Abstract
Brain-derived neurotrophic factor (BDNF) is upregulated in the hippocampus by antidepressant treatments, and BDNF produces antidepressant-like effects in behavioral models of depression. In our previous work, we identified genes induced by BDNF and defined their specific roles in hippocampal neuronal development and plasticity. To identify genes downstream of BDNF that may play roles in psychiatric disorders, we examined a subset of BDNF-induced genes also regulated by 5-HT (serotonin), which includes the neuropeptide VGF (nonacronymic). To explore the function of VGF in depression, we first investigated the expression of the neuropeptide in animal models of depression. VGF was downregulated in the hippocampus after both the learned helplessness and forced swim test (FST) paradigms. Conversely, VGF infusion in the hippocampus of mice subjected to FST reduced the time spent immobile for up to 6 d, thus demonstrating a novel role for VGF as an antidepressant-like agent. Recent evidence indicates that chronic treatment of rodents with antidepressants increases neurogenesis in the adult dentate gyrus and that neurogenesis is required for the behavioral effects of antidepressants. Our studies using [3H]thymidine and bromodeoxyuridine as markers of DNA synthesis indicate that chronic VGF treatment enhances proliferation of hippocampal progenitor cells both in vitro and in vivo with survival up to 21 d. By double immunocytochemical analysis of hippocampal neurons, we demonstrate that VGF increases the number of dividing cells that express neuronal markers in vitro. Thus, VGF may act downstream of BDNF and exert its effects as an antidepressant-like agent by enhancing neurogenesis in the hippocampus.
Keywords: antidepressant, BDNF, neuropeptide, proliferation, serotonin, transcription
Introduction
Depression is a complex disorder often resulting, in part, from exposure to chronic stress and is associated with a significant decrease in the volume of the hippocampus (Videbech and Ravnkilde, 2004; Neumeister et al., 2005). Recent evidence suggests that the well documented delay in antidepressant efficacy (Adell et al., 2005) may be attributable to neural adaptive mechanisms to reverse the damage of stress in the hippocampus including changes in synaptic plasticity, neurogenesis, and synaptogenesis (Castren, 2005; Dranovsky and Hen, 2006; Warner-Schmidt and Duman, 2006). These modifications of neuronal function most likely require the transcription of new molecules. Studies show that antidepressants, which primarily enhance 5-HT levels in the synapse also affect transcription of a number of genes including synapse-associated proteins and neuropeptides (Alfonso et al., 2005; Yamada and Higuchi, 2005; Holmes et al., 2006; Iwata et al., 2006; Sairanen et al., 2007). Furthermore, chronic antidepressant treatment upregulates the transcription factor cAMP response element binding protein (CREB) (Nibuya et al., 1996; Sairanen et al., 2005) and overexpression of CREB is sufficient to induce antidepressant-like effects in animal models of depression (Wallace et al., 2004), indicating the importance of transcription for antidepressant actions.
One CREB-activated gene implicated in depression is the neurotrophin BDNF. BDNF gene expression is reduced in the hippocampus in animal models of depression (Smith et al., 1995; Murakami et al., 2005; Gronli et al., 2006). In contrast, BDNF protein levels are increased in postmortem samples from human psychiatric patients treated with antidepressants (Chen et al., 2001; Karege et al., 2005). Moreover, BDNF is induced by selective serotonin reuptake inhibitors and repeated electroconsulsive seizure in animals (Nibuya et al., 1995; Altar et al., 2003; Russo-Neustadt et al., 2004). Finally, BDNF is required for the behavioral effects of antidepressants (Saarelainen et al., 2003; Monteggia et al., 2004), and BDNF itself has been shown to exert antidepressant-like effects in several models of depression (Siuciak et al., 1997; Shirayama et al., 2002; Hoshaw et al., 2005). BDNF may be involved in the antidepressant-induced reversal of hippocampal atrophy and cell loss by promoting the regrowth of damaged nerve fibers or the production of new neurons (Castren et al., 2007; Tanis et al., 2007). Indeed, overexpression or infusion of BDNF in the adult rat results in newly generated cells in the subgranular layer of the dentate gyrus and forebrain (Benraiss et al., 2001; Pencea et al., 2001; Scharfman et al., 2005).
In addition to these long-term effects of BDNF on neuronal development and neurogenesis, BDNF is also known to affect acute regulation of synaptic transmission (Lohof et al., 1993; Kang and Schuman, 1995; Levine et al., 1995; Gottschalk et al., 1998; Lessmann, 1998; Messaoudi et al., 1998; Sairanen et al., 2007). However, it remains to be revealed how a single trophic molecule can exert such a diverse temporal continuum of effects. BDNF may mediate its many actions through the expression of downstream molecules. We successfully used transcriptional profiling after neurotrophin treatment to identify novel roles for genes in neuronal function (Thakker-Varia et al., 2001; Alder et al., 2003). Among the many classes of genes induced by BDNF, our studies reveal that synaptic vesicle proteins and neuropeptides are important regulators of hippocampal function (Thakker-Varia et al., 2001; Alder et al., 2003, 2005; Ring et al., 2006). We recently discovered a role for the neuropeptide VGF in mediating synaptic activity of hippocampal cells (Alder et al., 2003). In this report, we show that the neuropeptide VGF is upregulated by both BDNF and 5-HT treatment and that VGF protein in the hippocampus is reduced in animals subjected to behavioral models of depression. We also demonstrate that VGF infusions reduce depressive-like behaviors in the forced swim test (FST) paradigm, and VGF enhances proliferation of hippocampal progenitor cells. Those newly born cells survive for at least 21 d and differentiate into neurons. Together, our studies suggest a novel function for VGF in the pathophysiology of depression.
Materials and Methods
Preparation of hippocampal neuronal cultures.
Hippocampi were obtained from time-mated embryonic day 18 rats [Sprague Dawley; Hilltop Laboratories (Scottsdale, PA)] and killed by CO2 asphyxiation in accordance with institutional guidelines for care and use of animals. Pooled tissue from each litter was mechanically triturated in Eagle's minimum essential medium with glucose and 7.5% fetal bovine serum and plated on poly-d-lysine-coated Petri dishes at 350,000 cells/dish. Cultures were maintained in serum-free medium at 37°C in a 95% air/5% CO2 humidified incubator as described previously (Thakker-Varia et al., 2001) and contained virtually pure neurons.
Peptides and chemical reagents.
VGF peptide (“TLQP” 62 aa C-terminal amidated peptide) was custom synthesized by Biopeptide (San Diego, CA). BDNF (50 ng/ml) (Peprotech, Rocky Hill, NJ) was used for transcriptional studies, because this dose is within the physiological range and was effective in previous electrophysiological and transcriptional profiling studies (Thakker-Varia et al., 2001; Alder et al., 2003). 5-HT (Sigma, St. Louis, MO) was used at 1 μm for transcriptional studies as determined by a dose–response study. The 5-HT receptor agonist used was 5-carboxamindotryptamine (5-CT) (1 μm; Sigma), which activates 5-HT1A, 5-HT1B, 5-HT1D, 5-HT5, and 5-HT7 receptors. Serotonin receptor antagonists used were N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY100635) against 5-HT1A and (R)-3-(2-(2-(4-methylpiperidin-1-yl)-ethyl)pyrrolidine-1-sulfonyl)phenol (SB269970) against 5-HT7 (10 μm; Sigma). Methyl-9-(S)-12(R)-epoxy-1H-diindolo[1,2,3-fg: 3′2′1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-2,3,9,10,11,12-hexahydro-10-(R)-hydroxy-9-methyl-1-oxo-10-carboxilate (K252a) was used to block troporelated kinase (trk) receptors (200 nm; Calbiochem, La Jolla, CA). Cells were pretreated with antagonists for 30 min before addition of agonist. Imipramine hydrochloride (10 mg/kg; Sigma) (Naudon et al., 2007) or vehicle was administered daily to rats by intraperitoneal injections for 21 d and tissue was collected 1 d after the last injection.
RNA isolation.
Total cellular RNA was prepared from hippocampal cultures (10 d in vitro) by the guanidine isothiocyanate method followed by cesium chloride gradients (Chomczynski and Sacchi, 1987). Removal of chromosomal DNA contamination from total cellular RNA was performed and the RNA was quantitated by absorbance at 260 nm (Thakker-Varia et al., 2001).
Real-time RT-PCR.
cDNA (200 μl) was prepared from 2 μg of RNA using random primers and SuperScript II reverse transcriptase. Twenty-five microliter PCRs were then performed using gene specific primers designed by Primer Express software and TaqMan MGB probes (Applied Biosystems 7000 Sequence Detection System; Applied Biosystems, Foster City, CA). Duplicate wells were included for each condition and primer pair. Primers specific to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as an internal control. Data analysis was performed according to the protocol provided by Applied Biosystems (Alder et al., 2003).
SDS-PAGE and Western blot analysis.
