Abstract
Most active processes by immune cells including adhesion, migration, and phagocytosis require the coordinated polymerization and depolymerization of filamentous actin (F-actin), which is an essential component of the actin cytoskeleton. This review focuses on a newly characterized hematopoietic cell-specific actin regulatory protein called hematopoietic protein-1 [Hem-1, also known as Nck-associated protein 1-like (Nckap1l or Nap1l)]. Hem-1 is a component of the “WAVE [WASP (Wiskott-Aldrich syndrome protein)-family verprolin homologous protein]” complex, which signals downstream of activated Rac to stimulate F-actin polymerization in response to immuno-receptor signaling. Genetic studies in cell lines and in mice suggest that Hem-1 regulates F-actin polymerization in hematopoietic cells, and may be essential for most active processes dependent on reorganization of the actin cytoskeleton in immune cells.
Keywords: Hem-1, NAP1l, Rac, WASP-family verprolin homologous protein, Actin cytoskeleton
1. Introduction
Reorganization of the actin cytoskeleton is essential for many aspects of an immune response to infection, including the formation of the immunological synapse (IS), cell polarization, adhesion of immune cells to the vascular endothelium, migration and extravasation to sites of invasion in response to chemokines, formation of phagosomes and phagocytosis of foreign agents, and destruction of ingested microbes by the production of reactive oxygen species (ROS) (see [1–5] for review). At the heart of these processes is the active, coordinated, directional polymerization and depolymerization of filamentous actin (F-actin), which is initiated in part by members of the Rho family of guanosine triphosphatases (GTPases) (Cdc42, Rho, and Rac), and their downstream target complexes including WASP (Wiskott–Aldrich syndrome protein) and WAVE (WASP-family verprolin homologous protein) family proteins (see [6] for review). The importance of coordinated reorganization of the actin cytoskeleton in immune and hematologic cells is underscored by several diseases in humans where this process is disrupted. For example, mutations in the WASP gene in humans results in Wiskott–Aldrich Syndrome, a severe X-linked immunodeficiency disease characterized by thrombocytopenia, eczema, and recurrent infections due to defects in immune cell migration, proliferation, and survival (see [7,8] for review). Similarly, a dominant-negative mutation in the human Rac2 gene results in a phagocyte immunodeficiency characterized by recurrent infections and the absence of pus in inflamed tissues, due to impaired neutrophil migration and production of superoxides [9]. As discussed below gene-targeted mutations in mice have further defined specific and essential roles of F-actin regulators such as Rac, Rho, and their effector proteins in many aspects of hematopoietic cell biology. This review will focus on the biology and molecular functions of the hematopoietic cell-specific Rac-effector protein Hem-1 [hematopoietic protein-1 (also known as Nck-associated protein 1-like (NAP1l or Nckap1l)] in the development and function of hematopoietic cells. The relative hematopoietic cell-specific expression of Hem1, and the specific abilities of Hem1 disruption in mice to selectively inhibit the function of immune cells [10], suggest that the generation of pharmacologic inhibitors of Hem-1 protein may provide a specific method of inhibiting immune cell survival and function in autoimmune diseases, and/or for blocking the metastasis and invasion of hematopoietic cell-derived cancers, processes which are also dependent on re-organization of the actin cytoskeleton [11].
2. Rho-family GTPases and the actin cytoskeleton
Rho family GTPases are activated downstream of multiple immuno-receptor types including B and T cell antigen receptors (BCR and TCR), chemokine and cytokine receptors, and Toll-like receptors (TLRs) (see Fig. 1 and [12,13] for detailed reviews). In response to stimulation, guanine nucleotide exchange factors (GEFs) convert Rho family members from an inactive GDP-bound state to the active-GTP-bound form, which results in a cascade of signaling events leading to cytoskeletal reorganization, proliferation, survival, adhesion, and transcription. Conversely, GTPase-activating proteins (GAPs) stimulate the hydrolysis of bound GTP to GDP, which switches off active Rho (see [14,15] for review). Thus far, biochemical and genetic studies indicate that important Rho-GEFs in immune cells include Dock2 (dedicator of cytokinesis 2), SWAP70 (switch associated protein 70), Vav (Vav1, 2, and 3), and Tiam1 (T-cell lymphoma invasion and metastasis 1), whereas immunologically important Rho-GAPs include Bcr (breakpoint cluster region), and RacGAP1 (see [13] for review).
Fig. 1.
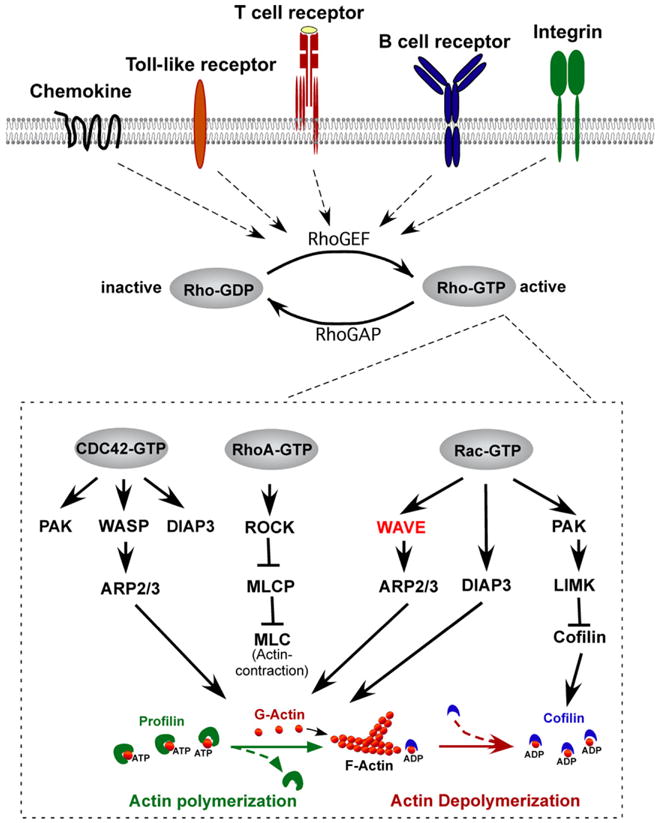
Rho family members regulate F-actin polymerization in response to immuno-receptor signaling. Rho-GTPases cycle between an inactive, GDP-bound state and the active, GTP-bound state. Interaction of immuno-receptors (i.e. chemokine, Toll-like receptor, T cell receptor, B cell receptor, integrin) with ligand activates Rho guanine nucleotide exchange factors (GEFs) such as Vav, which catalyze the release of GDP from Rho, allowing GTP to bind. Conversely, Rho-GTPase activating proteins such as Rho-GAP cause GTP to be hydrolyzed back to the inactive GDP-bound form. Major Rho family members in immune cells include CDC42, Rac, and RhoA. CDC42-GTP specifically binds and activates WASP, which facilitates Arp2/3-mediated nucleation of ATP-bound G-actin, which is released from Profilin. Rac-GTP specifically binds and activates the WAVE complex which also facilitates Arp2/3-mediated F-actin nucleation. Both Rac-GTP and CDC42-GTP also activate DIAP3, which is required for nucleation of unbranched actin filaments, and PAK-dependent LIM kinase, which stabilizes actin polymerization by preventing Cofilin from severing ADP-bound actin and promoting actin depolymerization. RhoA-GTP contributes to actin regulation by activating ROCK (Rho-associated coiled-coil-containing kinase), which inhibits myosin light chain phosphatase (MLCP) resulting in increased phosphorylation of myosin light chains (MLCs), which bind actin and stimulate contraction at the “tail” during migration (see [13] for review).
