Abstract
It is usually assumed that stressful life events interfere with our ability to acquire new information. However, many studies suggest that stressful experience can enhance processes involved in learning. The types of learning that are enhanced after stressful experiences include classical fear and eyeblink conditioning, as well as processes related to learning about threatening stimuli. Stressful life experiences do seem to interfere with processes involved in memory, often expressed as deficits in the retention or retrieval of information that was acquired prior to and was unrelated to the stressful experience. The trends are limited, as are their implications, because most studies examine adult males, yet the effects of stress on learning processes are influenced by age and sex differences. With respect to mechanisms and anatomical substrates, the effects of stress on learning are usually dependent on the action of stress hormones in combination with neuronal activities within the hippocampus, amygdala, the bed nucleus of the stria terminalis, and the prefrontal cortex.
Keywords: memory, sex differences, neurogenesis, hippocampus, amygdala
INTRODUCTION
The effects of acute stressful experience on subsequent learning are diverse and vary in their direction, strength, and occurrence. The variability in the types of responses is attributable, at least in part, to organismal properties, such as age, sex differences, and species. The responses are also dependent on the types of stressful events that occur, the length and intensity of those experiences, and finally, on the type of learning that is assessed. Because of this, there is no consistent or simple relationship between stress and learning. In this review, the effects of stressful experience on learning abilities in human and nonhuman animals are reviewed. Changes that occur across the lifespan are highlighted, as are effects that differ between males and females. Finally, the brain substrates and neuronal mechanisms that underlie the effects of stress on learning are assessed, with an emphasis on glucocorticoids and changes that occur within the hippocampus, amygdala, bed nucleus of the stria terminalis, and prefrontal cortex.
STRESS EFFECTS ON PROCESSES OF LEARNING AND MEMORY
The Effects of Acute Traumatic Stress on Learning and Memory in Humans
It is understandable that there would be numerous types of effects of stress on learning because there are so many types of stressful events that animals can experience in their lives. In humans, the effect of two types of stressful experience are most often studied—those that occur naturally during a severe trauma or those that are experimentally induced and often are not as stressful, such as public speaking or social interaction. The effects of traumatic experience on processes involved in learning abilities are discussed first. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; Am. Psychiatric Assoc. 2000), a traumatic stressor is an event that includes “actual or threatened death or serious injury, or other threat to one’s physical integrity or others.” Traumatic events include military combat, violent personal assault, being kidnapped, being taken hostage, terrorist attack, torture, incarceration as a prisoner of war or in a concentration camp, natural or manmade disasters, severe automobile accidents, or being diagnosed with a life-threatening illness. The ways in which traumatic stress can affect learning processes tend to fall into three categories: the learning abilities that exist before and during the traumatic experience (i.e., intelligence), memory processes that are affected during the experience itself (memory for the trauma and related stimuli), and the more incidental effects of the trauma on subsequent learning abilities.
There have been numerous reports that intelligence and general learning abilities at the time of trauma can predict whether a person will develop the pathological symptoms associated with posttraumatic stress disorder (PTSD) (Buckley 2000). In particular, several studies have reported a negative correlation between intelligence and the likelihood that a person will develop PTSD, such that war veterans with low IQ are more likely to develop symptoms of PTSD (Pitman et al. 1991). In one study, veterans from Operation Desert Storm were subjected to tests of attention, verbal memory, and visuospatial memory after returning home from the war (Vasterling et al. 1997). There was no relationship between PTSD and attention, but those that developed PTSD were more likely to have low scores on tests of verbal memory, which is thought to represent a more crystallized form of intelligence. This relationship between verbal memory and PTSD was apparently not affected by the amount or severity of the trauma but instead was a reflection of the degree of verbal memory skills prior to combat exposure. Thus, the data suggest that pretrauma abilities predict how the stressful event will affect future abilities, some of which include processes involved in learning and memory.
Several attempts have been made to directly assess associative learning in humans who have been exposed to a traumatic life event. One of the most common procedures involves classical eyeblink conditioning. In the standard paradigm, a human is presented with an airpuff to the eye, which elicits an eyeblink as an unconditioned response (UR). When the airpuff is immediately preceded by an auditory stimulus as the conditioned stimulus (CS), humans learn the association between the tone and the airpuff and blink in response to the tone, in anticipation of the airpuff. This blink is the conditioned response (CR) and is used as an indirect measure of learning. Performance of this task has been assessed in patients that have experienced trauma, and at least one study reports deficits in conditioning (Ayers et al. 2003). Interestingly, the deficits occurred in groups that experienced trauma associated with combat, irrespective of whether they suffered from PTSD, although medication may have contributed to the deficits. Aside from this effect, there are few demonstrations that learning per se is impaired by traumatic experience. Rather, it appears that processes involved in memory recall and retrieval are more vulnerable. For example, veterans with PTSD were able to acquire new information at the time of encoding; however, when asked to recall that information later, they expressed deficits (Yehuda et al. 1995). These effects of trauma on memory function are not limited to war stress but extend to other stressors such as childhood abuse (Bremner et al. 1995).
In the studies discussed thus far, the stimuli embedded in the learning tasks were unrelated or at least not directly associated with the trauma itself, yet humans react to stimuli that are related to stressful events differently than they react to stimuli that are unrelated to the stressful event. Some studies find that PTSD patients respond to threatening stimuli faster than to unthreatening stimuli (Bryant & Harvey 1995, 1997), although others suggest no bias exists (Trandel & McNally 1987). One of the more popular methods of assessing information processing after a stressful experience is a modified Stroop test, in which people are presented with words that are either threatening or neutral. The words are presented in colors, and the subject is asked to name the color of the word. In one study, rape victims with PTSD responded slower when naming the color of rape-related words than when naming neutral words; this bias did not occur in rape victims without PTSD or in women that had not been raped (Foa et al. 1991). This delayed recall of threat words has also been observed in patients that were victims of motor vehicle accidents and combat (Bryant & Harvey 1995). Thus, these studies suggest that humans respond to the cues related to trauma at a slower rate than to cues that are unrelated to trauma. These findings can be interpreted in at least one of two ways; one is that the victims are avoiding the threatening stimuli, which retards their response. The other is that the victims are paying more attention to the threat-related cues, which retards their response. Most studies seem to support the later explanation. In an implicit memory test, PTSD subjects showed a facilitated response for the threatening words, which suggests that the words had been encoded correctly and perhaps even in a facilitated manner at the time of training (Kaspi et al. 1995).
The Effects of Acute Social Stress on Learning and Memory in Humans
The relationship between stress and learning in humans that suffer from PTSD is complex and not easily summarized. It is also critical to note that most humans who experience severe trauma recover completely and do not suffer the consequences of PTSD (Bonanno 2004, McNally 2003). As such, an analysis of patients with mental disorders may not reveal the natural relationship between stress and learning. Minimally, the effects of stress on learning and memory in healthy humans must be considered. That said, it is difficult to study the effects of stress on learning in humans that are not victims of trauma. Some studies have used aversive stimuli such as shocks to induce stress in humans, but the practice is infrequent; more importantly, the degree of stress induced by mild shock is much different from that during trauma such as rape or combat. Often, anticipation of shock is used, as well as more benign experiences of public speaking, social interaction, and exposure to arousing pictures. A sample of findings from these types of manipulations is presented.
The effects of stressful experience on classical conditioning have been examined in humans. In one study, participants were exposed to emotionally arousing photographs that depicted pleasant, neutral, or unpleasant scenes. The participants were then classically conditioned using an eyeblink response as the measure of performance (Grillon & Hill 2003). In general, their responses were unaffected. However, about 50 years ago, Spence and colleagues conducted a series of studies in which they manipulated anxiety or took advantage of endogenous differences and measured performance during eyelid conditioning. In general, they found that greater degrees of anxiety were associated with increases in performance and that emotionally responsive subjects emitted more learned responses (Spence & Beecroft 1954, Spence & Goldstein 1961, Spence & Taylor 1951). Thus, if anything, arousing and stressful situations seem to enhance performance during classical conditioning.