Hippocampal neurons plated at 350,000 cells per 35 mm dish or flash-frozen hippocampal tissue were solubilized in lysis buffer (20 mm Tris, pH 8, 0.5% Triton X-100, 0.5% SDS, protease inhibitor tablet, 1 mm PMSF, and 0.5 mm vanadate). The protein content was determined using the BCA protein assay kit (Pierce Chemical, Rockford, IL). Samples were subjected to 8–12% gradient SDS-PAGE and transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, Bedford, MA). The PVDF membranes were incubated with primary antisera to VGF (1:1000; Steve Salton 5902), Arc (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and Actin (1:500; Santa Cruz Biotechnology) (Alder et al., 2003) followed by anti-rabbit or goat HRP-conjugated IgG (1:5000). In some experiments, membranes were also probed with a commercial VGF antibody (R15; Santa Cruz Biotechnology). The immunopositive bands were visualized by chemiluminescence using the ECL detection kit (NEN, Boston, MA) and quantitated on a Bio-Rad (Hercules, CA) Gel Doc 2000 (Thakker-Varia et al., 2001).
Learned helplessness.
Adult male rats (300–400 g) were placed in two electrically linked shuttle boxes (46 × 19 × 18 cm) located within two illuminated (7.5 W bulb) sound-attenuating chambers (69 × 69 × 53 cm). The shuttle boxes were divided in half by a wall with a doorway (7.5 cm2). A scrambled shock generator delivered shocks (1 mA) through the floor grid and walls of the apparatus. Animals placed in the shuttle box (controllable condition) could learn to escape the shock by traversing the apparatus once. Crossing through the shuttle box doorway tripped a balance switch and thereby terminated the shock exposure in both boxes simultaneously. Animals placed in the other shuttle box (uncontrollable condition) could not escape the shock and thus could not establish control over the stressor. Because the shuttle boxes were electrically linked, the yoked control was exposed to the same number and amount of shock as was the animal that could learn to escape. Each day, animals were exposed to 30 trials (30 s maximum shock duration; 1 mA; 60 s intratrial interval) of training over the course of 7 d. The latency to escape was recorded and used as a measure of performance. After each training session, rats were returned to their home cage for 24 h (Shors et al., 1989; Leuner et al., 2004b). Naive controls were also included. For protein expression studies, subjects were killed on day 7, 1 h after their last session. Hippocampi and cerebelli were dissected immediately and flash frozen. Protein was extracted from the tissue and subjected to Western blot as described above (Alder et al., 2003).
Forced swim test.
On the first day of training, C57BL/6 mice (4 months old; 30 g) were placed in a container with water at a depth of 17 cm (25°C) for 6 min. At this depth, the mice could not touch the bottom with their hindlimbs or tail (Dalla et al., 2004). For protein expression studies, 1 d after training, hippocampi and cerebelli were dissected immediately and flash frozen. Protein was extracted from the tissue and subjected to Western blot. Controls consisted of naive animals. Alternatively, to test whether VGF can reverse the effects of depression (Siuciak et al., 1997; Hoshaw et al., 2005), VGF or saline was infused intrahippocampally 4 h after the first training session to allow time for recovery. Mice received anesthetic doses of tribromoethanol in amyl alcohol (Avertin) (0.375 mg/g; Sigma) and a 30 gauge injection needle attached to a Hamilton syringe was used to deliver the peptide. Coordinates were as follows: anterior–posterior, −1.9 mm with respect to bregma; lateral, ±1.5 mm; ventral, −1.5 mm with respect to the surface of the skull (Heinrich et al., 2006). Mice receive bilateral infusions of 100 ng in 1 μl of VGF in sterile saline delivered manually over 1 min. The needle was left in place for 1 min after delivery to allow for diffusion of the neuropeptides (Wanisch et al., 2005). Controls consisted of saline infusion (negative control) or BDNF (500 ng; positive control). Three and 6 d after infusion, the mice were placed back into the water for 6 min and the sessions were videotaped. The time spent immobile as defined by a lack of movement other than what is necessary to keep the head above water was scored (Duman et al., 2007). The behavior was videotaped and also scored by an observer blinded to treatment. The location of infusion site was confirmed by visual inspection after the animal was killed and only data from mice in which the infusion site was at the hippocampus were included.
Open field test.
The open field apparatus consisted of a (40 × 40 × 49 cm) clear Plexiglas arena with a white floor marked with tape dividing it into 12 equal quadrants (Chen et al., 2006). Separate sets of mice were used for FST and open field testing. Three and 6 d after intrahippocampal VGF infusion as described above, a single mouse was placed into the center of the open field and its behavior was videotaped. During the 15 min of open field performance, the number of crossings of the quadrants with all four paws was scored (Zomkowski et al., 2006). The apparatus was washed with water and ethanol in between mice.
[3H]Thymidine assay in vitro
Hippocampal cells were plated in 24-multiwell plates (100,000 cells/well), treated at time of plating with VGF at concentrations of 10, 3, 1, 0.1, 0.03 μm in triplicate and exposed to [3H]thymidine (1 μCi/ml) for the last 4 h of 48 h incubations. Cells were harvested and collected for scintillation counting (Lu and DiCicco-Bloom, 1997).
Bromodeoxyuridine double-labeling immunocytochemistry in vitro.
Hippocampal cells (350,000 cells per 35 mm dish) were treated with VGF (3 μm) at the time of plating. Bromodeoxyuridine (BrdU) (10 μm) was added to cells at 48 h, and cultures were fixed in 4% paraformaldehyde 4 h or 6 d later. Neurons were then incubated with the following primary antibodies: nestin (1:250; Hybridoma Bank, University of Iowa, Iowa City, IA), neuronal class III β-tubulin (TuJ1; 1:500; Covance, Princeton, NJ), glial fibrillary acidic protein (GFAP) (1:400; Chemicon, Temecula, CA), or Tau (1:500; Sigma). Secondary antibodies consisted of Alexa Fluor 594 (red) goat anti-mouse or goat anti-rabbit (1:1000) after which cells were briefly refixed. The cells were then treated with 2N HCl followed by rat anti-BrdU (1:100; Accurate Chemical and Scientific, Westbury, NY) and Alexa Fluor 488 (green) goat anti-rat (1:1000). The cells were coverslipped with Fluoromount G and the immunostaining was visualized on a Zeiss (Oberkochen, Germany) inverted fluorescence microscope at 40× (Wagner et al., 1999).
BrdU labeling in vivo.
Surgical implantation of guide cannulas was performed by Zivic Laboratories (Pittsburgh, PA). Adult male rats (215–375 g) were implanted bilaterally with 22 gauge steel guide cannulas (Plastics One, Roanoke, VA) in the dorsal hippocampus above the dentate gyrus (coordinates: anterior–posterior, −3.8 mm with respect to bregma; lateral, ±2.0 mm; ventral, −3.0 mm with respect to the surface of the skull). The patency of the guide cannulas was maintained with dummy cannulas. The rats were allowed to recover from surgery for 7 d. VGF (4 μg in 2 μl) or saline was delivered manually by a Hamilton syringe connected to a 28 gauge internal cannula, which protruded slightly beyond the guide cannula, and the injection cannula was left in place for 1 min after delivery to allow for diffusion of the neuropeptide (Babri et al., 2007). VGF was infused daily for 7 d and the animals were weighed every day at the time of injection.
Immediately after the last intrahippocampal infusion with VGF or saline, animals received BrdU (100 mg/kg) intraperitoneally, and rats were perfused transcardially with 4% paraformadehyde 2 h or 21 d later. Brains were dissected and postfixed overnight. The location of cannulas was confirmed by visual inspection, and only data from animals in which the infusion site was at the hippocampus were included. The tissue was sectioned using a vibratome (40 μm) coronally in a 1:12 series, mounted on charged poly-lysine-coated slides and processed immunochemically. Briefly, slides were immersed in citrate buffer (0.01 m) and microwaved. Tissue was treated with trypsin and 2N HCl. BrdU antibody (1:200; Becton Dickinson, Franklin Lakes, NJ) was applied overnight followed by goat anti-mouse biotinylated secondary antibody (1:200). Staining was visualized with DAB/nickel-cobalt (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) and cresyl violet counterstaining (Cheng et al., 2001). DAB-positive cells in the subgranular zone (SGZ) plus granular cell layer (GCL) and hilus (H) were counted in every 12th section, with an average of 10 sections per region in each animal (Leuner et al., 2004a). Cells were counted as being in the SGZ plus GCL when they are in the GCL or within two cell bodies width of it. Cells were counted as hilar when they are beyond two cell bodies of the GCL. To estimate the total number of BrdU+ cells per dentate gyrus, counts were multiplied by 12.
For identification of BrdU+ cell types in vivo, tissue was obtained as above and treated with sodium citrate buffer (SSC) plus formamide. The slides were then treated with 2N HCl and incubated overnight with rat anti-BrdU (1:100; Accurate Chemical and Scientific) plus either GFAP (1:400; Chemicon) or NeuN (1:200; Chemicon). The slides were incubated in donkey anti-rat biotin-SP followed by fluorescein-DTAP-streptavidin and finally donkey anti-mouse rhodamine red X (Jackson ImmunoResearch Laboratories, West Grove, PA) (Leuner et al., 2004a). The slides were coverslipped with Fluoromount G (Southern Biotech, Birmingham, AL) and the immunostaining was visualized on a Zeiss Apotome microscope with Z plane projection to verify double labeling.
