Abstract
Approximately one-third of people who recover from a stroke require some form of assistance to walk. Repetitive task-oriented rehabilitation interventions have been shown to improve motor control and function in people with stroke. Our long-term goal is to design and test an intensive task-oriented intervention that will utilize the two primary components of constrained-induced movement therapy: massed, task-oriented training and behavioral methods to increase use of the affected limb in the real world. The technological component of the intervention is based on a wearable footwear-based sensor system that monitors relative activity levels, functional utilization, and gait parameters of affected and unaffected lower extremities. The purpose of this study is to describe a methodology to automatically identify temporal gait parameters of poststroke individuals to be used in assessment of functional utilization of the affected lower extremity as a part of behavior enhancing feedback. An algorithm accounting for intersubject variability is capable of achieving estimation error in the range of 2.6–18.6% producing comparable results for healthy and poststroke subjects. The proposed methodology is based on inexpensive and user-friendly technology that will enable research and clinical applications for rehabilitation of people who have experienced a stroke.
Index Terms: Gait parameters, stroke rehabilitation therapy, wearable sensors
I. Introduction
Stroke is the leading cause of disability in the United States [1]. It is estimated that 700 000 people in the United States experience a stroke each year with over 5 million Americans currently having suffered from one [2]. Approximately one-third of these individuals will be left with functional limitations as a result of their stroke [3]. Initially after a stroke, two-thirds of individuals cannot walk or require assistance to walk. After three months, one-third of individuals who experience a stroke still require some form of assistance or are not able to walk [3].
Many of those who do regain walking ability do not have sufficient locomotor capacity for independent mobility in their community. Regaining the ability to walk is an important goal for individuals who have experienced a stroke [4]–[8] and it is often a primary focus of the rehabilitation of these individuals. Poststroke individuals who are independent walkers require less care and their level of disability is reduced as they are better able to participate in their societal roles [9]. As walking ability is a primary goal of clients and focus of rehabilitation, it is important that effective interventions are developed to improve walking ability in this population.
Current research suggests that rehabilitation strategies that are based on task-oriented, intensive training are necessary to induce use of dependent neurological reorganization in order to enhance motor and functional recovery after stroke [10], [11]. Dean et al. [12] found that individuals with chronic stroke who participated in a task-related lower extremity training program had a significantly greater improvement in locomotor capacity, compared to a control group that received upper extremity training. Yang et al. [13] had similar results where task-oriented interventions were designed to increase the strength of the affected lower extremity in a functionally relevant way and to provide repetitive walking practice under various conditions.
Locomotor training utilizing a body weight support and treadmill systems is another task-oriented intervention that has a growing body of evidence to support its effectiveness [14]–[17]. Although these studies have demonstrated that interventions utilizing task-oriented, intensive training can improve walking ability in people with stroke, these rehabilitation strategies may be missing a key training component that could promote further recovery of locomotor capacity, behavioral enhancing strategies. Behavioral enhancing strategies are a key component of constraint-induced movement therapy (CIMT), which has been shown to be effective in improving upper extremity motor control and functional use of the affected limb in real-world situations in people with stroke [18]–[20].
Adherence enhancing behavioral strategies include a variety of techniques that are used to assist the patient in taking responsibility for actively engaging in the intervention strategy and transferring gains from the clinic to increasing use of the affected limb in a real-world setting. Elements of this transfer package include recording use of the affected limb in a diary, performing home practice of functional tasks, and problem solving with the rehabilitation therapist around barriers [20].
A key component of adherence enhancing behavioral strategies is the ability to monitor the use of the affected limb in the patient’s home and community. This is necessary so the patient can gain an accurate view of their use of the limb and it provides important information for the rehabilitation therapist to assist with problem solving in order to overcome barriers to use. Incorporating two of the three components of CIMT therapy into a comprehensive rehabilitation strategy aimed at improving walking ability and lower extremity function poststroke may be beneficial. Incorporating adherence enhancing behavioral strategies with repetitive task-oriented gait interventions is feasible and may enhance the effects of massed, task-oriented interventions; however, to the best of our knowledge this combined intervention strategy has not been reported in the literature.
There is a strong need for developing systems that enable the evaluation and progress of the therapy in free-living conditions, capable of accurate monitoring and comparing the performance of the affected limb versus the unaffected one during walking. The measurement of temporal gait parameters such as percentage of time in swing and stance provides important information on the symmetry of the person’s walking patterns. These measures provide an accurate assessment of motor recovery after stroke [21] and can be used as feedback to the patient and therapist. Here, we propose the use of a shoe-based wearable sensor system consisting of pressure sensors and accelerometers to accurately detect temporal gait parameters in people with stroke. This system has been successfully used for automatic monitoring of posture allocation and activities in healthy [22] and poststroke individuals [23].
This study is organized as follows: Section II describes a background on different sensors used for gait parameters analysis. In Section III, the methodology used to obtain gait parameters in poststroke individuals is described in detail. Results are presented in Section IV. Sections V and VI, respectively, present discussion and conclusion.
II. Background
The methodology proposed here for estimation of temporal gait parameters in poststroke individuals is based on direct measures of pressure distribution under the feet when performing walking activities. Several works have proposed different approaches for the task of gait analysis using different sensors such as force-sensitive resistors (FSR) [24], [25] and/or kinematic sensors like accelerometers [24], [25] and gyroscopes [24]–[27].