A commonly used stressor in human studies is the Trier Social Stress Test, in which humans are asked to organize and perform a five-minute speech in front of observers while being videotaped. They are then asked to conduct several minutes of mental arithmetic. In one study, adult men were exposed to a list of words and then asked to perform the speech and the mental arithmetic tasks. After the stressful experience, the men were unable to recall as many emotionally arousing words as those who did not experience social stress (Kuhlmann et al. 2005). There was no effect of the stressful experience on cued recall, working memory, or attention. In another study, exposure to the stressful event was associated with poor recognition of pleasant, but not unpleasant, words; recall was unaffected (Domes et al. 2004). Studies using public speaking as a stressor have presented mixed results. In one study, there was no effect of public speaking on performance about 30 minutes later when using such measures as verbal learning, digit memory spans, and recall. Similarly, there was no effect on learning or recall of words presented either before or after a psychosocial stressor (Wolf et al. 2002). Others find enhanced performance after public speaking. For example, one study found that public speaking increased performance during a dichotic listening test, in which subjects were instructed to attend to the presentation of auditory stimuli delivered to one ear versus the other (Al’Absi et al. 2002). The effect size was not large, but most of the effects of public speaking stress are not. It would be surprising if the effects were large, since dramatic changes in learning abilities in response to minor fluctuations in the stress response would not be very adaptive.
The effects of stress on memory in nonpatient populations seem to depend on not only the quality and intensity of the stressful experience but also on what is asked to be remembered. Using a more intense stressor than public speaking, Cahill and colleagues have reported some interesting findings (Cahill et al. 2003). Participants first were exposed to slides that were emotionally arousing or neutral. Immediately afterward, they were asked to immerse their arm in a bucket of ice-cold water for several minutes. This type of stressor is perceived as very aversive and elicits robust increases in stress hormones, such as cortisol. One week after the stressful event, the participants were asked to recall the pictures that they had seen prior to the stressor. Those that experienced the stressor recalled more of the arousing pictures and in greater detail than did those that did not experience the stressor. The acute stressor did not affect the recall of neutral words. Thus, exposure to a relatively intense stressful experience in humans was associated with enhanced recall of information that was arousing in nature. These results are perhaps similar to those observed in humans with PTSD, who tend to express a bias toward remembering stimuli that are threatening and/or related to the traumatic event from which they suffered.
The Effects of Acute Stress on Learning and Memory in Nonhuman Animals
The effects of a stressful experience on learning processes in nonhuman animals are most often studied in one of two ways. The first is considered an acute stressful event and often consists of brief shocks or swimming for tens of minutes. The second is a more chronic stressful event, usually consisting of hours of restraint each day for several weeks. Other procedures include naturalistic stressors such as predator odors and social dominance manipulations, again under acute and chronic situations, respectively. The effects of stressful experience on learning in laboratory animals, as in humans, are assessed at different times, sometimes during the acquisition of new information but most often during the recall or retrieval of memories that were acquired prior to the stressful event.
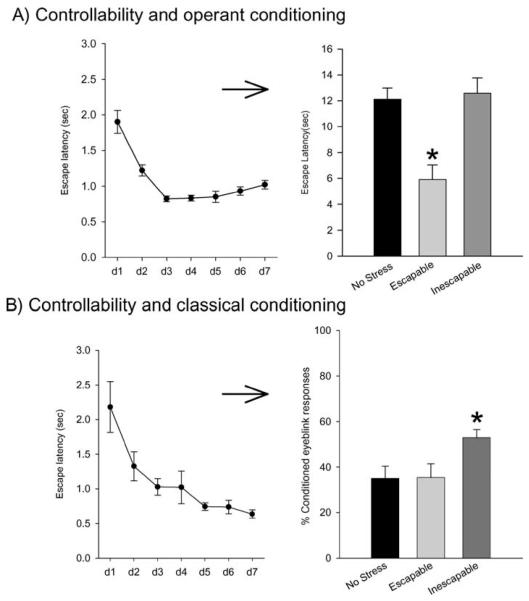
The most well-known and investigated stress/learning phenomenon is that of learned helplessness (Overmier & Seligman 1967, Seligman & Maier 1967). In this manipulation, animals are exposed to a series of shocks, typically footshocks, from which they can learn to escape. A yoked control animal is exposed to the same amount and numbers of shocks but cannot escape. Thus, in this manipulation, two animals are exposed to shocks, but one animal learns to control the amount of stress that it receives and is therefore considered to have established control over the stressful events. With this type of stressor, it has been shown that animals that cannot establish control over the shock are impaired in their ability to learn to escape a shock in another task. For example, during the stressful event manipulation, one animal would learn to escape a shock by running once through a door to the other side of a shuttle box (Figure 1A). A yoked control animal would not be able to escape but would be exposed to the same amount of stress as the escapable stress animal. Subsequently, both animals would be trained in a new context on a task in which they could learn to escape a shock by running back and forth across a shuttle box. The animal that had learned to escape by running through the door rapidly learns to run back through the door and escape the shock. The animal that learned that escape was not possible would not move as much upon exposure to the new learning situation and thus would not learn to escape (Figure 1A). This effect of not learning to escape after being exposed to inescapable stress is termed “learned helplessness” and has been associated with a number of negative symptoms in humans such as passivity, sleeping and eating disturbances, and depressive-like behavior. The important point is that the animals exposed to the uncontrollable stress learn that their responses are ineffectual.
Figure 1.
The effects of controllable versus uncontrollable stress on “learning” depend on what the animal can learn. (A) Adult male rats were trained each day for seven days (d1–d7) on an operant conditioning task in which they could learn to escape from a mild footshock. The graph to the left shows response times (mean latency in seconds ± SEM) for rats that could escape. These rats were yoked to animals that could not escape but were nonetheless exposed to the same amounts of shocks. One day after this manipulation, all animals were trained on a similar task except that they had to cross the shuttle box twice in order to terminate the shocks. The new task was also conducted in a new context. As shown, those that were exposed to the inescapable stress in the first phase did not learn to escape in the second phase of training, whereas those that were exposed to the escapable stress rapidly learned to escape (Shors et al. 2005). Rats that were not pretrained on the escape task (No Stress) performed moderately well, but not as well as those that learned to escape. Asterisk denotes a significant change in response relative to the response from the No Stress group. (B) As represented in Figure 1A, adult male rats were trained each day for seven days (d1–d7) on an operant conditioning task in which they could learn to escape from a mild footshock. The graph on the left shows response times (mean latency in seconds ± SEM) for rats that could escape. These rats were yoked to animals that could not escape but were nonetheless exposed to the same amounts of shocks. One day after this manipulation, all animals were trained with a classical eyeblink conditioning task using a trace paradigm in which the conditioned stimulus and the unconditioned stimulus are separated slightly in time (500 ms). The graph on the right shows the percentage of conditioned responses in all groups, including a group of animals that were not exposed to the escape training. Only the animals exposed to the uncontrollable stress responded differently, and they responded with a greater percentage of conditioned responses (Leuner et al. 2004b). Asterisk denotes a significant change in response relative to the response from the No Stress group.
There has been much discussion about learned helplessness and whether it represents a deficit in learning or performance (Maier & Jackson 1979, Minor et al. 1991). It is certainly the case that animals that cannot learn to escape are impaired later on a task in which escape is possible (Figure 1A). It is also the case that they do not move much when being trained because they learned that movement did not alleviate the shock. This is in part why the failure to learn is called “learned helplessness”: Animals exposed to the inescapable shock have “learned” to be helpless. Interestingly, animals exposed to inescapable stress show enhanced learning (or at least performance) during training on other tasks that are not as dependent on overt movement. For example, male rats that are exposed to inescapable stressful events emit more learned responses during classical conditioning using either a fear response or eyeblinks as the dependent measure (Figure 1B) (Leuner et al. 2004b, Maier 1990). Neither of these types of conditioning is affected by exposure to the same amount of escapable stress. This combination of findings illustrates the futility of identifying one type of stress effect on learning. Clearly, exposure to the very same type of stressful experience, in this case inescapable footshocks, can either impair or enhance performance during various learning tasks. Minimally, the effects of stress on learning depend on what the animal is asked to learn and what it learned before.
