Abstract
To associate events that are disparate in time, the brain must record, retain and perhaps even reflect on the individual events themselves. Aspects of such learning can be probed with trace conditioning, during which an animal learns to associate events that are temporally distant from one another. For decades, we have known that the formation of so-called trace memories (in which one stimulus is associated with a second stimulus that is discontinuous and later in time) depends on the hippocampal formation. Recent findings indicate that the hippocampus is crucial for the initial acquisition of trace memories but not for their expression or long-term storage. More recent findings implicate neurogenesis, synaptogenesis and awareness in the formation of trace memories.
‘Every memory we have is finally of ourselves. If the memory of an experience is flawed, there is a rift in the continuity of self. There is less of us with each depleted memory.’ – Don DeLillo (The New Yorker, 2003)
The mechanisms whereby the brain associates experiences that are temporally distant from one another have yet to be elucidated, and understandably so. These mechanisms must account not only for the formation of associations between events, of which we know little, but also for remembering the individual stimulus events themselves so as to establish their temporal relationships with subsequent events later in time. In its most simple form, such learning is known as trace conditioning [1]. This article will describe trace memories, some of the learning processes that underlie their acquisition and expression, and what is known about the neuroanatomical substrates that underlie these learning processes, with special emphasis on the hippocampal formation and neurogenesis within the dentate gyrus. Evidence that trace memories are associated with synaptogenesis in the hippocampus will be discussed, as will the potential role of awareness in this form of learning.
Traces in time
In a typical classical conditioning paradigm, a conditioned stimulus (CS) such as a tone is followed by an unconditioned stimulus (US) such as shock or food. Characteristically, conditioned responding (which is indicative of learning) is strongest when the CS is immediately followed by the US or when they overlap slightly in time. This type of learning is known as delay conditioning because there is a temporal delay between the onsets of the two stimuli [1] Figure 1a). This terminology might seem a bit of a misnomer because there is no delay between the stimuli other than in time. The crucial distinction is that the stimuli are always temporally continuous. Learning the association becomes much more difficult when one stimulus precedes the other but they do not overlap in time. This type of learning is known as trace conditioning (Figure 1b). The phenomena of delay and trace conditioning can be illustrated with classical eyeblink conditioning, in which an auditory CS such as a tone is followed by a US (Figure 1a,b), which is usually an air puff or eyelid shock, either of which causes the animal to blink as an unconditioned response (UR). When these two stimuli are presented in a delay-type configuration (Figure 1a), animals readily learn the association and blink in response to the tone, thus emitting the learned or conditioned response (CR). When the two stimuli are separated in time in a trace-like paradigm (Figure 1b), animals require many more trials to learn the association but eventually blink in response to the CS, before the US. The amount of time over which an animal can effectively associate temporally incongruous stimuli differs with the specifics of the conditioning protocol, the system that is being used to process the information (e.g. autonomic or somatic) and the species [2]. During eyeblink conditioning, a gap of > 0.5 s is difficult for a rat or a mouse to bridge [3], whereas during fear and heart-rate conditioning, in which a tone CS is followed by a footshock US, the two stimuli can be associated when separated by many seconds [4,5]. One might imagine that we often learn to associate events over much longer intervals than these and that these processes could be valuable even in everyday life. For example, say you park your car each day in the same place, but then one day you park in a new spot. You return to find a ticket for parking on Tuesday, the day for street cleaning. After a ticket or two, you are unlikely to park in the new spot on Tuesdays again.
Figure 1.