Of the 23 related mammalian Rho family GTPases, only a few of the more prototypic members (including Rac1, Rac2, Cdc42, RhoA, RhoH, and RhoG) have conclusively been shown to be important for the development and/or function of innate and acquired immune cells. For example, Rac1 and/or Rac2 deficiency results in defective IL-2 production, migration, and adhesion of mature T cells (see [13] for review); impaired dendrite formation and dendritic cell function [16]; and impaired adhesion, migration, phagocytosis, and superoxide production by neutrophils (see [17] for review). In addition, both Rac and Rho-deficiency result in defects in the development of hematopoietic cells, including impaired T and B cell development [18–22] and defective proliferation, migration, and adhesion of hematopoietic stem and progenitor cells (HSC/Ps) [23–25]. Altogether, these studies (and many other studies not mentioned here) indicate that Rho-family members differentially control cytoskeletal reorganization, and the processes controlled thereof, in both acquired and innate immune cells.
3. Rho family members control F-actin polymerization through WASP and WAVE complexes
Molecular ‘hints’ as to the molecular targets utilized by Rho-members to control the actin cytoskeleton were initially defined through genetic and biochemical studies of Rho-orthologs in Drosophilia and Caenorhabditis elegans, as well as through biochemical studies in mammalian cell lines. Two molecular adaptor complexes in particular, called WASP and WAVE, have emerged as focal points in the control of actin polymerization in hematopoietic cells (see [8,26,27] for review). Actin dynamics depend on the ability of monomeric actin (G-actin) to switch to its filamentous form (F-actin), a process which is dependent on the nucleation activity of the actin regulatory complex (Arp2/3), which initiates the formation of new actin filaments on the barbed end of preexisting filaments from a pool of ATP-actin monomers stored in association with Profilin (Fig. 1). This growth pushes the membrane forward, while capping proteins antagonize this process by binding the barbed ends, thus terminating elongation and promoting branching. As actin filaments age, the actin depolymerizing factor Cofilin binds to ADP-actin, resulting in the severing of actin filaments and dissociation of actin monomers, thus creating new barbed ends that serve as new sites for elongation.
The Arp2/3 complex is itself activated by “nucleation promoting factors (NPFs)”, which include the WASP complex [WASP or N-WASP, in association with WIP (WASP-interacting protein)] (Fig. 2A) and the WAVE complex (Fig. 2B), which consists of Sra1 (specifically Rac-associated protein 1), Hem [Hem-1 (also known as Nap1l (Nck-associated protein 1-like), or Hem-2 (also known as Nap1 (Nck-associated protein-1)], Abi [Abelson interactor 1 or 2 (Abi1 or Abi2)], WAVE (WAVE1, WAVE2, or WAVE3), and HSPC300 (hematopoietic stem/progenitor cell protein 300) [28]. The WASP complex is basally inactive due to auto-interaction of the VCA (Verprolin-homology, cofilin-homology, and acidic) region with the CRIB (CDC42/Rac-interactive binding) domain, which results in a “closed” autoinhibitory conformation (Fig. 2A, left). Following immuno-receptor activation, GTP-bound CDC42 preferentially binds the CRIB domain, which results in a “open” conformational change allowing the VCA region to bind G-actin and the Arp2/3 complex, resulting in F-actin polymerization [29– 31] (Figs. 1 and 2A, right). Similar to WASP, the WAVE complex also is intrinsically inactive (Fig. 2B) [32]. GTP-bound Rac preferentially binds (indirectly) the WAVE-homology domain (WHD) of WAVE, which also stimulates Arp2/3-induced F-actin polymerization by a less-defined mechanism. Stoichiometry studies indicate that activated Rac binds directly to Sra1 [33], which binds to Hem (Hem-1 or Hem-2) [28]. Hem interacts with Abi1/2, which binds HSPC300 and the WHD domain of WAVE (Fig. 2B). WAVE activation requires the simultaneous interaction of WAVE with Rac-GTP, lipid vesicles containing acidic phospholipids [including phosphatidylinositol (3,4,5) triphosphate(PIP3)], and phosphorylation of WAVE by kinases [34] including Abl [35,36], cell division protein kinase 5 (Cdk5) [37], mitogen-activated protein kinases (MAPKs) [38], and Casein kinase 2 [39]. WAVE activation may occur by relocalization of the WAVE complex from the cytosol to the plasma membrane where it interacts with IRSp53 (insulin receptor tyrosine kinase substrate p53), PIP3, and activated Rac [40]; or by release from an autoinhibitory conformation (more akin to WASP activation) regulated by an interaction between the VCA domain of WAVE and Sra1 [41] (Fig. 2B, left), which is relieved by interaction with activated Rac (Fig. 2B, right).
Fig. 2.
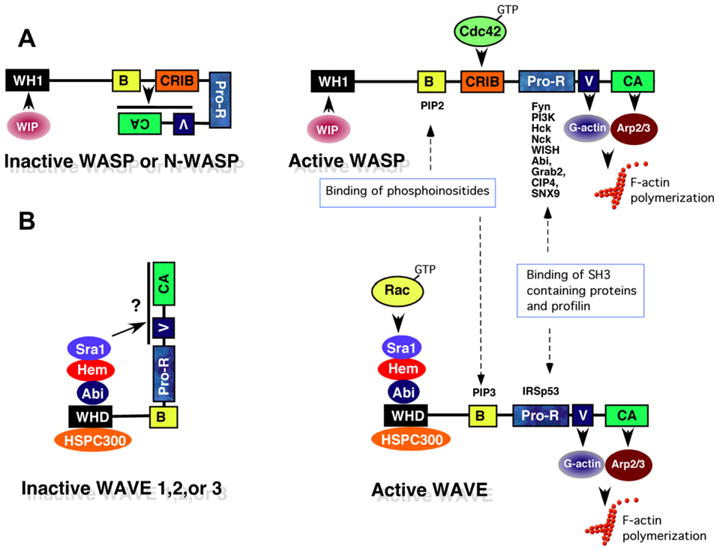
Activation of WASP and WAVE complexes by Rho-family GTPases. (A) Schematic diagram of the WASP complex. WASP [WASP or Neural WASP (N-WASP)] is basally inactive due to interaction of the basic region (B) and GTPase binding domains (CRIB) with the C-terminal verprolin homology/cofilin-homology/acidic region (VCA) domain, which prevents access to the Arp2/3 complex and G-actin. The interaction of Wip (WASP-interacting protein) with the WASP homology 1 domain (WH1) is believed to stabilize the inhibitory conformation. Following immunoreceptor activation, active Cdc42 (GTP-Cdc42) and PIP2 bind the CRIB and B domains respectively, thus releasing the VCA domain to bind G-actin and the Arp2/3 complex, which stimulates the branching of F-actin. In addition, the proline-rich region (Pro-R) of WASP acts as an adaptor protein by binding SH3-domain containing proteins (Fyn, PI3K, and Nck) which contribute to cellular activation, and Profilin which helps recruit actin monomers. (B) Schematic diagram of the WAVE complex. The WAVE complex is composed of pentameric subunits including Sra1, Hem (1 or 2), Abi (1 or 2), HSPC300 and WAVE (1, 2, or 3). Similar to WASP, the WAVE complex is also basally inactive, perhaps due to an interaction of the C-terminal VCA region with Sra1, which inhibits accessibility with G-actin and the Arp2/3 complex. Upon immunoreceptor stimulation, Rac-GTP binds to the WAVE complex via Sra1, which in the presence of PIP3 and IRSp53, may result in a structural change allowing the VCA region to bind G-actin and the Arp2/3 complex, resulting in F-actin polymerization. The binding of IRSp53 with the proline rich region of WAVE also enhances the ability to stimulate Arp2/3-driven actin polymerization, perhaps by recruiting WAVE to the plasma membrane [27,49].
