In addition to participation in the stress response, there is considerable literature suggesting that glucocorticoids and catecholamines modulate learning and memory processes. In the case of glucocorticoids, exogenous administration enhances some processes of learning (Sandi and Rose, 1997; Roozendaal and McGaugh, 1997b), whereas removal via adrenalectomy (ADX) has been associated with learning impairments (Conrad and Roy, 1995; Pugh et al., 1997; Vaher et al., 1994). In previous studies, we found a positive correlation between basal levels of glucocorticoids during training and acquisition of trace memories in male rats (Wood et al., 2001). The medullary substances epinephrine and norepinephrine have also been associated with enhanced performance on learning tasks, whereas antagonism of their receptors can be detrimental (Gamaro et al., 1997; Leon, 1998; Roozendaal et al., 1996).
Because of the important role that these two adrenal systems—the medulla and cortex—play in the stress response and the potential impact on learning, we examined their role in enhanced learning after exposure to the stressor of brief intermittent tailshocks. In a series of five experiments, we tested whether the presence of adrenal hormones is necessary and sufficient for induction and expression of the facilitation of associative learning after exposure to an acute stressful event. Procedures and details of individual experiments are presented below.
Methods
Subjects
Sprague–Dawley rats (250 –350 g) were housed in groups of five prior to surgery and individually postoperatively to prevent head stage damage. Food and water were provided ad libitium with a 14-h light followed by 10-h dark cycle. Light onset occurred at 7 AM with testing between 9 AM and 6 PM.
Electrode surgery
Rats were fitted with head stages attached to electrodes as previously described (Servatius et al., 1994). Under anesthesia, four electrodes were implanted subcutaneously to emerge through the eyelid; two were used to deliver a periorbital stimulation and two to record electromyographic (EMG) activity, which was used as an indirect measure of eyeblinks. Postoperatively, rats were injected intramuscularly with 300,000 units of penicillin and provided Children’s Tylenol diluted 1:100 in drinking water for 48 h.
Adrenalectomy and demedullation
Rats were anesthetized with sodium pentobarbital (50 mg/kg) and adrenal glands removed bilaterally through small dorsal incisions. Following surgery, ADX rats were provided 0.9% saline and tap water. In Experiments 2 and 4, ADX rats had access to either water or saline supplemented with corticosterone (25 μg/ml). ADX rats were tested within 1 week of surgery. During demedullation (Demed), a small incision was made in the adrenal gland and pressure was placed on the gland until the medulla popped out, leaving the cortex intact. Rats assigned to sham surgery were subjected to the same surgical procedure except that the adrenal glands or medulla were left intact. Demed rats were given 4 weeks of recovery between surgery and training, allowing time for the adrenal cortex to regenerate and produce corticosterone.
Corticosterone injection
Corticosterone (Sigma) was dissolved in absolute ethanol and diluted in 0.9% saline to 5 mg/kg concentration with 5% ethanol. This dose induced plasma levels similar to those observed after an acute stressor (Sandi et al., 1996). Groups of rats were injected with corticosterone (CORT) or vehicle and sacrificed 30 min later with trunk blood collected. Additional groups were injected and trained 30 min or 24 h later and sacrificed with trunk blood collected.
Stress and conditioning procedure
Head stages were connected to a cable that allowed free movement within the conditioning chamber. Eyelid EMG activity was filtered to pass 0.3–1.0 KHz, amplified (10,000) with a differential AC amplifier, and passed through a 16-bit A/D card. All rats were acclimated to the conditioning apparatus for 1 h with the ventilating fans and house lights operating. In the absence of any conditioning stimuli, spontaneous blink rate was recorded. A total of 100 samples of 550 ms were recorded over ~1 h. A blink during a sampling period was scored as a response.
Stressed rats were transferred to a separate room, restrained in Plexiglas holders, and exposed to 90 1-s, 1-mA, 60-Hz shocks to the tail, after which they were returned to their home cages. Unstressed rats were returned to their home cages for 24 h. Rats were returned to the conditioning apparatus and spontaneous eyeblinks were again recorded for ~20 min. They were then exposed to 10 white noise stimuli (320 ms, 80 dB, ITI: 20 ± 10 s). If an eyeblink occurred during the first 80 ms of the stimulus, it was scored as a sensitized response to the CS. Rats were classically conditioned with 300 trials of paired stimuli. The intertrial interval was randomized between 20 and 30 s and every 10 trials consisted of a CS-alone (80 dB, 5 ms rise and fall time), followed by four paired trials, a US-alone, and four paired trials. During trace conditioning, a 250-ms CS and 100-ms US were separated by a 500-ms trace interval. During delay conditioning, an 850-ms CS overlapped and coterminated with a 100-ms US. The interstimulus interval was 750 ms during both delay and trace conditioning.