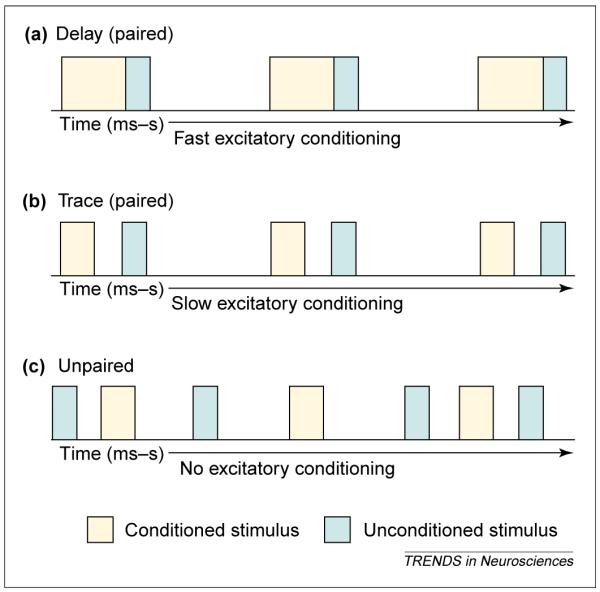
Temporal relationships between stimulus events mediate the ability to acquire associative memories. The conditioned stimulus (CS) is depicted in blue and followed by an unconditioned stimulus (US) in yellow. (a) Delay conditioning is represented as two stimuli that immediately follow one another. The stimuli can also overlap in time as long as the US follows the CS. In either case, the association is usually acquired rapidly, is not associated with awareness and, as such, is considered a procedural memory. (b) Trace conditioning is represented as two stimuli that are not contiguous in time. The stimuli are typically separated from one another by a trace interval, often referred to as the ‘gap’. This association is more difficult to learn and because of its dependence on the hippocampal formation [3,13,14] and association with awareness is often considered a declarative memory [51]. (c) Explicitly unpaired stimuli are presented one after another but the time between stimuli is random and unpredictable. After repeated exposure to these stimuli, an animal learns that the CS is followed by a US but does not know when the US will occur other than that it will not occur during the CS, and so the animal does not display excitatory responses to the CS. As illustrated, the stimulus events and their relationships to one another are remarkably similar between trace and unpaired conditioning, emphasizing the amazing ability of animals to detect predictive relationships among stimuli as they encounter them in their environment.
Although the time when events occur is important for trace conditioning, the crucial issue is not so much the time between the events but rather the length of the entire learning episode (the trial duration relative to the time between events). As illustrated in Figure 1b, the animal trained with trace conditioning can associate the first stimulus (the CS) with the second stimulus (the US) but could also associate the US with the next CS (essentially what is forward versus backward conditioning). In most animal conditioning protocols, the intertrial interval is much longer than the interstimulus interval and is randomized, thus enhancing probability that the CS and US will be associated with each other rather than the US and the next CS. Indeed, the longer the intertrial interval, the faster the learning [6,7].
Bridging the gaps
Related to the issue of trial duration during trace conditioning is the issue of context because the ‘gap’ between the CS and the US typically consists of the same context as that between the US and the next CS. As early as 1965, Kamin showed that animals more readily acquired the CS–US association if the temporal gap between the CS and the US (the trace interval) was replaced with another stimulus – a so-called filler, one distinct from that used during the intertrial interval [8]. In addition to enhancing the distinction between the two contexts, the presence of a gap filler could enhance learning via serial conditioning, because the first stimulus (the CS) could be paired with a second CS (the filler) and finally with the US. The enhanced performance could also reflect some stimulus generalization, as the CS and the fillers tend to be more similar than the CS and the context that is typically presented in the temporal gap [9] (although see Ref. [10]). Importantly, the strength of the learned response is related to amount of overlap between the CS and the stimulus filling the gap [11]. A complete or even partial overlap of the CS with the gap filler produced less conditioning, suggesting that contextual and second-order conditioning, and even configural learning, could be involved [12]. Whatever the explanation, it is clear that there is more to acquiring trace memories than simply maintaining memory traces over time.