Cleaved caspase-3 immunocytochemistry. Rats implanted with bilateral intrahippocampal cannulas as described above received VGF or saline injections (2 μl/d for 7 d) and tissue was perfused 21 d after the last injection (Gould and Tanapat, 1997). Sections (12 μm) throughout the hippocampus were incubated in cleaved caspase-3 (Asp175) antibody (1:200; Cell Signaling, Danvers, MA) overnight at 4°C followed by goat anti-rabbit biotin (1:1000). Staining was visualized with DAB/nickel-cobalt (Vectastain Elite ABC kit; Vector Laboratories). Slides were counterstained for Nissl using toluidine blue O and coverslipped with Permount.
Results
5-HT regulates a subset of BDNF-induced genes through an independent pathway
To begin to elucidate mechanisms of BDNF as an antidepressant, we examined the transcriptional regulation by 5-HT of BDNF-induced genes (Alder et al., 2003; Ring et al., 2006). Genes previously identified as BDNF-regulated were selected for this analysis based on their categorization as either immediate-early genes (IEGs), synapse-associated markers, or neuropeptides. Of 20 BDNF-induced genes selected, 8 were significantly upregulated by 3 h 5-HT treatment as shown by real time RT-PCR (Table 1). We detected upregulation of IEGs such as c-fos, early growth response protein (EGR), and activity-regulated cytoskeletal-associated protein (Arc), which are recognized to play a key role in the cascade of molecular changes leading to modifications in neural plasticity (Lanahan and Worley, 1998). We also detected changes in the synapse-associated protein neuronal pentraxin, which is a protein involved in synapse remodeling (Abad et al., 2006). Another gene upregulated by BDNF and 5-HT is the serine proteinase inhibitor plasminogen activator inhibitor (PAI), which blocks the proteolytic cleavage of proBDNF (Pang et al., 2004). Finally, we observed increased expression of neuropeptides including VGF, Substance P (SP), and Neuropeptide Y (NPY). NPY and SP but not VGF have been implicated in psychiatric disorders (Redrobe et al., 2002b; Heilig, 2004; Chahl, 2006; Karl and Herzog, 2007). The regulation of gene expression was dose dependent such that the response plateaued at 1 μm 5-HT. In addition, a number of genes induced by BDNF were not affected by 5-HT exposure including somatostatin, prepronociceptin, thyrotropin releasing hormone, period, synaptotagmin, and synaptojanin, indicating the specificity of the coregulation. The common genes regulated by both BDNF and 5-HT were the focus of all subsequent studies.
Table 1.
5-HT and BDNF induce a common set of genes
| Gene name | Abbreviation | Accession no. | 1 μm 5-HT | 50 ng/ml BDNF | 5-HT + BDNF |
|---|---|---|---|---|---|
| c-fos proto-oncogene | cfos | X06769 | 2.25 ± 0.23* | 3.65 ± 0.58* | 5.80 ± 1.04** |
| Early growth response protein 1 | EGR | M18416 | 1.41 ± 0.14* | 10.13 ± 1.79* | 9.75 ± 2.29 |
| Activity-regulated cytoskeletal-associated | Arc | U19866 | 1.70 ± 0.16* | 42.32 ± 4.37* | 52.59 ± 7.30 |
| Neuronal pentraxin 1 | Ptx | NM153735 | 1.92 ± 0.24* | 10.11 ± 3.60* | 10.36 ± 5.30 |
| Plasminogen activator inhibitor 2 | PAI | X64563 | 2.37 ± 0.43* | 62.17 ± 3.84* | 56.33 ± 12.38 |
| VGF protein precursor | VGF | M60525 | 1.67 ± 0.17* | 16.90 ± 3.74* | 13.33 ± 2.68 |
| Substance P | SP | NM012666 | 2.05 ± 0.35* | 4.41 ± 1.09* | 3.24 ± 0.84 |
| Neuropeptide Y precursor | NPY | M20373 | 1.73 ± 0.17* | 2.33 ± 0.35* | 3.39 ± 0.63 |
Hippocampal cultures were treated with 1 μm 5-HT, 50 ng/ml BDNF, or a combination of 5-HT and BDNF for 3 h followed by real-time RT-PCR analysis of genes induced by BDNF. All samples were first normalized to GAPDH and then represented as a ratio of vehicle controls ± SE (n = 4).
*p < 0.05, significantly different from control (t test);
**p < 0.05, significantly different from BDNF (ANOVA).
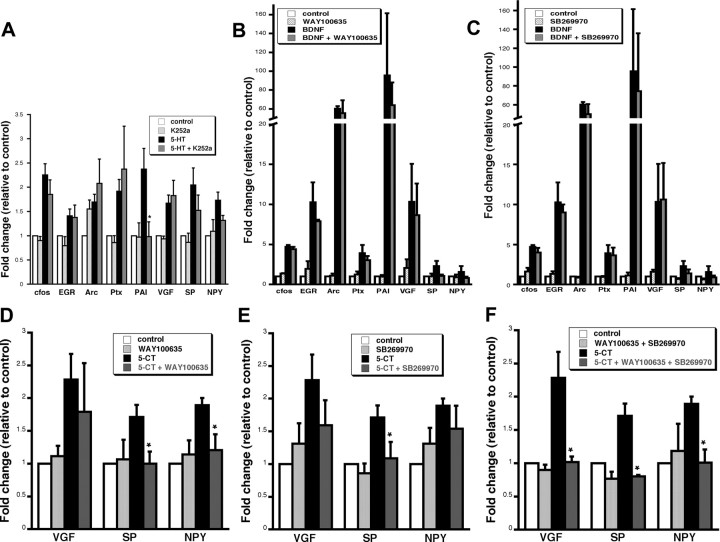
To explore the interaction between 5-HT and BDNF pathways on transcription, the effectiveness of 5-HT and BDNF separately and in combination was assayed. 5-HT was a more modest stimulator of transcription than BDNF when used separately. In addition, the combination of the two treatments did not significantly increase mRNA levels of the genes relative to BDNF alone, indicating that there is no synergistic interaction between the 5-HT and BDNF pathways on transcription (Table 1). To determine whether trk receptor activation is required for 5-HT-mediated gene expression, hippocampal cells were pretreated with the trk receptor antagonist, K252a, before 5-HT exposure at a concentration we previously showed to be effective at blocking transcription induced by BDNF (Alder et al., 2003). With the exception of PAI, no effect on transcription was observed for the panel of genes (Fig. 1A), indicating that trk activation is not necessary for 5-HT-induced transcription. Conversely, to determine whether 5-HT receptor activation is required for BDNF-mediated gene expression, hippocampal cells were pretreated with 5-HT1A (WAY100635) or 5-HT7 receptor antagonist (SB269970) before BDNF treatment. The overall trend among the entire panel of genes studied indicated no effect of 5-HT1A or 5-HT7 antagonists on BDNF-induced transcription. Although Substance P and NPY both showed some decrease in BDNF-induced transcription by the inhibitors, these data were not statistically significant (Fig. 1B,C). Together, these data suggest that 5-HT and BDNF stimulate transcription independently.
Figure 1.
5-HT regulates transcription independent of BDNF and requires differential activation of either 5-HT1 and/or 5-HT7. A, Average gene expression in hippocampal cells preincubated with the trk receptor antagonist K252a (200 nm) for 30 min before 5-HT treatment (1 μm) followed by real time RT-PCR for a panel of genes. Only one gene, PAI, showed a significantly lower response to 5-HT plus K252a than 5-HT alone. *5-HT plus K252a is significantly different from 5-HT alone (p < 0.05, ANOVA; n = 4). B, C, Average gene expression in hippocampal cells preincubated with 5-HT receptor antagonists for 30 min before BDNF treatment (50 ng/ml) followed by real-time RT-PCR for a panel of genes. B, 5-HT1A antagonist (WAY100635; 10 μm). C, 5-HT7 receptor antagonist (SB269970; 10 μm). No statistical difference was detected between BDNF plus receptor antagonists and BDNF alone for any of the genes (p > 0.05, ANOVA; n = 3). D–F, Average gene expression in neurons preincubated with inhibitors to 5-HT receptor subtypes before 5-CT (1 μm) treatment followed by real-time RT-PCR for the neuropeptides VGF, SP, and NPY. D, 5-HT1A antagonist (WAY100635; 10 μm). E, 5-HT7 antagonist (SB269970; 10 μm). F, Combination of WAY100635 plus SB269970. One group of cells in each experiment was treated with inhibitor alone and showed no significant change in expression relative to vehicle control. *Significant reduction of transcription in the presence of inhibitor plus 5-CT relative to 5-CT alone (p < 0.05, ANOVA; n = 3). All samples were first normalized to GAPDH and then represented as an average ratio of vehicle controls ± SE.
The receptor requirement for 5-HT-induced transcription was studied using receptor antagonists and the neuropeptides VGF, SP, and NPY as prototype genes. The 5-HT analog 5-CT, which activates the 5-HT1A,B,D, 5-HT5, and 5-HT7 receptors, induced transcription of VGF, SP, and NPY (2.28 ± 0.39-, 1.71 ± 0.18-, and 1.89 ± 0.11-fold of control, respectively; n = 3; p < 0.05, ANOVA). The involvement of specific 5-HT receptors was assayed using the 5-HT1A antagonist (WAY100635) and 5-HT7 antagonist (SB269970) in combination with 5-CT as an agonist. Transcription of NPY requires activation of 5-HT1A, whereas SP requires activation of either 5-HT1A or 5-HT7 and VGF requires activation of both 5-HT1A and 5-HT7 (Fig. 1B–D). In contrast, agonists and antagonists of the 5-HT2 receptor did not affect transcription of any of the neuropeptides (data not shown). These findings suggest that transcription of each gene requires activation of specific 5-HT receptors.