To determine the occurrence of a conditioned response, the maximum EMG response that occurred during the baseline (250 ms prior to CS onset) was added to four times its standard deviation. Responses that exceeded that value and had a width of at least 3 ms were considered eyeblinks. Eyeblinks were considered conditioned responses (CRs) when they occurred 250 –1000 ms after CS onset on CS-alone trials and 250 ms after CS onset but prior to US onset (250 –750 ms after CS onset) on paired trials. Performance was computed as a percentage of eyeblinks that occurred during each 100 trials (90 in which a CS was delivered). Analysis of variance (ANOVA) with repeated measures, planned comparisons, and Newman–Keuls were used for analysis.
Radioimmunoassay
After training, all rats were anesthetized with sodium pentobarbital and decapitated. Trunk blood was collected between 1600 and 1900 h, when corticosterone levels are relatively high. Blood was collected in heparinized tubes and centrifuged and plasma was stored at −80°C. Hormone levels were determined using Coat-A-Count radioimmunoassay for rat corticosterone with a detection limit of 5.7 ng/ml.
Results
Experiment 1: ADX, stress, and trace conditioning
In the first experiment, ADX and sham-operated rats were either stressed or unstressed and trained 24 h later with 300 trials of trace conditioning. There were four groups as follows: ADX exposed to stress (n = 7); ADX not exposed to stress (n = 9); sham-operated controls exposed to stress (n = 8); and sham-operated controls not exposed to stress (n = 9). The results were analyzed using ANOVA with independent variables of stressor exposure (stress vs no stress) and surgical treatment (ADX vs sham-operated) as well as a repeated measure of training blocks (three blocks of 100 trials each). There was a two-way interaction between ADX and stressor exposure on percentage of CRs during the 300 trials of trace conditioning [F(1,29) = 6.63; P < 0.05] (Fig. 1). Post hoc analysis revealed that the sham-operated rats exposed to stress emitted more CRs than any other group (P < 0.001). Also, performance in ADX rats exposed to stress was not different from that in ADX rats not exposed to stress (P = 0.89). Performance of unstressed ADX rats did not differ from that of unstressed sham-operated rats and thus ADX did not affect conditioning itself (P = 0.89). Neither ADX nor stress altered spontaneous blink rate (P = 0.90, P = 0.29, respectively) or responses to the CS prior to training (P = 0.90, P = 0.70, respectively).
Fig. 1.
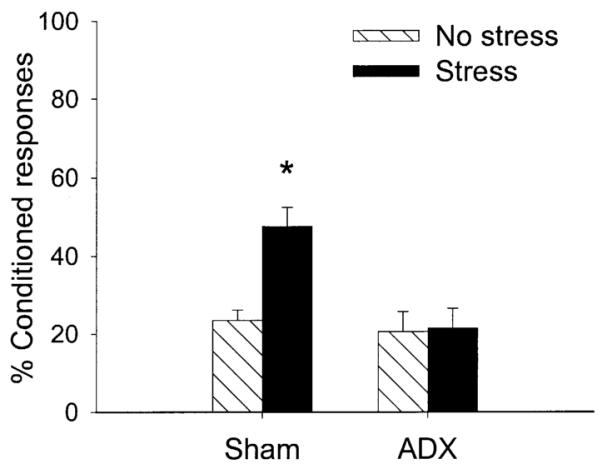
The percentage CRs in unstressed (No stress) or stressed (Stress) rats during trace conditioning. Groups were either adrenalectomized (ADX) or exposed to sham surgery (Sham). ADX prevented the enhancement of conditioning 24 h after stressor exposure.
As expected, ADX reduced CORT levels [F(1,29) = 316.97; P < 0.001]. There was no effect of the stressor on CORT levels >24 h after its cessation (P = 0.68) nor an interaction with ADX (P = 0.65). Levels were 132.63 ± 4.91 ng/ml for the unstressed sham group, 139.19 ± 14.65 for the stressed sham group, and less than 1 ng/ml for both ADX groups.