Anatomy of a trace
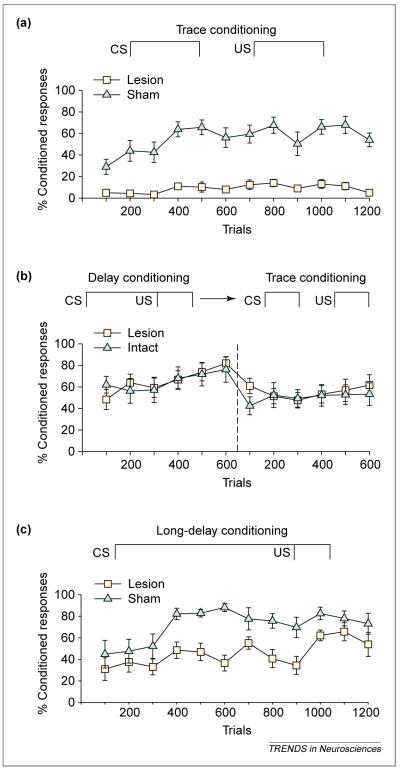
One of the interesting features of trace conditioning is its dependence on the hippocampal formation. Nearly 20 years ago, Solomon et al. found that rabbits subjected to aspiration lesions of the entire hippocampal formation could not acquire the trace conditioned response [13]. Similarly, we found that rats with excitotoxic lesions to the hippocampus did not acquire the trace eyeblink response – even after > 1000 trials [3,14] Figure 2a). The same type of lesion had no impact on acquisition of the conditioned response when the animal was trained with a delay conditioning procedure in which the CS and US overlapped and co-terminated (Figure 2b). Crucially, however, the lesioned animals that had learned the delay conditioned response could then perform the conditioned response when a trace interval was placed between the CS and the US (Figure 2b). To test whether this ‘transfer’ of the CR from delay to trace conditioning simply reflected the fact that the animal learned to blink at a specific time after the CS, irrespective of the when the US occurred, we altered the interstimulus intervals between delay and trace conditioning. Even with this manipulation, animals that had learned the response during delay conditioning could perform the trace response and effectively moved the CR to accommodate a new temporal relationship [14]. These data indicate that animals with lesions of the hippocampus can learn to emit a previously learned response at a new time, despite speculation that the hippocampus is necessary for response timing. More crucially, they indicate that the hippocampus is necessary for acquiring the trace association if the stimuli are initially separated in time but not if the relationship between those stimuli is already known.
Figure 2.
Formation of trace memories requires an intact hippocampus. (a) Eye-blinks that were detected during the temporal gap were considered conditioned responses and are represented as a percentage of responses during training. As shown, animals with excitotoxic lesions of the hippocampus emitted virtually no conditioned responses even after > 1000 trials of trace eyeblink training [14]. (b) However, animals with lesions to the hippocampus can learn the conditioned response when a delay training protocol is used. In this experiment, the intervals between the conditioned stimulus (CS) and the unconditioned stimulus (US) were the same except that the CS was temporally contiguous and overlapped slightly with the US. After training with delay conditioning (dashed line), animals with hippocampal lesions could perform the trace conditioning task. (c) As the stimulus relationships become more difficult to learn, the hippocampus becomes involved. Using a long-delay paradigm in which the CS and the US are continuous in time but the time between stimuli onsets is extended, animals with lesions to the hippocampus are learning-impaired. These animals eventually learned and, moreover, were able to perform the trace conditioned response by moving the response to accommodate the new onset time of the US (data not shown) [14]. All three panels were generated using data from Ref. [14].
That the hippocampus is involved in formation, but not the expression, of trace memories might be important for understanding the behavior of H.M., the most studied patient in neuroscience. In the 1950s, H.M. was subjected to hippocampectomy to relieve severe epileptic seizures, after which he lost his ability to form many types of new memories, mostly those that were declarative in nature [15,16]. Oddly, H.M. readily acquired a trace memory task using the eyeblink response, casting some doubt on the premise that the hippocampus is necessary for trace conditioning [17]. However, H.M. was trained with and learned a delay conditioning task before trace conditioning. Thus, his ability to perform the trace response is consistent with data in the rat; once the animal learns the association between the two stimuli, the hippocampus is no longer necessary for the acquisition of trace memories.