The stability of the WAVE complex appears to be interdependent on the presence of individual WAVE protein components, since elimination of single subunits generally results in degradation of all WAVE protein components [10,42–45] and disruption of F-actin polymerization. Hence, the phenotypic changes in organisms following disruption of individual WAVE components are often attributed to loss of the entire WAVE complex (as discussed below). However, in some cases, disruption of individual WAVE complex proteins have directly been associated with impaired localization of actin-regulatory and/or signaling proteins (rather than degradation of the WAVE complex) [46,47].
The WAVE complex is highly conserved in many multicellular organisms including Arabidopsis thaliana, Dictyostelium discoideum, C. elegans, and Drosophila melanogaster, but appears to be absent in yeast. This suggests that the WAVE complex may have evolved to allow cells from multicellular organisms migrate, physically interact with each other or foreign agents (in the case of immune cells), and to undergo morphogenesis in response to environmental cues [48]. Whereas the importance of WASP in the immune system is well characterized (see [8,26] for review), the roles and importance of the ubiquitous WAVE complex in immune function are only now beginning to be unveiled (detailed below).
4. Hem-1 is a hematopoietic cell-specific component of the WAVE complex
Although the majority of WAVE complex proteins including Hem-2 are expressed in multiple tissues [49], Hem-1 is expressed almost exclusively in hematopoietic cells [10]. Indeed, Hromas et al. originally cloned Hem1 based on hybridization to transcripts from hematopoietic cell lines [50], whereas Hem2 was identified as a transcript enriched in the mouse brain [51].
Real-time PCR analyses of Hem family expression in multiple mouse tissues indicated that Hem1 is predominantly expressed in hematopoietic and urogenital tissues, whereas Hem2 is more widely expressed in brain, heart, kidney, muscle, and embryo, but is not expressed in hematopoietic cells [10]. A comparison of cDNA and protein sequences from multiple species suggests that Hem family members are evolutionarily conserved in Drosophila (KETTE) [52], C. elegans (GEX-3) [53], Dictyostelium (Nap1) [54], and Arabidopsis (Nap1) [55]. Although the protein structure of Hem-family members does not contain any known domains, there are multiple conserved hydrophobic regions and cysteine residues, consistent with protein-interaction domains and possible cAMP/cGMP phosphorylation sites [50]. However, the significance of Hem-1 phosphorylation is unknown.
Immunoprecipitation of Hem-1 protein from lysates derived from the human promyelocytic leukemia cell line human promyelocytic leukemia cells-60 (HL-60) using either internal or C-terminal anti-Hem1 antibodies reveals that Hem-1 associates with WAVE complex proteins including WAVE2, Abi1, and CYFIP1/2 [56]. In addition, both the internal and C-terminal anti-Hem1 antibodies immunoprecipitated other polarity proteins including myosin light chain phosphatase, two different Rho-specific GTPase activating proteins (GAPs) (Rho-GAP4 and Myosin IXB), and Vps34 [a class III phosphatidylinositol-3-kinase (PI3K)], each of which have been implicated in excluding myosin from the leading edge of migrating cells. These results suggest that Hem-1 may also associate with myosin regulatory complexes (in addition to the WAVE actin-regulatory complex), and may have additional functions in inhibiting myosin-based contraction at the leading edge of migrating cells [43].
Several lines of evidence suggest that dysregulation of Hem-1 could be involved in disease processes in humans. The human Hem1 gene (also known as Nckap1l) is located on chromosome 12q13.1, a region of occasional chromosome translocations in hematopoietic cell cancers and a rare folic acid fragile site, Fra 12a [50]. High Hem1 expression is associated with a poor prognosis in human chronic lymphocytic leukemias (CLL), and downregulation of Hem1 in patient CLL cells resulted in a significant increase in fludarabine-mediated killing [57].
5. Hem family members regulate actin polymerization, morphogenesis, and immunity
Initial clues as to the biological functions of Hem family members were revealed from genetic and biochemical studies of Hem-orthologs, which suggested general roles in cytoskeletal organization, cell migration, and cell shape, which are important for proper morphogenesis during development [51–54] (see Table 1). For example, hypomorphic mutations in the Drosophila Kette gene result in axonal defects which “phenocopy” mutations in Drosophila CDC42 (Dcdc42) and Drosophila Rac1 (Drac1), both of which have been implicated in regulating cytoskeletal organization and axonal growth [52]. Loss of GEX-3 in C. elegans results in failed morphogenesis due to impaired cell migration [53]. Disruption of the Hem-ortholog NapA in Dictyostelium results in diminished actin polymerization and small pseudopod formation, due to loss of SCAR (a WAVE ortholog) function [54].
Table 1.
Hem family orthologues, expression patterns, and null allele phenotypes.
| Species | Hem family (% homology relative to hHem1)b | Expressiona | Associated proteins | Hem null allele | References |
|---|---|---|---|---|---|
| Drosophila melanogaster | Kette (47%) | Embryonic tissue, head | Sra-1, Scar, Abi, HSPC300 | Impaired actin cytoskeleton, defective axon projections | [44,51,52] |
| Caenorhabditis elegans | GEX-3 (36%) | Embryo, L1/L2 larva stages | GEX1, GEX-2, Abi-1 | Impaired morphogenesis and cell migration | [53] |
| Dictyostelium discoideum | Nap1 (24%) | Ubiquitous | PIR121, Scar, Abl-interactor homolog, Brick1 | Impaired actin polymerization, small pseudopods, decreased adhesion and multicellular development | [54] |
| Arabidopsis thaliana | Nap1 (22%) | Flower, root, seed, silique | Klunker, Scar2, BRK1 | Disrupted F-actin polymerization and trichome morphology | [55] |
| Mammals |
Hem1 [NCKAP1L (NAP1-like)] |
Hematopoietic cells | Abi1/2, PIR121, HSPC300, WAVE1/2, Myosin IXB, Vps34, RhoGAP4, catalytic subunit of myosin light chain phosphatase | Impaired actin polymerization, phagocytosis, migration in neutrophils; impaired T cell development, activation, adhesion; impaired B cell development; regenerative anemia; increased HSC/Ps in peripheral blood | [10,43] |
| Homo sapiens | − hHem1 (100%) | ||||
| Mus musculus | − mHem1 (94%) | ||||
|
Hem2 [NCKAP1(NAP1)] |
Ubiquitous | Abi1/2, PIR121, HSPC300, WAVE1/2 | Mid-gestation embryonic lethal; failed neural tube closure, impaired neuronal differentiation | [59,60] | |
| Homo sapiens | − hHem2 (58%) | ||||
| Mus musculus | − mHem2 (58%) |
Gene expression is based on NCBI UniGene website and the listed references.