Experiment 2: ADX with basal CORT, stress, and trace conditioning
This experiment is essentially a replication of the previous one except that low levels of CORT were provided in the drinking water of ADX rats and rats were trained for a second day of 300 trials (total of 600 trials). There were four groups as follows: ADX exposed to the stressor (n = 7); ADX not exposed to the stressor (n = 7); sham-operated controls exposed to the stressor (n = 7); and sham-operated controls not exposed to the stressor (n = 8). Using ANOVA, there was a three-way interaction between ADX (ADX vs sham), stressor exposure (stress vs no stress), and percentage CRs over 600 trials (six blocks of 100 trials) of trace conditioning [F(5,100) = 3.04; P < 0.05] (Fig. 2). Exposure to the stressor of inescapable tailshocks enhanced the number of CRs in the sham-operated group (P < 0.005) but did not affect the number of CRs in ADX rats even in the presence of low levels of CORT (P = 0.40) (Fig. 2). As in Experiment 1, ADX did not affect overall conditioning (P = 0.17). There was no effect of ADX or stress on spontaneous blink rate (P = 0.29, P = 0.73, respectively) or response to the CS prior to training (P = 0.60, P = 0.89, respectively).
Fig. 2.
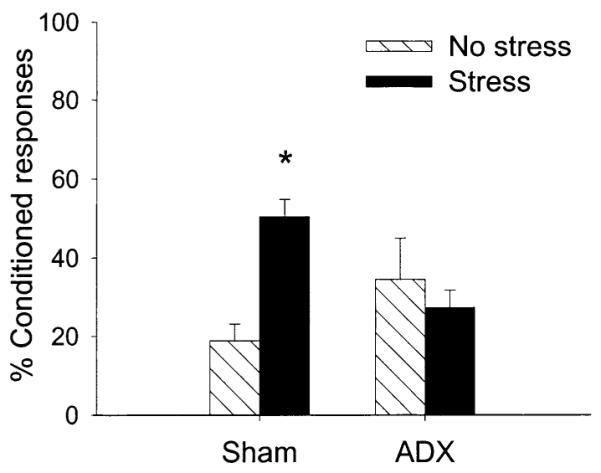
The percentage CRs in unstressed (No stress) or stressed (Stress) rats during trace conditioning. Groups were either adrenalectomized (ADX) or exposed to sham surgery (Sham). Basal levels of corticosterone were provided in the drinking water. Nonetheless, ADX prevented the enhancement of conditioning 24 h after stressor exposure.
CORT levels were 310.87 ± 72.50 ng/ml for the sham unstressed group and 332.70 ± 49.48 ng/ml for the sham stressed group, 55.25 ± 11.76 ng/ml for the ADX stress group and 41.71 ± 7.41 ng/ml for the ADX unstressed group. Although levels were reduced in the ADX rats that were provided CORT in the drinking water [F(1,25) = 33.29; P < 0.0001], they were nonetheless higher than those in rats without replacement in Experiment 1 and similar to levels that prevent ADX-induced cell death in the dentate gyrus of the hippocampal formation (Sloviter et al., 1989). Stressor exposure did not alter CORT levels in either ADX or sham-operated rats at the time of sacrifice, which was more than 48 h later (P = 0.84, P = 0.74, respectively).
Experiment 3: demedullation, stress, and trace conditioning
This experiment used similar procedures as in Experiment 1 except that only the adrenal medulla was removed rather than the entire adrenal gland. Rats were exposed to 300 trials of trace conditioning. There were four groups as follows: Demed rats exposed to the stressor (n = 6); Demed rats not exposed to the stressor (n = 7); sham-operated controls exposed to the stressor (n = 6); and sham-operated controls not exposed to the stressor (n = 6). Following ANOVA, there was a two-way interaction between stress (stress vs no stress) and trials of training (three blocks of 100 trials) on the percentage CRs [F(2,42) = 5.49; P < 0.01]. Stress enhanced the rate of acquisition in both stress groups irrespective of demedullation (Fig. 3). Demedullation alone did not affect the percentage CRs (P = 0.71) or rate of conditioning (P = 0.93). Neither demedullation nor stressor exposure altered spontaneous blink rate (P = 0.26, P = 0.23, respectively) or responding to the CS prior to training (P = 0.09, P = 0.60, respectively).
Fig. 3.