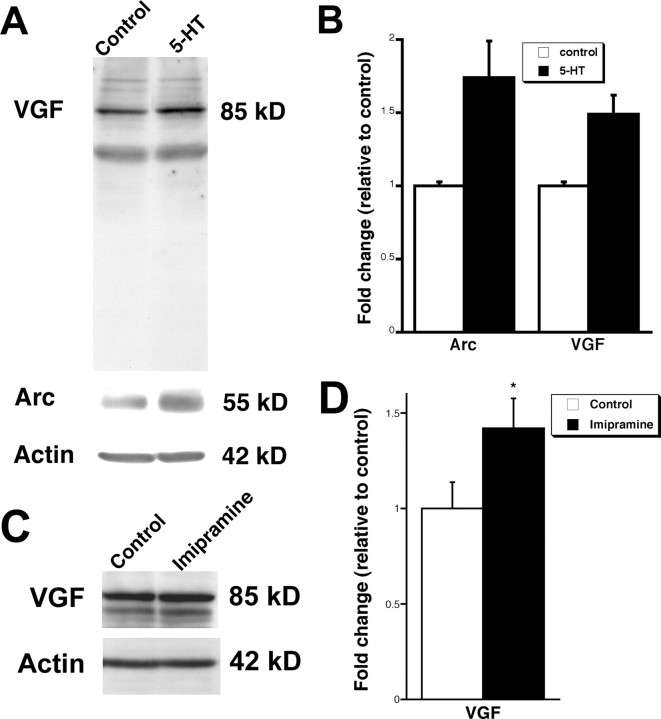
Western blot analysis was performed to determine whether transcriptional regulation of the IEG Arc and the neuropeptide VGF by 5-HT is accompanied by protein translation. Hippocampal cultures treated with 5-HT for 6 h displayed a ∼1.5-fold increase in expression of both Arc and VGF (Fig. 2A,B), indicating that the protein products of the induced genes are also upregulated by 5-HT. VGF protein sometimes appeared as a doublet in Western blots (Hahm et al., 1999). Furthermore, whereas the VGF antibody detects some other bands, the intensity of these bands did not change with the treatment, indicating the specificity of the antibody. To test whether chronic antidepressant treatment affects VGF expression in the hippocampus, rats received daily injections of imipramine for 21 d. Western blot analysis revealed a ∼1.4-fold increase in VGF protein expression in the hippocampus of imipramine-treated rats compared with rats injected with vehicle (Fig. 2C,D). Together, these results indicate that VGF is induced both in vitro and in vivo after treatments that elevate serotonin levels.
Figure 2.
5-HT and imipramine upregulate Arc and VGF protein levels. A, Western blot analysis of Arc and VGF expression after 5-HT (1 μm) exposure of hippocampal cultures for 6 h. B, Quantitation of protein levels indicates that Arc and VGF are upregulated. The bars represent average protein expression ± SE (n = 2). Data are first normalized to actin to control for protein loading, and data are expressed as a fold of expression in vehicle controls. C, Representative Western blot of VGF expression in the hippocampus after intraperitoneal injection of imipramine (10 mg/kg) for 21 d. D, Quantitation of protein levels indicates VGF is upregulated. The bars represent average protein expression ± SE (n = 4, 6). Data are first normalized to actin, which controls for protein loading, and data are expressed as a fold of expression in vehicle-injected rats. *p < 0.05, unpaired t test.
VGF is downregulated in animal models of depression and produces antidepressant-like effects
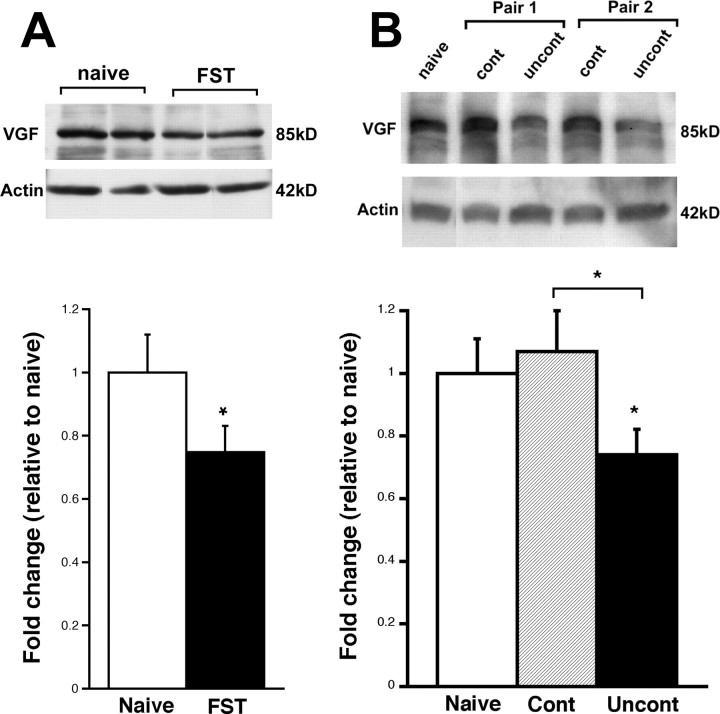
To investigate the potential role of VGF in depression, we first determined levels of VGF in the hippocampi of animals subjected to a depression paradigm, FST. Mice underwent FST for 5 min and expression of the 5-HT- and BDNF-regulated protein, VGF, was examined in the hippocampi of the mice 24 h later by Western blot analysis. We found a decrease of ∼20% in the protein levels of VGF in the mice exposed to FST relative to naive mice (Fig. 3A). Because no effect was observed in the cerebellum (0.93 ± 0.09-fold of naive; n = 6; p > 0.05, unpaired t test), this effect on neuropeptide expression may be specific to the hippocampus. We also used another depression paradigm, learned helplessness (LH), to demonstrate a similar phenomenon. Rats exposed to 30 trials of mild footshock for 7 d showed a decrease in the latency to escape the shock (controllable). In contrast, the yoked littermates who were unable to control the stressor (uncontrollable) did not attempt to escape after the first day of training and performed poorly on a subsequent test (FR2) demonstrating their inability to learn to escape the shock (Shors et al., 2007). These observations suggest that the animals subjected to uncontrollable stress exhibit a form of learned helplessness that is an accepted model of depression. The expression of VGF in the hippocampus was examined on the last day of training. The rats subjected to uncontrollable stress show significant decreased in VGF protein expression compared with naive rats, as well as compared with rats subjected to controllable stress (Fig. 3B). These data suggest that the decrease in VGF expression in the animals subjected to uncontrollable stress is attributable to the depression paradigm specifically. Together, these data suggest that VGF protein is downregulated in animal models and suggest that the neuropeptide may play a role in the pathogenesis of depression.
Figure 3.
VGF expression is decreased in two models of depression. A, Representative Western blot of VGF protein expression in naive rodents and those subjected to FST. Each lane represents one animal. All data are normalized to actin expression. The bars represent average VGF protein levels relative to naive ± SE (n = 4, 5). *p < 0.05, unpaired t test. B, Western blot of VGF protein expression in a representative naive animal and two representative pairs of animals subjected to controllable or uncontrollable stress. All data are normalized to actin expression. The bars represent average VGF protein expression ± SE in rodents subjected to controllable stress or uncontrollable stress (n = 7) relative to naive rats (n = 3). *p < 0.05, ANOVA.
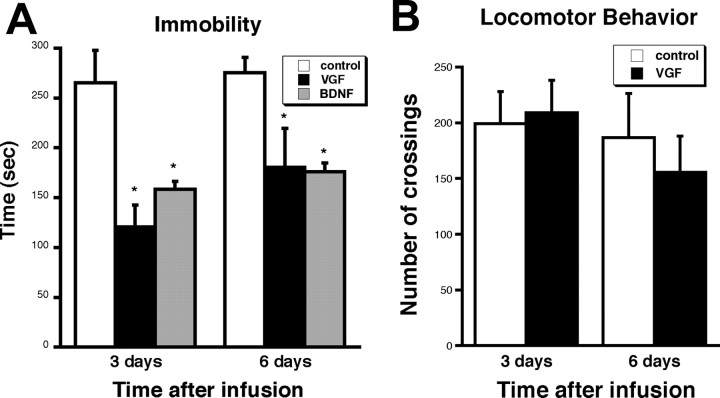
The effectiveness of VGF as an antidepressant-like agent was explored by introducing the neuropeptide via intrahippocampal infusions into mice and subjecting the mice to FST. Mice were preexposed to FST, followed several hours later by VGF injection bilaterally by stereotaxic surgery. This technique was used to see whether VGF can reverse the effect of depression as has been done for other growth factors (Siuciak et al., 1997; Hoshaw et al., 2005). BDNF was used as a positive control and saline as a vehicle control. Three and 6 d after surgery, the mice were scored for time spent immobile in the FST. The time an animal spends immobile is indicative of a state of helplessness and depressive-like behavior. Our data reveal that mice infused with VGF exhibited an approximately twofold decrease in immobility both 3 and 6 d after surgery (Fig. 4A). In a separate set of animals, there was no change in the number of crossings in the open field test after infusion of VGF (Fig. 4B), indicating that the effect on immobility is not attributable to a nonspecific effect on locomotor function. We also observed that the presurgery behavior for each injection group was similar, suggesting that the differences detected after surgery were attributable to the neuropeptide rather than differences in the individual mice. Furthermore, there was no change in behavior over the time course of the experiment in saline infused animals, demonstrating that repeated exposure to the FST does not affect their behavior. Finally, there was no difference in weight gain in the VGF-infused mice relative to control mice, suggesting that there was no significant effect on metabolism (data not shown) from the intrahippocampal infusion of VGF.