Saremi et al. [26] tested an integrated system of five accelerometers to determine if these sensors could offer an option for acquisition of spatiotemporal gait parameters in healthy and hemiplegic individuals. Comparison of the acceleration system was performed with a commercial footswitch that has shown high reliability in gait parameters’ estimation. No statistically significant differences for spatiotemporal measures of gait were found between both systems, which suggest valid and reliable measures of gait for each lower extremity. However, measures were only reliable for speeds from 0.5 to 1.8 m/s and walking speed was accurate as long as walking was continuous.
On the other hand, Aminian et al. [24] suggest the use of gyroscopes located in any segment of the body, as long as its axis is parallel to the mediolateral axis, to estimate spatial gait parameters in addition to temporal parameters, in young and elderly subjects using wavelet transformation to find gait events, i.e., Heel-strike and Toe-off. With these sensors, the angular rate signal is less noisy than the one from accelerometers since acceleration is the derivative of velocity and involves higher frequency components. This system was compared to a two-FSR array by thresholding the pressure signals obtained while walking at the exact time of Heel-strike and Toe-off. The authors reported no significant error observed for Toe-Off detection, while a 10-ms average delay was observed between Heel-strike obtained from gyroscopes and the FSR sensor. However, the static threshold for FSR measurements can modify the results of the performance obtained from the gyroscopes; also, the system worked under the assumption that subjects had a symmetrical stride length, which could be not true in real-life situations or in people with stroke or other neurological disorders.
A similar approach was used by Salarian et al. [27] where body attached gyroscopes were used to estimate spatiotemporal parameters of gait in patients with Parkinson’s disease (PD) where angular velocity of shank was used to estimate gait events of Heel-strike and Toe-off. Error estimation of this system was obtained in comparison with a motion-capture system where a human scorer carefully examined the recorded video. Error results obtained for Heel-strike and Toe-off were −8.7 ± 12.5 and −2.9 ± 26.8 ms, respectively. Gait cycle and stance times error reported were 2.2 ± 23.2 and 5.9 ± 29.6 ms, respectively.
A more elaborated wearable system was proposed by Bamberg et al. [25] named GaitShoe, where accelerometers, gyroscopes, pressure sensors, bend sensors, and electric field height sensors were used for quantitative gait analysis in healthy and Parkinsonian subjects. The sum of four FSR sensors was used here to obtain the Heel-strike by setting the first time point after the local maxima that exceeds the previous by more than 0.005 kg; the Toe-off was set at the first time point after the local minima within 0.005 kg of the following point. This method proved to be highly successful with average rms error results of −6.7 ± 22.9 ms for Heel-strike detection and −2.9 ± 16.9 ms for Toe-off detection.
In summary, although several works have focused on gait analysis, there is still a need for development and testing of the methodologies that perform well in stroke individuals who can exhibit highly asymmetrical gait patterns. In addition to asymmetry, the gait patterns of poststroke individuals also exhibit high degree of intersubject variability and thus require adaptation to individual traits. In previous work, we reported the development of a wearable shoe-based device to detect and identify different postures and activities of poststroke subjects by monitoring signals extracted from five FSR sensors located in the insole of a shoe, and a 3-D accelerometer located at the heel [23]. The methodology described in [23] enables monitoring of activity levels expressed categorically (e.g., sitting, standing, and walking). The methodology for estimation of temporal gait parameters proposed in this study enables the assessment of functional utilization and motor recovery of the affected extremity in stroke patients during locomotion.
III. Methodology
A. Sensor Description
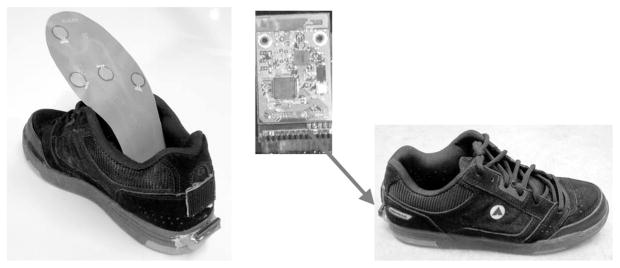
A detailed description of the wearable shoe-based sensor system can be found in [22] and [23]. Each shoe comprises five FSR sensors (Interlink, Inc.), and a 3-D accelerometer based on MEMS technology (LIS3D02AS). The FSR sensors were located in different foot contact points, the heel, the heads of the metatarsal bones, and the great toe, soldered into a flexible printed circuit board (PCB); these sensors were used to capture variations of pressure in the plantar area at all times (see Fig. 1). The FSR sensors respond approximately linearly to pressure exerted by the feet in different postures (standing, sitting) or activities (walking) [23]. However, the quantitative measure of the pressure is not of interest for this study, but the qualitative measure is.
Fig. 1.
FSR sensors located on a flexible PCB and wireless circuit and an accelerometer located at the back of the shoe.
