Abstract
To ensure survival, parasitic wasps of Drosophila have evolved strategies to optimize host development to their advantage. They also produce virulence factors that allow them to overcome or evade host defense. Wasp infection provokes cellular and humoral defense reactions, resulting in alteration in gene expression of the host. The activation of these reactions is controlled by conserved mechanisms shared by other invertebrate and vertebrate animals. Application of genomics and bioinformatics approaches is beginning to reveal comparative host gene expression changes after infection by different parasitic wasps. We analyze this comparison in the context of host physiology and immune cells, as well as the biology of the venom factors that wasps introduce into their hosts during oviposition. We compare virulence strategies of Leptopilina boulardi and L. heterotoma, in relation to genome-wide changes in gene expression in the fly hosts after infection. This analysis highlights fundamental differences in the changes that the host undergoes in its immune and general physiology in response to the two parasitic wasps. Such a comparative approach has the potential of revealing mechanisms governing the evolution of pathogenicity and how it impacts host range.
5.1. INTRODUCTION
Parasitic wasps act as foundation species in natural ecosystems (LaSalle and Gauld, 1993). Leptopilina, Asobara and Ganaspis are part of a large group of insects making up more than 20% of all insect species. Thus, even though the parasitoid lifestyle is somewhat atypical, it is by no means unusual. Parasites hijack the body of the developing larva, taking over its resources and reprogramming the development of a single parasite within the host’s body. In large measure, the natural success of parasitic wasps is due to the presence of virulence factors in the venom glands of female wasps. Virulence factors can be proteins, or in some cases, mutualists, microparasites or microbial symbionts. The nature of the virulence factors in parasitic wasps of Drosophila, as well as the relationships between virulence factors and their insect hosts (wasps), or wasp hosts (Drosophila), remain largely unknown. Because virulence factors represent the interface of host–pathogen interactions and are subject to natural selection, they are likely to shape the genetic structures of both the host and parasite populations. In this chapter, we review the immune competence of the host, and the nature of putative virulence factors and virus-like particles produced in venom glands of L. boulardi, L. heterotoma and L. victoriae. We also address the relationship of virulence strategies to cellular and molecular changes, especially as they relate to host defense in Drosophila.
5.1.1. Parasitism by Leptopilina spp
The cosmopolitan genus Leptopilina (Nordlander, 1980) consists of three species groups: heterotoma (five described species, including L. heterotoma and L. victoriae), boulardi (three described species) and longipes (five described species; Allemand et al., 2002; Schilthuizen et al., 1998). As evident from considerable coverage in accompanying chapters, the biology of L. boulardi and L. heterotoma is particularly well studied. These parasitic wasps infect (or superinfect) second-to-early third-instar stages of Drosophila larvae. Infection causes a slight delay in host development (Kopelman and Chabora, 1984; Schlenke et al., 2007).
One of the clearest responses to wasp infection is encapsulation of the wasp egg or the early embryo. This innate immune reaction has been documented in many invertebrates (Brehélin, 1985), and is likely to be a universal defense mechanism in animals to combat large foreign bodies. Encapsulation of wasp eggs is relatively easy to observe in whole Drosophila larvae because the larval cuticle is transparent, and encapsulation is often accompanied by melanization (see Chapter 4 by Nappi et al.). While the dead, dark encapsulated wasp in the host hemocoel has fascinated biologists for a number of years, only recently we have learned that wasp infection also activates the humoral pathways in fly larvae (Schlenke et al., 2007; Wertheim et al., 2005). Humoral responses in insects include localized melanization and systemic induction of antimicrobial peptides (AMPs). These innate immune responses in Drosophila have thus far been characterized mainly in the context of microbial (bacterial and fungal) infections of the adult fly. Many molecules that are involved in nonself recognition, core components of the NF-κB pathways (Toll and IMD) and the effector molecules that limit infection in vivo are now known (see Lemaitre and Hoffmann, 2007 for a recent comprehensive review). However, the way in which wasp eggs are recognized in the host hemocoel, and various functions of antimicrobial peptides or other effector molecules, specifically in host defense against wasps, are not known. In the three species of Leptopilina discussed here (L. boulardi and the sister species L. heterotoma and L. victoriae), these immune responses are avoided, blocked, or suppressed, complicating the understanding of specific host responses.
We and others have studied host physiology, genetics and genomics in response to infections by L. heterotoma/L. victoriae and L. boulardi to probe similarities and differences in their host range and virulence strategies. Because these species present different virulence strategies, host responses differ dramatically: L. heterotoma/L. victoriae produce 300-nm wide virus-like particles (VLPs) within specialized long glands (also called venom glands). These particles are deposited along with the eggs. VLPs bind to host lamellocytes, become internalized and promote lamellocyte lysis (Morales et al., 2005; Rizki and Rizki, 1984, 1990, 1992, 1994). L. heterotoma/L. victoriae infection also leads to apoptosis of hemocytes in the circulation and in the lymph gland, respectively (Chiu and Govind, 2002). Different strains of L. heterotoma tested on Drosophila melanogaster are consistently highly virulent. Furthermore, morphologically identical 300-nm VLPs have been reported from at least three independently isolated strains of this wasp.
L. boulardi, in contrast, exhibits substantial intraspecific variation with respect to virulence on D. melanogaster spp. (Dupas et al., 1996; see also Chapter 6 by Poirié et al. and Chapter 11 by Dupas et al.) and venom content. Even though venom glands of L. boulardi-17 and G486 strains produce VLPs (Dupas et al., 1996; Schlenke et al., 2007), their ability to provoke encapsulation is different, the former strain being more virulent. In addition, filamentous viruses (Varaldi et al., 2006; see Chapter 13 by Varaldi et al.) are also found in venom glands of some L. boulardi strains. Regardless, unlike L. heterotoma/L. victoriae venom, lytic or apoptotic effects of L. boulardi venom on host hemocytes has not been reported.
A second major difference between heterotoma/L. victoriae and L. boulardi has to do with the location of the wasp eggs soon after oviposition: while eggs of L. heterotoma or L. victoriae are found floating freely in host hemolymph, L. boulardi eggs are often found attached to host tissues. This difference suggests that, in addition to active suppression affecting hemocytes, L. boulardi also employs a passive or evasive method of protecting its eggs from complete hemocyte encapsulation (Melk and Govind, 1999; Rizki et al., 1990). Together, these differences in the proteins, particles and other secretions of the venom gland appear to confer unique properties on Leptopilina spp. (and perhaps even to individual strains), resulting in unique host responses.