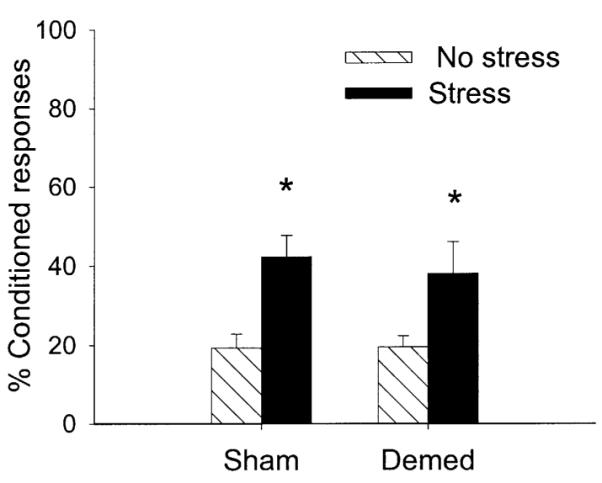
The percentage CRs in unstressed (No stress) or stressed (Stress) rats during trace conditioning. Groups were either adrenal demedullated (Demed) or exposed to sham surgery (Sham). Demed did not affect the enhancement of conditioning 24 h after stressor exposure.
As expected, demedullation did not alter plasma levels of CORT (P = 0.35) nor did stressor exposure >24 h after its cessation, when trunk blood was collected (P = 0.40). Levels were 199.58 ± 13.64 ng/ml for the sham no stress group, 220.83 ± 12.63 ng/ml for the sham stress group, 184.69 ± 22.35 ng/ml for the Demed no stress group, and 197.67 ± 25.60 ng/ml for the Demed stress group.
Experiment 4: ADX, stress, and delay conditioning
This experiment was similar to Experiment 2 except rats were trained with the hippocampal-independent task of delay rather than the hippocampal-dependent task of trace conditioning. Twenty-four h after stressor exposure, they were trained with 300 trials, and 300 more trials on the next day. As in Experiment 2, ADX rats were provided CORT in their drinking water. Rats were grouped as follows: ADX rats exposed to the stressor (n = 6); ADX rats not exposed to the stressor (n = 8); sham-operated controls exposed to the stressor (n = 7); and sham-operated controls not exposed to the stressor (n = 7). Following ANOVA, there was a two-way interaction between surgery (ADX vs sham) and stressor exposure (stress vs no stress) on the percentage CRs [F(1,20) = 5.52; P < 0.05] (Fig 4). Again, stressor exposure enhanced conditioning in the rats exposed to a sham surgery; conditioning in ADX rats exposed to the stressor was not different from that in ADX rats not exposed to the stressor (P = 0.48) (Fig. 4). Neither ADX nor stressor exposure altered spontaneous blink rate (P = 0.50, P = 0.43, respectively) or induced responses to the CS prior to training (P = 0.68, P = 0.98, respectively).
Fig. 4.
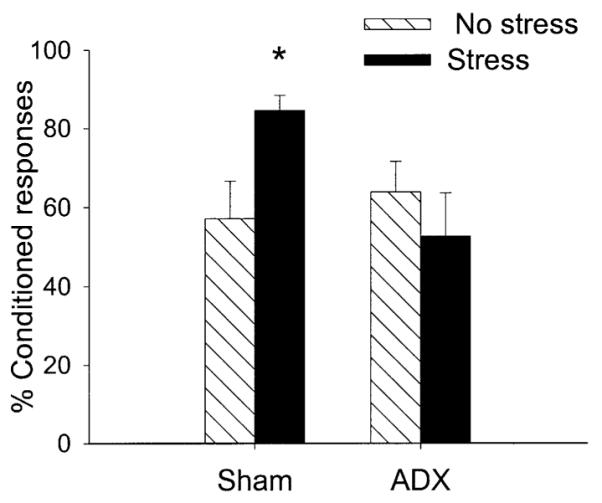
The percentage CRs in unstressed (No stress) or stressed (Stress) rats during delay conditioning. Groups were either adrenalectomized (ADX) or exposed to sham surgery (Sham). ADX prevented the enhancement of conditioning 24 h after stressor exposure.