The accelerometer was mounted on the heel at the back of the shoe together with the battery, power switch, and wireless board in a rigid PCB (see Fig. 1). Data were sampled from these sensors at 25 Hz with a 12-bit ADC and sent to a portable computer system using a wireless intelligent sensor and actuator network (WISAN) wireless link [28] and stored on a hard drive. The choice of sampling frequency was made as a design tradeoff between battery life and physical size, both defined by power consumption during sampling and wireless transmission and time resolution of temporal gait parameters. The proposed algorithms should perform equally well for higher sampling frequencies [22]. The combination of these sensors has been successfully used to automatic recognize different postures and activities of stroke patients [23]. In this study, the variations of pressure captured by the FSR sensors were used to detect the temporal gait parameters.
The integrated sensors add no significant weight to the shoe (a five-pad sensor insole with connector weighs 17 g) and do not cause observable interference with normal motion, posture, or normal activities. Subjects who participated in the data collection expressed no discomfort or apparent change of walking behavior while wearing the shoe sensors. The wireless sensor system is also inexpensive: the cost of parts per shoe-sensor pair is less than U.S.$ 100 in single quantities and can scale down substantially in mass production.
B. Signal Processing
Data obtained from the FSR sensors was used to estimate the following temporal gait parameters: cadence, step time, cycle time, percentage of gait cycle in swing for each lower extremity, percentage of gait cycle in stance for each lower extremity, percentage of gait cycle in single limb support for each lower extremity, and percentage of gait cycle in double limb support for each lower extremity. These temporal gait parameters are most relevant for clinical practice [29], [30] as they provide a method of assessing gait symmetry and serve as an accurate measure of motor recovery after stroke [21].
First, for each subject’s foot the sum of all five FRS sensors was calculated as
| (1) |
This signal provides enough information to obtain accurate detection of Heel-strike and Toe-off location in time as discussed in the next sections.
The graph on top in Fig. 2 shows signals from all five FSR sensor locations (heel, left, middle, and right metatarsal, and great toe); it also shows, in the bottom graph, the waveform of the sum of all of them as in (1). For these signals, no significant noise was present in the obtained sumFSR signal and no signal preprocessing or denoising was performed. It can be argued that only the sensors located at the heel and the great toe could provide the same information [24], but it has been observed in the laboratory that compared to accelerometer the sumFSR signal is less sensitive to irregularities of lower extremity motion of paraplegic gait.
Fig. 2.
FSR sensor signals of one of the wearable shoe-based sensors of a poststroke subject; the graph on top shows the signals obtained from each of the FSR sensor locations. The graph at the bottom shows the sumFSR signal, that is, the sum of the five signals obtained from the FSR sensors.
C. Heel-Strike and Toe-Off Detection
Heel-strike and Toe-off events are critical in computation of gait parameters. The proposed algorithm for detection of these events is based purely on FSR signals as methods based on inertial sensors may present significant differences between unaffected and affected limb in subjects with gait abnormalities due to stroke [26].
We use the terms Heel-strike and Toe-off as these are commonly used to describe different cycles of gait. The shoe sensor will detect initial contact no matter what portion of the foot first strikes the ground (corresponds to Heel-strike of normal gait) and end of stance phase no matter what portion of the foot leaves the ground (corresponds to Toe-off of normal gait). These detected variables are used to calculate the different temporal gait parameters.
Heel-strike and Toe-off events may not be applicable to patients with severe gait abnormalities (dragging of the foot, for example); however, patients with stroke who are not able to clear their foot in swing to some degree are not going to be able to walk independently so would not have their gait parameters assessed. In order to walk independently, the foot needs to clear the ground in swing if only to a small degree. The shoe sensor would be able to detect this period of no contact compared to the foot being on the ground and calculate the gait parameters.
For the data extracted from each one of the shoe-based wearable sensor, a threshold to detect Heel-strike and Toe-off events is calculated by defining the average maxima and minima of the sumFSR signal. For the sumFSR signal, all the local maxima and local minima are obtained. The averages of these data points define maxima and minima thresholds as
| (2) |
| (3) |
where Maxa , a = 1,2,…,k, are the local maxima data points found and Minb , b = 1,2,…,l, are the local minima data points found.
The difference between the average values defines the threshold to obtain the Heel-strike (H) and Toe-off (T) as
| (4) |
Equation (4) defines the threshold τ that detects Heel-strike and Toe-off events in the sumFSR signal. This threshold is set within the range of variation of the sumFSR signal by the means of the proportional factor α that adjusts the threshold as a fraction of the difference between the average maximum and the average minimum of the signal to compensate for interindividual variability in pressure levels.
The value of α is obtained through leave-one-out training and validation to produce the lowest relative error of temporal gait parameters for the dataset. Once this value is calculated, it can be used to adjust the threshold τ of any new subject. It was observed experimentally that a value of α = 0.1725 gives the best temporal gait parameters detection across all subjects and all experiments.