Memories of the trace
How long is the hippocampus involved in the performance and retention of trace memories once they are learned? It appears to be days, at most. Animals trained on trace conditioning and lesioned just one day later emit virtually no learned responses, whereas those submitted to lesions one month after training perform as they did before the lesion [18]. Recently, an amazing set of studies demonstrated that one week after training, the hippocampus is not essential for performing the trace response, whereas the prefrontal cortex becomes so [19]. Others have reported that the prefrontal cortex is involved in performance of the conditioned eyeblink response, regardless of whether the lesion is performed before or after training [13,20]. In addition, the deep nuclei of the cerebellum are crucial for establishing and maintaining the conditioned response, again irrespective of when the lesion or manipulation occurs [21,22]. So it is clear that more than the hippocampus is necessary for trace conditioning.
But what does the hippocampus do during trace conditioning? More specifically, how does it ‘know’ what information must be processed and maintained for establishing a hippocampus-dependent trace memory versus that for the delay-type memory? Initially, it doesn’t. In fact, the hippocampus responds to conditioning stimuli of all sorts and not just those involving a trace. For example, pyramidal neurons in area CA1 increase their activity in response to stimuli embedded in a delay, as well as a trace, paradigm [23,24]. Furthermore, recent data indicate that trace or delay memories increase the density of dendritic spines in the hippocampus [25]. The increase was evident on basal dendrites of pyramidal cells in area CA1 but not on the more intensely studied apical dendrites or on granule neurons in the dentate gyrus. The increase in spine density appears specific to learning, at least to the extent that the presence of an NMDA antagonist prevented both learning and the increase in spine density. However, since the increase was observed after both delay and trace conditioning (Figure 1a,b), the spines are not preferentially affected by stimuli that are disassociated in time but, rather, respond to stimuli irrespective of the temporal contiguity. It is noted that spine density was not altered by exposure to explicitly unpaired stimuli (Figure 1c). Thus, the spines apparently detect and respond to stimuli that are temporally related, but the response is not limited to those that are discontinuous in time.
Given the crucial role that the hippocampus plays in the formation of declarative and episodic memories [26–30], it is not surprising that its cells respond to stimuli regardless of their temporal contiguity. Indeed, finding cells that preferentially respond to stimuli that are separated by a trace interval seems vastly more unlikely. However, new neurons in the adult hippocampus appear especially sensitive to the formation of trace memories. Their potential role in the formation of trace memories is discussed in detail in the following section.
Neurogenesis and the survival of trace memories
In the past five years or so, it has become accepted that the brain continues to produce new neurons throughout adulthood [31–35]. The vast majority of these are produced in the hippocampus, and their numbers are significant and estimated to be > 5000 per day [36,37]. The neurons arise from progenitor cells in the subgranular zone of the dentate gyrus and, as they mature, they migrate to the granule cell layer. Over the course of a week, the new neurons establish axonal projections and connections to CA3 pyramidal cells [38]. They are capable of generating action potentials and thus appear as functional neurons [39]. Because these new neurons are experientially nal̈ve yet can establish connections with cells that are not, they possess properties that would be useful for detecting novelty and perhaps encoding new information, as suggested for nonmammalian species [40].
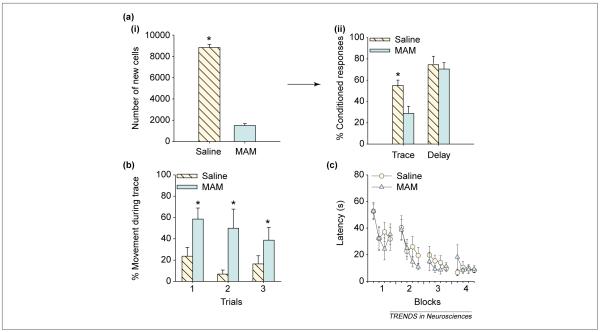
To evaluate the role of these new neurons in memory formation, we reduced their production to ~ 20% of normal using an anti-mitotic agent [41] Figure 3a). Animals were then trained on several different learning tasks, some requiring the hippocampus and others not, some including a trace interval and others not. Animals in which neurogenesis was greatly reduced did not acquire the trace eyeblink conditioning task, although they readily learned the same task using a delay-type paradigm (Figure 3a). Similarly, animals in which neurogenesis was greatly reduced had impaired ability to learn a trace fear conditioning task [42] Figure 3b). These animals expressed minimal conditioned responses during the trace interval or even when the footshock US would have occurred, suggesting that they had very little memory for the aversive event or at least could not remember when it would occur. Depletion of the population of new neurons did not disrupt other types of hippocampus-dependent learning, such as that evident in a spatial navigation task using the Morris water maze (Figure 3c) or in contextual fear conditioning [42,43]. Thus, the reduction in neurogenesis was not associated with a general decrement in learning or even a preferential decrement in hippocampus-dependent learning. Rather, it appears that the presence of new neurons in the adult hippocampus is preferentially associated with the formation of trace memories.
