Abstract
Background.
Secondary hyperparathyroidism (sHPT) represents an adaptive response to progressively impaired control of calcium, phosphorus and vitamin D in chronic kidney disease (CKD). It is characterized by parathyroid hyperplasia and excessive synthesis and secretion of parathyroid hormone (PTH). Parathyroid hyperplasia in uremic rats can be prevented by calcium-sensing receptor (CaSR) activation with the calcimimetic cinacalcet (Sensipar®/Mimpara®); however, it is unknown, how long the effects of cinacalcet persist after withdrawal of treatment or if cinacalcet is efficacious in uremic rats with established sHPT.
Methods.
We sought to determine the effect of cinacalcet discontinuation in uremic rats and whether cinacalcet was capable of influencing parathyroid hyperplasia in animals with established sHPT.
Results.
Discontinuation of cinacalcet resulted in reversal of the beneficial effects on serum PTH and parathyroid hyperplasia. In rats with established sHPT, cinacalcet decreased serum PTH and mediated regression of parathyroid hyperplasia. The cinacalcet-mediated decrease in parathyroid gland size was accompanied by increased expression of the cyclin-dependent kinase inhibitor p21. Prevention of cellular proliferation with cinacalcet occurred despite increased serum phosphorus and decreased serum calcium.
Conclusions.
The animal data provided suggest established parathyroid hyperplasia can be reversed by modulating CaSR activity with cinacalcet and that continued treatment may be necessary to maintain reductions in PTH.
Keywords: calcimimetics, cinacalcet, parathyroid hormone, parathyroid hyperplasia, secondary hyperparathyroidism
Introduction
Disturbances in mineral metabolism that are secondary to chronic kidney disease (CKD), such as hypocalcemia, hyperphosphatemia and impaired synthesis of 1,25-dihydroxyvitamin D3 (calcitriol), result in excess parathyroid hormone (PTH) secretion that is a characteristic of secondary hyperparathyroidism (sHPT) [1, 2]. In contrast to low levels of parathyroid gland proliferation and growth in normal adults, hyperparathyroidism secondary to CKD is characterized by abnormally increased parathyroid cell proliferation [3]. Increased parathyroid cell proliferation has also been observed in patients with primary hyperparathyroidism with demonstrable parathyroid hyperplasia [4]. Although molecular mechanisms controlling parathyroid cell proliferation and gland size are not yet clear, the endogenous cyclin-dependent kinase (CDK) inhibitor p21, which inhibits progression from the G1 to the S phase of the cell cycle [5, 6], may play a role in decreasing parathyroid cell proliferation [7] and attenuating parathyroid hyperplasia [8].
Parathyroid cell proliferation in uremic, as well as non-uremic animals, is aggravated by hypocalcemia, phosphorous retention and vitamin D deficiency, underscoring the importance of controlling these factors [9]. In addition, the calcium-sensing receptor (CaSR), the vitamin D receptor (VDR) and Klotho/fibroblast growth factor (FGF) receptor 1 are downregulated, rendering the parathyroid gland more resistant to serum calcium, calcitriol and FGF-23 further exacerbating parathyroid hyperplasia [3, 10, 11]. Although parathyroid cell proliferation is increased in mice with systemic deletion of the VDR [12], parathyroid proliferation was not significantly altered when VDR site-specific deletion was limited to the parathyroid gland [13]. Thus, effects of vitamin D on parathyroid hyperplasia are mediated by indirect mechanisms distinct from activation of parathyroid VDR, such as the calcitropic effects of vitamin D. This is further supported by the ability of calcium rescue diets to correct sHPT in mice with systemic VDR deletion [14, 15]. Although FGF-23 can decrease PTH expression and FGF-23 resistance develops with parathyroid hyperplasia, there is no evidence of FGF-23 regulation of parathyroid gland proliferation [10, 11, 16].