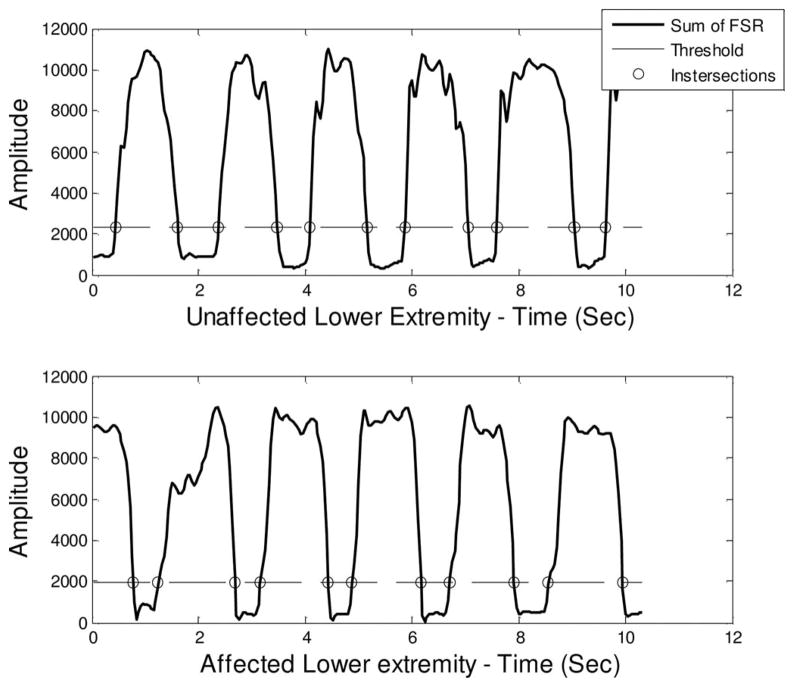
The intersection points of the threshold τ with the sumFSR signal correspond to H (Heel-strike) and T (Toe-off) points (see Fig. 3). To discriminate detection of H from T, a simple criterion is introduced: immediate points located previously to a local minima are considered T and those immediately located after a local minima are considered H. For each foot, left (L) and right (R), the numbers of H and T are the same in order to consider only complete steps and be able to calculate more precisely the temporal gait parameters:
Fig. 3.
Heel-strike and Toe-off detection for unaffected (top) and affected (bottom) lower extremity of a poststroke subject.
Left: HLi , TLi for i = 1,2,…n number of left steps,
Right: HRj , TRj for j = 1,2,…m number of right steps.
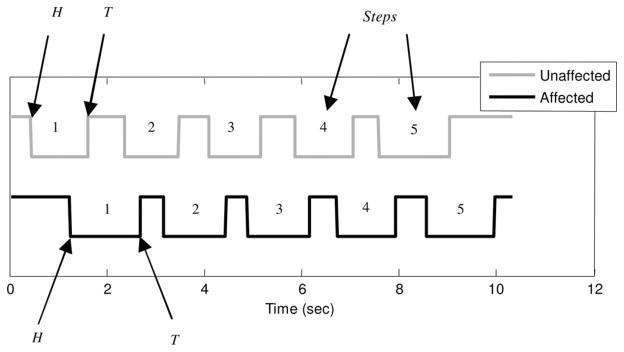
After all HL, TL, HR, and TR points are identified (see Fig. 4), these are used to obtain the corresponding temporal gait parameters (see Table I).
Fig. 4.
Heel-strike and Toe-off detected from the shoe-based wearable sensor of a poststroke subject.
TABLE I.
Temporal Gait Parameters Definition
| Left | Right | ||
|---|---|---|---|
Calculation of the temporal gait parameters from detected Heel-strike and Toe-off for each foot. Notations HLi and HRi (TLi and TRi) represent the time of the ith Heel-strike (Toe-off) event for the left and right foot, respectively. From top to bottom, the parameters are: gait cycle time, step time, stance, swing, single support, and double support.
D. Calculation of Temporal Gait Parameters
From each H and T events detected, different temporal gait parameters were computed. First, cadence temporal gait parameter was obtained as the average of both feet detection as
| (5) |
where Nmax and Nmin are the number of maxima and minima on the sumFSR signal, respectively; Tmax and Tmin are the time between the first and the last maxima and minima, respectively. The rest of the gait parameters were obtained separately for each lower extremity as described in Table I.
E. Validation
Data collection was performed on a group of 16 healthy human subjects (8 males and 8 females) and 7 poststroke subjects (2 males and 5 females). These subjects were selected to reflect a diverse adult population and compare proposed algorithm performance in healthy and poststroke individuals. The university Institutional Review Board approved the study and each subject provided informed consent. Subject characteristics for both groups are shown in Table II.
TABLE II.
Subjects Demography
| Healthy | Post-stroke | |
|---|---|---|
| Age | 25±6.5 years (range 18–44) | 60.4±10.6 years (range 40–76) |
| BMI | 26.7±6.5 kg/m2 (range 18.1–39.4) | 31.7±7.8 kg/m2 (range 26.1–48.3) |
| Shoe size (US) | 7–11 | 8–10.5 |
With the poststroke subjects, four had a left cortical cerebrovascular accident (CVA) with right hemiparesis, one a right cortical CVA with left hemiparesis, one a brainstem CVA that resulted in right hemiparesis, and one a cerebellar CVA. Five subjects ambulated without an assistive device, one subject used a cane, and one used a hemiwalker. Two subjects used an ankle foot orthotic (AFO) and five did not. None of the healthy subjects used an assistive device or AFO.
The healthy subjects performed two experiments where they were asked to walk over a GAITRite commercial test system (CIR Systems, Inc.). This commercial system provides reliable automated means of measuring spatial and temporal parameters of gait consisting on an electronic walkway with a useful area of 61 × 366 cm (24 × 144 in) connected to a Windows-based PC.