% homology based on amino acids.
In mammals, RNA interference (RNAi)-mediated knockdown of Nckap1 (Hem2) in a melanoma cell line revealed that NAP1 is essential for Rac-dependent formation of foot-like structures called lamellipodia in response to growth factor stimulation [58]. Gene-targeted disruption of Nckap1 in mice results in early-mid embryonic lethality (embryonic day 11.5) due to severe neurolation defects, failure of neural tube closure, and impaired neuronal differentiation [59,60]. To examine the role for Hem-1 in hematopoietic cells, Weiner et al. utilized RNAi technology to “knockdown” Hem-1 protein in HL-60 cells, which resulted in dramatic impairment of chemoattractant-induced actin polymerization, polarity, and chemotaxis [43]. In addition, they found that Hem-1 protein normally polarizes towards the leading edge of activated cells, and that Hem1 knockdown prevented the exclusion of activated myosin from the leading edge. Interestingly, Rac1 activation and phosphatidylinositol-(3,4,5)-tris-phosphate production were weakened following knockdown of Hem1, indicating that Hem-1 may modulate a Rac/F-actin-mediated feedback circuit that organizes the leading edge. In a subsequent study, Weiner et al. utilized total internal fluorescence (TIRF) microscopy to visualize the localization of Hem-1 conjugated to yellow-fluorescent protein (Hem-1-YFP) in the HL-60 cells. They elegantly showed that Hem-1 initially accumulates on the membrane as small foci, which then burst into outwardly propagating waves concentrated near the leading edge [56]. Furthermore, they showed that the morphology and speed of the leading edge advance correlates with the most peripheral Hem-1 waves. These results collectively suggest that Hem-1 may stimulate waves of actin nucleation at the leading edge of cells, which may drive cell polarization, morphogenesis, and migration during chemotaxis.
6. Disruption of Hem-1 in mice results in multiple defects in hematopoietic cell development and function
The in vivo functions of Hem-1 in higher organisms were described in studies by Park et al., whereby they utilized an N-ethyl-N-nitrosourea (ENU) chemical mutagenesis strategy in mice [61] to generate a novel mouse strain containing a nonsense mutation in the Hem1 gene (Nckap1l), which results in the absence of Hem-1 protein [10] (see also [62]). Mice lacking Hem-1 (Nckaplm1Iri/Nckaplm1Iri or Hem1−/−) are viable and fertile (although female mice exhibit reduced fertility). Relative to normal wildtype (WT) littermate control mice, Hem1−/− mice exhibit significantly altered representations of circulating immune cells in peripheral blood, including moderate leukocytosis with marked neutrophilia and lymphopenia. Hem1−/− mice also present with a myriad of gross lesions including amyloid accumulations and focal mineralization along liver lobe margins, membrano-proliferative glomerulopathy, and occasional diffuse inflammatory involvement of the liver, epididymis, mesentery, pleura, and heart. Hem1−/− spleens typically are enlarged and filled with proliferating myeloid and erythroid progenitors indicative of extramedullary hematopoiesis. While the exact cause of splenomegaly is unknown, bone marrow (BM) from Hem1−/− mice contains significantly reduced CFU-GM (colony forming units-granulocyte and monocyte), and CFU-GEMM (CFU-granulocyte/erythroid/macrophage/monocyte) relative to WT BM, whereas there is a dramatic increase in CFU-GM, CFU-GEMM, and BFU-E (burst forming units-erythroid) in spleen and peripheral blood from Hem1−/− mice relative to WT mice. This correlates with a significant increase in Lin−Sca1+cKit− hematopoietic stem/progenitor (HSC/Ps) cells in the spleens and peripheral blood of Hem1−/− mice relative to WT mice. These results suggest that the extramedullary hematopoiesis observed in Hem1−/− mice may be due to increased mobilization of Hem1−/− HSC/Ps from the BM to the spleen and blood. Consistent with this notion, mice deficient in Rac1 and Rac2 also exhibit massive mobilization of HSC/Ps from the BM to peripheral blood due to decreased adhesion of HSC/Ps to the BM microenvironment, resulting in their release into circulation [23].
Mice deficient in Hem-1 also exhibit altered red blood cell (RBC or erythrocyte) indices including regenerative anemia with reticulocytosis (increased RBC progenitors) characterized by abnormal erythrocyte morphology. Hem1−/− RBCs also exhibit increased fragility compared to wildtype RBCs, as measured by increased percent lysis in serial hypotonic NaCl solutions relative to wildtype RBCs [10]. Collectively these results suggest that Hem1−/− RBCs are more susceptible to damage from various stressors (oxidation, shear stress, and inflammation), and are less deformable than wild-type erythrocytes. Interestingly, mice deficient in Rac1 and Rac2 also present with regenerative anemia due to decreased F-actin capping and altered erythrocyte cytoskeletal reorganization [63], which is consistent with Hem-1 acting downstream of Rac to regulate formation of the actin cytoskeleton in red blood cells.
7. Hem-1 is essential for neutrophil and macrophage migration and phagocytosis
When compared to wildtype littermate mice, Hem1−/− mice exhibited a ∼25-fold increase in the numbers of circulating neutrophils, which correlated with severe defects in the in vitro migration of Hem1−/− neutrophils in response to the tri-peptide chemoattractant fMLP (formyl-methionyl-leucyl-phenylalanine) [10]. Hem1−/− neutrophils also showed significantly reduced and disorganized F-actin polymerization, which clearly polarized toward the leading edge in wildtype neutrophils. These findings in primary neutrophils are highly consistent with results from Weiner et al. in HL-60 cells, which collectively suggest that Hem-1 is required for F-actin polymerization, polarization, and migration in mature neutrophils [43].
Hem1−/− neutrophils and macrophages also exhibited severe defects in the phagocytosis of fluorescently-labeled E. coli, consistent with an essential role for F-actin polymerization in the formation of phagosome structures [10]. While in vivo host-defense studies were not performed in Hem1 null mice, the mice were noted to exhibit increased susceptibility to natural infection by opportunistic bacteria such as Pasteurella pneumotropica, which is present in most Specific Pathogen Free (SPF) animal facilities. The overall phenotype of Hem1 null neutrophils is similar to neutrophils from mice deficient in the Rac-GEF Vav, which exhibit defective adhesion, migration, and phagocytosis [64,65], and neutrophils from Rac2-deficient mice [66,67], which also exhibit impaired neutrophil migration and phagocytosis. Surprisingly, Hem-1 null macrophages produced normal or increased levels of proinflammatory cytokines and chemokines such as IL-1β, TNF-α, MIP-2, KC, and IL-23 in response to Toll-like receptor stimulation, suggesting that nuclear factor-kappaB (NF-κB)-dependent production of cytokines proceeds independently of Hem-1 and F-actin polymerization [10].