The other type of learning that is studied frequently in laboratory animals is spatial navigation and memory for locations in space. This type of learning is frequently measured using two types of tasks, the Morris water maze task and the radial maze task. In the Morris water maze, an animal is placed in a pool of water from which it cannot escape. As it explores, the animal eventually locates a hidden platform just under the surface of the water. After reaching the platform and perching itself there, the animal learns its location by learning the spatial relationship of cues around the outside of the maze. Upon reentry into the maze, the animal locates the platform using those cues and the way in which the platform is positioned relative to its location. This task has been used extensively because it is dependent on the hippocampus, a brain structure involved in learning and one that possesses cells that are specialized to encode spatial location (Riedel et al. 1999). It is also a useful and relatively easy task for assessing performance and learning in rodents. The effects of stress on this type of learning are somewhat varied and depend on the type of stressor, but even more so on when the stressor is experienced. For example, animals that are exposed to a stressful event of brief intermittent tailshocks learn to navigate in the Morris water maze and do so at the same rate as animals that are unstressed (Kim et al. 2005). There are also examples of enhanced learning in the Morris water maze after a stressful experience. For example, male rats learn faster after having had a stressful aggressive encounter with another male (Buwalda et al. 2005). However, stressed animals can express deficits later in their ability to recall where the platform was located (Kim et al. 2005). Similarly, if animals are exposed to brief shocks after they are trained in the Morris water maze, they express a memory deficit for the platform location (de Quervain et al. 1998). Interestingly, this effect is time limited since the deficit occurred only in animals that were exposed to the stressor 30 minutes before the retention test and not in those that were stressed two minutes or four hours before. In the end, the effects of stress on performance in the Morris water maze are mixed: In general, exposure to a stressful event tends to impair retention and/or retrieval but not learning per se.
In addition to the Morris water maze, a land maze with arms radiating from the center is used. In this task, the rat is food deprived and is given the opportunity to locate food pellets in the arms of the maze. The most efficient strategy is to enter an arm once and not again after the food has been consumed. In the typical eight-arm radial maze, animals use spatial cues in the room to remember which arms they have already entered. In one study, animals were exposed to brief intermittent tailshocks and trained in the radial maze task 24 hours later (Shors & Dryver 1992). Although the stressed animals did accrue more errors (reentries into arms that had already been debaited), a closer analysis of the data revealed that the stressed animals did know the spatial locations of the food pellets; they were simply taking the pellets to a safe arm (their first arm entry) and consuming the food there instead of in a novel location. This type of strategy is consistent with how animals might behave in a naturalistic situation after confrontation with a predator or a potential threat. Eventually, they would need to venture out to obtain food, but would likely consume it in a location that that was not associated with that threat. Therefore, in this instance, what looks to be a learning deficit is likely not one, but rather an adaptive response to a threatening stimulus event. For other types of stressors, such as restraint, the results are mixed. After 7 days consisting of hours of restraint each day, the behavior of animals in the radial maze is unaffected, whereas after 13 days, they seem to perform better, and after 21 days, they accrue more errors (Luine et al. 1996). Finally, there are reports that stress can specifically alter learning that is dependent on the hippocampus but not learning that is independent of the structure. For example, exposure to a predator after training reduced performance in a working-memory version of the radial maze task (Woodson et al. 2003). Again, most studies find that a stressful experience is associated with deficits in the retrieval of information but not deficits in learning itself.
It is most often assumed that stress has a negative impact on learning processes. But as shown above, the negative effects tend to occur upon retrieval of information that has already been learned, with relatively few demonstrations that stress impairs learning itself. What about the effects that stress has on new learning? Some investigators report enhancements, at least in adult male animals. For example, two hours of restraint stress as well as the more chronic experience of hours each day for 21 days enhanced the amount of conditioned fear that animals expressed toward the context (Conrad 1999, Cordero et al. 2003). There are also reports that exposure to inescapable stressful events enhances the conditioning of fine motor responses such as the eyeblink response (Figure 2) (Shors et al. 1992). The enhanced effect of stress occurs under numerous conditions, stressors, and paradigms. For example, the enhancement occurs in response to brief intermittent tailshocks and swim stress (Shors 2001). It occurs during training on tasks that are dependent on the hippocampus, such as trace conditioning, as well as during training on tasks that are not dependent on the hippocampus. The effect has a protracted time course in that the increase in performance is evident immediately after exposure to the stressful event but also persists for days. To be specific, if an animal is exposed to the stressful event and then placed in its home cage for a day or two, it will emit more conditioned responses upon exposure to the new training situation (Shors & Servatius 1997). This effect appears to be related to the initial acquisition and not to the retention or performance of the conditioned response since exposure to the stressor during training does not alter the expression of the conditioned response (Shors 2001) or the magnitude of the unconditioned response (Servatius et al. 2001). In some studies, the rate of acquisition is increased, and in most, asymptotic performance is also enhanced. Thus, it cannot be stated with certainty that this phenomenon represents a learning effect, although the data are certainly suggestive. Finally, and as noted previously, the effect is dependent on the absence of control, because conditioning in animals that can learn to control the stress is unaffected, whereas conditioning in animals that cannot establish control is enhanced (Figure 1B) (Leuner et al. 2004b).
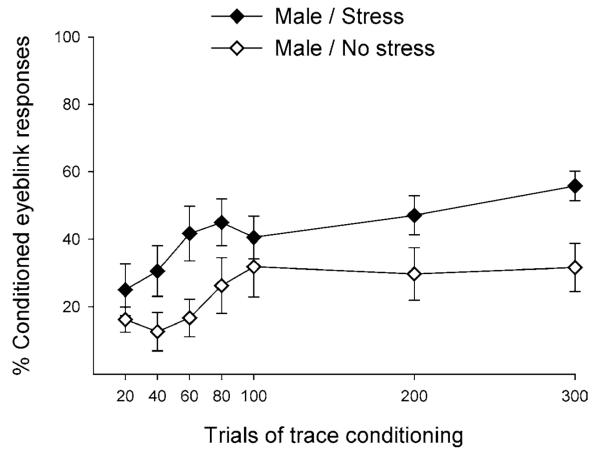
Figure 2.
Exposure to an acute stressful event enhances subsequent responses to an associative learning task. Adult male rats were exposed to an acute stressor of brief, low-intensity tailshocks over 30 minutes and were trained 24 hours later on the classically conditioned eyeblink task. The trace paradigm was used, which consisted of a white-noise conditioned stimulus (CS) that predicted the occurrence of an eyelid stimulation unconditioned stimulus (US). The CS and US were separated by a 500-ms trace interval. Rats exposed to the stressor emitted a greater percentage of conditioned responses (eyeblink responses during the trace interval) to the CS than did animals that were not exposed to the stressful event (Hodes & Shors 2005a).
STRESS AND LEARNING AS A FUNCTION OF AGE AND SEX DIFFERENCES
Sex Differences in Stress Effects on Learning
The effects reported up to this point are primarily those of adult males and suggest that, if anything, stressful experience tends to enhance new learning but reduces performance at the time of memory retrieval. In other words, if the stressful event occurs before the acquisition of new information, the learning is either unaffected or enhanced. However, if the stressful experience occurs after the information has been acquired, the retrieval of that information can be disrupted, and performance is oftentimes impaired. These findings suggest that negative effects of stress may be confined to disrupting information that has already been encoded. However, some studies suggest that stress can impair learning and that memory can be enhanced by stress (Nielson et al. 2005). Many of these reports, interestingly enough, involve differences that arise either because of gender or because of age.
Some years ago now, we reported sex differences in how animals respond to stress. As described, males that are exposed to the uncontrollable stressors of either brief intermittent tailshocks or swim stress tend to outperform males that are unstressed. Females, on the other hand, show the opposite pattern (Wood & Shors 1998). Thus, upon exposure to the same uncontrollable stressful events, their ability to acquire the classically conditioned eyeblink response is compromised. This effect, like that in male animals, is dependent on the absence of control; conditioning in females that could learn to control the shock during escape training is unaffected (Leuner et al. 2004b). Also like the effect in males, the effect in females is evident during training on the hippocampal-dependent task of trace conditioning as well as the hippocampal-independent task of delay conditioning. In summary, the effects of a stressful experience on new learning are very different in males versus females, and in some cases, the effects are opposite.