GAITRite measures were used as the gold standard to evaluate the methodology described in this paper since it has been proven to have a strong reliability in temporal gait parameter definition for young and elder [29], and for healthy and stroke individuals [30]. At the same time, subjects were wearing the sensor-based shoes described in Section III-A.
Subjects with poststroke condition performed similar experiments, where they were asked to walk in two different manners: walking comfortably and walking as fast as they could. Both of these experiments were repeated for four times. For all the experiments of the data collection, all subjects walked a distance of 17 feet (approximately two steps before and after the length of the GAITRite sensor mat). Unlike GAITRite, the shoe-sensor system has no limitation on the distance.
IV. Results
Gait data from two types of subjects, healthy and poststroke, were collected and processed by the proposed algorithm to detect temporal gait parameters and compare to the GAITRite system. The results are shown in Tables III and IV.
TABLE III.
Average Values Across all Healthy Subjects
| Healthy Subjects
| ||||||
|---|---|---|---|---|---|---|
| GAITRite® | FSR Sensors | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| Cadence (Step/sec) | 1.31 | 1.2 | 1.42 | 1.24 | 1.13 | 1.35 |
|
Left foot |
||||||
| GAITRite® | FSR Sensors | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| Step time (Sec) | 0.59 | 0.56 | 0.63 | 0.63 | 0.58 | 0.69 |
| Cycle time (Sec) | 1.17 | 1.06 | 1.28 | 1.17 | 1.07 | 1.28 |
| Swing % | 35.65 | 32.81 | 38.49 | 37.58 | 34.52 | 40.64 |
| Stance % | 60.77 | 56.07 | 65.47 | 59.17 | 54.54 | 63.8 |
| S supp % | 35.77 | 32.9 | 38.64 | 36.27 | 33.37 | 39.17 |
| D supp % | 24.75 | 22.32 | 27.18 | 22.58 | 20.11 | 25.05 |
|
Right foot |
||||||
| GAITRite® | FSR Sensors | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| Step time (Sec) | 0.61 | 0.58 | 0.64 | 0.61 | 0.58 | 0.65 |
| Cycle time (Sec) | 1.16 | 1.06 | 1.27 | 1.21 | 1.14 | 1.28 |
| Swing % | 36.01 | 33.1 | 38.92 | 37.18 | 36.18 | 38.18 |
| Stance % | 60.43 | 55.73 | 65.12 | 62.82 | 61.83 | 63.82 |
| S supp % | 35.89 | 33 | 38.77 | 37.07 | 36.13 | 38.02 |
| D supp % | 25.1 | 22.68 | 27.52 | 26.06 | 24.53 | 27.59 |
TABLE IV.
Average Values Across all Poststroke Subjects
| Post-stroke Subjects
| ||||||
|---|---|---|---|---|---|---|
| GAITRite® | FSR Sensors | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| Cadence (Step/sec) | 1.07 | 0.95 | 1.18 | 1.00 | 0.92 | 1.07 |
|
Affected Lower Extremity |
||||||
| GAITRite® | FSR Sensors | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| Step time (Sec) | 0.67 | 0.59 | 0.74 | 0.65 | 0.59 | 0.71 |
| Cycle time (Sec) | 1.34 | 1.25 | 1.44 | 1.37 | 1.26 | 1.48 |
| Swing % | 32.80 | 30.58 | 35.02 | 33.73 | 31.67 | 35.80 |
| Stance % | 67.20 | 64.98 | 69.42 | 66.88 | 64.79 | 68.98 |
| Single support % | 34.17 | 32.36 | 35.97 | 34.66 | 32.74 | 36.59 |
| Double support % | 32.82 | 29.94 | 35.71 | 31.60 | 29.17 | 34.03 |
|
Unaffected Lower Extremity |
||||||
| GAITRite® | FSR Sensors | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| Step time (Sec) | 0.68 | 0.61 | 0.74 | 0.69 | 0.62 | 0.76 |
| Cycle time (Sec) | 1.34 | 1.24 | 1.44 | 1.37 | 1.25 | 1.48 |
| Swing % | 34.22 | 32.38 | 36.06 | 34.26 | 32.18 | 36.35 |
| Stance % | 65.79 | 63.95 | 67.62 | 65.75 | 63.66 | 67.84 |
| S supp % | 32.88 | 30.61 | 35.14 | 32.08 | 29.85 | 34.31 |
| D supp % | 33.26 | 30.42 | 36.11 | 33.99 | 30.78 | 37.21 |
For healthy subjects, the statistical t-test using a confidence value of 95% was performed to compare data recorded with the GAITRite system and the shoe-based wearable sensor; no significant difference in the mean across all subjects for cadence (p > 0.35) and for parameters calculated for each lower extremity (p > 0.18) was observed.
Results from the statistical t-test with a 95% confidence for poststroke subjects also did not show significant difference between GAITRite and the shoe-based wearable sensor for cadence (p > 0.29) and for parameters calculated for each lower extremity (p > 0.51).
Relative error from the shoe-based wearable sensor related to the GAITRite results was calculated for both types of subjects as
| (6) |
where “Gaitrite” represents the GAITRite reported gait parameters used as the gold standard and “Shoe” represents the gait parameters obtained from the shoe-based wearable sensor.