Figure 3.
Formation of trace memories is impaired when the population of newly generated neurons is depleted. (a) (i) New cells in the hippocampus were labeled with bromodeoxyuridine (BrdU), a thymidine analog that labels into dividing cells [56]. Animals received daily injections of either an anti-mitotic agent (MAM) or saline for two weeks. The number of cells labeled after treatment for groups injected with either MAM or saline is presented here as group averages. Using neuron specific markers, it was determined that the vast majority of BrdU-labeled cells were neurons. (ii) The percentage of conditioned (learned) eyeblink responses during training. Animals treated with MAM emitted many fewer learned responses during trace conditioning than during delay eyeblink conditioning [41]. Asterisk indicates a significantly different result. Using data from Ref. [41]. (b) Fear conditioning is represented as a decrease in movement during the trace interval after repeated presentations of a tone conditioned stimulus (CS) and a footshock unconditioned stimulus (US). After two weeks of treatment with MAM and corresponding depletion of the pool of new neurons, animals expressed much less fear (more movement) during the trace interval, and showed reduced anticipation of the US [42]. Thus, their ability to learn the trace fear response was impaired. Asterisks indicate significantly different results. Using data from Ref. [42]. (c) Latency to reach the platform during training on the spatial navigation version of the Morris water maze. As in (a) and (b), rats were treated with MAM or saline for two weeks and then trained. With very few new neurons, the animals readily learned the spatial navigation task [42]. These results are consistent with a recent study showing that nearly complete elimination of adult-generated cells does not alter spatial maze learning [43]. Using data from Ref. [42].
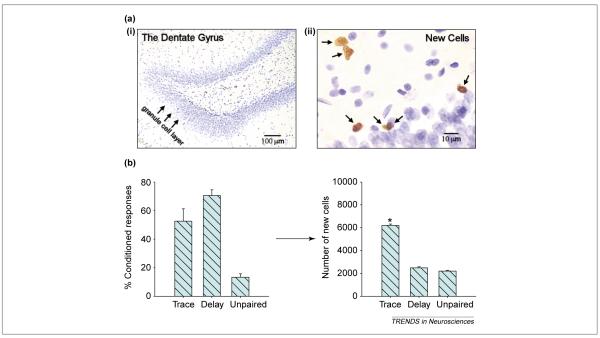
Many adult-generated neurons in the hippocampus, indeed the majority, die within weeks of their birth and do not survive to establish connections with other cells or brain regions [36,38]. What might be their function during this short window of time? Perhaps they are used to process novel stimuli, and if the neurons are not used or exposed to stimuli in a temporally related manner, they die. If this general scenario were true, then cells exposed to trace conditioning stimuli as they establish themselves in the granule cell layer should survive longer. To test this hypothesis, cells born on a particular day were treated with bromodeoxyuridine (BrdU; Figure 4a), which labels cells in the S phase of mitosis [36]. After one week, groups of animals were trained on trace or delay eyeblink conditioning. Other groups were exposed to the same number of stimuli but presented in an explicitly unpaired manner (Figure 1c). Once the animals learned the association, the number of new cells that remained was counted and the percentage of cells with neuron-specific markers was determined. Many more neurons – over twice as many – remained in animals that had acquired the trace memory (Figure 4b). These data indicate that exposure to the trace conditioning procedure can rescue newly generated neurons from death. Learning the delay conditioning task did not alter cell survival and, thus, learning in and of itself is not sufficient to rescue newly generated neurons from death. Exposure to explicitly unpaired stimuli also did not enhance their survival (Figure 4b). Again, these findings suggest that newly generated neurons in the hippocampus are sensitive to the formation of trace memories.