Figure 4.
VGF intrahippocampal infusions decrease immobility in FST. A, Mice were injected bilaterally with saline, VGF (100 ng), or BDNF (500 ng) and scored on FST (6 min) performed 3 and 6 d postsurgery. The bars represent average time spent immobile for all animals ± SE (n = 5). *p < 0.05, t test. B, Mice were also scored for locomotor behavior in the open field test. The bars represent the average number of quadrant crossings ± SE in a 15 min time period scored 3 and 6 d after surgery (n = 5). *p < 0.05, t test.
VGF promotes proliferation in hippocampal cells
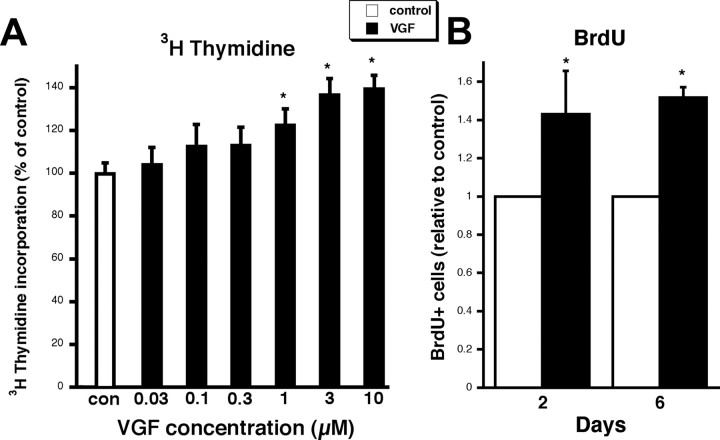
To explore the mechanism by which VGF acts as an antidepressant-like agent, we studied the effect of the neuropeptide on proliferation of hippocampal cells. Freshly plated hippocampal cultures were treated with VGF and DNA synthesis was quantitated by [3H]thymidine incorporation. A dose-dependent increase in [3H]thymidine incorporation was observed at 48 h (Fig. 5A). Several other neuropeptides tested such as nociceptin and SP did not induce proliferation of the hippocampal precursors (data not shown), indicating the specificity of the effect by VGF. BrdU incorporation studies were also performed to quantitate cell proliferation. Hippocampal cells were treated with VGF or vehicle at time of plating, BrdU was added to cells at 48 h, and cells were fixed 2 or 6 d later. A ∼50% increase in DNA synthesis by VGF was observed at both 2 and 6 d (Fig. 5B), thus validating the [3H]thymidine incorporation experiments and suggesting that the proliferating cells survive to at least 6 d in vitro. There was no significant difference in the total number of cells in VGF-treated cultures relative to controls (48 h, 1.08 ± 0.05; 6 d, 1.10 ± 0.06-fold of control; n = 5; p > 0.05, ANOVA) consistent with the fact that BrdU-positive cells represent only ∼5% of the total cell number in control dishes and therefore should not influence total cell number. The expression of cell cycle genes was determined in the VGF-treated cultures using real-time RT-PCR. mRNA for cell-dividing control protein 2 (Cdc2) (also known as Cdk1), which promotes M-phase transition required for completion of mitosis (Wang et al., 2005), was upregulated after 48 h of exposure to VGF (1.43 ± 0.09 relative to control; n = 3; p < 0.05, ANOVA). Therefore, VGF promotes the expression of some of the activators of cell cycle progression, supporting the hippocampal cell proliferation data.
Figure 5.
VGF enhances DNA synthesis in hippocampal cultures. A, VGF increases [3H]thymidine incorporation in hippocampal cultures in a dose-dependent manner. Hippocampal neurons were treated with VGF in concentrations ranging from 0.03 to 10 μm or vehicle control from time of plating to 48 h. Cells were then loaded with 1 μCi/ml [3H]thymidine at 37°C for 4 h. Data are expressed as mean percentage of control ± SE (n = 8). *p < 0.05, ANOVA. B, Hippocampal cells were treated with VGF (3 μm) or vehicle control at time of plating. BrdU was added to cells at 48 h and cells were fixed 2 or 6 d later. The bars represent fraction of total cells that were BrdU+ expressed as an average fold change relative to control ± SE. Approximately 200–700 total cells were counted per dish, and there were four dishes per condition per experiment. Each bar represents a cell count from three independent experiments (∼2400–8400 total cells). *p < 0.05, ANOVA.
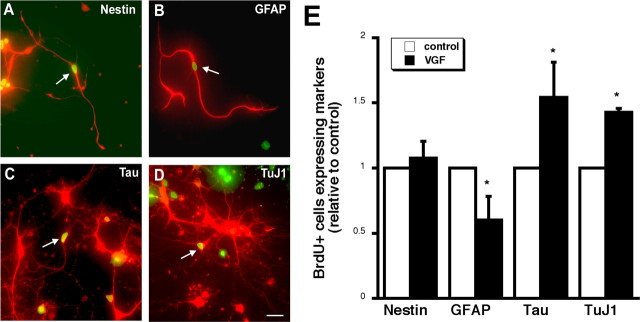
To reveal the identity of the hippocampal cells proliferating in the presence of VGF in vitro, double immunocytochemistry experiments were performed. Characterization of BrdU-positive (BrdU+) cells in the hippocampal cultures treated with vehicle or VGF was assessed after 6 d of differentiation by examining the colocalization of BrdU with cell type-specific markers. Two cytoskeletal markers expressed in neural progenitors were used: nestin and GFAP (Fig. 6A,B). In addition, two cytoskeletal markers of mature neurons were localized: Tau and neuronal class III β tubulin (TuJ1) (Fig. 6C,D). BrdU+ cells coexpressed not only nestin and GFAP after VGF treatment, but also Tau and TuJ1 indicating that some of the proliferating progenitor cells had begun to differentiate into mature neurons after 6 d in vitro. To begin to determine whether VGF influences the differentiation into neurons of the proliferating cells, the percentage of BrdU+ cells that express different cell specific markers were assayed after the cells were allowed to differentiate for 6 d. No change in the percentage of BrdU+ cells expressing nestin was observed but a decrease in the percentage of BrdU+ cells expressing GFAP after VGF treatment relative to control was seen. Moreover, there was an increase in the percentage of BrdU+ cells expressing either Tau or TuJ1 (Fig. 6E). These findings indicate that VGF treatment enhances the proliferation of progenitor cells that differentiate into neurons and suggest that VGF may play a role in neurogenesis.
Figure 6.
VGF promotes the differentiation of neuronal cells. A–D, BrdU and cell-specific markers colocalize after 6 d of differentiation. Freshly plated hippocampal cells were treated with 3 μm VGF or vehicle control at time of plating. BrdU was added to cultures at 48 h, and cells were fixed 6 d later. The arrows indicate double-labeled cells with a BrdU+ nucleus (green) and nestin (A), GFAP (B), Tau (C), and TuJ1 (red) (D). Scale bar, 50 μm. E, Quantitation of BrdU+ cells expressing cell-specific markers after 6 d of differentiation in vitro. Data represent fraction of BrdU+ cells coexpressing cell-specific markers including the precursor markers nestin and GFAP and the mature neuronal markers Tau and TuJ1 (∼50 BrdU+ cells/dish; n = 3). *p < 0.05, ANOVA.
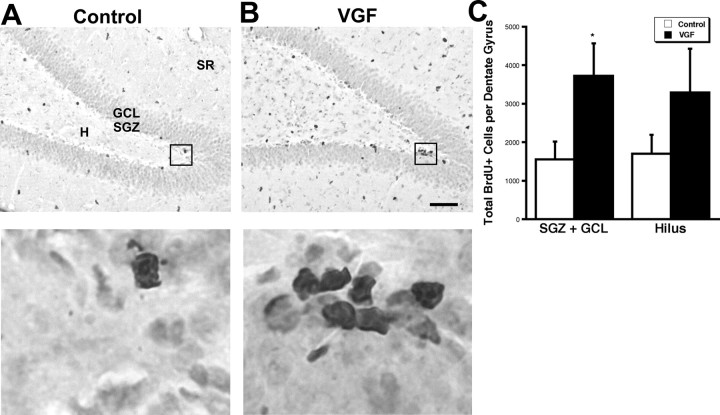
The effect of VGF on proliferation of precursor cells in the dentate gyrus was explored in vivo. VGF was delivered by intrahippocampal infusion into adult male rats for 7 d followed immediately by a single BrdU injection. The animals were killed 2 h later (Malberg et al., 2000), and the number of BrdU+ cells was quantitated in the dentate gyrus in which adult neurogenesis occurs. There was a significant increase in BrdU+ cells in the SGZ plus GCL and a trend toward more BrdU+ cells in the hilus (Fig. 7A–C). These data suggest that VGF positively affects the proliferating cell population in vivo.