CORT levels were 248.55 ± 23.26 ng/ml for the sham no stress group, 285.50 ± 27.23 for the sham stress group, 50.17 ± 10.03 ng/ml for the ADX no stress group, and 45.18 ± 11.48 ng/ml for the ADX stress group. As in the second experiment, ADX reduced corticosterone levels [F(1,24) = 126; P < 0.001], and they were slightly elevated by the CORT in the drinking water. Again, stressor exposure did not affect CORT levels >48 h after its cessation (P = 0.42).
Experiment 5: stress levels of CORT and trace conditioning
In the final experiment, intact rats were injected with stress levels of CORT (5 mg/kg) or the vehicle and trace conditioned either 30 min (CORT n = 6; vehicle n = 7) or 24 h after the injection (CORT n = 7; vehicle n = 6). Groups were exposed to 300 trials on one day and 300 more trials on the next day. Following ANOVA, there was a two-way interaction between the injection procedure (CORT vs vehicle) and the time since injection (30 min vs 24 h) on the percentage CRs during 600 trials of training [F(1,22) = 4.46; P < 0.05]. Rats exposed to a bolus of exogenous CORT emitted more CRs when trained 30 min later than any of the other groups (P < 0.05) (Fig. 5). These rats were exposed to 300 more trials of training the next day. They emitted more CRs during the last block of trials (P < 0.01), but their CORT levels were no longer elevated. Thus, the high rate of responding after stressor exposure is likely a result of learning rather than an increase in performance of the motor response in the presence of high levels of CORT.
Fig. 5.
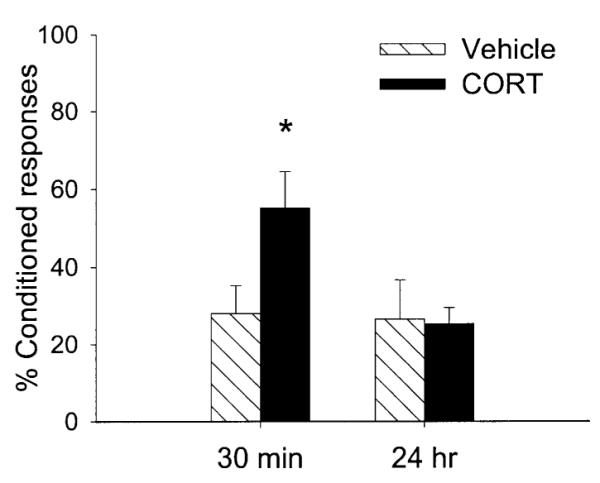
The percentage CRs in rats injected with 5 mg/kg of corticosterone and trained either 30 min later (30 min) or a day (24 h) later with trace conditioning. Exogenous corticosterone enhanced conditioning 30 min but not 24 h later. This dose produced plasma CORT levels similar to those observed in rats exposed to acute swim stress or intermittent tailshocks (Shors, 2001).
To verify that the injection of CORT would achieve stress levels in the blood, additional groups were injected with 5 mg/kg of CORT (n = 5) or the vehicle (n = 5), not trained, and sacrificed 30 min later. Injection of CORT enhanced plasma levels 30 min later relative to those injected with saline [F(1,8) = 233.45; P < 0.001]. Levels were 402.00 ± 9.26 ng/ml after CORT injection and 211.20 ± 8.38 ng/ml after vehicle injection. These levels are similar to those observed minutes after exposure to the stressor of intermittent tailshocks or inescapable swimming, both of which enhance classical eyeblink conditioning (Shors, 2001).
Discussion
Exposure to an acute stressful experience greatly enhances classical conditioning of the eyeblink response in the male rat (Servatius and Shors, 1994; Shors et al., 1992). This effect is observed within minutes to 24 h after the stressful event has passed (Beylin and Shors, 1998; Shors, 2001). Here we hypothesized that the major stress hormones glucocorticoids were involved and perhaps necessary for enhancing the formation of associative memories after acute stressful experience. To test this hypothesis, glucocorticoids were manipulated under various conditions and rats were later exposed to the acute stressor of intermittent tailshocks or were left undisturbed. One day later, male rats were trained on the hippocampal-dependent task of trace eyeblink conditioning or the hippocampal-independent delay procedure. Removal of the adrenal glands prevented the stress-induced enhancement of trace and delay conditioning (Figs. 1 and 4) as it also did when basal levels of CORT were maintained after ADX (Figs. 2 and 4). Although the results from these studies suggest that glucocorticoids are involved in the stress effect on conditioning, they are inconclusive since the adrenal glands produce other substances such as epinephrine. To rule out the contribution of substances within the adrenal medulla, we removed the structure while leaving the adrenal cortex intact (adrenal demedullation). As illustrated in Fig. 3, exposure to the stressful event enhanced trace conditioning even in the absence of the adrenal medulla. Thus, adrenal glucocorticoids appear to be critically involved in the stress-induced enhancement of trace and delay conditioning in the male rat.