How general is this phenomenon, and does it apply to other types of conditioning or learning situations? This question is difficult to address because many of the tasks that are used rely on exploratory behavior, which has different patterns in male animals than in females. Some studies find that females are more exploratory than are males after being exposed to shocks, are more likely to enter a compartment where they were previously shocked, and are less likely to freeze in a context previously associated with shock (Beatty 1979, Maren et al. 1994, Shors 1998). Thus, a stressful experience can alter behaviors differently in males than in females. In fact, it was reported decades ago that females did not express helplessness behavior (Kirk & Blampied 1985, Steenbergen et al. 1989). Females were trained to either escape a footshock or they could not escape. They were then trained on a task in which they could escape the shocks. As noted earlier, male rats that learn they cannot escape do not learn the new task very well. Females, on the other hand, move more than males do after they have been exposed to inescapable shocks. When they encounter the new learning situation involving escape, they move and consequently learn, or are not as impaired as males are. It is difficult in the end to know whether these findings represent learning effects or differences in performance that nonetheless affect the expression of learned responses.
Acute Stressful Experience and Learning from Gestation Through Puberty
Most humans experience stressful events throughout their lives, but those that occur during pregnancy are a concern not only for the mother but also for the off-spring. Surprisingly, only a few studies have found that stress during pregnancy has consequences for learning in the offspring once they become adults. In one study, pregnant female rats were restrained daily for one week before giving birth. Apparently, the male offspring expressed more latent inhibition as adults (Bethus et al. 2005). Interestingly, the investigators found no effect of the stressful experience on latent inhibition in females. Another study found that prenatal stress increases fear conditioning in male rats as adults (Griffen et al. 2003). Many more studies have been directed at the period immediately after birth, usually by manipulating contact with the mother (Levine et al. 1956). Typically, the offspring are removed from their mothers, often for hours each day; a control group is not removed. Their responses to learning opportunities are assessed in adulthood. Overall, the data suggest that male rats are more vulnerable than are females to this manipulation. Male offspring that were separated from their mothers on gestational day nine showed enhanced learning during an active avoidance task and a spatial water maze task as adults (Lehmann et al. 1999). Females were unaffected. Others have found that stress during the juvenile period can alter learning abilities, at least in males. For example, animals that were stressed chronically for four weeks expressed a deficit in performance in the spatial water maze task as adults (Isgor et al. 2004). In another study, male rats that were isolated from other rats between 15–21 days after birth emitted more errors in a working memory task as adults (Sandstrom & Hart 2005). In yet another study, monkeys were socially isolated for brief periods during early development (postnatal weeks 17–27) and then tested as adults (Parker et al. 2005). The monkeys that had been isolated and therefore stressed during development made fewer errors using a response-inhibition testing paradigm. Most intriguing are the additive effects of multiple stressors on new learning. In one study, rats were exposed to a stressful event as juveniles and then again as adults. The ones that were exposed to both stressful events outperformed those that were exposed to just one stressful event in their lives. However, those that were exposed to two stressors as adults were not as affected as were those exposed to one stressor as juveniles and one as adults (Avital & Richter-Levin 2005). Thus, stressful experience was shown to have a positive influence on performance and new learning in the water maze, and the effect was dependent on the stage of life in which the stressor occurred.
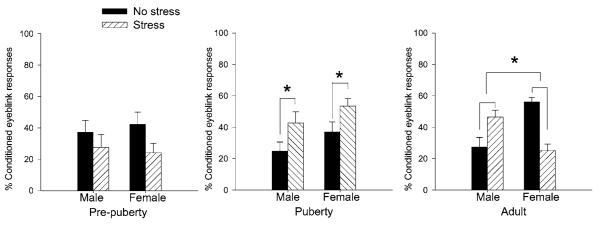
We recently examined the effects of acute stressful experience on learning in males and females before, during, and after puberty (Figure 3) (Hodes & Shors 2005a). Animals were stressed with brief intermittent tailshocks and 24 hours later were trained with the classically conditioned eyeblink response using a trace paradigm. We found no effect of stress on conditioning before puberty. This was surprising in some ways since this period is well beyond the so-called stress hyporesponsive period, a time when the stress response is diminished. In fact, these prepubescent animals had robust stress responses as measured by the release of the stress hormones, the glucocorticoids. Nonetheless, there was no effect of the stressful event on new learning. During puberty, however, both males and females that were previously exposed to the stressful event emitted more learned response during training. In adulthood, the sex differences in response to stress emerged; adult males that were exposed to the stressful event emitted more learned responses, whereas females emitted fewer learned responses after exposure to the same stressor. These data indicate that the detrimental effect of stress on learning in females emerges in adulthood and is not evident before or during puberty. Thus, the way in which stress affects learning abilities is not only dependent on the sex of the animal but also on the stage of life during which it occurs.
Figure 3.
Stress during puberty and its effect on subsequent learning. Male and female rats of different ages were exposed to an acute stressor of brief intermittent tailshocks and were trained 24 hours later on a classical eyeblink conditioning task using a trace paradigm. Conditioning in males and females that were stressed and trained before puberty was unaffected by exposure to the stressful event. In contrast, conditioning in males and females that were stressed and trained during puberty was enhanced. As adults, however, sex differences in conditioning and the response to stress emerged. Specifically, females emitted fewer conditioned responses after exposure to the stressful event, especially if they were trained in proestrus (Hodes & Shors 2005a).
Stress and Learning from Sexual Maturity Through Motherhood
It is clear that stress can affect learning differently in males than in females, and in some cases can even have opposite effects on learning in males and females. Moreover, the effects of stress on learning in females often depend on the stage of estrus during which the stress and/or training occur (Shors et al. 1998). For the effects of stress on classical eyeblink conditioning, it appears that females in proestrus are most affected. That is, females that are stressed and then trained in proestrus emit many fewer conditioned responses than do females that are unstressed. However, if they are in proestrus and not exposed to a stressful event, females emit more conditioned responses than do females in any other stage of estrus and than do males. Thus, females in proestrus learn best but are also most susceptible to the negative consequences of a stressful experience. Similar results have been reported in human studies. In fact, in 19 out of 20 separate experiments, women emitted more conditioned eyeblink responses than did men (Spence & Spence 1966). Stages of the menstrual cycle were not monitored. Nonetheless, a very consistent sex difference in classical conditioning was apparent, and it was one similar to that observed in male and female rats. Also, the effects of anxiety on conditioning seem to interact with sex differences in humans. During differential conditioning, anxious men expressed more excitatory conditioning for the positive cue, whereas anxious women expressed greater excitation for both negative and positive cues (Spence & Farber 1954). A more recent study found that a stressful experience of public speaking and mental arithmetic enhanced fear conditioning in males yet had no effect on acquisition in females (Jackson et al. 2005). Overall, the amount of data is insufficient at this time to draw strong conclusions about how stress affects learning differently in males and females, much less to answer why. This is in part because females are usually excluded from animal studies, whereas in human studies, they are included but often are not distinguished from males during data analysis. Fortunately, this practice is beginning to change (Cahill 2005).