5.2. THE HOST RANGE OF L. BOULARDI AND L. HETEROTOMA
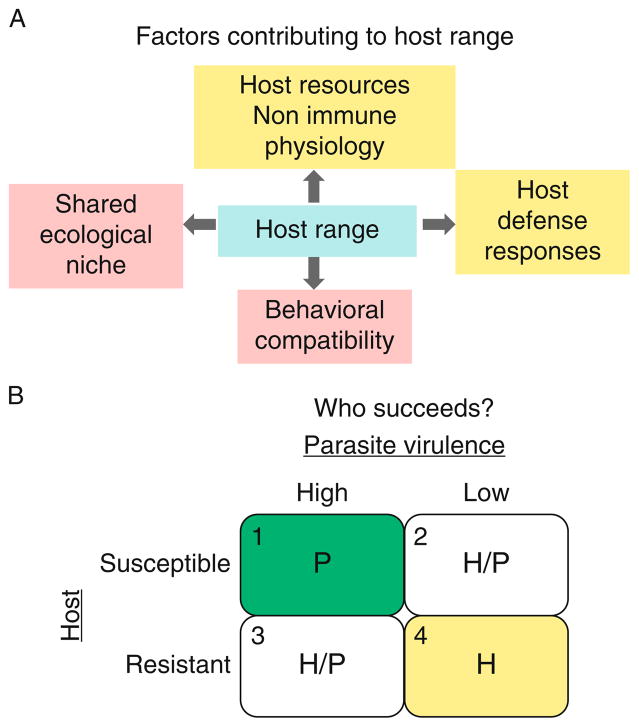
A host range or spectrum is the total variety of species that a parasite infects in nature. Factors contributing to this spectrum include shared ecosystem, behavioral compatibility and the ability of the host to mount a robust immune response. The strength of the immune response depends on the genetic factors that directly control production of immune effector cells and molecules. The success of this response also rests on the host’s general health and physiology (tolerance to pathogens) of the healthy host (Fig. 5.1A; e.g., Ayres et al., 2008). The complex interactions of the genetic and physiological factors within the host and with the parasite dictate the outcome in each infection (Fig. 5.1B). Thus, a parasite is a generalist if it successfully infects a number of related or unrelated hosts. In contrast, a specialist parasite succeeds on one or few host species in nature.
FIGURE 5.1.
Host range and susceptibility. (A) A confluence of ecological, behavioral and host factors contributes to host range. (B) In the event of infection, success of a parasite will depend on the combination of specific individual host and parasite factors, as shown. Host resistance (immune mechanisms), host resources (nonimmune physiological factors that enable host defense) versus mechanisms of evasion and/or virulence on the part of the parasitoid. Clear outcomes of either the parasite [quadrant 1; “high virulence” parasite (e.g., L. heterotoma) infecting “low resistance” host] or the host [(quadrant 4; “low virulence” parasites (e.g., A. tabida) infecting a “high resistance” host)] can be predicted. However, in case of high virulence/high resistance or low virulence/low resistance (quadrants 2 and 3), specific outcomes may be more difficult to predict.
Infection by wasps is known to modify host development: a parasite can take control of host development, generally by delaying it. This delay allows the parasite to utilize host resources optimally. For example, the polydnavirus of the ichneumonid parasitoid, Campoletis sonorensis (CsV) was found to be the only component of its calyx fluid responsible for causing developmental arrest of its host Heliothis virescens (Dover et al., 1987). This developmental delay is attributed to a reduction of ecdysteroid titers that actually occurs because of partial degeneration of the prothoracic glands. Thus, infection introduces factors that modify aspects of host physiology, other than host immunity, that facilitate parasite success.
In laboratory infection experiments involving 18 Drosophila spp., L. boulardi strain-17 was found to be far less infectious than L. heterotoma strain-14 (Schlenke et al., 2007). L. boulardi-17 succeeded (>90% wasp emergence) only on D. melanogaster, and was only moderately successful (~ 50% wasp emergence) on D. mauritiana, D. sechellia and D. simulans, all of which are closely related species of the melanogaster group. L. heterotoma-14 showed higher success on these hosts.
The difference in infection outcomes on D. yakuba, D. santomea and D. teissieri was stark: whereas L. boulardi-17 failed (0–1% wasp emergence) on these three closely related hosts, L. heterotoma-14 succeeded with greater than 60% wasp emergence. L. boulardi-17 infection induced clear encapsulation in these hosts (Schlenke et al., 2007; Fig. 5.2A); the capsules are similar to those formed in D. melanogaster, showing typical aggregation and melanization of hemocytes (Schlenke et al., 2007).
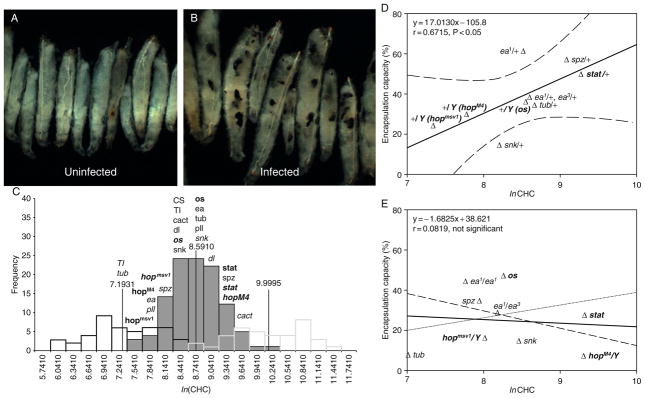
FIGURE 5.2.
Hemocyte-mediated encapsulation depends on both hemocyte concentrations and hemocyte differentiation. (A) and (B) L. boulardi-17 infection induces massive encapsulation in D. yakuba. (A) Uninfected and (B) infected third-instar larvae. (C) Hemocyte concentration in D. melanogaster larvae (log normal transformation of hemocyte concentrations) shows a normal distribution. Empirically determined range of normal hemocyte concentration in staged third-instar larvae is shown (vertical lines). Values of hemocyte concentration from heterozygotes and mutants were derived from outcrossed animals. Genotype of mutants is italicized (allele name, where used, is superscripted), heterozygotic genotypes are not. Genotypes shown in bold pertain to JAK-STAT signaling components (os, outstretched; hop, hopscotch; STAT, STAT92E), whereas those that are not bold relate to Toll signaling (snk, snake; ea, easter; spz, spatzle; Tl, Toll; tub, tube; pll, pelle; cact, cactus. CS, Canton S is a wild-type strain). (D,E) Direct correlation of encapsulation capacity with hemocyte concentration in wild-type and control values (D). This correlation is violated in mutant animals. Genotypes are referred to as in legend above. Note: Figures modified from Sorrentino et al. (2004) with permission from the publisher.
Of the remaining 11 species tested, L. heterotoma-14 succeeded on six species (>30% wasp emergence), whereas L. boulardi-17 infection was successful on only two species.