Figure 4.
The formation of trace memories increases the survival of adult-generated neurons. (a) The dentate gyrus of the hippocampal formation (i) and new cells within the granule cell layer (ii) that were labeled with bromodeoxyuridine (BrdU). (b) Animals were injected with one dose of BrdU to label cells generated in the dentate gyrus of the hippocampal formation [36]. One week later they were trained on trace or delay eyeblink conditioning or exposed to unpaired stimuli. One day after training, the number of cells, the vast majority of which were neurons, was increased in animals that were exposed to a trace conditioning paradigm (Figure 1b) relative to those exposed to delay conditioning (Figure 1a) or explicitly unpaired stimuli (Figure 1c). Thus, the formation of trace memories preferentially rescued neurons in the adult hippocampus from death. Asterisk indicates a significantly different result. Using data from Ref. [36].
Proliferation of neurons and memory traces
It is important at this point to distinguish between the possible effects of learning on proliferation versus survival of adult-generated neurons in the hippocampus. In our initial study, we did not observe any effect of trace conditioning on proliferation; the number of new neurons did not increase during the training experience [36]. In any case, it would be difficult to imagine how a learning-induced increase in neuronal production would affect learning itself because it takes more than a week for the new neurons to become incorporated into the granule cell layer and establish connections. However, it is certainly conceivable that forms of experience that involve learning and alter proliferation could affect future behaviors. Indeed, many of the reported effects of experience on neurogenesis such as environmental enrichment [44], stress [35,45,46] and even spatial water-maze training [47] could, if maintained over time, have a significant impact on the structure and circuitry of the hippocampus, thereby affecting learning processes and some behavioral outputs. For example, we showed that exposure to spatial water-maze training enhanced the survival of newly generated neurons, but when the cells were depleted, animals could still learn the water-maze task [36,42]. Thus, new neurons are affected by the acquisition of spatial memories but are not necessary for their acquisition. In addition, it was recently reported that spatial learning is enhanced in aged animals that possess large numbers of adult-generated neurons. These data do not indicate that the newly generated neurons are being used for spatial learning but, rather, that their presence is indicative of learning abilities [48]. As a final example, we have recently shown that exposure to trace conditioning enhances the survival of newly generated cells for months after training and beyond the time when the hippocampus is used for learning. Specifically, animals were injected with BrdU and trained one week later on trace conditioning, as in the previous study [36], and then sacrificed at differing time points after they had learned. Learning the trace conditioning task greatly enhanced the number of cells that remained even two months later [49]. Thus, it appears that once the cells are rescued from death by learning, they become permanently, or at least persistently, incorporated into the dentate gyrus. Because the hippocampus is no longer necessary for maintaining the trace memory at that time, they appear to have adopted another, as yet undetermined, role. The overall point here is that these new neurons are extremely sensitive to new experience. Teasing out the effects of experience on their production and survival from their putative function in learning processes is proving a major challenge.
Traces of awareness and difficulties
Perhaps more surprising than reports that neurogenesis is involved in trace conditioning are those suggesting that consciousness might be also be involved [50]. In one report, Clark and Squire tested humans with and without damage to their hippocampal formation during delay and trace eyeblink conditioning [51]. As expected, those with damage to the hippocampus acquired the delay but not the trace memory. In healthy controls, however, learning the trace memory was associated with levels of awareness. At first glance, these data may seem self-evident: of course awareness of the association would be related to learning. But critically, level of awareness did not relate to performance during delay conditioning (see Ref. [50] for differences). In an ingenious set of experiments, Clark et al. went on to test these ideas using what is known as the ‘gambler’s fallacy’ [52]. In it, the CS (tones) and US (air puffs to the eye) used during eyeblink conditioning are presented in strings such that the probability of a US occurring after a CS is always 0.5 and independent of string length. The subjects are then asked to predict whether or not they think a US will occur on the next trial. Subjects tend to expect a US after hearing several CSs in succession – which is the gambler’s fallacy because the occurrence of the US is unrelated to string length. The crucial observation was that learning in subjects trained with a delay conditioning protocol (in which the CS and US were temporally continuous and overlapped slightly) was not influenced by their expectation. By contrast, learning the trace task was influenced by expectation such that when expectancy was high, the probability of a CR increased, and when expectancy was low, the probability of a CR decreased. These data support the notion that formation of trace memories might involve, or at least be enhanced by, awareness.