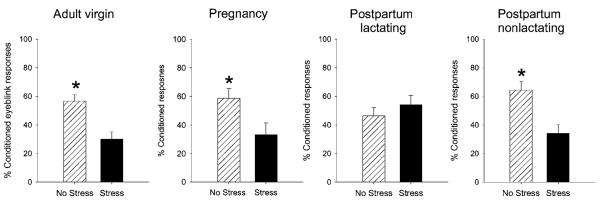
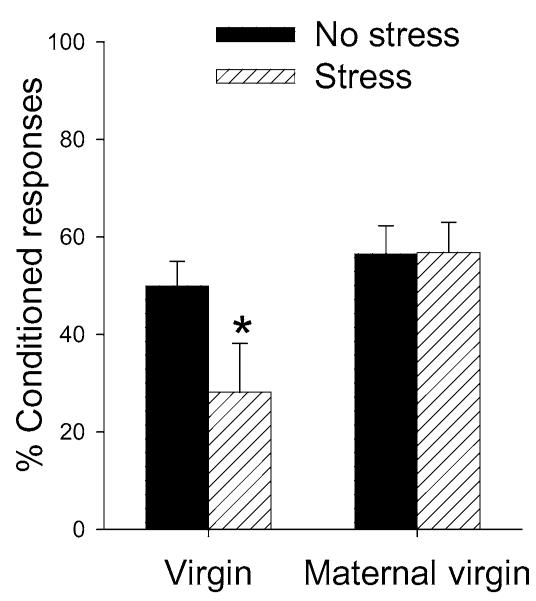
Females respond differently to stressful experiences during specific stages of life. The period immediately after giving birth is one of those times. In general, postpartum females (including women) are less anxious while they are lactating and show blunted responses to stressful experience (Carter et al. 2001). Consistent with these effects, female rats that are lactating do not express a deficit in conditioning after stressor exposure, whereas those that are cycling or pregnant express a profound deficit (Figure 4) (Leuner & Shors 2005). This effect is dependent on the presence of the offspring, since the deficit will reemerge if the mothers are separated from their offspring. Also, the effect can be induced in virgins that behave maternally (Figure 5). This process, known as “maternal sensitization,” occurs when virgin females are exposed to offspring of other females. After several days, the virgin females begin to take care of the offspring and express many of the behaviors associated with motherhood. Amazingly enough, in the absence of giving birth, these virgins become resistant to the negative consequences of stressful experience. Thus, learning in females that are induced to act like mothers becomes resistant to stress, as it does in the postpartum female. The way in which the expression of maternal behavior, induced either by birth or by exposure to off-spring, alters the response to stress is unknown, and why learning abilities would be protected is probably unknowable. Obviously, new mothers must learn many new behaviors and associative responses, and must do so oftentimes under threatening conditions. The point here is that the effect of stress on learning changes across the lifespan, particularly across the life of a female.
Figure 4.
The female response changes from pregnancy to postpartum. Females were exposed to an acute stressful event and trained 24 hours later on the classically conditioned eyeblink response using a trace paradigm. As shown previously, stress reduced the percentage of conditioned responses in adult females that were cycling. Stress also reduced conditioning in females that were pregnant and therefore not cycling. However, stress did not alter conditioning in females that were lactating. Upon removal of their offspring, lactation ceased and these females emitted a smaller percentage of conditioned responses after exposure to the stressful event (Leuner & Shors 2005).
Figure 5.
The female response to stress is absent in virgins that express maternal behavior. Virgin females were induced to behave maternally with repeated exposure to offspring. While behaving in this way, they were exposed to an acute stressful event and 24 hrs later trained on the classically conditioned eyeblink response using a trace paradigm. Conditioning in the maternal virgins was not affected by exposure to the stressful event and thus their response was similar to females that are postpartum and lactating (Leuner & Shors 2005). This response (or lack thereof) is contrasted with that of virgin females that were not exposed to offspring; they emitted many fewer conditioned responses after exposure to the stressor. Asterisk denotes a significant change in response relative to the response from the No Stress group.
One of the most significant changes in life is the cessation of reproductive potential, a process that occurs most overtly in women but is also experienced in the lives of men. It is often assumed that stress will have more of an impact on the aged than on the young, and that the everyday stressors that occur in life can interfere with cognitive processing in the elderly. However, very few studies have examined how stress affects learning in aged animals. We recently examined the effects of acute stressful experience using brief intermittent tailshocks on classical eyeblink conditioning (Hodes & Shors 2005b). As discussed above, exposure to this event enhances conditioning in males but interferes with performance in females. In aged rats, however, there was no effect of stress whatsoever on conditioning. Thus, the enhancing effect of stress on conditioned responding in males did not occur and the deficits in females did not occur. These findings suggest that the older animals become immune to the effects of stress on this type of conditioning, irrespective of whether the effect is positive or negative. It is perhaps worth noting that before puberty, there are also no observable effects of acute stress on this type of learning (Figure 3). Therefore, the effects of stress on new learning of this response seem to emerge with sexual maturity and diminish with the loss of reproductive potential.
HORMOMES, BRAIN MECHANISMS, AND ANATOMICAL SUBSTRATES
The Glucocorticoid Connection
Glucocorticoids are most often associated with stress and are important for their interaction with learning processes. These stress hormones are released in response to nearly every stressful event that a person encounters in life and their effects on the central nervous system are nothing short of profound. Mostly, they are anti-inflammatory and thus are critical for reestablishing and maintaining home-ostasis after a stressful experience. Because glucocorticoids have direct effects on processes involved in learning and memory, they are studied in their own right. Readers are referred to current reviews and analyses of the glucocorticoid literature (Het et al. 2005, Lupien et al. 2004, McEwen & Wingfield 2003, Roozendaal 2002) that are not discussed here because of space limitations. Rather, the focus here is on studies that tested whether glucocorticoids are necessary for the effects of a stressful experience on learning and memory.
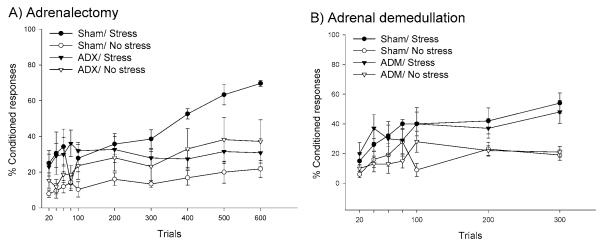
The methods available to address this issue are not feasible for use in humans; thus, these studies have been conducted in laboratory animals. Probably the most conclusive method is to remove the adrenal gland via an adrenalectomy. This surgical manipulation removes the sole source of glucocorticoids. However, it also removes a source of epinephrine since epinephrine is also released from the adrenal medulla within the glands. Thus, to be sure that the effects observed after adrenalectomy are due to the absence of glucocorticoids, adrenalectomy studies should be followed up by adrenal demedullations, in which the adrenal medulla is removed, leaving the adrenal cortex and the source of glucocorticoids intact. This approach has been used to determine whether the enhancing effect of acute stress on classical eyeblink conditioning is dependent on the presence of glucocorticoids (Beylin & Shors 2003). As shown in Figure 6A, adrenalectomy did not affect the overall level of conditioning, but did prevent the enhanced conditioning in response to an acute stressful experience of intermittent tailshocks. However, removal of the adrenal medulla did not prevent the enhanced responding after stress. Together, these data indicate that the effect of stress on classical eyeblink conditioning is dependent on the presence of stress hormones released from the adrenal cortex, i.e., glucocorticoids. Although necessary, these studies do not address whether the presence of stress levels of the hormones are sufficient to enhance learning. To address this issue, we conducted a subsequent study in which intact male rats were injected with a bolus of corticosterone at a concentration similar to that induced by stressful experience. Conditioning was increased while the glucocorticoid levels were elevated. However, once the hormone levels returned to baseline, the enhanced conditioning did not occur. In other words, conditioning in animals that were injected with the glucocorticoids and trained for the first time 24 hours later was unaffected. This is in contrast to the effects of an actual stressful event, where the enhanced learning occurs days after the stressful event has ceased and long after glucocorticoid levels have returned to baseline. Together, these findings indicate that glucocorticoids are necessary but not sufficient for the persistent increase in eyeblink conditioning that occurs in males after an acute stressful experience.
Figure 6.
The presence of glucocorticoids is necessary but not sufficient for the persistent increase in learning after stressful experience. (A) The graph shows the percentage of conditioned responses in animals that were adrenalectomized (ADX) and provided basal levels of corticosterone through their drinking water. The other groups were exposed to a sham surgery. The group that was stressed and trained emitted a greater percentage of conditioned responses than did the unstressed groups, unless they were adrenalectomized. There was no effect of stress on trace conditioning in the ADX group (Beylin & Shors 2003). (B) The effects of stress on trace conditioning were examined in rats after their adrenal medulla was removed. In this experiment, adrenal demedullation did not prevent the enhancing effect of stress on conditioning, a finding that indicates the effect is mediated by the presence of glucocorticoids released from the adrenal cortex (Beylin & Shors 2003).