The ability of L. heterotoma-14 to infect diverse hosts of different sizes and developmental times successfully is quite remarkable (Schlenke et al., 2007). It implies that the egg and/or the factors that are introduced with the egg, interfere with host development and defense in a “species-nonspecific” manner. Part of this strategy is likely to involve the deadly effects that L. heterotoma infection unleashes on host hemocytes and hematopoiesis, a strategy not shared by L. boulardi. The host range of L. victoriae on laboratory-raised fly hosts has not been studied. Because L. heterotoma and L. victoriae share similar virulence mechanisms, especially their lethal effects on host hemocytes, it will be interesting to compare the ability of L. victoriae to succeed on these same species that L. heterotoma infects.
Because of the dramatic differences in host range in laboratory-raised hosts (Schlenke et al., 2007) and hosts in natural habitat (Carton et al., 1986), L. boulardi is characterized as a “specialist,” whereas. L. heterotoma is a “generalist.” It is worth noting that L. boulardi-17 infection induces encapsulation in many species (Fig. 5.2A and B; Schlenke et al., 2007), whereas L. heterotoma-14 infection does not. This difference underscores the importance of using the optimal wasp/host pair to study encapsulation. More importantly, it highlights the contribution of the encapsulation reaction in host defense across different Drosophila spp.
5.2.1. Infection by L. boulardi-G486 triggers stage-specific hematopoiesis in third-instar hosts
The encapsulation reaction (Fig. 5.2A and B) is complex, highly controlled and quite effective. Successful encapsulation requires coordinated interactions of three hemocyte types, namely plasmatocytes, crystal cells and lamellocytes. While the encapsulation reaction is triggered by oviposition, it is not known what factors limit the hemocytic response and what determines its timely termination.
The hemocoel of normal uninfected third-instar larvae is populated mainly by plasmatocytes. These small, phagocytic cells (Fig. 5.3H and I) consume bacteria, scavenge dead cells and secrete antibacterial peptides and extracellular matrix components (Fessler et al., 1994; Rizki and Rizki, 1984). Crystal cells make up less than 5% of all hemocytes in circulation and synthesize substrates and enzymes for melanization reactions.
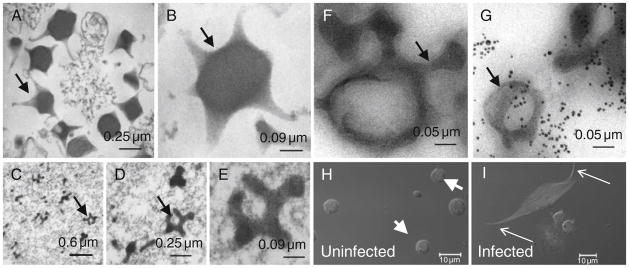
FIGURE 5.3.
Virus-like particle (VLP) structure and biogenesis. (A,B) Sections of gradient-purified VLPs from L. victoriae. Notice the somewhat irregular pentagonal/hexagonal organization of the VLP core that extends into projections. (C–E) Section of a region from the lumen of L. victoriae long gland with immature VLPs at different magnifications. (F,G) Association of p40 antigen within and around immature VLPs of L. heterotoma (arrows point to immuno-gold particles linked to secondary antibody (G)). Sample in panel (F) was not treated with the primary antibody. (H and I) L. victoriae infection induces lamellocytes to alter morphology. Hemocytes from hemolymph of normal (H) or infected hosts (I) stained with rhodamine-phalloidin (F-actin) and Hoechst (blue). Normal hemolymph has 10-μm wide spherical plasmatocytes (arrowheads) but very few, if any, lamellocytes. None are present in this panel. Infected lamellocytes exhibit altered, spindle-shaped morphology.
The lymph gland is a small organ at the anterior dorsal region (behind the brain through the ring gland, in the anterior abdominal segments) of the larva. It houses hematopoietic progenitors of the adult fly (Govind, 2008; Holz et al., 2003; Lemaitre and Hoffmann, 2007; Martinez-Agosto et al., 2007; Meister and Lagueux, 2003).
When faced with parasitization by L. boulardi-G486 the fly larva triggers differentiation of lamellocytes and crystal cells (Lanot et al., 2001; Sorrentino et al., 2002). Furthermore, a limited burst of mitosis follows shortly after infection (Sorrentino et al., 2002), suggesting that both cell division and differentiation of lymph gland progenitors are required for encapsulation. These changes, observed in the lymph glands of third instar, but not of second-instar hosts, are almost always accompanied by dispersal of the anterior lobes themselves. In this dispersal response, the continuous basement membrane that lines (and possibly holds) the cells of the anterior lobes is disrupted (Sorrentino et al., 2004). Lamellocytes and their precursors are also present in the posterior hematopoietic compartment. Cells from this location also contribute to host defense (Markus et al., 2009).
A link between host development and immune competence was confirmed in genetic experiments using mutant hosts in which development was blocked during mid-to-late larval stages. Drosophila strains where ecdysone levels are low (ecdysoneless) or ecdysone signaling is blocked (nonpupariating allele of the transcription factor broad), were infected by L. boulardi-G486. The encapsulation response in such hosts was severely compromised: (1) the postinfection mitotic amplification in the lymph glands of third-instar ecdysoneless hosts was absent; (2) there was a reduction in crystal cell maturation in the lymph gland; and (3) there was also a reduction in the postinfection circulating lamellocyte concentration (Sorrentino et al., 2002). These results suggested that development of the precursors continues through larval instars, a prediction that was confirmed in subsequent studies of lymph gland cell populations in which precursor population was distinguished from differentiating cells (Jung et al., 2005). It appears then that these parasitic wasps are able to infect larval hosts while the hosts are still immune incompetent, while giving themselves almost the entire third larval instar to program their own development. The infection cycle is therefore developmentally aligned with the development of the host. Conversely, lymph gland precursors for lamellocytes are available to divide and differentiate at precisely the time that these wasps are able to recognize and infect their hosts.
Sorrentino et al. (2002) also predicted the existence of an ecdysone-activated pathway that potentiates precursors of effector cell types to respond to parasitization by proliferation and differentiation. This prediction regarding hormonal control of immune cell development remains to be explored at the molecular level.