In general, tasks that are more difficult to acquire tend to depend on the hippocampus. For example: learning with visual cues in a water maze does not depend on the hippocampus, whereas learning with spatial cues does [53]; discriminating between two tones does not but learning a reversal of the discrimination does [54]; and delayed nonmatching to sample with a short test interval does not but with a long interval does [27]. Similarly, delay conditioning does not depend on the hippocampus, whereas trace conditioning does. So how might awareness relate to task difficulty? Some report that the degree to which awareness predicts performance depends more on the complexity of the task than on the temporal relationship between stimuli [50]. Recently, we tested whether task difficulty is important for the known dependence of trace conditioning on the hippocampus [14]. To do this, we increased the duration of the CS nearly twofold, so that it overlapped with the US much later in time (a long delay task). Using such parameters, it becomes much more difficult to use the CS onset to predict when the US will occur, even if they are temporally continuous or overlap at some point in time. Animals with lesions of the hippocampus had great difficulty acquiring the association with a long delay. They did eventually exhibit some learning but only after 1000 trials (Figure 2c). Thus, the hippocampus could play a role in learning tasks that are most difficult and most assisted by awareness.
Thinking outside the trace
That the hippocampus participates in learning temporal relationships is not a new idea. However, learning something even as seemingly straight forward as a trace memory involves more than learning about time [55]. Nonetheless, it is clear that the hippocampus is crucial for establishing trace memories – provided that the association is not already known. Is it a coincidence that neurogenesis is so prevalent in the hippocampus and adult-generated neurons are so receptive to the formation of trace memories? I think not. Is it a coincidence that the hippocampus seems to be involved in aspects of awareness from which trace conditioning seems to benefit? Perhaps not. But whether the various correlates of trace conditioning – neurogenesis, synaptogenesis, awareness and difficulty – relate causally to one another remains very much an open question. In the mean time, we persist in making memories of ourselves, as noted by a famous author whose name escapes me.
Acknowledgements
This work was supported by National Institute of Mental Health (R01– 59970; R01–59740) and the National Science Foundation (IBN0217403). Special thanks to E. Gould, B. Leuner and L. Matzel for comments on a previous version of the manuscript.