Others have examined the contribution of glucocorticoids to the effects of stress on learning by using a classically conditioned preference for morphine. Exposure to uncontrollable, but not to controllable, stress enhances the rewarding effects of drugs of abuse. Specifically, a stressful experience enhances an animal’s ability to learn and remember where the rewarding effects of morphine were experienced (Der-Avakian et al. 2005). This effect of stress on learning is also dependent on the presence of adrenal hormones, since adrenalectomy eliminated the enhanced preference for the morphine context. Like the effects of stress on eyeblink conditioning, these findings indicate that glucocorticoids are usually necessary for stress effects on learning, However, in their study, Der-Avakian et al. (2005) found the presence of glucocorticoids to be important during the morphine experience and not during the stressor. Thus, the release of glucocorticoids during the stressful event was not as important as the release during the training experience.
The previous two studies indicated that the enhancing effects of stress on learning are dependent on the presence of glucocorticoids. What about the more negative effects that stress has on memory? A stressful experience can impair retrieval of information that was acquired before the stressful event (de Quervain et al. 1998). The release of glucocorticoids also seems necessary for this effect, since rats that were administered a drug that inhibits the synthesis of glucocorticoids did not express the memory deficit. In this case, the effect could be mimicked with an injection of glucocorticoids; therefore, the hormones were necessary and sufficient.
These few examples illustrate the many ways that the hypothalamic-pituitary adrenal response to stress interacts with processes involved in learning and memory. In general, glucocorticoid levels correlate with stress effects on learning. The existence of this relationship is not surprising because more stressful experiences result in both the release of more glucocorticoids and the increased likelihood to induce changes in learned responses. Despite the existence of this correlation, it does not necessarily mean that glucocorticoids are playing a critical role, and in some cases, they are playing none. For example, female rats have very high levels of glucocorticoids, particularly after exposure to a stressful event, yet the detrimental effect of stress on subsequent learning does not depend on their presence (Wood et al. 2001). In summary, the role that glucocorticoids play in the effects of stress on learning is complicated. Their presence appears to be necessary for most effects, although the critical period varies depending on the learning situation. There are also significant differences in the hypothalamic-pituitary adrenal response to stress between species and even the sexes.
The Hippocampal Formation and Correlations Within
The hippocampus is the brain structure most often associated with stress and learning. This is in part because it possesses an abundance of glucocorticoid receptors (McEwen & Wallach 1973). Two types reside there: type I or mineralocorticoid receptor, which is occupied at low basal levels of the hormone, and type II or glucocorticoid receptor, which is occupied during stressful times. Type I receptors are heavily concentrated in the hippocampus, whereas the stress-related type II receptors are especially prevalent in other brain regions, such as the hypothalamus and prefrontal cortex (Reul & De Kloet, Lupien & Lepage 2001). Nonetheless, the hippocampus remains a structure of interest because it is involved in and is even necessary for some types of learning. Moreover, it is extremely sensitive to stressful experiences.
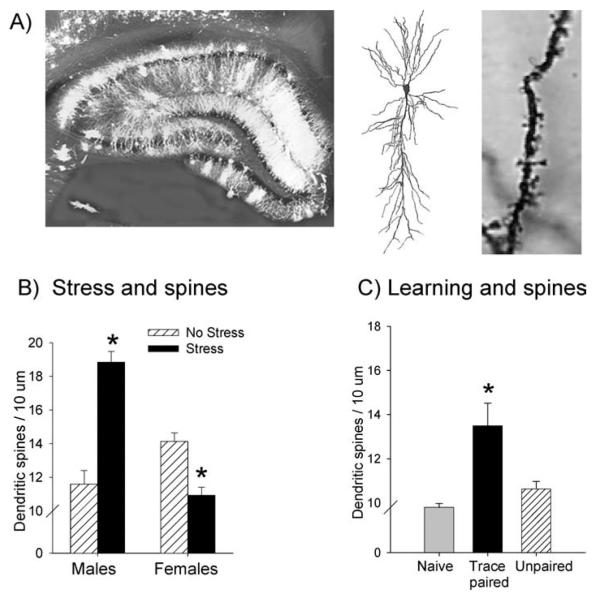
Relatively few attempts have been made to show that the hippocampus is necessarily involved in stress effects on learning. This is in part because the structure is involved in some types of learning and therefore its disruption will affect learning, even in the absence of stress. For example, it has been proposed that the stress-induced increase in trace conditioning is dependent on the hippocampus, but this has not been shown because disrupting the hippocampus would disrupt learning itself. There are, however, numerous correlations between stress effects on learning and changes within the hippocampal formation. For example, exposure to 21 days of restraint reduces the branching of CA3 pyramidal cells in the hippocampus (Wantanabe et al. 1992). The reduction in synaptic connections correlates with performance deficits in the Y maze (Conrad 1999). In other studies, it has been shown that exposure to the acute stressful event that enhances classical eyeblink conditioning also increases the excitability of pyramidal cells in area CA1 of the hippocampus. The increase persists for at least 24 hours and thus would be evident during the time when animals are being trained. These results suggest a positive relationship between cell excitability and new learning after stressful experience. Similarly, it has been shown that exposure to the stressor that enhances trace conditioning increases the density of dendritic spines in the hippocampus (Figure 7B) (Shors et al. 2001a). Again, this effect suggests that an increase in spine density may relate to the increase in learning. The effect of stress on spine density is quite different in the female hippocampus, where exposure to the same stressor reduces spine density. Since acute stress enhances conditioning in males but impairs it in females, these data represent another positive correlation between the presence of dendritic spines and classical eyeblink conditioning. It would be interesting to know whether exposure to the stressor decreases cell excitability in the female hippocampus. If so, this would suggest a causal relationship between these various phenomena; however, the relationships would still be only correlational. It is nonetheless noted that this type of learning is associated with changes in both cell excitability and spine density. In males that are trained with eyeblink conditioning, neuronal activity increases as does the number of spines on dendrites in area CA1 (Figure 7C) (Berger et al. 1983, Leuner et al. 2003, McEchron & Disterhoft 1999). Thus, a stressful experience induces changes in neuronal function and structures that are affected by learning itself, indicating a convergence on common substrates.
Figure 7.
The presence of dendritic spines is affected by stressful experience and learning. (A)A photomicrograph illustrates the massive amount of dendritic branching that exists within the hippocampal formation. To the right is a CA1 pyramidal cell and dendritic spines on those cells. (B) It was determined, using Golgi staining techniques, that exposure to an acute stressful experience increases the presence of dendritic spines in the hippocampus of the male rat. Exposure to the same stressor reduces spine density in females. These responses to stress are represented in the graph as the mean number of spines along 10 um of a dendrite in area CA1 of the hippocampus, as well as in representative photomicrographs (Shors et al. 2001a, 2004). Asterisk denotes a significant change in response relative to the response from the No Stress group. (C) The presence of spines also increased in response to learning the classically conditioned eyeblink response, at least in males. Thus, spine density in the hippocampus correlates with learning after stress and is enhanced by learning.
There has been much discussion about how neurogenesis may contribute to the effects of stress on learning. It has been established that the adult brain continues to produce new neurons throughout life, and many of those are produced in the dentate gyrus of the hippocampus (Cameron et al. 1995, Gould et al. 1997, van Praag et al. 2002). The production of new cells is especially sensitive to stressful experience; stressors such as social dominance, maternal deprivation, and predator odors all reduce the production of new cells in this brain region (Kosorovitskiy & Gould 2004, Mirescu et al. 2004, Tanapat et al. 2001). These cells have also been associated with some types of learning and thus it is tempting to conclude that stress effects on learning are mediated by changes in neurogenesis. This is probably not a viable explanation, at least not for the effects of acute stress on new learning. Most studies indicate that the new cells do not become involved in learning for at least a week after they are born (Gould et al. 1999, Leuner et al. 2004a). Thus, a change in cell production in response to acute stress would most likely not affect cells that are involved in learning. Also, the data to date suggest that the new neurons respond to relatively few types of learning situations (Shors 2004). It is possible, however, that chronic stressful experiences over weeks or months could alter the production of cells to such an extent that changes in learned behaviors would emerge.