To this point, the results suggested that the stress-induced release of glucocorticoids is necessary for the enhanced conditioning after stress, but do not address whether it is sufficient. In a final experiment, we tested whether exposure to stress levels of CORT would enhance conditioning. Male rats were injected with one dose of CORT (5 mg/kg) and 30 min later, serum levels were similar to those observed after exposure to the acute stressor of intermittent tailshocks (Sandi et al., 1996; Shors, 2001). Interestingly, the one injection with CORT enhanced trace conditioning, but only when trained minutes after the injection, not when initially exposed to training 24 h later (Fig. 5). Thus the stress-induced enhancement of conditioning can be mimicked by an acute exposure to glucocorticoids, but the effect does not persist, as it does after exposure to the acute tailshock stress. As a group, the animals that were trained minutes after the injection and in the presence of stress levels of glucocorticoids continued to emit more conditioned responses even the next day, when glucocorticoid levels were no longer elevated from the injection. Thus, the presence of stress levels of glucocorticoids is not necessary for continued expression of the enhanced conditioning. These results suggest that exposure to glucocorticoids does not just enhance performance of the response, but rather does enhance learning. In summary, these various data indicate that while insufficient, glucocorticoids are at the time of stressor exposure nonetheless necessary for the persistent enhancement of learning after stress.
How would glucocorticoids act to enhance learning and memory formation? Glucocorticoids have two known receptors, the mineralocorticoid (Type I) and stress-related glucocorticoid (Type II) (Reul and de Kloet, 1985). Hormones act on these intracellular receptors to initiate changes in protein synthesis and transcription. This mode of action is potent and can have effects on many systems, but requires some time to engage (minutes to hours). Since exposure to stress can enhance conditioning within minutes as did the one injection of corticosterone, mechanisms other than the more traditional nuclear ones may be involved. For example, glucocorticoids have rapid effects on physiological activity and behavior that have been suggested to be mediated by effects on membrane fluidity or membrane receptors (Armanini et al., 1990; Orchinik, 1998; Orchinik et al., 1991; Venero and Borrell, 1999). It has been suggested that these rapid effects initiate fast responses while activity at nuclear receptors initiates more persistent ones (Christ and Wehling, 1998; Falkenstein et al., 2000; Orchinik, 1998; Sandi et al., 1997). With respect to the data presented here, the relatively rapid effect of corticosterone exposure on conditioning could occur via effects on membrane properties (Joëls and de Kloet, 1989; Karst and Joels, 1991), whereas the more persistent effect of stressor exposure likely requires additional factors for activation such as glutamate release, NMDA receptor activation, or increased occupancy of or changes in GR and MR receptors, all of which occur in response to acute stress and can interact with learning processes (Deak et al., 1999; Kim et al., 1996; Shors and Mathew, 1998; Venero et al., 1999). It is also possible that these relatively fast effects occur via interactions among glucocorticoids, neurotransmitters, and second messenger systems.
Even when identified, it is still not obvious how additional factors would interact with glucocorticoids to produce such a long-term change in learning ability. Anatomical changes are a consideration since they represent a means for long-term modification in brain substrates that could in turn affect function. We have recently reported that exposure to the stressor of intermittent tailshocks that enhances memory formation also enhances the density of dendritic spines 24 h later (Shors et al., 2001). The increase was observed on apical dendrites in area CA1 of the hippocampus, but not on dendrites in cortical regions. Spines are sources of excitatory input that exist at many if not most synapses in the hippocampus (Shephard and Harris, 1998). Because they can form quickly and persist for days, they are considered viable candidates for substrates of memory formation (Moser, 1999; Woolley et al., 1990). At the neuroanatomical level, they provide a means whereby associations between stimulus representations could form. Indeed, we have recent data to suggest that training with both delay and trace conditioning increases the observation of dendritic spines in area CA1 of the hippocampus (Leuner et al., 2002). If synaptic spines are involved in learning, an increase in their availability after a stressful experience may provide an anatomical substrate for enhancing the formation of new memories should the opportunity arise. Such a mechanism would be sustained for several days after the stressful experience, when the chance of another encounter would be high. In addition to its adaptive value, such a process could persist without a sustained increase in stress hormones, which can be detrimental to hippocampal function (Magarinos et al., 1997; McEwen, 2000; Sapolsky, 1996, 1999; Stein-Behrens et al., 1994; Woolley and McEwen, 1992).