Figure 7.
Intrahippocampal infusion of VGF increases BrdU+ cells in the SGZ plus GCL. A, B, Saline (A) or VGF (4 μg) (B) was infused daily via a cannula into the hippocampi of adult male rats for 7 d followed by a single BrdU injection (100 mg/kg). The animals were killed 2 h later. BrdU+ cells (black) are visible in the subdivisions of the dentate gyrus including the hilus (H), subgranular zone (SGZ), granule cell layer (GCL), and stratum radiatum (SR) in coronal sections. Scale bar, 80 μm. Enlarged images of boxed regions are shown below. C, Quantitation of total BrdU+ cells in the various regions of the dentate gyrus. The bars represent average BrdU+ cells ± SE (n = 7, 9). *p < 0.05, t test.
To determine whether the newly born cells induced by VGF survive, we quantitated the number of BrdU+ cells 21 d after the BrdU injection. We observed a trend toward more BrdU+ cells in the subgranular and granular cell layer in VGF-treated animals compared with saline controls. A statistically significant increase in BrdU+ cells was detected in the hilus of VGF-treated rats relative to controls. We therefore conclude that the newly born cells induced by VGF in the dentate gyrus survive at least 21 d (Fig. 8A–C). Previous studies have shown that a single injection of 2 μl of saline into the hippocampus caused no damage or change in cell proliferation in the dentate gyrus (Gould and Tanapat, 1997). To confirm that the increase in proliferating cell number in the dentate gyrus is not attributable to damage induced either by repeated (2 μl/d every day for 7 d) infusions or VGF itself, we performed immunocytochemistry for cleaved caspase-3 (Fig. 8D,E). This antibody detects cells undergoing apoptosis, and in addition we used Nissl stain to reveal degenerating cells. The guide cannula is positioned above the dentate gyrus, and we detected small numbers of caspase-3-positive cells and darkly stained small and rounded cell bodies as is described for degenerating cells (Gould and Tanapat, 1997) in the immediate vicinity of the implanted cannula. This damage around the guide cannula was comparable in both saline- and VGF-treated animals. The internal cannula used to deliver saline or VGF protrudes slightly beyond the guide cannula and there are several caspase-3+ cells in that region in both treatments. Importantly, the dentate gyrus and the cell layers surrounding it do not appear damaged in either saline- or VGF-treated animals. There are minimal caspase-3+ cells in the dentate gyrus after either saline or VGF infusions. Therefore, repeated infusions do not cause damage and VGF does not induce more cell death than saline. Thus, the increase in BrdU+ cells in the dentate gyrus seen in the VGF-treated rats is most likely attributable to proliferation induced by the neuropeptide rather than degenerating cells. Finally, the differentiation of the BrdU+ cells was examined after 21 d in vivo. We observed that most of the BrdU+ cells in the dentate gyrus coexpressed the nuclear neuronal marker, NeuN. Very few of the BrdU+ cells colocalized with the precursor and astrocytic marker GFAP (Fig. 8F–K). These findings suggest that VGF-induced proliferating progenitor cells differentiate into mature neurons.
Figure 8.
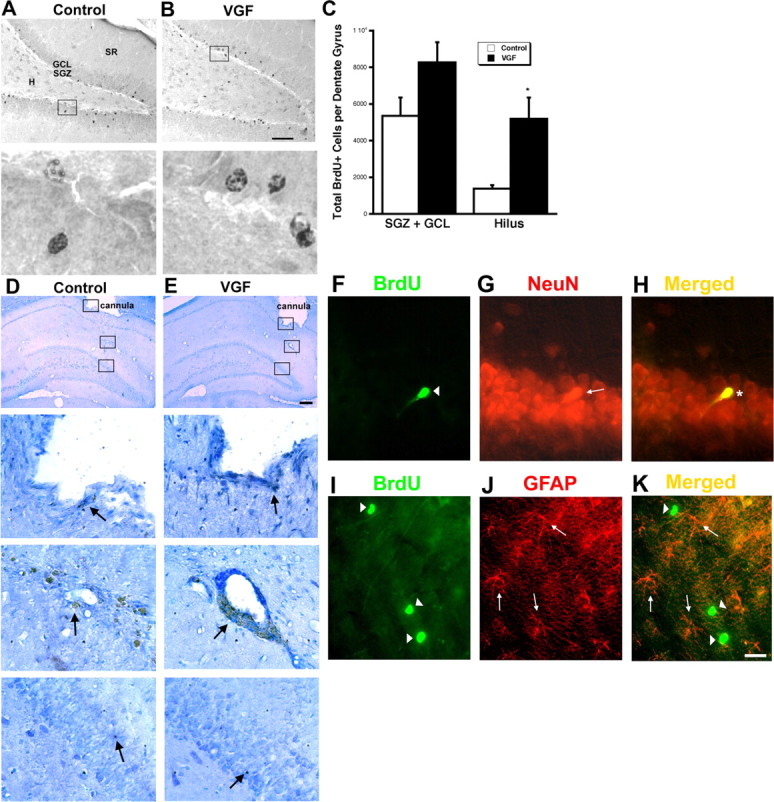
VGF-induced BrdU+ cells survive to 21 d and differentiate primarily into neurons. A–C, VGF-induced BrdU+ cells survive to 21 d. A, B, Saline (A) or VGF (4 μg) (B) was infused daily via a cannula into the hippocampi of adult male rats for 7 d followed by a single BrdU injection (100 mg/kg). The animals were killed 21 d later. BrdU+ cells (black) are visible in the subdivisions of the dentate gyrus including the hilus (H), subgranular zone (SGZ), granule cell layer (GCL), and stratum radiatum (SR) in coronal sections. Scale bar, 80 μm. Enlarged images of boxed regions are shown below. C, Quantitation of total BrdU+ cells in the various regions of the dentate gyrus. The bars represent average BrdU+ cells ± SE (n = 8). *p < 0.05, t test. D, E, Repeated infusions of saline or VGF do not cause damage to the dentate gyrus. Saline (D) or VGF (4 μg) (E) was infused daily via a cannula into the hippocampi of adult male rats for 7 d. The animals were killed 21 d later and processed for cleaved caspase-3 immunohistochemistry. Location of guide cannula is shown at low magnification (arrowhead). Scale bar, 150 μm. Enlarged images of boxed regions showing tip of cannula, site of infusion, and dentate gyrus, respectively, are shown below. Caspase-3-positive cells are indicated by arrows. F–H, BrdU+ cells that survive primarily express neuronal markers. F, BrdU+ cells (green; arrowhead). G, NeuN+ cells (red; arrow). H, Merged image showing colocalization (yellow; asterisk). I–K, BrdU+ cells that survive do not express glia markers. I, BrdU+ cells (green; arrowheads). J, GFAP+ cells (red; arrows). K, Merged image showing no double-labeled cells (yellow). Scale bar, 20 μm.
Discussion
Transcriptional regulation by BDNF and 5-HT
Our aim in the present study was to identify potential molecular mediators of the effects of BDNF on depression. The significance of BDNF in the context of psychiatric disorders has been emerging over the past decade. However, it remains to be elucidated how BDNF can exert diverse effects ranging from antidepressant-like activity to modulating synaptic plasticity and neuronal development. BDNF may mediate its many actions through the expression of downstream molecules. Both BDNF and antidepressants, which have effects on several molecular pathways including enhancing 5-HT levels in the synapse, have been shown to induce transcription (Thakker-Varia et al., 2001; Alder et al., 2003; Alfonso et al., 2005; Yamada and Higuchi, 2005; Ring et al., 2006). BDNF upregulates several classes of genes including synaptic vesicle proteins and neuropeptides (Thakker-Varia et al., 2001; Alder et al., 2005; Ring et al., 2006). Neuropeptides known to be induced by BDNF include, among others, VGF, NPY, SP, and Nociceptin (Nawa et al., 1994; Eagleson et al., 2001; Alder et al., 2003; Ring et al., 2006). The fact that 5-HT does not regulate expression of all BDNF-induced genes suggests that BDNF is involved in numerous cellular processes, whereas 5-HT may have a more defined role in the hippocampus. The common set of specific genes we identified as regulated by both 5-HT and BDNF relate to neuronal activation, synaptic remodeling, and neuropeptides, and parallels those classes of genes altered by various antidepressant treatments (Newton et al., 2003; Altar et al., 2004; Alfonso et al., 2005; Yamada and Higuchi, 2005; Ploski et al., 2006; Conti et al., 2007).