8. Hem-1 regulates lymphocyte development, adhesion, and migration
Since Hem-1 null mice are also lymphopenic, Park et al. examined the roles of Hem-1 in the development of B and T cells in the bone marrow and thymus. T cell development was significantly impaired in Hem1−/− mice at the CD4−CD8−CD25+CD44− double-negative 3 (DN3) to CD4+CD8+CD25−CD44− double-negative 4 (DN4) cell transition, mediated by successful in-frame TCRβ chain rearrangement and formation of the pre-T cell receptor complex (pre-TCR) [10] (see Fig. 3A). This phenotype suggests a role for Hem-1 and cytoskeletal reorganization during T cell development, and parallels the phenotype seen in mice deficient in other actin regulators including Vav[68], Rac [20], and Rho [19,69,70]. Likewise, B cell development was impaired at the pro-B cell and T0/T1 stages in Hem-1 null mice relative to wildtype mice (Fig. 3B and unpublished), which is similar to mice deficient in RacGAP [71], Vav [72] and Rac1/2 [18]. Transplant of Hem1−/− HSC/Ps into lethally irradiated Rag2−/−γc−/− mice does not rescue the development of Hem1−/− B cells or T cells, indicating that the defects in lymphocyte development are autonomous to bone marrow derived cells [10]. These results suggest essential roles for Hem-1 in T and B lymphocyte development, and are highly consistent with Hem-1 controlling cytoskeletal organization during lymphocyte development downstream of Vav and Rac.
Fig. 3.
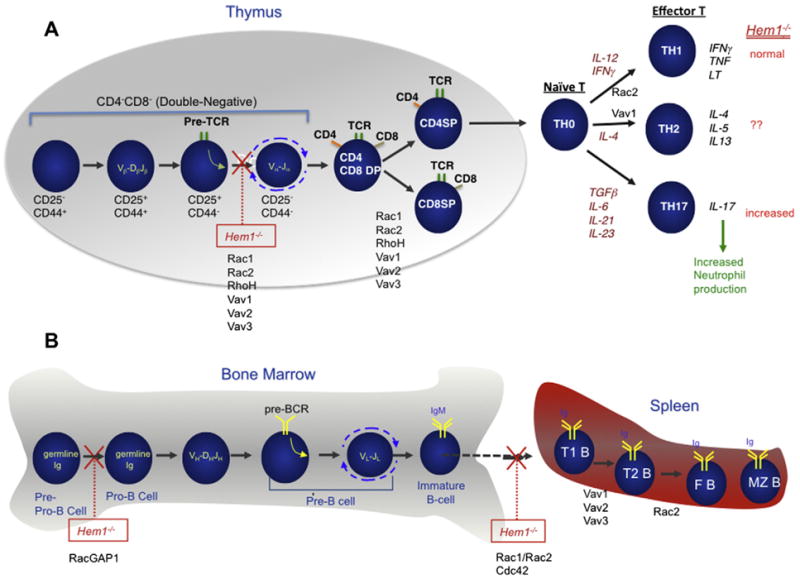
Roles of Hem-1 in lymphocyte development and function. (A) Schematic diagram of T cell development in the thymus and periphery. Successful in-frame rearrangement of T cell receptor β chain genes [V(variable)β-D(diversity)βJ(joining)β] in CD4−CD8− [double-negative (DN)] cells results in formation of the pre-TCR complex, which signals expansion, rearrangement of TCRα chain genes (Vα–Jα), and differentiation to the CD4+CD8+ double-positive (DP) cell state. Following in-frame TCRα chain rearrangement, TCRαβ expressing DP thymocytes undergo “positive selection” based on MHC class I- or against MHC class II-restriction, which drives selection of mature CD8+CD4− single-positive (SP) and CD4+CD8− SP cells respectively. SP cells then migrate into secondary lymphoid tissues, in preparation for encountering foreign antigens. DN cells are further subdivided into different stages of development based on expression of CD25 and CD44. Upon T cell activation, naïve CD4 T cells differentiate into distinct CD4 T cell subsets based on the local cytokine milieu. CD4 effector T cells (TH1, TH2, and TH17) produce their unique set of signature cytokines. Gene-targeted mutations of Hem-1, Rac (1 and/or 2), RhoH, or Vav (1,2, and/or 3) in mice result in arrest or impaired differentiation at different stages of T cell development in the thymus and periphery [10,13,74]. (B) Schematic diagram of B cell development in the bone marrow (and fetal liver). The earliest committed B cell progenitor (called a pre-pro-B cell) has its immunoglobulin (Ig) heavy and light chain genes in germline (unrearranged) configuration. The Ig heavy chain genes rearrange first (VH-DHJH) in pro-B cells. Following successful in-frame VH-DHJH rearrangement, the pre-B cell receptor (pre-BCR) is formed on the surface of pre-B cells, resulting in proliferation, differentiation and immunoglobulin light (IgL) chain gene rearrangement (VL-JL). Following successful VL-JL chain gene rearrangement, immature B cells leave the bone marrow for the spleen and lymph nodes where cells further develop into transitional B cells (T1 and T2) and mature B cells (follicular and marginal zone). Inhibition of Hem-1, Rac (1 and 2), RacGAP1, or Vav (1, 2, and 3) function results in arrest or impaired differentiation at different stages of B cell development [10,13].
Analysis of peripheral T cells from Hem-1 null mice revealed that T cell activation, proliferation, survival, adhesion, and F-actin capping were also impaired following loss of Hem-1 [10]. However, production of interleukin-2 (IL-2) and interferon γ appeared normal in Hem-1 null mice relative to wildtype mice, whereas interleukin-17 production was significantly increased due to increased numbers of TH17 cells (Fig. 3A). Whereas the exact cause of the shift towards increased TH17 cells is unknown, other studies have shown that impaired migration of neutrophils from the circulation into peripheral tissues results in decreased phagocytosis of neutrophils by tissue macrophages and increased IL-23 production [73], which stimulates increased generation of TH17 cells. These results collectively suggest that Hem-1 is required for F-actin polymerization, adhesion, and perhaps immune synapse formation in peripheral T cells. Other studies have also shown that disruption of cytoskeleton regulating proteins such as Vav [74,75], Rho [21], Rac [76], HS1 [77], Dynamin [78], Abi1/2 [46], WASP [79] and Wave2 [47] also result in impaired actin polymerization and immune synapse formation in T cells or T cell lines. However, whereas loss of Vav, WASP, and Rac2 impairs Ca2+ signaling and IL-2 production, Park et al. found that cytokine production proceeds normally in the absence of Hem-1 (as they found in innate immune cells), which may suggest a bifurcation of signals from Vav and Rac upstream of Hem-1.
To examine how loss of Hem-1 impinges on the signaling pathways that control F-actin polymerization, Park et al. examined the activation of selected signaling pathways in T cells and neutrophils from Hem1 null and wildtype mice. Src family protein tyrosine kinases (Ptks), Syk family Ptk's, Erk, and Rac1 were represented and activated normally in thymocytes and T cells from Hem-1 null mice relative to wildtype mice following TCR stimulation, suggesting that membrane-proximal signaling was not affected by loss of Hem-1. In contrast, protein components of the WAVE complex including Wave2, Abi1, Abi2, and specifically Rac associated 1 (Sra-1) were largely degraded in Hem-1 null T cells and thymocytes relative to wildtype cells, both before and after TCR stimulation. Similarly, activation of neutrophils from Hem1 null mice with fMLP resulted in equivalent total tyrosine phosphorylation, Erk phosphorylation, and Rac1 activation, while levels of Wave2, Sra1, Abi2, and Abi1 proteins were significantly reduced relative to wildtype neutrophils [10]. These results collectively suggested that Hem-1 is required for the stabilization of the WAVE complex proteins in primary neutrophils and T cells, and are consistent with many other studies in HL-60 cells [43], Jurkat T cells [46,47] and lower organisms which indicate that loss of one WAVE complex member results in the degradation of the other members (see [49] for review).