Exposure to stress levels of glucocorticoids either endogenously or exogenously is not exclusively an enhancing stimulus for learning. In fact, there are several reports that glucocorticoids impair processes of retrieval and in some cases acquisition, at least during spatial navigation learning (de Quervain et al., 1998; Diamond and Rose, 1994; Kim et al., 2001; Kim and Diamond, 2002). In other studies, exposure to stress levels of glucocorticoids immediately after training enhanced long-term retrieval but not acquisition (Sandi et al., 1997), again during performance of a spatial navigation task. Some of these seemingly conflicting effects of glucocorticoids are likely attributable to very different types, times, or lengths of stressor exposure or concentrations of CORT administration, not to mention measurement of different learning tasks and processes within those tasks. In the present experiments, we concentrated on one learning paradigm, that of classical eyeblink conditioning, using slight variations in stimulus presentation to differentially engage the hippocampus. We also focused exclusively on acquisition of these learned responses. The present data indicate that glucocorticoids are necessary for enhancing acquisition of these associations, but they do not permit us to draw conclusions about how glucocorticoids affect learning processes in general. In fact, such attempts are likely futile, given the relatively ubiquitous presence of the hormone, not to mention the complexity of learning processes themselves.
It is oftentimes proposed that glucocorticoids affect memory formation via the hippocampal formation. This is based in part on the structure’s relatively high density of CORT receptors (McEwen and Wallach, 1973). However, it is MR receptors that appear most concentrated in the hippocampus (Reul and de Kloet, 1985). Thus, the link between stress-induced glucocorticoids and memory in the hippocampus may be overemphasized, as recently suggested (Lupien and Lepage, 2001). There are several studies highlighting a role for the amygdala. For example, the enhancing effects of glucocorticoids on inhibitory conditioning were prevented by lesions to the basolateral nucleus of the amygdala (Roozendaal and McGaugh, 1997a). We observed that the enhancement of eyeblink conditioning after stressor exposure was ameliorated by blocking NMDA receptor antagonism specifically in the basolateral and not the central nucleus of the amygdala (Shors and Mathew, 1998). The blockade was only effective when instituted during the stressor and not afterwards, suggesting that some critical aspects of the induction process occur in the amygdala during the stressful experience.
ADX prevented the stress effect on conditioning irrespective of whether or not the task was dependent on the hippocampus. Thus, this particular effect of stress on learning is not limited to hippocampal-dependent types. Others have found that activity in the hippocampus can modulate learning that is independent of the structure. For example, glucocorticoids injected directly into the hippocampus enhance inhibitory conditioning, a task that is not dependent on the hippocampus (Roozendaal and McGaugh, 1997a). The data presented here do not illuminate whether the hippocampal formation is critically involved in the stress-induced enhancement of conditioning. They do, however, place the relationship between glucocorticoids and learning in a relatively unusual and positive light. That is, acute exposure to stressful experience can transiently engage glucocorticoids to induce a persistent and positive outcome— that of enhanced memory formation.
Acknowledgments
This work was supported by a National Science Foundation Fellowship to A.V.B., and NIMH (59970), NSF (IBN0217403), and National Alliance for Research on Schizophrenia and Depression (NARSAD) to T.J.S.