5.2.2. Does the concentration of circulating hemocytes have a bearing on successful encapsulation?
While virulence factors interfere with encapsulation, variabilities in the host’s immune physiology and cells are also likely to contribute to the wasp/fly outcome. Hemocytes normally circulate freely in the body cavity and do not clump or spread inside the hemocoel. Yet, upon introduction of parasites into the hemocoel, some of these cells flatten and become adhesive to form a capsule around the nonself entity. Sorrentino et al. (2004) characterized circulating hemocyte concentration (CHC) from wild-type third-instar hosts to examine if hemocyte density has a bearing on wasp encapsulation. They found that: (1) the control mean raw CHCs exhibit a nearly sevenfold range of values; (2) the distribution of wild-type Canton-S or control (n = 110) CHC values does not follow a normal distribution. Instead, when the CHC values were converted to natural logarithms of raw CHC values (ln CHC), the frequency distribution showed a normal distribution (Fig. 5.2C). Using L. boulardi-G486 to infect developmentally staged animals, they reported a significant correlation between hemocyte concentration and encapsulation capacity among wild-type larvae and larvae heterozygous for mutations in the JAK-Stat92E and Toll-NF-κB pathways (Fig. 5.2D).
5.2.3. Toll-NF-κB and JAK-STAT signaling in encapsulation
JAK-STAT92E and Toll-NF-κB signaling control cellular physiology, proliferation and/or differentiation. Activated by extracellular cytokines, these signaling pathways mediate cellular and systemic responses to infection. Many core components of these pathways (that are responsible for relaying information from the membrane to the nucleus) have been identified (see Fig. 5.4). Highly conserved in metazoan animals, JAK kinases, transcription factors NF-κB/Rel or STAT proteins and their inhibitors (IκB and PIAS, respectively) also regulate aspects of mammalian hematopoiesis (Baker et al., 2007; Bottero et al., 2006; Martinez-Agosto et al., 2007).
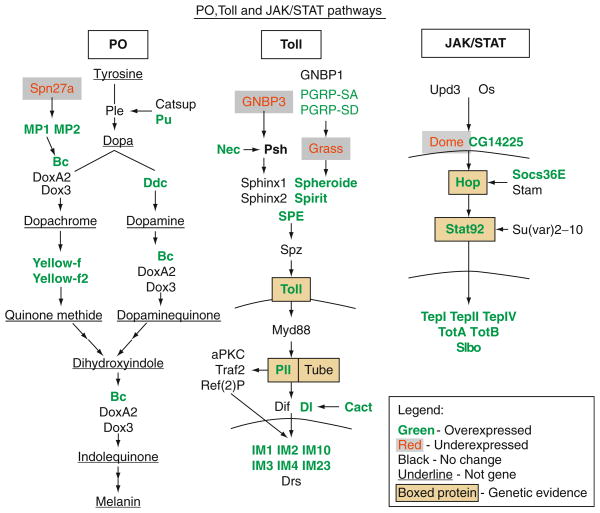
FIGURE 5.4.
Components and targets of pro-phenol oxidase, Toll-Dorsal/Dif and JAK/STAT pathways. Genes whose expression is modulated by L. boulardi-17 infection (up- or downregulated) are shown. The Toll-Dorsal/Dif and JAK/STAT pathway components that were tested in genetic experiments (Sorrentino et al., 2004) for their requirement for a robust encapsulation response are also shown (genetic evidence). Note: Figure modified from Schlenke et al. (2007).
Heterozygous animals carrying loss-of-function (recessive) mutations in Toll, tube or pelle show CHC in the normal range. However, homozygous larvae carrying loss-of-function mutations in these “dorsal group” genes (Toll5BRE, tube238 and pellerm8) have significantly reduced circulating hemocyte concentrations (Qiu et al., 1998). These mutants are severely compromised in their ability to mount an effective encapsulation response (Sorrentino et al., 2004; Fig. 5.2E). Loss of function in IκB/cactus results in the opposite effect reflected in increased CHC outside of the normal range (Qiu et al., 1998). Affected mutants show microtumors. The genetic lesion results in overproliferation and constitutive lamellocyte differentiation of the hematopoietic tissue, which in turn encapsulates self tissue.
The situation with components of the JAK-STAT signaling is different. Here heterozygous or mutant larvae deficient in Hopscotch-Stat92E signaling (outstretched0, hopscotchM4, hopscotchmsv1, stat92EHJ) exhibit ln CHC in the control range (Fig. 5.2E). Yet infection of loss-of-function mutant animals affecting hop or STAT genes affects encapsulation; mutant lymph gland progenitors are unable to differentiate into lamellocytes (Sorrentino et al., 2004).
The genetic regulation of lamellocyte differentiation is not entirely clear. The JAK-STAT signal appears to be essential for holding hematopoietic progenitors in their immature state within the lymph gland (Krzemień et al., 2007).
While L. boulardi-G486 provokes a substantial and measurable cellular immune response in D. melanogaster, this is not true for L. heterotoma. Oviposition by L. heterotoma has different effects on the hematopoietic system of D. melanogaster (see Section 5.3). Encapsulation can be observed in L. victoriae-infected D. melanogaster hosts, presumably because the L. victoriae venom acts at a slower rate in vivo than that of L. heterotoma (Chiu and Govind, 2002; see Section 5.3).
5.3. ORIGIN OF L. HETEROTOMA/L. VICTORIAE VLPs AND THEIR EFFECTS ON HOST HEMOCYTES
5.3.1. An actin-lined canal system controls biogenesis and release of virulence factors
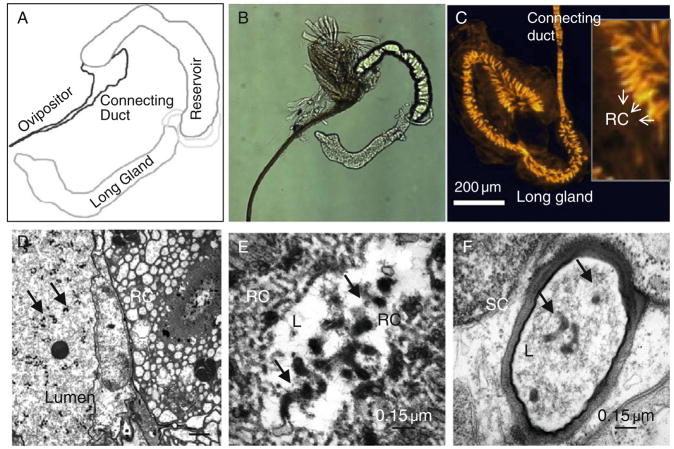
The venom apparatus in the female wasp is associated with the reproductive tract and produces some of the factors that accompany the egg. The gland has three main regions: the venom (or long) gland, the reservoir and the ovipositor (Fig. 5.5A and B). The venom gland is the site of production of VLP precursors and other secreted factors. These factors are secreted into the gland lumen, pass through a connecting duct, into the reservoir, where they mature further. The venom fluid is also stored in the reservoir until the female injects it with its eggs. The ovipositor is structurally sharp and chitinous, and capable of extending outside the wasp abdomen.
FIGURE 5.5.