9. Model of Hem-1 functions in the innate and acquired immune system
Studies derived from Hem-1 orthologues in lower organisms, and from Hem-1 studies in HL-60 cells, Jurkat T cells, and Hem-1 null mice, suggest a general model for the functions of Hem-1 in F-actin polymerization in immune cells (see Fig. 4). Hem-1 is a cytosolic adaptor or “scaffold” protein, which interacts with Sra1 and Abi (1 or 2) as part of the WAVE complex (which also includes WAVE and HSPC300). Upon activation of immune cells by growth factors, cytokines, chemokines, Toll-like receptors, and antigen receptors (Fig. 4), the intrinsically-inactive WAVE complex interacts with active GTP-bound Rac, which allows a conformation change to occur in the WAVE protein (although this is still controversial) thus providing a binding site for the Arp2/3 actin nucleating proteins and G-actin. Recruitment of Arp2/3 allows the initiation of actin filament growth and branching from G-actin monomers. “Waves” of Hem-1 and WAVE complex-induced activation of Arp2/3 provide a “moving front” of F-actin polymerization, which results in the protrusion and lamellipodia that form at the leading edge of motile cells. In contrast, the contraction of the “tail” of moving cells may occur independently of Hem-1 by a Rho-dependent mechanism (see Fig. 1). The Hem-1-dependent actin cytoskeleton also likely interacts with the cytoplasmic portion of integrins thus forming focal adhesions that result in integrin activation and cell-adhesion (see [80] for review), and contributes to immune synapse formation in T cells. WAVE2 has also recently been shown to activate integrins by recruiting and activating Abl kinase and the CrkL-C3G complex, which activates the integrin regulatory guanosine triphosphate Rap1. These results suggest that Hem-1 might also activate integrins via Rap1 [47]. Hem-1-dependent actin polymerization may also be required for the protrusion of the cytoplasmic membrane that surrounds foreign organisms during phagocytosis (see [5] for review). A potential role for Hem-1 (and the actin cytoskeleton) in immune cell survival and differentiation is also inferred from studies in Hem-1 deficient mice and of orthologues in lower organisms, although the mechanism has not been defined.
Fig. 4.
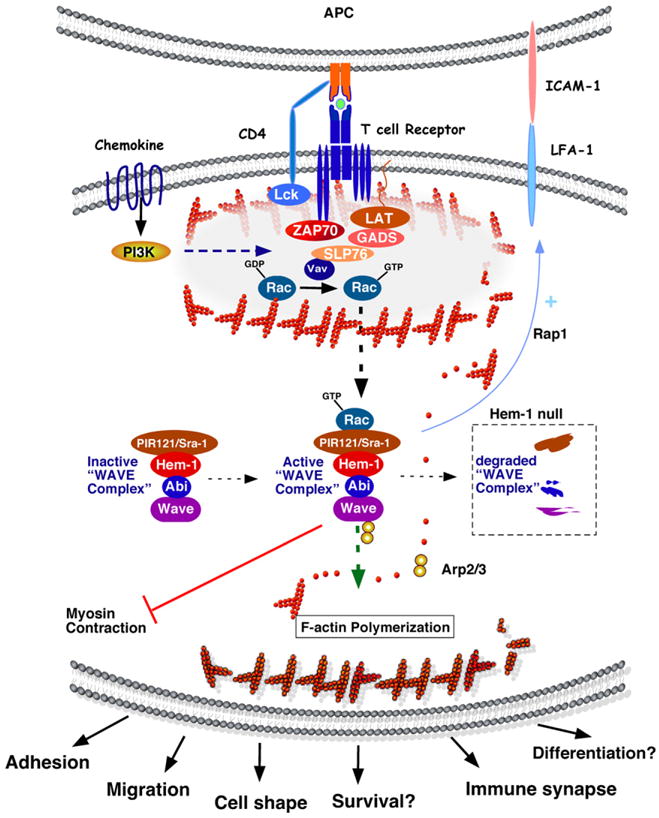
Model of Hem-1 functions in F-actin polymerization during T cell activation. T cell activation is induced by engagement of T cell receptor (TCR) complex on T cells with antigen peptide presented by major histocompatibility complexes (MHCs) on the surface of antigen presenting cells (APCs). Interaction of co-stimulatory molecules (CD28 and ICOS) on T cells with B7 family members on APCs (not shown) prevents anergy, thereby ensuring optimal activation. TCR-mediated signaling cascades are initiated by activation of the Src-family protein tyrosine kinase Lck which phosphorylates tyrosine residues in both CD3 complex and ZAP70. This results in recruitment of the adaptor proteins LAT, GADS, and SLP76, which are phosphorylated by ZAP70. LAT and SLP76 serve as scaffolds for adaptor molecules to regulate the F-actin polymerization via the Vav GEF, which activates Rac molecules. PI3K-mediated signaling facilitates cell survival, proliferation, and cytokine production in cooperation with TCR-mediated signaling. Interaction between LFA-1 and ICAM-1 is crucial for immune synapse formation. In immune cells, the WAVE complex [which consists of WAVE (1 or 2), Abi (1 or 2), Hem-1, Sra1, and HSPC300] is basally inactive. Upon T cell activation, activated Rac binds and activates the WAVE complex, thus promoting Arp2/3-mediated F-actin polymerization from monomeric G-actin. Hem-1 [also known as Nck-associated protein-1-like (NAP1L)] is a hematopoietic cell specific adaptor molecule which helps recruit and maintain a stable WAVE complex. Loss of Hem-1 (in Hem-1 null mice) destabilizes the interactions of WAVE complex subunits, resulting in WAVE protein degradation and impaired F-actin polymerization, which results in deficient adhesion (LFA-activation), immune synapse formation, migration, differentiation, and survival [10,83]. The WAVE complex (including Hem-1) may also directly control cell adhesion in a Rap1-dependent manner [47]. Hem-1 has also been implicated in inhibiting myosin contraction at the leading edge of migrating cells, which may contribute to membrane protrusion [43].
Remarkably, the production of NF-κB-dependent pro-inflammatory cytokines proceeds normally in the absence of Hem-1, which is in contrast to knockouts of WASP, Rac, and Vav, which generally result in inhibition of IL-2 production. These results collectively suggest that Hem-1 functions to selectively control processes dependent on F-actin polymerization (such as adhesion, migration, and phagocytosis), whereas NF-κB-dependent production of cytokines occurs independent of Hem-1. The imbalance between a functional pro-inflammatory (cytokine) arm and a dysfunctional effector arm, in both lymphocytes and phagocytes from Hem1 deficient mice, results in a hyper-stimulated yet largely ineffective immune response.
10. Concluding remarks
Despite recent progress in our understanding of WAVE complex proteins and Hem-family members in lower organisms and vertebrates, there are still many questions regarding the roles of Hem-1 in hematopoietic cell development and function. These questions include the following:
Does Hem-1 have WAVE-independent functions? Although the functions of Hem-1 appear to be closely associated with the WAVE complex, some experimental evidence suggests that Hem-1 may also function independently of WAVE. For example, in HL-60 cells, Hem-1 exists in much greater abundance than WAVE2, and is also found in high-molecular weight pools that exclude the WAVE complex. In addition, Hem-1 associates with proteins that regulate myosin activity, including myosin light chain phosphatase, the RhoGAPs RhoGA4 and Myosin IXB, and PI3 kinase Vps34 (among other proteins). These results suggest that Hem-1 may have both WAVE-dependent and -independent functions, and that the complicated phenotypes of Hem-1 deficient mice may reflect disruption of both functions.