References
- Armanini MP, Hutchins C, Stein BA, Sapolsky RM. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- Berry SD, Swain RA. Water deprivation optimized hippocampal activity and facilitates nictitating membrane conditioning. Behav. Neurosci. 1989;105:202–209. doi: 10.1037//0735-7044.103.1.71. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav. Neurosci. 1998;112:1327–1338. doi: 10.1037//0735-7044.112.6.1327. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Talk AC, Gandhi CC, Wood GE, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal incongruity or task difficulty? Neurobiol. Learn. Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Christ M, Wehling M. Cardiovascular steroid actions: swift swallows or sluggish snails? Cardiovasc. Res. 1998;40:34–44. doi: 10.1016/s0008-6363(98)00147-3. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Roy EJ. Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middleaged rats. Hippocampus. 1995;5:1–15. doi: 10.1002/hipo.450050103. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Cotter CS, Fleshner M, Watkins LR, Maier SF, Spencer RL. Long-term changes in mineralocorticoid and glucocorticoid receptor occupancy following exposure to an acute stressor. Brain Res. 1999;847:211–220. doi: 10.1016/s0006-8993(99)02050-8. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann. NY Acad. Sci. 1994;30:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones—A focus on rapid, non-genomic effects. Pharmacol. Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- Gamaro GD, Denardin JD, Michalowski MB, Catelli D, Correa JB, Xavier MH, Dalmaz C. Epinephrine effects on memory are not dependent on hepatic glucose release. Neurobiol. Learn. Mem. 1997;68:221–229. doi: 10.1006/nlme.1997.3787. [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER. Effects of glucocorticoids and norepinephrine on the excitability of the hippocampus. Science. 1989;245:1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- Karst H, Joels M. The induction of corticosteroid actions on membrane properties of hippocampal CA1 neurons requires protein synthesis. Neurosci. Lett. 1991;130:27–31. doi: 10.1016/0304-3940(91)90219-j. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Neurosci. Rev. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc. Natl. Acad. Sci. USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J. Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M. Catecholaminergic contributions to early learning. Adv. Pharmacol. 1998;42:961–964. doi: 10.1016/s1054-3589(08)60907-2. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2002 doi: 10.1523/JNEUROSCI.23-02-00659.2003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behav. Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Prog. Brain Res. 2000;122:25–34. doi: 10.1016/s0079-6123(08)62128-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wallach G. Corticosterone binding to the hippocampus: nuclear and cytosol binding in vitro. Brain Res. 1973;57:373–386. doi: 10.1016/0006-8993(73)90143-1. [DOI] [PubMed] [Google Scholar]
- Moser E. Making more synapses? a way to store information? Cell Mol. Life Sci. 1999;55:593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munich A, Guyre PM, Holbrook NJ. Physiological function of glucocorticoids in stress and their relation to pharmacological actions. Endocrinol. Rev. 1984;1:339–376. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Orchinik M. Glucocorticoids, stress, and behavior: shifting the timeframe. Horm. Behav. 1998;34:320–327. doi: 10.1006/hbeh.1998.1488. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Pugh RC, Tremblay D, Fleshner M, Rudy JW. A selective role of corticosterone in contextual-fear conditioning. Behav. Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- Reul JHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 1997a;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh J. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol. Learn. Mem. 1997b;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Carmi O, McGaugh J. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proc. Natl. Acad. Sci. USA. 1996;93:1429–1433. doi: 10.1073/pnas.93.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP. Training-dependent biphasic effects of corticosterone in memory formation for a passive avoidance task in chicks. Psychopharmacology. 1997;133:152–160. doi: 10.1007/s002130050385. [DOI] [PubMed] [Google Scholar]
- Sandi C, Venero C, Guaza C. Novelty-related rapid locomotor effects of corticosterone in rats. Eur. J. Neurosci. 1996;8:794–800. doi: 10.1111/j.1460-9568.1996.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;97:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress and their adverse neurological effects: relevance to aging. Exp. Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behav. Neurosci. 1994;108:1101–1106. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- Shephard GMG, Harris KM. Three-dimensional structure and composition of CA3/CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol. Learn. Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol. Learn. Mem. 1997;67:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. NMDA receptor antagonism in the basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. J. Learn. Mem. 1998;5:220–230. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Solomon PR, van der Schaaf ER, Thompson RF, Weisz D. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Mattson MP, Chang I, Yeh M, Sapolsky RM. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 1994;14:5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaher PL, Luine V, Gould E, McEwen BS. Adrenalectomy impairs spatial memory in rats. Ann. NY Acad. Sci. 1994;746:407. doi: 10.1111/j.1749-6632.1994.tb39269.x. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur. J. Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Wilson LM, Wilson JR, Dicara LV. Facilitation of Pavlovian conditioned cardiodecelerations following preshock in immobilized rats. Physiol. Behav. 1975;15:653–658. doi: 10.1016/0031-9384(75)90115-8. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males but impairs conditioning in females through activational influences of ovarian hormones. Proc. Natl. Acad. Sci. USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav. Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuations in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