Table V shows the relative error obtained for the healthy subjects. Table VI shows relative error obtained for subjects poststroke, separated by types of experiments, e.g., walking comfortably and walking fast.
TABLE V.
Relative Error for Healthy Subjects
| Healthy subjects
| |||
|---|---|---|---|
| Parameter | Relative Error % | 95% CI | |
| Cadence | 10.40 | 8.40 | 12.50 |
| Step time | 18.40 | 14.80 | 22.10 |
| Cycle time | 3.10 | 2.40 | 3.90 |
| Swing % | 6.40 | 5.30 | 7.40 |
| Stance % | 3.60 | 2.90 | 4.40 |
| S supp % | 5.50 | 4.30 | 6.70 |
| D supp % | 10.90 | 8.30 | 13.60 |
TABLE VI.
Relative Error for Poststroke Subjects
| Comfortable Walking | Fast Walking | |||||
|---|---|---|---|---|---|---|
| Parameter | Relative Error % | 95% CI | Relative Error % | 95% CI | ||
| Cadence | 9.5 | 5.2 | 13.8 | 8.8 | 4.8 | 12.8 |
| Step time | 18.67 | 10.2 | 27.2 | 15.4 | 11.1 | 19.7 |
| cycle time | 2.70 | 1.6 | 3.8 | 2.6 | 1.7 | 3.4 |
| swing % | 8.56 | 5.9 | 11.2 | 10.7 | 7.8 | 13.7 |
| stance % | 3.37 | 2.7 | 4.0 | 5.2 | 3.7 | 6.8 |
| S supp % | 7.78 | 5.3 | 10.3 | 9.9 | 7.1 | 12.6 |
| D supp % | 10.3 | 8.3 | 12.2 | 12.4 | 9.5 | 15.3 |
Across poststroke subjects, a statistical t-test with 95% confidence was performed to compare the relative error obtained between experiments from comfortable and fast walking. There was no significant difference between both types of experiments across all parameters calculated (p > 0.21), except for stance percentage (p = 0.01).
V. Discussion
Results obtained from the shoe-based wearable sensor show that the methodology proposed here is able to accurately identify temporal aspects of the gait cycle in both healthy people and individuals with stroke using only the FSR signals.
Because of the limited number of subjects enrolled for the study, besides the average relative error of calculated temporal gait parameters, 95% confidence intervals are reported as part of the results. A statistical t-test using a confidence interval of 95% was performed to compare data recorded with the GAITRite system and the shoe-based wearable sensor. There was no significant difference in cadence (p > 0.35) and temporal gait parameters calculated for each lower extremity (p > 0.18) in healthy subjects. There was also no significant difference in cadence (p > 0.29) and for temporal gait parameters calculated for each lower extremity (p > 0.51) in subjects with stroke.
The relative error for these temporal aspects of the gait cycle, except for the step time, are acceptable for practical purposes, and comparable to other methods, i.e., 3.1% versus 2.2% error for gait cycle time and 3.6% versus 5.9% error for stance compared to the results reported in [27]. Values of relative error up to 10% for cadence and double support can be explained by the way they are calculated, where Heel-strike and Toe-off from both lower extremities are used and their respective error is carried to the final calculation. The temporal gait parameters’ mean values obtained from the healthy subjects are similar to the ones previously reported in the literature [24], [26], [27], and [29].
Computation of temporal gait parameters using only FSR signals is proposed since pressure measurements from the insole of a shoe involve a more direct representation of the walking behavior. When using accelerometers the angular rate signal tends to be noisy since acceleration is the derivative of velocity and involves higher frequency components [21]. As discussed in the literature, with the use of gyroscopes it is possible to estimate spatial gait parameters in addition to temporal parameters as long as its axis is parallel to the mediolateral axis. However, it is important to notice that the use of gyroscopes requires more sophisticated techniques for Heel-strike and Toe-off detection, i.e., wavelet transform, finite-impulse response, etc., since gait events are transitory signals that cannot be properly enhanced by simple traditional signal processing. Also, gyroscopes are more sensitive to temperature and mechanical shock that may be significant in nonlaboratory conditions [24]. The constraint when using FSR sensors is that they are limited only to the estimation of temporal parameters. Our current research is focused also on the accurate estimation of spatial parameters like walked distance and walking speed of a subject by using data extracted from the accelerometer located at the heel of the shoe-based wearable sensor.
For detection of Heel-strike and Toe-off, the proposed methodology adjusts a threshold τ based on the pressure behavior of the sum of all five FSR sensors located in the shoe-based wearable sensor. In [24] and [25], a fixed threshold over a signal obtained from FSR sensors was used to detect Heel-strike and Toe-off. The adjustable threshold in the proposed algorithm is less sensitive to variability of subject’s weight, gait asymmetry, and intersubject variability due to different levels of hemiparesis from poststroke condition.
However, one of the concerns is the impact of mechanical and temperature drifts of the sensors over long periods of data collection in free-living conditions. If significant variations during long recordings are to be observed, the threshold τ has to be readjusted over shorter windows of fixed time length over the complete data collected.