Could Hem-1 have broader effects on transcription and/or other nuclear functions? Recent studies suggest that nuclear actin may control the transcription of certain target genes regulated by myocardin-related transcription factors (MRTFs) and serum response factors (SRFs) (see [81] for review). In addition, nuclear actin is involved in nucleocytoplasmic shuttling (see [82] for review). Could Hem-1 and WAVE modulate nuclear signaling and transcription in immune cells?
What transcription factors and DNA elements control the expression of Hem1? Hem1 is expressed almost exclusively in hematopoietic cells, yet the regulatory elements controlling Hem1 expression are not defined. University of California Santa Cruz (UCSC) Genome browser predicts binding sites for a number of transcription factors in the Hem1 (Nckap1l) promoter including Evi-1, Foxc1, Rfx1, Max, Mif-1, Foxo3b, Mef2a, LHX3b, and Pou6F1. The significance of these sites or other transcriptional regulatory elements is unclear.
Does Hem-1 modulate the biology of hematopoietic stem cells? Rac1 and Rac2 control many aspects of HSC function including proliferation, survival, and adhesion of HSCs within the bone marrow niche. Hem1−/− HSCs appear to be released from the bone marrow environment into circulation, which implies that the adhesion of Hem1−/− HSCs to the BM niche is impaired. However, the exact roles of Hem-1 in HSC function have not been addressed.
Is Hem-1 important for the invasion and metastasis of hematopoietic cell cancers? Actin polymerization is important for the adhesion, migration, and invasion of tumor cells, as well as normal immune cells (see [11] for review). An interesting question is whether inhibition of Hem-1 might provide a mechanism to inhibit the invasion and metastasis of hematopoietic cell cancers.
The hematopoietic cell specific expression of Hem1, and the relatively low toxicity of complete Hem1 disruption in mice (i.e. viable and fertile), suggests that acute inhibition of Hem-1 function may provide a mechanism of acutely disrupting the “WAVES” of actin cytoskeletal reorganization, which are essential for adhesion, migration, survival, and the endocytic/phagocytic properties of most immune cells. Ultimately, the generation of an inducible tissue-specific Hem1 targeted-deletion model in mice will be required to test the acute consequences/benefits of inhibiting Hem-1 function in autoimmunity and cancer models.
Abbreviations
- Cdc42
cell division control protein 42
- Rho
Ras homolog gene family member
- Rac
Ras-related C3 botulinum toxin substrate
- Tiam1
T-cell lymphoma invasion and metastasis 1
- Bcr
breakpoint cluster region
- IL
Interleukin
- Sra-1
specifically Rac associated 1
- HSPC300
hematopoietic stem/progenitor cell protein 300
- Cdk5
cell division protein kinase 5
- MAPK
mitogen-activated protein kinase
- IRSp53
insulin receptor tyrosine kinase substrate p53
- Nap1
Nck-associated protein 1
- HL-60
human promyelocytic leukemia cells-60
- Lin
lineage
- TNF
tumor necrosis factor
- MIP
macrophage inflammatory proteins
- NF-κB
nuclear factor-kappaB
- Rag
recombinase activating gene
- Syk
spleen tyrosine kinase
- cAMP/cGMP
cyclic adenosine monophosphate/cyclic guanosine monophosphate
Contributor Information
Heon Park, Email: heonp@uw.edu.
Maia M. Chan, Email: maiac@uw.edu.
Brian M. Iritani, Email: biritani @uw.edu.
References
- 1.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 2.Fooksman DR, et al. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo A, Frenette PS. Leukocyte podosomes sense their way through the endothelium. Immunity. 2007;26:753–755. doi: 10.1016/j.immuni.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Mulloy JC, Cancelas JA, Filippi MD, Kalfa TA, Guo F, Zheng Y. Rho GTPases in hematopoiesis and hemopathies. Blood. 2010;115:936–947. doi: 10.1182/blood-2009-09-198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notarangelo LD, Miao CH, Ochs HD. Wiskott–Aldrich syndrome. Curr Opin Hematol. 2008;15:30–36. doi: 10.1097/MOH.0b013e3282f30448. [DOI] [PubMed] [Google Scholar]
- 8.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 9.Williams DA, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- 10.Park H, et al. A point mutation in the murine Hem1 gene reveals an essential role for Hematopoietic protein 1 in lymphopoiesis and innate immunity. J Exp Med. 2008;205:2899–2913. doi: 10.1084/jem.20080340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–2111. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 15.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 16.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 17.Weston VJ, Stankovic T. Rac1 and Rac2 GTPases in haematopoiesis. Bioessays. 2004;26:221–224. doi: 10.1002/bies.20017. [DOI] [PubMed] [Google Scholar]
- 18.Walmsley MJ, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 19.Galandrini R, Henning SW, Cantrell DA. Different functions of the GTPase Rho in prothymocytes and late pre-T cells. Immunity. 1997;7:163–174. doi: 10.1016/s1074-7613(00)80519-1. [DOI] [PubMed] [Google Scholar]
- 20.Gomez M, Tybulewicz V, Cantrell DA. Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat Immunol. 2000;1:348–352. doi: 10.1038/79808. [DOI] [PubMed] [Google Scholar]
- 21.Corre I, Gomez M, Vielkind S, Cantrell DA. Analysis of thymocyte development reveals that the GTPase RhoA is a positive regulator of T cell receptor responses in vivo. J Exp Med. 2001;194:903–914. doi: 10.1084/jem.194.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo F, Cancelas JA, Hildeman D, Williams DA, Zheng Y. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood. 2008;112:1767–1775. doi: 10.1182/blood-2008-01-132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Y, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 24.Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, Pennington J, Williams DA. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Dong B, Siminovitch KA. Contributions of Wiskott– Aldrich syndrome family cytoskeletal regulatory adapters to immune regulation. Immunol Rev. 2009;232:175–194. doi: 10.1111/j.1600-065X.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurisu S, Takenawa T. The WASP and WAVE family proteins. Genome Biol. 2009;10:226. doi: 10.1186/gb-2009-10-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautreau A, Ho HY, Li J, Steen H, Gygi SP, Kirschner MW. Purification and architecture of the ubiquitous Wave complex. Proc Natl Acad Sci USA. 2004;101:4379–4383. doi: 10.1073/pnas.0400628101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgs HN, Pollard TD. Activation by Cdc42 and PIP(2) of Wiskott–Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J Cell Biol. 2000;150:1311–1320. doi: 10.1083/jcb.150.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 31.Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150:1299–1310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derivery E, Lombard B, Loew D, Gautreau A. The Wave complex is intrinsically inactive. Cell Motil Cytoskeleton. 2009;66:777–790. doi: 10.1002/cm.20342. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, et al. P140Sra-1 (specifically Rac1-associated protein) is a novel specific target for Rac1 small GTPase. J Biol Chem. 1998;273:291–295. doi: 10.1074/jbc.273.1.291. [DOI] [PubMed] [Google Scholar]