The Amygdala, Bed Nucleus, and Connections Between
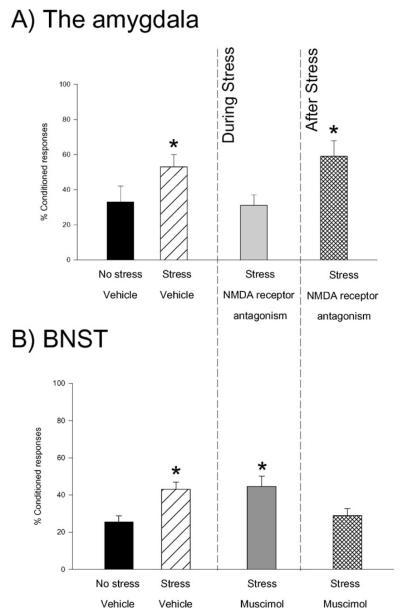
The amygdala has been associated with stress and learning almost as much as the hippocampus. It is involved in aspects of emotional responses as well as learning about fearful events. In general, the amygdala allows emotional events to be remembered, and remembered well (McGaugh 2004). Many studies have focused on memory retrieval and its interactions with glucocorticoids in the amygdala, and as noted above, this literature is not reviewed here. There are also those that have examined whether the amygdala is necessary for the stress effects on learning and memory. For example, the enhancing effect of stress on classical eyeblink conditioning could be prevented by blocking N-methyl-D-asparate (NMDA) receptors in the amygdala (Figure 8) (Shors & Mathew 1998). This effect was locally specific because antagonism in the basolateral, but not the central, nucleus was effective. Also, the effect was temporally specific because antagonism during, but not after, the stress was effective. These data suggest that a stressful experience activates NMDA receptors in the amygdala during the experience itself, which then induces more long-term changes in processes involved in learning. Others have shown a similar dependence. Recall that acute stress can reduce retention for spatial location in the Morris water maze. This effect can be prevented by inactivating the amygdala during, but not after, the stressful event (Kim et al. 2005). Thus, it would appear that the amygdala is involved in stress effects on learning and memory, whether they are enhancements in learning or deficits in memory.
Figure 8.
The amygdala and bed nucleus of the stria terminalis (BNST) are involved, but at different times. (A) The percentage of conditioned responses in groups of male rats is shown. They were either unstressed or stressed in the presence of a vehicle or an N-methyl-D-aspartate (NMDA) receptor antagonist in the basolateral nucleus of the amygdala. Exposure to the stressor enhanced the percentage of conditioned responses in groups that were injected with a vehicle or if they were injected with the antagonist after the stressful event was over. The group that was injected with the NMDA receptor antagonist prior to the stressful event did not express an increase in conditioned responses (Shors & Mathew 1998). Asterisk denotes a significant change in response relative to the response from the No Stress group. (B) As described above, exposure to the stressor enhanced the percentage of conditioned eyeblink responses in males. However, inactivation of the BNST during the stressful event did not prevent the enhanced conditioning, whereas inactivation during training was effective (Bangasser et al. 2005). Asterisk denotes a significant change in response relative to the response from the No Stress group.
One of the major outputs of the amygdala is the stria terminalis. Some years ago, it was shown that lesions of this structure prevent the effects of glucocorticoids on memory processes, thereby suggesting that the “information” in the amygdala was being relayed to afferent structures (McGaugh 2004, Roozendaal & McGaugh 1996). The primary target of the stria terminalis is the bed nucleus, which is a structure known to be involved in stress, anxiety, and sex differences. Recent studies have indicated its involvement in anxiety (Walker & Davis 1997). Thus, the bed nucleus is a structure that can maintain a state of anxiety in animals, a state that would likely alter an animal’s ability to learn and remember. Consistent with this idea, the stress-induced increase in classical eyeblink conditioning is prevented by inactivating the bed nucleus (Bangasser et al. 2005). However, inactivation was only effective if it was done during the learning experience and not during the stressful event (Figure 8). The data suggest that the BNST is critical for maintaining the effects of stress for several days in case a new learning situation arises in which the increased responding can be used. It appears that the effect of stress on this type of learning is induced by activity within the amygdala, but that the response is maintained by changes in activity elsewhere, including within the bed nucleus of the stria terminalis.
Considering the Prefrontal Cortex
The prefrontal cortex is involved in cognitive processes that are generally referred to as executive functions. Recently, a number of studies have suggested that the prefrontal cortex may be involved in stress effects on learning (Birnbaum et al. 2004). One of the more persuasive studies involves the effects of uncontrollable versus controllable stress on subsequent fear conditioning and escape behaviors. As discussed above, exposure to uncontrollable, but not to the same amount of controllable, stress enhances fear conditioning and impairs escape performance in a shuttle box escape task. However, neither effect occurred if the prefrontal cortex was inactivated during the stressful experience (Amat et al. 2005). Others have found correlations between stress effects on learning and changes in the prefrontal cortex. For example, exposure to a predator odor can disrupt memory for a spatial location, and the deficit correlates with the expression of a neural cell adhesion molecule in the prefrontal cortex (Sandi et al. 2005). Others report that retrieval of fearful memories increases activity in the prefrontal cortex (Bremner et al. 1999). This area of the brain is not as well delineated in the rat as it is in primates, making a direct comparison between species difficult. Nonetheless, given the involvement of the prefrontal cortex in executive function, its high concentration of stress hormone receptors, and its elaborate network of connections to most brain regions, a role in stress and learning is nearly assured.
CONCLUSION
In the end, is stress good or bad for learning? Intuitively, most would answer that it is bad. However, a slightly different view emerges from the literature. Overall, exposure to an acute stressful event tends to enhance learning of new information, if there is an effect at all, whereas exposure to a similar stressful event tends to impair the retrieval of information that has already been acquired. Although the implications are compelling, they are limited because most studies have been done exclusively in adult male animals. When age and sex differences are considered, these trends are nonexistent, and an even more diverse repertoire of responses emerges. This is especially so in females as they change their reproductive status. This should not be that surprising, since changes in learning abilities will have different consequences for survival during different phases of life, and oftentimes ones that cannot be anticipated. Nonetheless, a malleable stress response that interacts with learning is what one would expect from a complex biological system that must respond rapidly and appropriately to a changing environment. As such, it is unlikely that one mechanism or even a handful of mechanisms can account for the diversity of behavioral responses to stressful experience.