Venom apparatus, canals and contents. (A) and (B) A schematic (A) that corresponds to the whole mount (phase, B) of a dissected sample of a venom apparatus from L. heterotoma. Different parts of the organ are labeled. (C) Phalloidin staining of the venom (long) gland from L. heterotoma reveals the scope and organization of a supra-cellular canal system. Made of individual secretory units (one per secretory cell), VLP precursors and other constituent proteins make their way, initially through the rough portion of the canal system, present in the cytoplasm of the large secretory cell itself. This portion (panel inset) is composed of membranous folds of actin-rich microvilli that give the structure a rough appearance. Rough canal (RC) loses the folds and narrows into the smooth canal, which opens into the gland lumen. (D–F) Transmission electron micrographs of the venom gland. (D) Rough canal in cross section, a cell of the intimal layer and immature VLPs are seen in this low magnification view of the gland. (E and F) Cross-sections of the rough (E) and smooth (F) canals of L. victoriae venom glands. Both these structures contain VLP precursors (arrows).
The venom gland is composed of a peripheral layer of large secretory cells and an internal intimal layer of narrow cells. Both cell layers are concentric to the gland lumen. We recently discovered a specialized system of canals in venom glands of five parasitoid wasps that are quite different in their infection strategies (Ferrarese et al., 2009). This supracellular system of canals is made up of individual secretory units lined with bundles of filamentous actin. Each unit has two (continuous) parts: (a) the proximal rough canal, which originates within the secretory cell and is organized into brush border morphology; and (b) the smooth canal, which is narrower and passes through the intimal cells opening into the gland lumen. Three-dimensional reconstruction of fluorescently labeled canals and cell nuclei reveals that the canal system occupies the whole organ. Each secretory cell has one canal that is oriented roughly perpendicular to the venom gland lumen (Fig. 5.5C). This analysis, at the light microscopy level is reinforced by observations at higher magnification using transmission electron micrographic (TEM) methods (Chiu et al., 2006; Ferrarese et al., 2009; Morales et al., 2005; Fig. 5.5D–F). Based on localization of p40 to the microvilli of the rough canals, within the smooth canals and in the gland lumen, and its close association with VLP precursors (Fig. 5.3F and G) in immunostaining experiments, we have proposed that the canal system is adapted for efficient trafficking of the molecular components from secretory cells to the lumen (Ferrarese et al., 2009). p40 is a putative virulence factor of L. heterotoma VLPs (Chiu et al., 2006, see below).
Remarkably, structures with a very similar organization of actin-lined canals were observed in three Leptopilina spp. and Ganaspis xanthopoda, parasitoids of Drosophila spp., as well as in Campoletis sonorensis, an ichneumonid parasitoid of Heliothis virescens. These observations suggest that the novel supracellular canal system may be a shared trait of venom glands in parasitic wasps. This system appears to be essential for efficient biogenesis and delivery of virulence factors (Ferrarese et al., 2009).
5.3.2. The nature of L. victoriae and L. heterotoma VLPs
Wasp venom is fluid-like, composed of a variety of proteins and microscopic entities (bacteria, viruses or virus-like particles). Mature VLPs of L. victoriae and L. heterotoma (Fig. 5.3A and B) are pentagonal and hexagonal in shape, with varying numbers of spike-like appendages (Chiu et al., 2006; Morales et al., 2005). Through a silver stain gel analysis of purified VLPs obtained from L. heterotoma and L. victoriae, we showed that mature VLPs of these two closely related wasps are composed of at least four major proteins. Of these, p40 and p47.5 are the most abundant in the respective species (Chiu et al., 2006). An antibody raised against purified L. heterotoma VLPs recognizes p40 and cross reacts with p47.5 of L. victoriae.
Using fluorescence light microscopy and immuno-EM methods, we could track biogenesis of VLPs from secretory cells of the venom gland, all the way into the host hemocytes using the anti-p40 antiserum (Chiu and Govind, 2002; Chiu et al., 2006; Ferrarese et al., 2009): (1) VLP precursors (p40) are produced in the perinuclear region of secretory cells; (2) p40 moves from the perinuclear region toward the membrane microvilli region, also known as the “rough” canal; (3) It is delivered into the venom gland lumen via the smooth canal (Fig. 5.5D–F). VLP precursors and partially assembled particles continue to undergo assembly within the venom gland lumen (e.g., compare morphologies in Fig. 5.3C–E with Fig. 5.3A and B). Additional morphological changes are observed in sections from regions adjacent to the connecting duct and from within the reservoir, where they eventually mature into VLPs (Chiu et al., 2006; Morales et al., 2005).
The presence of VLPs has also been reported in different L. boulardi strains including L. boulardi-G486, although their biogenesis, structure and mechanism of action are not well understood. L. boulardi venom induces cytoskeletal changes affecting function of lamellocytes (Dupas et al., 1996; Labrosse et al., 2005). Regardless, it is clear that the population of symbiotic/microbial structures formed and residing within parasitoid venom gland can profoundly modulate parasitoid–host interaction. Their characterization will clarify the nature of this interaction and shed light on the evolution of Drosophila spp.
5.3.3. The lethal effects of L. heterotoma/L. victoriae VLPs on host hemocytes
The VLPs of both L. heterotoma and L. victoriae have spikes with knobs at the end extending from the center core (Chiu et al., 2006; Rizki and Rizki 1994; Fig. 5.3A and B). Immuno-EM staining of mature VLPs from both species shows that p40 and p47.5 proteins are largely located in the periphery and along the spike-like structures of VLPs (Fig. 5.3F and G; Chiu et al., 2006). In scanning electron microscope (SEM) preparations, VLPs aggregate with each other via VLP spikes. VLPs also attach to the lamellocyte membrane via these extensions.
Infection by L. heterotoma and L. victoriae (but not L. boulardi) results in a concerted and active deletion of larval hemocytes: first, infection leads to the apoptosis of the larval lymph glands (Chiu and Govind, 2002). This effect was observed in an in situ terminal deoxynucleotidyltransferase-mediated 2′-deoxyuridine 5′-triphosphate nick-end labeling (TUNEL) assay. Factors that trigger these changes in the lymph gland are also not fully known.
Second, few cells that are able to differentiate into lamellocytes undergo rapid lysis, but first assume a bipolar morphology (Fig. 5.3H and I). In vitro, venom extracted from either L. heterotoma or L. victoriae induces lamellocytes (from microtumor-bearing hopTum-l larvae) to assume bipolar morphology. Such bipolar cells have p40/p47.5 localized within them. Significantly, anti-p40 antibody specifically neutralizes this cellular transformation (almost completely for L. heterotoma VLPs and by more than half for L. victoriae VLPs) implicating these proteins in VLP-lamellocyte recognition or binding (Chiu et al., 2006). Bipolar cells remain TUNEL negative when induced in vitro (Chiu and Govind, 2002).