Serum calcium as sensed by the CaSR, a G protein-coupled receptor located on the parathyroid gland, is a critical negative regulator of PTH secretion and resultant serum levels [17] and the CaSR is thought to play a key role in parathyroid cell proliferation and hyperplasia [18]. Calcimimetics including R-568, AMG 641 [19] and cinacalcet (Sensipar®/Mimpara®) act as allosteric modulators of the CaSR to increase sensitivity to endogenous calcium [19–21], thereby shifting leftward the set point for calcium-regulated PTH secretion [20, 22]. In dialysis patients with sHPT, cinacalcet simultaneously lowers plasma PTH, serum calcium and serum phosphorus [22]. Preclinical studies have shown that cinacalcet prevents parathyroid cell proliferation and reduces gland weight in an animal model of sHPT [5/6 nephrectomized rats (5/6 Nx)] [23], consistent with studies using the calcimimetic R-568 [24, 25]. Clinically, patients treated with cinacalcet have been shown to exhibit reduced parathyroid glandular volume as visualized by ultrasound [26] and regression of parathyroid gland swelling [27]. Komaba and Fukagawa [28] subsequently reported that cinacalcet effectively decreases serum PTH and reduces parathyroid gland volume in patients with moderate to severe sHPT [29]. While the precise mechanism(s) of calcimimetic action on parathyroid gland size is currently unknown, both preclinical and clinical findings suggest that calcimimetic activation of CaSR is involved in controlling the development of the excessive cell proliferation that occurs in parathyroid hyperplasia. Preliminary findings suggest that calcimimetic-induced increases in p21, an inhibitor of cell cycle progression, may be involved in controlling parathyroid hyperplasia [1, 30].
The present report describes results from two separate experiments with cinacalcet in 5/6 Nx uremic rats. The purpose of the first experiment was to evaluate reoccurrence of sHPT (parathyroid gland hyperplasia and increased PTH) after discontinuation of treatment. The purpose of the second experiment was to evaluate the efficacy of cinacalcet in rats with established CKD (e.g. using a therapeutic paradigm whereby cinacalcet therapy was administered to rats several weeks after induction of uremia). Expression of p21 was evaluated to determine if calcimimetic-induced changes in p21 correlated with parathyroid hyperplasia during and after discontinuation of treatment.
Materials and methods
Animals and treatments
Experiments were performed under protocols approved by Amgen’s Internal Animal Care and Use Committee. Male Sprague–Dawley rats (300–350 g) purchased from Charles River Laboratories (Wilmington, MA) were pair-housed with a 12/12-h light/dark cycle and provided standard rat chow and water ad libitum. A rodent model of CKD was induced by 5/6 Nx, a two-step procedure reducing the original functional renal mass by five-sixths, as previously described [23]. Sham-operated animals underwent the same procedures without renal compromise.
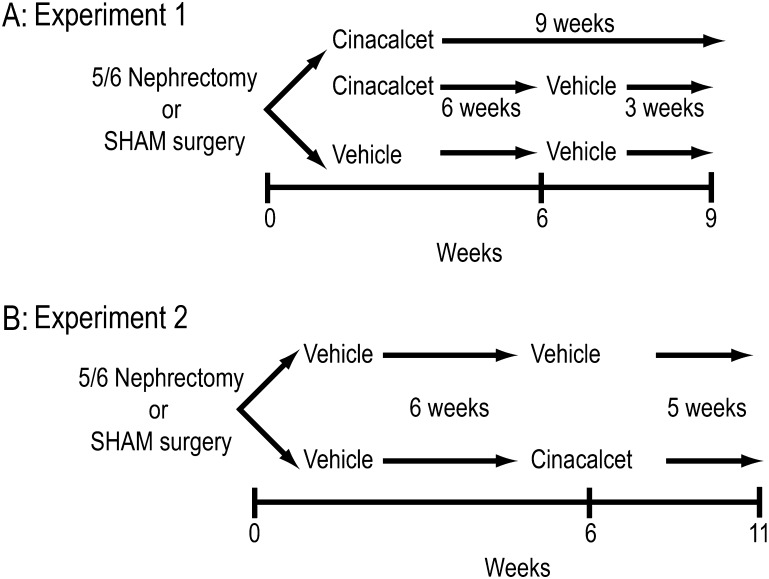
Animals were randomized into treatment groups based on normal distribution of body weights at baseline. Two separate experiments were performed with cinacalcet (Figure 1): (i) to evaluate reoccurrence of sHPT (parathyroid gland hyperplasia, increased PTH) after discontinuation of treatment and (ii) to evaluate the therapeutic paradigm of cinacalcet administration to rats with established CKD. Cinacalcet was formulated in 20% Captisol:water (vehicle) at Amgen, Inc. (Thousand Oaks, CA) to deliver 10 mg/kg (orally) in a l.5 mL/kg dose volume. Based on exposure levels, the equivalent human dose to 10 mg/kg is 60 mg per subject, which is a standard clinical dose [31].