Our data suggesting that trkB signaling is not required for 5-HT-induced transcription and that 5-HT receptor activation does not affect BDNF-mediated transcription indicates that BDNF does not act downstream of 5-HT or vice versa with regard to transcriptional activation. This finding may relate to the levels of transcription observed with the two factors used in this study; BDNF robustly induces expression of these genes, whereas the effect of 5-HT is relatively modest. In addition, BDNF and 5-HT do not have a synergistic effect on gene expression. Together, our data leads us to conclude that 5-HT and BDNF employ independent pathways to stimulate transcription, although they may eventually converge on a common mechanism to induce a common set of genes.
The specificity of the transcriptional regulation by 5-HT may be controlled at the receptor level requiring the activation of either 5-HT1 and/or 5-HT7 receptors. We did not detect involvement of 5-HT2 receptors, although they have been implicated in transcription of IEGs and BDNF in other cell types (Meller et al., 2002; Pei et al., 2004). Importantly, recent reports have shown that activation of 5-HT1A (Fricker et al., 2005) and 5-HT7 (Kvachnina et al., 2005) receptors leads to morphological changes and increased cell survival of hippocampal neurons. Both the higher transcriptional response of the cells to BDNF compared with 5-HT and the requirement of specific 5-HT receptor activation for transcription may be attributable to the levels of expression of these receptors in specific cell types in the hippocampus. Deciphering the cell populations in the hippocampus and the signaling cascades leading to transcription of the genes described in this study will help in understanding the involvement of the different receptor subtypes in the morphological and behavioral effects of 5-HT and BDNF.
VGF in depression models
Here, we found that 5-HT regulates the expression of a subset of BDNF-induced neuropeptides including VGF and NPY. NPY itself has been shown to have antidepressant-like activity. Intracerebroventricular injections of NPY or NPY receptor 1 (Y1) agonists significantly reduced immobility time in both the FST (Husum et al., 2000; Stogner and Holmes, 2000; Redrobe et al., 2002a) and LH paradigms (Ishida et al., 2007). However, the role of VGF in depression has not been explored. VGF is synthesized as a precursor polypeptide, which is processed during neuronal differentiation and becomes localized to large dense core vesicles in several areas in the brain including the dentate gyrus of the hippocampus (Salton et al., 2000). The receptor for VGF has not been identified, but genetic ablation of VGF results in deficits in energy balance and the regulation of homeostasis (Hahm et al., 1999, 2002). The VGF gene has a CREB binding site, which is necessary for its induction by other neurotrophic factors (Salton et al., 2000), and suggests a point of convergence for transcriptional regulation by 5-HT and BDNF. Furthermore, expression of VGF is induced by BDNF (Eagleson et al., 2001; Alder et al., 2003) as well as in models of neuronal activity such as learning, LTP, seizure, and synaptogenesis (Lombardo et al., 1995; Benson and Salton, 1996; Hevroni et al., 1998; Snyder et al., 1998; Alder et al., 2003; Newton et al., 2003; Altar et al., 2004). In support of our findings of an increase in VGF expression after imipramine treatment, an increase in VGF mRNA was observed in the hippocampus of animals after electroconvulsive therapy (Newton et al., 2003; Altar et al., 2004; Conti et al., 2007). However, fluoxetine treatment did not show a similar increase in VGF expression, possibly because the antidepressant treatment was only 2 weeks long (Conti et al., 2007). Consistent with these results, we also observed decreased VGF protein levels after both the LH and FST depression paradigms. Similar reductions in expression of another neuropeptide, NPY, was detected in these and other paradigms (S. Thakker-Varia and J. Alder, unpublished observations) (Sergeyev et al., 2005). Together, these findings implicate the involvement of VGF in affective disorders.
We demonstrate for the first time the effectiveness of VGF as an antidepressant-like agent in the FST behavioral model of depression. The FST paradigm has been used widely for its excellent pharmacologic predictive validity, and this model exhibits both acute and chronic response to antidepressant treatment (Dranovsky and Hen, 2006). In particular, growth factors such as BDNF and IGF have been shown to reverse the depression-like phenotype induced by preexposure to FST (Siuciak et al., 1997; Hoshaw et al., 2005). The decrease in immobility time in the FST induced by the dose of VGF used in this study was comparable with that reported for BDNF (Shirayama et al., 2002) and NPY (Husum et al., 2000; Stogner and Holmes, 2000), suggesting that VGF is as effective as those factors. In addition, the effects of VGF from a single infusion lasted up to 6 d as has been observed with BDNF and insulin growth factor (IGF) (Shirayama et al., 2002; Hoshaw et al., 2005), implying that VGF produces long-term and persistent changes that underlie the antidepressant behavioral responses. The requirement for VGF downstream of the actions of BDNF as an antidepressant-like agent, could be examined after the generation of adult hippocampal conditional VGF knock-out mice.
Role of VGF in proliferation of hippocampal progenitors
It is now well established that antidepressants enhance neurogenesis in the dentate gyrus, perhaps to replace those cells damaged by stress. Indeed, the decrease in proliferation of progenitor cells within the SGZ after depression paradigms can be rescued by antidepressant treatments (Malberg and Duman, 2003). 5-HT, acting through the 5-HT1A receptor, also enhances neurogenesis (Santarelli et al., 2003; Banasr et al., 2004). Finally, it has been demonstrated that neurogenesis is actually necessary for the behavioral effects of antidepressants in a rodent model of depression using x-irradiation of the hippocampus to block de novo proliferation (Santarelli et al., 2003; Jiang et al., 2005). We show here for the first time that the neuropeptide VGF enhances neurogenesis of hippocampal cells indicating a possible mechanism for actions of VGF as an antidepressant-like agent. There is no evidence of cell death or damage induced by VGF, and the survival of our cells both in vitro and in vivo is consistent with what has been reported for other antidepressant treatments (Malberg et al., 2000). Furthermore, our double-labeling studies in vitro suggest that VGF may actually influence the differentiation of neurons over glia and our data in vivo confirm that the proliferating cells differentiate into neurons, which also parallels reports with other antidepressants (Malberg et al., 2000; Nakagawa et al., 2002; Scharfman et al., 2005). Our study is the first to link VGF to proliferation; however, another neuropeptide, NPY, has been shown to influence proliferation in hippocampal cells via the Y1 receptor (Howell et al., 2003, 2005). Other neuropeptides induced by BDNF and 5-HT such as SP had no effect on proliferation suggesting that BDNF and 5-HT may act cooperatively to affect other processes in addition to neurogenesis in the hippocampus. The role of VGF in controlling the rate of endogenous neurogenesis in the dentate gyrus could be investigated in future studies using VGF conditional knock-out mice. Furthermore, the identification of the 5-HT receptor subtypes involved in the neurogenesis process and the determination of whether the VGF-enhanced neurogenesis is required for the antidepressant-like actions of VGF remain to be demonstrated.
The role of growth factors such as BDNF, bFGF (basic fibroblast growth factor), VEGF (vascular endothelial growth factor), and IGF-1 in the proliferation and survival of hippocampal cells has been explored (Wagner et al., 1999; Aberg et al., 2000; Schanzer et al., 2004). Overexpression or infusion of BDNF in the adult rat results in newly generated cells in the SGZ of the dentate gyrus and forebrain (Benraiss et al., 2001; Pencea et al., 2001; Scharfman et al., 2005). Interestingly, studies in wild-type and BDNF knock-out mice suggest that, whereas antidepressants increase turnover of hippocampal neurons, BDNF is required for the long-term survival of newborn neurons (Sairanen et al., 2005). Our studies suggest that not only does the BDNF-induced molecule, VGF, promote the proliferation of progenitor cells but also that those newly born cells survive to at least 21 d and primarily differentiate into neurons.
Enhanced neurogenesis is one possible mechanism of antidepressant agents. Another theory of antidepressant actions proposes that the problem underlying depression is abnormal activity-dependent neuronal communication. BDNF may therefore exert its antidepressant-like effects by enhancing synaptic plasticity and neuronal connections (Duman and Monteggia, 2006; Castren et al., 2007). As we have previously demonstrated increased synaptic activity after VGF treatment of hippocampal cultures (Alder et al., 2003), this mechanism may also contribute to the antidepressant-like actions of VGF. The short-term effects of antidepressant-like agents could be explained by their actions on neuronal plasticity, whereas the longer-term outcome may be attributable to neurogenesis.
A model consistent with our findings combined with published studies suggests that antidepressants may increase 5-HT levels directly and/or activate the BDNF cascade. 5-HT and BDNF employ independent pathways to induce transcription allowing for redundancy in the system; however, they may converge on a common transcription factor such as CREB. This transcriptional activation is dependent on receptor subtypes and therefore may occur in a cell-specific manner within the hippocampus. Increased expression of one CREB-dependent gene VGF may enhance synaptic activity, thereby alleviating abnormal neuronal communication which results from the neuronal atrophy in the hippocampus during depression. VGF also induces proliferation of progenitor cells in the dentate gyrus. The newly born cells that survive may preferentially differentiate into neurons and therefore reverse the cell loss that occurs in the hippocampus during depression. In the future, the demonstration of the effectiveness of VGF as an antidepressant-like agent in additional models of depression that respond to chronic treatment would support the idea that the neurogenesis induced by VGF is contributing to its behavioral effects. The requirement of neurogenesis for antidepressant actions may be one of the reasons for the delay in the onset of clinical effects of current therapies. Understanding the molecular and cellular changes that underlie the actions of BDNF, 5-HT, and neuropeptides and how these adaptations result in antidepressant-like effects will aid in developing drugs that target novel pathways for major depressive disorders and may decrease the latency to onset of clinical effects.