References
- 1.Pavlov I. Conditioned Reflexes. Oxford University Press; 1927. pp. 197–275. [Google Scholar]
- 2.Gormezano I, et al. Twenty years of classical conditioning research with the rabbit. In: Praque JM, Epstein AN, editors. Progress in Psychobiology and Physiological Psychology. Academic; New York: 1983. [Google Scholar]
- 3.Weiss C, et al. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav. Brain Res. 1999;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 4.McEchron MD, et al. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.McEchron M, et al. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Balsam PD. Relative time in trace conditioning. Ann. N. Y. Acad. Sci. 1984;423:211–227. doi: 10.1111/j.1749-6632.1984.tb23432.x. [DOI] [PubMed] [Google Scholar]
- 7.Balsam PD, et al. Timing at the start of associative learning. Learn. Motiv. 2002;33:141–155. [Google Scholar]
- 8.Kamin L. Temporal and intensity characteristics of the conditioned stimulus. In: Prokasy WF, editor. Classical Conditioning: a Symposium. Appleton-Century-Crofts; New York: 1965. pp. 118–147. [Google Scholar]
- 9.Kaplan PS, Hearst E. Bridging temporal gaps between CS and US in autoshaping: insertion of other stimuli before, during and after CS. J. Exp. Psychol. Anim. Behav. Process. 1982;8:187–203. [PubMed] [Google Scholar]
- 10.Rescorla RA. Effects of a stimulus intervening between CS and US in autoshaping. J. Exp. Psychol. Anim. Behav. Process. 1982;8:131–141. [PubMed] [Google Scholar]
- 11.Kaplan PS. Bridging temporal gaps between CS and US in autoshaping: a test of a local context hypothesis. Anim. Learn. Behav. 1984;12:142–148. [Google Scholar]
- 12.Kehoe EJ. Conditioning with serial compound stimuli: theoretical and empirical issues. Exp. Anim. Behav. 1983;1:30–65. [Google Scholar]
- 13.Solomon PR, et al. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 14.Beylin AV, et al. The role of the hippocampus in trace conditioning: temporal incongruity or task difficulty? Neurobiol. Learn. Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- 15.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corkin S. What’s new with the amnesic patient H.M.? Nat. Rev. Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff-Pak DS. Eyeblink classical conditioning in H.M.: delay and trace paradigms. Behav. Neurosci. 1993;107:911–925. doi: 10.1037//0735-7044.107.6.911. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, et al. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace conditioned responses. Behav. Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- 19.Takehara K, et al. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J. Neurosci. 2002;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronforst-Collins M, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol. Learn. Mem. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- 21.Woodruff-Pak DS, et al. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- 22.Krupa DJ, et al. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 23.Berger TW, et al. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J. Neurophysiol. 1983;50:1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- 24.Weisz D, et al. Increased responsivity of dentate granule cells during nictitating membrane response conditioning in rabbit. Behav. Brain Res. 1984;12:145–154. doi: 10.1016/0166-4328(84)90037-8. [DOI] [PubMed] [Google Scholar]
- 25.Leuner B, et al. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark CEL, et al. Recognition memory for single items and for associations is similarly impaired following damage to the hippocampal region. Learn. Mem. 2002;9:238–242. doi: 10.1101/lm.51802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squire LR, Zola SM. Structure and function of declarative and non-declarative memory systems. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallenstein G, et al. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 29.Fortin NJ, et al. Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEchron M, et al. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J. Neurosci. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron HA, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 33.Gould E, et al. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakic P. Neurogenesis in adult primates. Prog. Brain Res. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- 35.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 36.Gould E, et al. Learning enhances adult neurogenesis in the adult hippocampal formation. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 37.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 38.Hastings N, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nottebohm F. Neuronal replacement in adult brain. Brain Res. Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- 41.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 42.Shors TJ, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madsen TM, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 44.Kempermann G, et al. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 45.Gould E, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanapat P, et al. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 47.Lemaire V, et al. Prenatal stress produced learning deficits associated with inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drapeau E, et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shors, et al. Associative memory formation persistently enhances the survival of newly-generated neurons in the adult hippocampus. Society for Neuroscience; Washington, DC: 2003. Program No. 936.16. 2003 Abstract Viewer/Itinerary Planner. [Google Scholar]
- 50.Knuttinen MG, et al. Awareness in classical differential eyeblink conditioning in young and aging humans. Behav. Neurosci. 2001;115:747–757. doi: 10.1037//0735-7044.115.4.747. [DOI] [PubMed] [Google Scholar]
- 51.Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 52.Clark RE, et al. Trace and delay eyeblink conditioning: contrasting phenomena of declarative and nondeclarative memory. Psychol. Sci. 2001;12:304–308. doi: 10.1111/1467-9280.00356. [DOI] [PubMed] [Google Scholar]
- 53.Riedel G, et al. Reversible neural activation reveals hippocampal participation in several memory processes. Nat. Neurosci. 1999;2:898–906. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 54.Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav. Brain Res. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: where’s the trace? Behav. Neurosci. 2001;115:1224–1238. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- 56.Miller MW, Nowakowski RS. Use of bromodeoxyuridineimmunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]