Finally, incubation of L. heterotoma or L. victoriae venoms induces apoptosis of mature circulating plasmatocytes in short-term cultures. These changes occur when circulating hemocytes from hopTum-l mutant larvae were treated with venom from either L. heterotoma or L. victoriae, in vitro. In such cultures, approximately 30% of the hemocyte population becomes TUNEL positive (2–4 days after infection). Interestingly, the overwhelming majority of the TUNEL-positive cells were plasmatocytes. Furthermore, immunofluorescence experiments revealed that VLPs are actually localized inside the cytoplasm of the TUNEL-positive hemocytes. These findings suggest that VLPs may play an important role in inducing apoptosis in circulating hemocytes of host larvae (Chiu and Govind, 2002). The exact mechanisms or effectors involved in either apoptosis of plasmatocytes or bipolar cell lysis of lamellocytes are not fully understood.
The lytic and apoptotic effects of L. heterotoma and L. victoriae venom were compared in vivo in which tumor-bearing hopTum-l larvae were infected by the wasps. The effect of L. heterotoma venom was stronger where the development of most tumors in the mutant animals was inhibited. In contrast, L. victoriae infection resulted in encapsulation of wasp eggs in larvae of the same genetic background. This difference in “strength” of the venom was also observed in in vitro bipolar lysis assays (Morales et al., 2005).
5.4. HOST GENE EXPRESSION CHANGES AFTER L. BOULARDI AND L. HETEROTOMA INFECTION
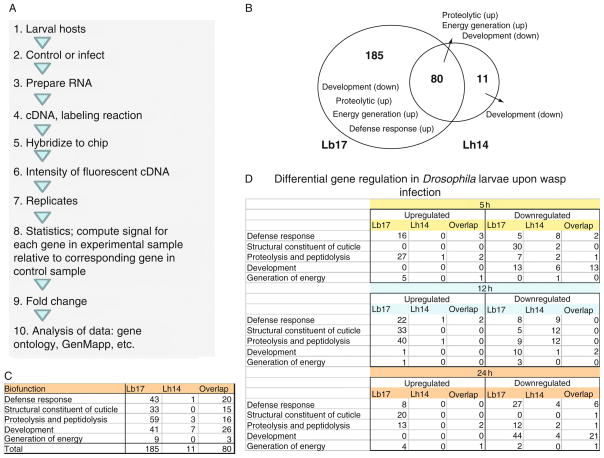
One approach to understanding the effects of different infection strategies on host physiology is to examine differences in the global gene expression patterns after L. boulardi-17 and L. heterotoma-14 infections. Schlenke et al. (2007) infected second-instar hosts for 2 h and harvested ribonucleic acid (RNA) from animals at 2–5, 9–12 or 21–24 h postinfection by either wasp for microarray analysis (Fig 5.6A).
FIGURE 5.6.
Host gene expression changes after L. boulardi-17 and L. heterotoma-14 infections. (A) Outline of the design and analysis of the microarray experiment as described in Schlenke et al. (2007). (B) Venn diagram showing number of genes whose expression is modulated at any of the three points after L. heterotoma-14 or L. boulardi-17 infections. Fold change in gene expression was calculated from the published data and analysis threshold was arbitrarily set (fold change ≤0.75 and ≥1.5). (C) Differentially expressed genes identified in panel (B) can be classified into five functional classes as shown. L. boulardi-17-infected larvae differentially express 265 genes compared to 91 genes in L. heterotoma-14-infected larvae. Eighty genes are expressed in the host after either infection. (D) The five functional categories of differentially expressed genes organized by timepoints. Total genes that are differentially expressed at each timepoint after L. boulardi-17 infection are 125, 136 and 163, and after L. heterotoma-14 infection are 42, 40 and 43, respectively. Not all genes are differentially regulated at all three timepoints for the same wasp infection, and therefore, the list of differentially regulated genes at one timepoint is different than the list of genes at another timepoint.
First, of the classes of genes that are most significantly upregulated by both wasp species are proteolysis and energy generation (mitochondrial electron transport, oxidative phosphorylation), while the gene functional class most significantly downregulated by both wasp species is development (Fig. 5.6B). Upregulation of a wide range of proteolytic genes is indicative that one common response to wasp parasitism is the activation of proteolytic cascades, which are believed to be important in extracellular signaling, hemolymph coagulation and humoral immune signaling. The dynamic between upregulation in energy generation and downregulation of development genes points to the host’s response to conserve energy by slowing down normal physiological activities and devote molecular machinery to mount an effective immune response. These changes in gene expression coincide with delayed pupariation of infected D. melanogaster hosts (2 days later than controls; see Schlenke et al., 2007).
Second, far smaller numbers of Drosophila genes are highly differentially regulated by L. heterotoma-14 than by L. boulardi-17 infection. In fact, the analysis of differentially expressed genes at all three timepoints reveals that hosts infected with L. boulardi-17 expressed twice as many genes compared to L. heterotoma-14 (Fig. 5.6B). Furthermore, this discrepancy is also evident by analyzing the number of genes that are differentially expressed at individual timepoints (Fig. 5.6D). The number of genes differentially regulated after L. boulardi-17 infection increase over time, while it remains the same after L. heterotoma-14 infection, widening the gap between two infections. Thus, gene expression in D. melanogaster is more robustly regulated in response to infection by the specialist L. boulardi-17 than to the generalist L. heterotoma-14.
Third, L. boulardi-17 infection leads to differential regulation of host gene expression, not only for those genes related to immune responses, but for a variety of other functional classes (Schlenke et al., 2007; also see Fig. 5.6C). Gene Ontology/GenMapp analysis of over 400 genes revealed differential expression of genes encoding recognition proteins, proteolytic enzymes, antimicrobial peptides and components of the Toll/NF-κB, JAK/STAT and the melanization cascade (Fig. 5.4). Gene activation of Toll/NF-κB and JAK/STAT pathway components is consistent with the genetic requirement of pathway components as discussed in Section 5.2.3. These results suggest that activation of cellular and humoral arms in the larval immune system is linked via these signaling pathways. Surprisingly, however, hosts infected by L. heterotoma-14 did not substantially modulate gene expression; with fewer than half as many genes affected.
Fourth, in contrast to the Toll, JAK/STAT and phenol oxidase (PO) pathways regulation of IMD, JNK and other described Drosophila immune pathways appears largely unaffected by wasp infection (Schlenke et al., 2007).