Footnotes
The Annual Review of Psychology is online at http://psych.annualreviews.org
LITERATURE CITED
- Al’Absi M, Hugdahl K, Lovallo WR. Adrenalcortical stress responses and altered working memory performance. Psychophysiology. 2002;39:95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Am. Psychiatric Assoc. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Am. Psychiatric Assoc.; Washington, DC: 2000. [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioral consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–73. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Ayers ED, White J, Powell DA. Pavlovian eyeblink conditioning in combatveterans with and without post-traumatic stress disorder. Integr. Physiol. Behav. Sci. 2003;38:230–47. doi: 10.1007/BF02688856. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is involved in the persistent increase in associative learning after stress. Behav. Neurosci. 2005 doi: 10.1037/0735-7044.119.6.1459. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW. Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Horm. Behav. 1979;12:112–63. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Berger TW, Rinaldi PC, Weisz DJ, Thompson RF. Neuronal substrate of classical conditioning in the hippocampus. Science. 1983;192:483–85. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Bethus I, Lemaire V, Lhomme M, Goodall G. Does prenatal stress affect latent inhibition? It depends on gender. Behav. Brain Res. 2005;158:331–38. doi: 10.1016/j.bbr.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing memory formation after stressful experience. Horm. Behav. 2003;43:124–31. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–84. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without post-traumatic stress disorder. Am. J. Psychiatry. 1999;156:1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Capelli S, Delaney R, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing threatening information in posttraumatic stress disorder. J. Traum. Stress. 1995;10:635–44. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- Buckley TC. Information processing and PTSD: a review of the empirical literature. Clin. Psychol. Rev. 2000;28:1041–65. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MHP, Veenema AH, Huininga M, de Boer SF, et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci. Biobehav. Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cahill L. His brain, her brain. Sci. Am. 2005;292:40–47. [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn. Mem. 2003;10:270–74. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulations of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995;15:4687–92. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog. Brain Res. 2001;133:241–49. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Conrad CD. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–18. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats: evidence for a role of corticosterone. Horm. Behav. 2003;44:338–45. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–90. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Will MJ, Bland ST, Deak T, Nguyen KT, et al. Surgical and pharmacological suppression of glucocorticoids prevents the enhancement of morphine conditioned place preference by uncontrollable stress in rats. Psychopharmacology. 2005;179:409–17. doi: 10.1007/s00213-004-2041-1. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Rimmele U, Reichwald U, Hautzinger M. Acute stress impairs recognition for positive words—association with stress-induced cortisol secretion. Stress. 2004;7:173–81. doi: 10.1080/10253890412331273213. [DOI] [PubMed] [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozak MJ, Mc-Carthy PR. Processing of threat-related information in rape victims. J. Abnorm. Psychol. 1991;100:156–62. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin AV, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the adult hippocampal formation. Nat. Neurosci. 1999;2(3):260–65. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LAM, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997;17(7):2492–98. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen WC, Skinner HD, Salm AK, Birkle DL. Mild prenatal stress in rats is associated with enhanced conditioned fear. Physiol. Behav. 2003;79:209–15. doi: 10.1016/s0031-9384(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Grillon C, Hill J. Emotional arousal does not affect delay eyeblink conditioning. Cogn. Brain Res. 2003;17:400–5. doi: 10.1016/s0926-6410(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–84. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hodes G, Shors TJ. Distinctive stress effects on learning during puberty. Horm. Behav. 2005a;48:163–71. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G, Shors TJ. Stress does not affect learning in aged males or females. Soc. Neurosci. 2005b Abstr. In press. [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biol. Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.08.002. In press. [DOI] [PubMed] [Google Scholar]
- Kaspi SP, McNally RJ, Amir N. Cognitive processing of emotional information in posttraumatic stress disorder. Cogn. Ther. Res. 1995;19:433–44. [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han J. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J. Neurosci. 2005;25:1532–39. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RC, Blampied NM. Activity during inescapable shock and subsequent escape avoidance learning: female and male rats compared. N. Z. J. Psychol. 1985;14:9–14. [Google Scholar]
- Kosorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J. Neurosci. 2004;24:6755–59. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J. Neurosci. 2005;25:2977–82. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol. Biochem. Behav. 1999;64:705–15. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–65. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 2004a;24:7477–81. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol. Psychiatry. 2004b;56:964–70. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Motherhood and memory: a resistance to stress. Horm. Behav. 2005 doi: 10.1016/j.yhbeh.2006.01.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Chevalier JA, Korchin SJ. The effects of early shock and handling on later avoidance learning. J. Personal. 1956;24:475–93. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Martinez C, Villegas M, Margarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol. Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2004;30:225–42. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behav. Brain Res. 2001;127:137–58. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced byinescapable shock. J. Exp. Psychol. Anim. Behav. Process. 1990;16:137–49. [PubMed] [Google Scholar]
- Maier SF, Jackson RL. Learned helplessness: All of us were right (and wrong). Inescapable shock has multiple effects. In: Bower B, editor. Advances in Learning and Motivation. Vol. 13. Academic; New York: 1979. pp. 155–215. [Google Scholar]
- Maren S, de Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eye-blink conditioned responses. J. Neurophysiol. 1999;78(2):1030–44. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wallach G. Corticosterone binding to the hippocampus: nuclear and cytosol binding in vitro. Brain Res. 1973;57:373–86. doi: 10.1016/0006-8993(73)90143-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu. Rev. Psychol. 2003;54:229–52. doi: 10.1146/annurev.psych.54.101601.145112. [DOI] [PubMed] [Google Scholar]
- Minor TR, Dess NK, Overmier JB. Inverting the traditional view of “learned helplessness.”. In: Denny MR, editor. Fear, Avoidance, and Phobias: A Fundamental Analysis. Erlbaum; Hillsdale, NJ: 1991. pp. 87–133. [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci. 2004;7:841–46. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Yee D, Erickson KI. Memory enhancement by a semantically unrelated emotional arousal source induced after learning. Neurobiol. Learn. Mem. 2005;84:49–56. doi: 10.1016/j.nlm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Seligman MEP. Effects of inescapable shock on subsequent escape and avoidance learning. J. Comp. Physiol. Psychol. 1967;63:23–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Shatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol. Psychiatry. 2005;57:848–55. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lowenhagen MJ, Macklin ML, Altman B. Pre-Vietnam contents of PTSD veteran’s service medical and personnel records. Compr. Psychiatry. 1991;32:1–7. doi: 10.1016/0010-440x(91)90018-8. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, De Kloet ER. Two receptor systems for corticosterone in the rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–12. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AGM, Roloff EVL, Martin SJ, et al. Reversible neural activation reveals hippocampal participation in several memory processes. Nat. Neurosci. 1999;2:898–906. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 2002;78:578–95. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh J. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Res. 1996;709:243–50. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, et al. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol. Psychiatry. 2005;57:856–64. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behav. Brain Res. 2005;156:289–96. doi: 10.1016/j.bbr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Maier SF. Failure to escape traumatic shock. J. Comp. Physiol. Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K. Stress facilitates acquisition of the classically conditioned eyeblink response at both long and short interstimulus intervals. Learn. Motiv. 2001;32:178–92. [Google Scholar]
- Shors TJ. Stress and sex effects on associative learning: for better or for worse. Neuroscientist. 1998;4:353–64. [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol. Learn. Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–56. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001a;21:6292–97. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Dryver E. Stress impedes exploration and the acquisition of spatial information in the eight-arm radial maze. Psychobiology. 1992;20:247–53. [Google Scholar]
- Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur. J. Neurosci. 2004;19:145–50. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Paczynski M, Mathew PR, Pickett J. Stages of estrus mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–23. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. NMDA receptor antagonism in the basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. J. Learn. Mem. 1998;5:220–30. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Edgecomb C, Sisti HM. Neurogenesis and depression: uncontrollable but not controllable stress reduces proliferation in the adult hippocampus. Neurosci. 2005 Abstr. In press. [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation (LTP) in rat hippocampus. Science. 1989;244:224–26. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol. Learn. Mem. 1997;67:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–39. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Spence KW, Beecroft RS. Differential conditioning and level of anxiety. J. Exp. Psychol. 1954;48:399–403. doi: 10.1037/h0057825. [DOI] [PubMed] [Google Scholar]
- Spence KW, Goldstein H. Eyelid conditioning performance as a function of emotion-producing instructions. J. Exp. Psychol. 1961;62:291–94. doi: 10.1037/h0043915. [DOI] [PubMed] [Google Scholar]
- Spence KW, Spence JT. Sex and anxiety differences in eyelid conditioning. Psychol. Bull. 1966;65:137–42. doi: 10.1037/h0022982. [DOI] [PubMed] [Google Scholar]
- Spence KW, Taylor J. Anxiety and strength of the UCS as determiners of the amount of eyelid conditioning. J. Exp. Psychol. 1951;42:183–88. doi: 10.1037/h0061580. [DOI] [PubMed] [Google Scholar]
- Steenbergen HL, Heinsbroek RPM, Van Haaren F, van de Poll NE. Sex-dependent effects of inescapable shock administration on behavior and subsequent escape performance in rats. Physiol. Behav. 1989;45:781–87. doi: 10.1016/0031-9384(89)90295-3. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LAM, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Trandel DV, McNally RJ. Perception of threat cues in post-traumatic stress disorder: semantic processing without awareness? Behav. Res. Ther. 1987;25:469–76. doi: 10.1016/0005-7967(87)90054-4. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–34. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Borges A, Sutker PB. Assessment of intellectual resources in Gulf War veterans: relationship to PTSD. Assessment. 1997;4:51–59. [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal pyramidal neurons. Brain Res. 1992;588:341–45. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, Reischies FM, Kirschbaum C. Moderate psychosocial stress appears not to impair recall of words learned 4 weeks prior to stressor exposure. Stress. 2002;5:59–64. doi: 10.1080/102538902900012332. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin A, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav. Neurosci. 2001;115:175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males but impairs conditioning in females through activational influences of ovarian hormones. Proc. Natl. Acad. Sci. USA. 1998;95:4066–71. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: working memory–specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn. Mem. 2003;10:326–36. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Keefe RSE, Harvey PD, Leven-good RA, Gerber DK, et al. Learning and memory in combat veterans with post-traumatic stress disorder. Am. J. Psychiatry. 1995;152:137–39. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]