Differences in gait are expected between healthy subjects and poststroke subjects. This can be observed in the results of Tables III and IV. Lower values in cadence are typical of stroke patients due to the asymmetric gait result of the affected extremity, and reflected in other temporal parameters, like lower swing and higher stance. The wearable shoe-based sensor described in this study was capable of successfully computing these gait parameters for both type of patients, and obtaining comparable results to GAITRite system used as a reference.
The use of our shoe-based wearable sensor may be able to provide accurate information for researchers on gait characteristics as people with stroke walk in their home and community in order to analyze the effectiveness of rehabilitation interventions designed to improve locomotion. In addition to providing outcomes, the data from the shoe-based wearable sensor could also be used as part of a telerehabilitation-based, behavioral enhancing feedback intervention to improve walking ability in people with stroke.
We are in the process of further developing the sensor system so that the data are wirelessly transmitted to a mobile phone that is worn by the user for preliminary processing and storage. The user could then view and receive feedback by looking at the mobile phone on steps taken and other gait characteristics. At the end of the day, these data could be wirelessly downloaded to a computer for further analysis and used for feedback, e.g., how many steps were taken, how symmetrical was the gait pattern. The data could be viewed simultaneously by the user and rehabilitation professional via the Internet. These data could be used to set goals to increase the amount of time in standing and walking and to decrease the amount of time in sitting. Based on the shoe-sensor data, user feedback, and progress toward these goals, the rehabilitation professional could remotely solve problem with the user on ways to remove barriers to increase physical activity and enhance social participation.
VI. Conclusion
An inexpensive wearable system consisting of five FSRs integrated in the insole of regular shoes was used to estimate temporal gait parameters in healthy and poststroke individuals. This system and the proposed methodology accurately estimated temporal gait parameters in both healthy individuals and people with poststroke condition by defining an adjustable threshold over the resulting signal collected of the FSR sensors for each subject’s foot. This shoe-based wearable sensor system may provide a way of monitoring walking activity and measuring motor recovery in the home and community in people with stroke who are undergoing rehabilitation therapy, and could be used to provide feedback on rehabilitation progress.
Acknowledgments
This work was supported by the NIH under Grant 1R15HD061006-01 “A Telemedicine Device for Rehabilitation of Lower Extremity Impairment after Stroke.”
Biographies

Paulo Lopez-Meyer received the B.E. degree in telecommunications engineering in 2003, and the M.E. degree in instrumentation engineering in 2005, both from the National Autonomous University of Mexico, Mexico City, and a Ph.D. degree in electrical and computer engineering from Clarkson University, Potsdam, NY, in 2010.
He is a Postdoctoral Fellow at the University of Alabama, Tuscaloosa. His research interests include the application of machine learning and pattern recognition techniques in the solution of real-life problems.

George D. Fulk received the B.A. degree in political science from Brandeis University Waltham, MA, in 1986, the M.S. degree from the University of Massachusetts, Lowell, and the Ph.D. degree from Nova Southeastern University, Ft. Lauderdale, FL, both in physical therapy in 1994 and 2005, respectively.
He is an Assistant Professor in the Department of Physical Therapy, Clarkson University, Potsdam, NY. His research interests include the application of rehabilitation engineering technologies to enhance motor recovery and quality of life after neurological injury and in outcome measures designed to assess clinically meaningful change in people with neurological disorders.

Edward S. Sazonov (M’02) received the Diploma of Systems Engineer from the Khabarovsk State University of Technology, Khabarovsk, Russia, in 1993 and the Ph.D. degree in computer engineering from West Virginia University, Morgantown, in 2002.
Currently, he is an Associate Professor in the Department of Electrical and Computer Engineering, University of Alabama, Tuscaloosa, and the Head of the Computer Laboratory of Ambient and Wearable Systems. His work has been supported by national (National Science Foundation, National Institutes of Health, National Academies of Science) and state agencies, and private industry. His research interests span bioengineering, computational intelligence, wireless, ambient and wearable devices. Applications include development of methods and wearable sensors for noninvasive monitoring of ingestion; methods and devices for monitoring of physical activity and energy expenditure; wearable platforms for rehabilitation of stroke patients and monitoring of the risk of falling in elderly; and, self-powered ambient sensors for structural health monitoring.
Contributor Information
Paulo Lopez-Meyer, Email: plopezmeyer@bama.ua.edu, Department of Electrical and Computer Engineering, University of Alabama, Tuscaloosa, AL 35487-0286 USA.
George D. Fulk, Email: gfulk@clarkson.edu, Department of Physical Therapy, Clarkson University, Potsdam, NY 13699 USA
Edward S. Sazonov, Email: esazonov@eng.ua.edu, Department of Electrical and Computer Engineering, University of Alabama, Tuscaloosa, AL 35487-0286 USA.