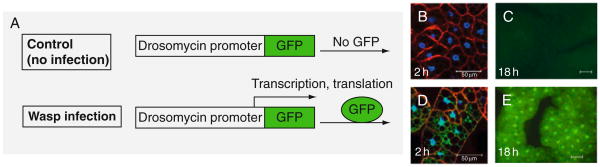
These results indicate that the Toll pathway is fundamentally important not only in regulating the antimicrobial response, but may also be the central regulator of the antiparasite response of insects. The systemic induction of AMP genes in the fat body cells was confirmed in an in vivo reporter assay. A host strain carrying a reporter transgene (Drosomycin promoter fused to GFP reporter, see schematic in Fig. 5.7A) was infected. Drosomycin is a specific target of the Toll pathway and its activation is easily detected in living animals using fluorescence microscopy (Ferrandon et al., 1998). Strong and uniform activation of this promoter was observed after L. boulardi-17 infection (Fig. 5.7B–E), but not after L. heterotoma-14 infection (Schlenke et al., 2007). The upregulation of several of the same effector molecules that are activated by microbial infections suggests that these effectors may also play a role in the antiparasite response or that they provide secondary protection from microbial infection.
FIGURE 5.7.
Wasp-induced fat body expression of Drosomycin-GFP reporter in vivo. (A) Reporter construct of Drosomycin-GFP designed to assay in vivo activation of the promoter (Ferrandon et al., 1998; Tzou et al., 2000). (B–E) Upon L. boulardi infection, Drosomycin-GFP expression is activated through the 24-h period of third-instar larval stages. Hosts were exposed to wasps for 24 h. Fat body samples were dissected 2 or 18 h after infection. Drosomycin-GFP was not expressed in the absence of infection (B, C), but was clearly expressed after infection (D, E).
The mechanism by which Toll pathway is activated is not known. However, genes encoding specific peptidoglycan recognition proteins are activated by L. boulardi-17 and L. heterotoma-14 infections and these might play a role in wasp egg recognition.
Differential activation of melanization by the two wasps was also observed in vitro from larval extracts after infection. Cytotoxic quinones, semiquinones and reactive oxygen species have been implicated in melanization and death of Drosophila parasites (Nappi and Vass, 1993; Nappi et al., 1995; see Chapter 4 by Nappi et al.).
5.5. CONCLUDING REMARKS
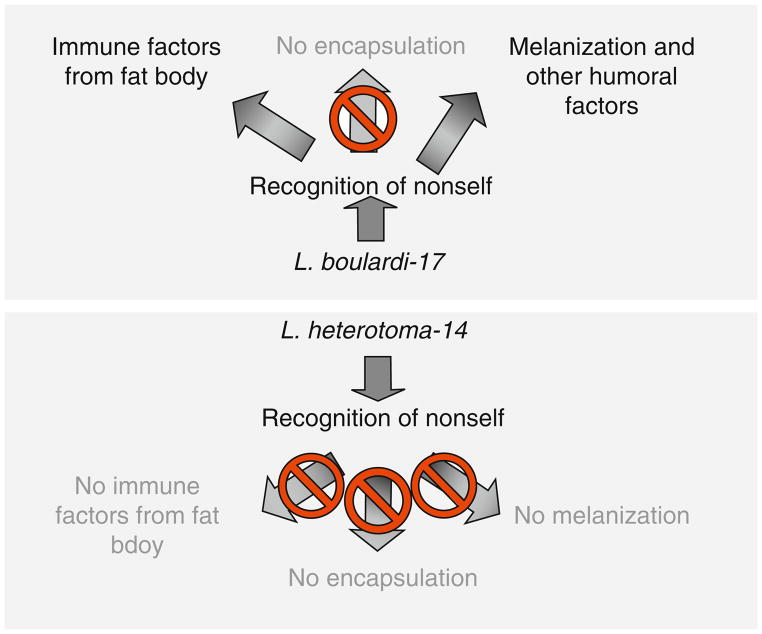
Parasitic wasps of Drosophila have evolved various strategies to maximize survival of their progeny. In this chapter, we have reviewed strategies of two wasps that are equally highly successful on D. melanogaster. Intriguingly, however, their ability to succeed on other Drosophila spp. is quite different. A key difference appears to lie in the lethal effects of venom components on the host hematopoietic system. While L. heterotoma-14 infection activates immune cells (hemocytes), it quickly disables all arms of the larval innate immune response. The immune-suppressive effect of L. boulardi-17 is more specific, where only wasp encapsulation is thwarted, while melanization and systemic production of antimicrobial peptide production both continue (Fig. 5.8). The ISm strain of L. boulardi produces Rac GTPase (called LbGAP) in its venom that affects lamellocyte morphology by targeting the host Rac1 and Rac2 proteins (Colinet et al., 2007; see Chapter 6 by Poirié et al.). It is not known whether L. boulardi-17 synthesizes LbGAP.
FIGURE 5.8.
Effects of L. boulardi and L. heterotoma on D. melanogaster. Differences in the activities of virulence strategies (active vs. passive) and factors (proteins affecting hemocyte morphology vs. viability) may account for differences in the activation of immune pathways after infection by L. boulardi-17 and L. heterotoma-14. In the former case, only encapsulation is abolished in D. melanogaster hosts. In the latter, all three aspects of immune responses are compromised.
A comparative approach applied to this three-part (symbiotic virus-like particles/wasp/fly) host–pathogen model system, that combines morphologic, molecular and genomics methods is beginning to provide a more comprehensive view of how this pathogen class succeeds in nature. Our knowledge of natural pathogens of Drosophila is still very limited. With application of molecular and proteomic methods, it is now feasible to explore the relationship of this well-characterized host with its natural parasites to understand how NF-κB signaling is activated, or remains repressed and how certain parasites of Drosophila have evolved to become highly virulent. The mechanisms of activation and immune suppression in related specific host–parasite pairs are expected to be shared. A systematic analysis of these mechanisms should provide insight into the nature and evolution of virulence.
Acknowledgments
Financial support from USDA (NRI/USDA CSREES 2006-03817 and 2009-35302-05277), NIH NIGMS S06 GM08168, GM056833-08 and G12-RR03060) and PSC-CUNY is gratefully acknowledged. We thank Felix Castellanos, Roberto Ferrarese and Jorge Morales for help with figures and colleagues for discussions. The L. heterotoma strain whose origin is not specified in the text was obtained from P. Chabora. G. xanthapoda and L. victoriae were obtained from P. Chabora and Jacques J.J.M. van Alphen, respectively.