- 34.Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–524. doi: 10.1016/j.molcel.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leng Y, Zhang J, Badour K, Arpaia E, Freeman S, Cheung P, Siu M, Siminovitch K. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc Natl Acad Sci USA. 2005;102:1098–1103. doi: 10.1073/pnas.0409120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuart JR, Gonzalez FH, Kawai H, Yuan ZM. C-Abl interacts with the WAVE2 signaling complex to induce membrane ruffling and cell spreading. J Biol Chem. 2006;281:31290–31297. doi: 10.1074/jbc.M602389200. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 38.Danson CM, Pocha SM, Bloomberg GB, Cory GO. Phosphorylation of WAVE2 by MAP kinases regulates persistent cell migration and polarity. J Cell Sci. 2007;120:4144–4154. doi: 10.1242/jcs.013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pocha SM, Cory GO. WAVE2 is regulated by multiple phosphorylation events within its VCA domain. Cell Motil Cytoskeleton. 2009;66:36–47. doi: 10.1002/cm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suetsugu S, Kurisu S, Oikawa T, Yamazaki D, Oda A, Takenawa T. Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP(3), and Rac. J Cell Biol. 2006;173:571–585. doi: 10.1083/jcb.200509067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ismail AM, Padrick SB, Chen B, Umetani J, Rosen MK. The WAVE regulatory complex is inhibited. Nat Struct Mol Biol. 2009;16:561–563. doi: 10.1038/nsmb.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolz JC, et al. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiner OD, et al. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunda P, Craig G, Dominguez V, Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Blagg SL, Stewart M, Sambles C, Insall RH. PIR121 regulates pseudopod dynamics and SCAR activity in Dictyostelium. Curr Biol. 2003;13:1480–1487. doi: 10.1016/s0960-9822(03)00580-3. [DOI] [PubMed] [Google Scholar]
- 46.Zipfel PA, Bunnell SC, Witherow DS, Gu JJ, Chislock EM, Ring C, Pendergast AM. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, Billadeau DD. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 49.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 50.Hromas R, Collins S, Raskind W, Deaven L, Kaushansky K. Hem-1, a potential membrane protein, with expression restricted to blood cells. Biochim Biophys Acta. 1991;1090:241–244. doi: 10.1016/0167-4781(91)90109-y. [DOI] [PubMed] [Google Scholar]
- 51.Baumgartner S, Martin D, Chiquet-Ehrismann R, Sutton J, Desai A, Huang I, Kato K, Hromas R. The HEM proteins: a novel family of tissue-specific transmembrane proteins expressed from invertebrates through mammals with an essential function in oogenesis. J Mol Biol. 1995;251:41–49. doi: 10.1006/jmbi.1995.0414. [DOI] [PubMed] [Google Scholar]
- 52.Hummel T, Leifker K, Klambt C. The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev. 2000;14:863–873. [PMC free article] [PubMed] [Google Scholar]
- 53.Soto MC, Qadota H, Kasuya K, Inoue M, Tsuboi D, Mello CC, Kaibuchi K. The GEX-2 and GEX-3 proteins are required for tissue morphogenesis and cell migrations in C. elegans. Genes Dev. 2002;16:620–632. doi: 10.1101/gad.955702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibarra N, Blagg SL, Vazquez F, Insall RH. Nap1 regulates Dictyostelium cell motility and adhesion through SCAR-dependent and - independent pathways. Curr Biol. 2006;16:717–722. doi: 10.1016/j.cub.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 55.Deeks MJ, Kaloriti D, Davies B, Malho R, Hussey PJ. Arabidopsis NAP1 is essential for Arp2/3-dependent trichome morphogenesis. Curr Biol. 2004;14:1410–1414. doi: 10.1016/j.cub.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 56.Weiner OD, Marganski WA, Wu LF, Altschuler SJ, Kirschner MW. An actin-based wave generator organizes cell motility. PLoS Biol. 2007;5:e221. doi: 10.1371/journal.pbio.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joshi AD, et al. ATM, CTLA4, MNDA, and HEM1 in high versus low CD38 expressing B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2007;13:5295–5304. doi: 10.1158/1078-0432.CCR-07-0283. [DOI] [PubMed] [Google Scholar]
- 58.Steffen A, Rottner K, Ehinger J, Innocenti M, Scita G, Wehland J, Stradal TE. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 2004;23:749–759. doi: 10.1038/sj.emboj.7600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokota Y, Ring C, Cheung R, Pevny L, Anton ES. Nap1-regulated neuronal cytoskeletal dynamics is essential for the final differentiation of neurons in cerebral cortex. Neuron. 2007;54:429–445. doi: 10.1016/j.neuron.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rakeman AS, Anderson KV. Axis specification and morphogenesis in the mouse embryo require Nap1, a regulator of WAVE-mediated actin branching. Development. 2006;133:3075–3083. doi: 10.1242/dev.02473. [DOI] [PubMed] [Google Scholar]
- 61.Appleby MW, Ramsdell F. A forward-genetic approach for analysis of the immune system. Nat Rev Immunol. 2003;3:463–471. doi: 10.1038/nri1109. [DOI] [PubMed] [Google Scholar]
- 62.David R. Rearranging the cytoskeleton. Nat Rev Immunol. 2009;9:8–8. [Google Scholar]
- 63.Kalfa TA, et al. Rac GTPases regulate the morphology and deformability of the erythrocyte cytoskeleton. Blood. 2006;108:3637–3645. doi: 10.1182/blood-2006-03-005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall AB, Gakidis MA, Glogauer M, Wilsbacher JL, Gao S, Swat W, Brugge JS. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Gakidis MA, et al. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts AW, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 67.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 68.Fujikawa K, et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costello PS, Cleverley SC, Galandrini R, Henning SW, Cantrell DA. The GTPase rho controls a p53-dependent survival checkpoint during thymopoiesis. J Exp Med. 2000;192:77–85. doi: 10.1084/jem.192.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henning SW, Galandrini R, Hall A, Cantrell DA. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada T, Kurosaki T, Hikida M. Essential roles of mgcRacGAP in multilineage differentiation and survival of murine hematopoietic cells. Biochem Biophys Res Commun. 2008;372:941–946. doi: 10.1016/j.bbrc.2008.05.170. [DOI] [PubMed] [Google Scholar]
- 72.Tedford K, Nitschke L, Girkontaite I, Charlesworth A, Chan G, Sakk V, Barbacid M, Fischer KD. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat Immunol. 2001;2:548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- 73.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Miletic AV, et al. Vav links the T cell antigen receptor to the actin cytoskeleton and T cell activation independently of intrinsic Guanine nucleotide exchange activity. PLoS One. 2009;4:e6599. doi: 10.1371/journal.pone.0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu H, Leitenberg D, Li B, Flavell RA. Deficiency of small GTPase Rac2 affects T cell activation. J Exp Med. 2001;194:915–926. doi: 10.1084/jem.194.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez TS, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gomez TS, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6:261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 81.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vartiainen MK. Nuclear actin dynamics – from form to function. FEBS Lett. 2008;582:2033–2040. doi: 10.1016/j.febslet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 83.Billadeau DD, Burkhardt JK. Regulation of cytoskeletal dynamics at the immune synapse: new stars join the actin troupe. Traffic. 2006;7:1451–1460. doi: 10.1111/j.1600-0854.2006.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