Footnotes
This work was supported by grants from the National Institute of Child Health and Human Development, National Alliance for Research on Schizophrenia and Depression, and the New Jersey Commission for Science and Technology. This paper is dedicated to the memory of our esteemed coauthor and colleague Ira B. Black. We are grateful to S. Salton for providing VGF antibody. We thank the following for their technical advice: C. Dalla for FST, T. Coyne for surgeries, C. Edgecomb for BrdU staining in vivo, E. DiCicco-Bloom for [3H]thymidine assays, and A. Falluel-Morel for caspase-3 immunostaining.
References
- Abad MA, Enguita M, DeGregorio-Rocasolano N, Ferrer I, Trullas R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-β and is overexpressed in dystrophic neurites in Alzheimer's brain. J Neurosci. 2006;26:12735–12747. doi: 10.1523/JNEUROSCI.0575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A, Castro E, Celada P, Bortolozzi A, Pazos A, Artigas F. Strategies for producing faster acting antidepressants. Drug Discov Today. 2005;10:578–585. doi: 10.1016/S1359-6446(05)03398-2. [DOI] [PubMed] [Google Scholar]
- Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ, Black IB. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23:10800–10808. doi: 10.1523/JNEUROSCI.23-34-10800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder J, Thakker-Varia S, Crozier RA, Shaheen A, Plummer MR, Black IB. Early presynaptic and late postsynaptic components contribute independently to brain-derived neurotrophic factor-induced synaptic plasticity. J Neurosci. 2005;25:3080–3085. doi: 10.1523/JNEUROSCI.2970-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA, Charles V, Palfreyman MG. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24:2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babri S, Badie HG, Khamenei S, Seyedlar MO. Intrahippocampal insulin improves memory in a passive-avoidance task in male wistar rats. Brain Cogn. 2007;64:86–91. doi: 10.1016/j.bandc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Salton SR. Expression and polarization of VGF in developing hippocampal neurons. Brain Res Dev Brain Res. 1996;96:219–228. doi: 10.1016/0165-3806(96)00108-3. [DOI] [PubMed] [Google Scholar]
- Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chahl LA. Tachykinins and neuropsychiatric disorders. Curr Drug Targets. 2006;7:993–1003. doi: 10.2174/138945006778019309. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Tao Y, Black IB, DiCicco-Bloom E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J Neurobiol. 2001;46:220–229. doi: 10.1002/1097-4695(20010215)46:3<220::aid-neu1004>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, Bilbe G, Hoyer D, Bartfai T. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur J Neurosci. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Fairfull LD, Salton SR, Levitt P. Regional differences in neurotrophin availability regulate selective expression of VGF in the developing limbic cortex. J Neurosci. 2001;21:9315–9324. doi: 10.1523/JNEUROSCI.21-23-09315.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, Kelley KA, Takahashi JS, Pintar JE, Roberts JL, Mobbs CV, Salton SR. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23:537–548. doi: 10.1016/s0896-6273(00)80806-5. [DOI] [PubMed] [Google Scholar]
- Hahm S, Fekete C, Mizuno TM, Windsor J, Yan H, Boozer CN, Lee C, Elmquist JK, Lechan RM, Mobbs CV, Salton SR. VGF is required for obesity induced by diet, gold thioglucose treatment, and agouti and is differentially regulated in pro-opiomelanocortin- and neuropeptide Y-containing arcuate neurons in response to fasting. J Neurosci. 2002;22:6929–6938. doi: 10.1523/JNEUROSCI.22-16-06929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heinrich C, Nitta N, Flubacher A, Muller M, Fahrner A, Kirsch M, Freiman T, Suzuki F, Depaulis A, Frotscher M, Haas CA. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006;26:4701–4713. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevroni D, Rattner A, Bundman M, Lederfein D, Gabarah A, Mangelus M, Silverman MA, Kedar H, Naor C, Kornuc M, Hanoch T, Seger R, Theill LE, Nedivi E, Richter-Levin G, Citri Y. Hippocampal plasticity involves extensive gene induction and multiple cellular mechanisms. J Mol Neurosci. 1998;10:75–98. doi: 10.1007/BF02737120. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neurosci Lett. 2006;408:1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger A, Gray WP. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- Husum H, Mikkelsen JD, Hogg S, Mathe AA, Mork A. Involvement of hippocampal neuropeptide Y in mediating the chronic actions of lithium, electroconvulsive stimulation and citalopram. Neuropharmacology. 2000;39:1463–1473. doi: 10.1016/s0028-3908(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Ishida H, Shirayama Y, Iwata M, Katayama S, Yamamoto A, Kawahara R, Nakagome K. Infusion of neuropeptide Y into CA3 region of hippocampus produces antidepressant-like effect via Y1 receptor. Hippocampus. 2007;17:271–280. doi: 10.1002/hipo.20264. [DOI] [PubMed] [Google Scholar]
- Iwata M, Shirayama Y, Ishida H, Kawahara R. Hippocampal synapsin I, growth-associated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience. 2006;141:1301–1313. doi: 10.1016/j.neuroscience.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol (Paris) 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Karl T, Herzog H. Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007;28:326–333. doi: 10.1016/j.peptides.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, Dityateva G, Schachner M, Voyno-Yasenetskaya TA, Ponimaskin EG. 5-HT7 receptor is coupled to Gα subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004a;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004b;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Rabacchi SA, Cremisi F, Pizzorusso T, Cenni MC, Possenti R, Barsacchi G, Maffei L. A developmentally regulated nerve growth factor-induced gene, VGF, is expressed in geniculocortical afferents during synaptogenesis. Neuroscience. 1995;65:997–1008. doi: 10.1016/0306-4522(94)00538-g. [DOI] [PubMed] [Google Scholar]
- Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc Natl Acad Sci USA. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Babity JM, Grahame-Smith DG. 5-HT2A receptor activation leads to increased BDNF mRNA expression in C6 glioma cells. Neuromolecular Med. 2002;1:197–205. doi: 10.1385/NMM:1:3:197. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudon L, Hotte M, Jay TM. Effects of acute and chronic antidepressant treatments on memory performance: a comparison between paroxetine and imipramine. Psychopharmacology (Berl) 2007;191:353–364. doi: 10.1007/s00213-006-0660-4. [DOI] [PubMed] [Google Scholar]
- Nawa H, Pelleymounter MA, Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pei Q, Tordera R, Sprakes M, Sharp T. Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004;46:331–339. doi: 10.1016/j.neuropharm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploski JE, Newton SS, Duman RS. Electroconvulsive seizure-induced gene expression profile of the hippocampus dentate gyrus granule cell layer. J Neurochem. 2006;99:1122–1132. doi: 10.1111/j.1471-4159.2006.04156.x. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002a;26:615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Quirion R. Neuropeptide Y (NPY) and depression: from animal studies to the human condition. Life Sci. 2002b;71:2921–2937. doi: 10.1016/s0024-3205(02)02159-8. [DOI] [PubMed] [Google Scholar]
- Ring RH, Alder J, Fennell M, Kouranova E, Black IB, Thakker-Varia S. Transcriptional profiling of brain-derived-neurotrophic factor-induced neuronal plasticity: a novel role for nociceptin in hippocampal neurite outgrowth. J Neurobiol. 2006;66:361–377. doi: 10.1002/neu.20223. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, O'Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Salton SR, Ferri GL, Hahm S, Snyder SE, Wilson AJ, Possenti R, Levi A. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21:199–219. doi: 10.1006/frne.2000.0199. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Fetissov S, Mathe AA, Jimenez PA, Bartfai T, Mortas P, Gaudet L, Moreau JL, Hokfelt T. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 2005;178:115–124. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SE, Cheng HW, Murray KD, Isackson PJ, McNeill TH, Salton SR. The messenger RNA encoding VGF, a neuronal peptide precursor, is rapidly regulated in the rat central nervous system by neuronal activity, seizure and lesion. Neuroscience. 1998;82:7–19. doi: 10.1016/s0306-4522(97)00280-7. [DOI] [PubMed] [Google Scholar]
- Stogner KA, Holmes PV. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur J Pharmacol. 2000;387:R9–R10. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Newton SS, Duman RS. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS Neurol Disord Drug Targets. 2007;6:151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21:6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanisch K, Tang J, Mederer A, Wotjak CT. Trace fear conditioning depends on NMDA receptor activation and protein synthesis within the dorsal hippocampus of mice. Behav Brain Res. 2005;157:63–69. doi: 10.1016/j.bbr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Yamada M, Higuchi T. Antidepressant-elicited changes in gene expression: remodeling of neuronal circuits as a new hypothesis for drug efficacy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:999–1009. doi: 10.1016/j.pnpbp.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Zomkowski AD, Santos AR, Rodrigues AL. Putrescine produces antidepressant-like effects in the forced swimming test and in the tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1419–1425. doi: 10.1016/j.pnpbp.2006.05.016. [DOI] [PubMed] [Google Scholar]