References
- Allemand R, Lemaitre C, Frey F, Bouletreau M, Vavre F, Nordlander G, et al. Phylogeny of six African Leptopilina species (Hymenoptera: Cynipoidea, Figitidea), parasitoids of Drosophila, with description of three new species. Ann Soc Entomol Fr. 2002;38:319–332. [Google Scholar]
- Ayres JS, Freitag N, Schneider DS. Identification of Drosophila mutants altering defense and endurance of to Listeria monocytogenes infection. Genetics. 2008;178:1807–1815. doi: 10.1534/genetics.107.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- Bottero V, Withoff S, Verma IM. NF-kappaB and the regulation of hematopoiesis. Cell Death Diff. 2006;13:785–797. doi: 10.1038/sj.cdd.4401888. [DOI] [PubMed] [Google Scholar]
- Brehélin M. Proceedings in Life Sciences. Springer-Verlag; Berlin; New York: 1985. Immunity in Invertebrates: Cells, Molecules, and Defense Reactions. [Google Scholar]
- Carton Y, Bouletreau M, van Alphen JJM, van Lenteren JC. The Drosophila parasitic wasps. In: Ashburner, Carson, Thompson, editors. The Genetics and Biology of Drosophila. Academic Press; London: 1986. pp. 347–394. [Google Scholar]
- Chiu H, Govind S. Natural infection of D. melanogaster by virulent parasitic wasps induces apoptotic depletion of hematopoietic precursors. Cell Death Diff. 2002;12:1379–1381. doi: 10.1038/sj.cdd.4401134. [DOI] [PubMed] [Google Scholar]
- Chiu H, Morales J, Govind S. Identification and immuno-localization of p40, a protein component of immune-suppressive virus-like particles from Leptopilina heterotoma. J Gen Virol. 2006;87:461–470. doi: 10.1099/vir.0.81474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet D, Schmitz A, Depoix D, Chrochard D, Poirié M. Convergent use of RhoGAP toxins by eukaryotic parasites and bacterial pathogens. PLoS Pathog. 2007;3:2029–2037. doi: 10.1371/journal.ppat.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover BA, Davies DH, Vinson SB. Dose-dependent influence of Campoletis sonorensis polydnavirus on the development and ecdysteroid titers of last-instar Heliothis virescens larvae. Arch Insect Biochem Physiol. 1987;8:113–126. [Google Scholar]
- Dupas S, Brehelin M, Frey F, Carton Y. Immune suppressive virus-like particles in a Drosophila parasitoid: significance of their intraspecific morphological variations. Parasitology. 1996;113:207–212. doi: 10.1017/s0031182000081981. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, et al. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998;17:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarese R, Morales J, Fimiarz D, Webb BA, Govind S. A supracellular system of actin-lined canals controls biogenesis and release of virulence factors in parasitoid venom glands. J Exp Biol. 2009;212:2261–2268. doi: 10.1242/jeb.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler LI, Nelson RE, Fessler JH. Drosophila extracellular matrix. Methods Enzymol. 1994;245:271–294. doi: 10.1016/0076-6879(94)45016-1. [DOI] [PubMed] [Google Scholar]
- Govind S. Innate immunity in Drosophila: pathogens and pathways. Insect Sci. 2008;15:29–43. doi: 10.1111/j.1744-7917.2008.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kopelman AH, Chabora PC. Immature stages of Leptopilina boulardi (Hymenoptera: Eucoilidae), a protelean parasite of Drosophila spp. (Diptera: Drosophilidae) Ann Ent Soc Am. 1984;77:264–269. [Google Scholar]
- Krzemień J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by posterior signaling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Labrosse C, Eslin P, Doury G, Drezen JM, Poirie M. Haemocyte changes in D. melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: a Rho-GAP protein as an important factor. J Insect Physiol. 2005;51:161–170. doi: 10.1016/j.jinsphys.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Post-embryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- LaSalle J, Gauld ID. Hymenoptera, their diversity and their impact on the diversity of other organisms. In: LaSalle Gauld., editor. Hymenoptera and Biodiversity. CAB International; Wallingford, United Kingdom: 1993. pp. 1–26. [Google Scholar]
- Lemaitre B, Hoffmann JA. The host defense of Drosophila melanogaster. Ann Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Markus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, Sipos B, et al. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci USA. 2009;106:4805–4809. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–580. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- Melk JP, Govind S. Developmental analysis of Ganaspis xanthopoda: a larval parasitoid of Drosophila. J Exp Biol. 1999;202:1885–1896. doi: 10.1242/jeb.202.14.1885. [DOI] [PubMed] [Google Scholar]
- Morales J, Chiu H, Plaza R, Oo T, Hoskins S, Govind S. Biogenesis, structure, and immune suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J Insect Physiol. 2005;51:181–195. doi: 10.1016/j.jinsphys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993;6:117–126. doi: 10.1111/j.1600-0749.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E, Frey F, Carton Y. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur J Cell Biol. 1995;68:450–456. [PubMed] [Google Scholar]
- Nordlander G. Revision of the genus Leptopilina Forster, 1869, with notes on the status of some other genera (Hymenoptera, Cynipoidea: Eucoilidae) Entomol Scand. 1980;11:428–453. [Google Scholar]
- Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- Rizki RM, Rizki TM. Selective destruction of a host blood cell type by a parasitoid wasp. Proc Natl Acad Sci USA. 1984;81:6154–6158. doi: 10.1073/pnas.81.19.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki RM, Rizki TM. Parasitoid virus-like particles destroy Drosophila cellular immunity. Proc Natl Acad Sci USA. 1990;87:8388–8392. doi: 10.1073/pnas.87.21.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16:103–110. doi: 10.1016/0145-305x(92)90011-z. [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM. Parasitoid-induced cellular immune deficiency in Drosophila. Primordial immunity: foundations for the vertebrate immune system. Ann N Y Acad Sci. 1994;712:178–194. doi: 10.1111/j.1749-6632.1994.tb33572.x. [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM, Carton Y. Leptopilina heterotoma and L. boulardi: strategies to avoid cellular defense responses of Drosophila melanogaster. Exp Parasitol. 1990;70:466–475. doi: 10.1016/0014-4894(90)90131-u. [DOI] [PubMed] [Google Scholar]
- Schilthuizen M, Nordlander G, Stouthamer R, van Alphen JJM. Morphological and molecular phylogenetics in the genus Leptopilina (Hymenoptera: Cynipoidea: Eucoilidae) Syst Ent. 1998;23:253–264. [Google Scholar]
- Schlenke TA, Morales J, Govind S, Clark AG. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 2007;3:1486–1501. doi: 10.1371/journal.ppat.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Melk JP, Govind S. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics. 2004;166:1343–1356. doi: 10.1534/genetics.166.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- Varaldi J, Petit S, Boulétreau M, Fleury F. The virus infecting the parasitoid Leptopilina boulardi exerts a specific action on superparasitism behavior. Parasitology. 2006;132:747–756. doi: 10.1017/S0031182006009930. [DOI] [PubMed] [Google Scholar]
- Wertheim B, Kraaijeveld AR, Schuster E, Blanc E, Hopkins M. Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol. 2005;6:R94. doi: 10.1186/gb-2005-6-11-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]