References
- 1.Gresham GE, Duncan PW, Stason WB, et al. Quick Reference Guide for Clinicians. Vol. 16. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; May, 1995. Post-Stroke Rehabilitation: Assessment, Referral, and Patient Management, Clinical Practice Guideline. Pub. No. 95-0663. [PubMed] [Google Scholar]
- 2.AHA. American Heart Association. Dallas, TX: 2008. [Google Scholar]
- 3.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: The copenhagen stroke study. Arch Phys Med Rehabil. 1995 Jan;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 4.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin North Amer. 1999 Nov;10:887–906. [PubMed] [Google Scholar]
- 5.Bohannon RW, Horton MG, Wikholm JB. Importance of four variables of walking to patients with stroke. Int J Rehabil Res. 1991;14:246–250. doi: 10.1097/00004356-199109000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Chiou II, Burnett CN. Values of activities of daily living. A survey of stroke patients and their home therapists. Phys Ther. 1985 Jun;65:901–906. doi: 10.1093/ptj/65.6.901. [DOI] [PubMed] [Google Scholar]
- 7.Bohannon R, Andrews A, Smith M. Rehabilitation goals of patients with hemiplegia. J Rehabil Res. 1988 Jun;11:181–184. [Google Scholar]
- 8.Harris JE, Eng JJ. Goal priorities identified through client-centred measurement in individuals with chronic stroke. Physiother Canada. 2004;56:171–176. doi: 10.2310/6640.2004.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandel A, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Prediction of walking function in stroke patients with initial lower extremity paralysis: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 2000 Jun;81:736–738. doi: 10.1016/s0003-9993(00)90102-3. [DOI] [PubMed] [Google Scholar]
- 10.Harvey RL. Motor recovery after stroke: new directions in scientific inquiry. Phys Med Rehabil Clin North Amer. 2003 Feb;14:S1–S5. doi: 10.1016/s1047-9651(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 11.Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin North Amer. 2003 Feb;14:S57–76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 12.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: A randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000 Apr;81:409–417. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Wang R, Lin K, Chu M, Chan R. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin Rehabil. 2006 Oct;20:860–870. doi: 10.1177/0269215506070701. [DOI] [PubMed] [Google Scholar]
- 14.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998 Jun;29:1122–1128. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 15.Plummer P, Behrman AL, Duncan PW, Spigel P, Saracino D, Martin J, Fox E, Thigpen M, Kautz SA. Effects of stroke severity and training duration on locomotor recovery after stroke: A pilot study. Neurorehabil Neural Repair. 2007 Mar;21:137–151. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 16.Hesse S, Bertelt C, Schaffrin A, Malezic M, Mauritz KH. Restoration of gait in nonambulatory hemiparetic patients by treadmill training with partial body-weight support. Arch Phys Med Rehabil. 1994 Oct;75:1087–1093. doi: 10.1016/0003-9993(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 17.Fulk GD. Locomotor training with body weight support after stroke: The effect of different training parameters. J Neurol Phys Ther. 2004 Mar;28:20–28. [Google Scholar]
- 18.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical. J Amer Med Assoc. 2006 Nov;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 19.Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, Morris D, Blanton S, Nichols-Larsen D, Clark PC. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: The EXCITE randomised trial. Lancet Neurol. 2008 Jan;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: Characterizing the intervention protocol. Europa Medicophysica. 2006 Sep;42:257–268. [PubMed] [Google Scholar]
- 21.Levin M, Kleim J, Wolf S. What do ‘motor’ recovery and ‘compensation’ mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 22.Sazonov E, Fulk G, Hill J, Schutz Y, Browning R. Monitoring of posture allocations and activities by a shoe-based wearable sensor. IEEE Trans Biomed Eng. 2010 Apr; doi: 10.1109/TBME.2010.2046738. Preprint article. [DOI] [PubMed] [Google Scholar]
- 23.Fulk GD, Sazonov E. Using sensors to measure activity in people with stroke. Top Stroke Rehabil. doi: 10.1310/tsr1806-746. to be published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aminian K, Najafi B, Büla C, Leyvraz P, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomechan. 2002 May;35:689–699. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 25.Bamberg SJ, Benbasat AY, Scarborough DM, Krebs DE, Paradiso JA. Gait analysis using a shoe-integrated wireless sensor system. IEEE Trans Inf Technol Biomed. 2007 Jul;12(4):413–423. doi: 10.1109/TITB.2007.899493. [DOI] [PubMed] [Google Scholar]
- 26.Saremi K, Marehbian J, Yan X, Regnaux J, Elashoff R, Bussel B, Dobkin BH. Reliability and validity of bilateral thigh and foot accelerometry measures of walking in healthy and hemiparetic subjects. Neurorehabil Neural Repair. 2006 Jun;20:297–305. doi: 10.1177/1545968306287171. [DOI] [PubMed] [Google Scholar]
- 27.Salarian A, Russmann H, Vingerhoets FJG, Dehollain C, Blanc Y, Burkhard PR, Aminian K. Gait assessment in Parkinson’s disease: Toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng. 2004 Aug;51(8):1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 28.Sazonov E, Krishnamurthy V, Schilling R. Wireless intelligent sensor and actuator network—A scalable platform for time-synchronous applications of structural health monitoring. Struct Health Monit. 2010 [Google Scholar]
- 29.Menz HB, Latt MD, Tiedemann A, Kwan MMS, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Post. 2004 Aug;20:20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 30.Stokic DS, Horn TS, Ramshur JM, Chow JW. Agreement between temporospatial gait parameters of an electronic walkway and a motion capture system in healthy and chronic stroke populations. Amer J Phys Med Rehabil/Assoc Acad Physiatrists. 2009 Jun;88:437–444. doi: 10.1097/PHM.0b013e3181a5b1ec. [DOI] [PubMed] [Google Scholar]