Fig. 1.
Experimental design. (A) Discontinuation. (B) Therapeutic paradigm.
Experiment 1: cinacalcet prevention and control of sHPT (parathyroid gland hyperplasia and increased PTH) and effects of discontinuation of treatment in vivo
Animals were randomly placed into one of the following five groups (n = 8–10 per group): 5/6 Nx administered (i) vehicle (20% Captisol:water per day, orally) or (ii) cinacalcet (10 mg/kg) once per day for 6 weeks (starting within 1 week after completion of the 5/6 Nx procedure) and then sacrificed; (iii) 5/6 Nx administered cinacalcet for 6 weeks followed by vehicle for 1–3 weeks and sacrificed, sham animals receiving either (iv) vehicle or (v) cinacalcet for 9 weeks and then sacrificed (Figure 1A). Blood samples were taken 4 h post-treatment at the times indicated for determination of blood chemistries [PTH, blood urea nitrogen (BUN), creatinine, phosphorus and total calcium]. Parathyroid weights, proliferating cell nuclear antigen (PCNA)- and p21-positive cells were determined in tissue samples obtained at sacrifice. The data on p21 from three groups (5/6 Nx vehicle 6 weeks, 5/6 Nx cinacalcet 6 weeks and 5/6 Nx cinacalcet 6 weeks followed by 3 weeks vehicle) were previously presented in summary form in a review article by Drueke et al. [1]. The data from these animals are reported here to allow for interpretation of the complete experiment.
Experiment 2: therapeutic administration of cinacalcet to rats with established CKD
To determine the effectiveness of cinacalcet on reversing parathyroid hyperplasia in established sHPT, 5/6 Nx animals were subdivided into groups in a separate study and all received vehicle for 6 weeks to allow uremia, sHPT and parathyroid hyperplasia to progress (Figure 1B). In this experimental therapeutic paradigm, uremic rats (6 weeks after 5/6 Nx) were treated with cinacalcet (10 mg/kg/day, orally) or vehicle once daily for 5 weeks and then sacrificed. Sham-operated rats treated with vehicle for the entire time period (11 weeks) were used as controls. Animal weights were monitored throughout the course of treatment to assess any treatment-related weight changes. Blood was collected for determination of serum chemistry profiles at 2 days prior to surgery and 4 h post-treatment at 6 and 11 weeks post-surgery.
Serum chemistry profiling
PTH levels were quantified according to the vendor’s instructions using rat PTH(1–34) immunoradiometric assay kits (Immutopics, San Clemente, CA). BUN, total calcium, phosphorus and creatinine were measured using a blood chemistry analyzer (AU 400; Olympus, Melville, NY).
Parathyroid histology
At sacrifice, dissected laryngo-tracheal complexes were stored 2–3 days in zinc-buffered formalin, transferred to 70% alcohol and trimmed. For histology, parathyroids were dissected from the thyroid, blotted dry and weighed (Sartorius BP211D balance; Gottingen, Germany). After processing and paraffin embedding, 5 μm sections were cut, placed onto charged slides (VWR Scientific, West Chester, PA) and immunostained using a PCNA staining kit (Zymed Laboratories, Inc., South San Francisco, CA) or a mouse anti-rat p21 monoclonal antibody (Santa Cruz, CA) according to vendors’ instructions. Parathyroid area from approximately the same level of individual parathyroid was determined by visualizing tissue samples at ×100 on a Leitz Laborlux microscope (Wetzlar, Germany) and the number of 0.01 mm2 grids (calibrated area measurement graticle) overlaying the central region of the parathyroid sections was counted by an observer blinded to treatment groups. Total parathyroid area was calculated by multiplying the number of grids by 0.01 mm2, after which the number of PCNA-positive or p21-positive cells in the gridded sectional area were counted and expressed as number of PCNA-positive cells/mm2 and p21-positive cells/mm2, respectively.
Statistics
Data are reported as mean ± SEM unless otherwise indicated. Overall, treatment effects were determined by analysis of variance (α = 0.05). Statistical significance for comparisons between a given treatment group with its corresponding vehicle control was determined by unpaired Student’s t-test (α = 0.05).
Results
Experiment 1: cinacalcet prevention and control of sHPT (parathyroid gland hyperplasia and increased PTH) and effects of discontinuation of treatment
Cinacalcet—prevention of elevated serum PTH and parathyroid hyperplasia in uremic rats did not persist after treatment withdrawal.
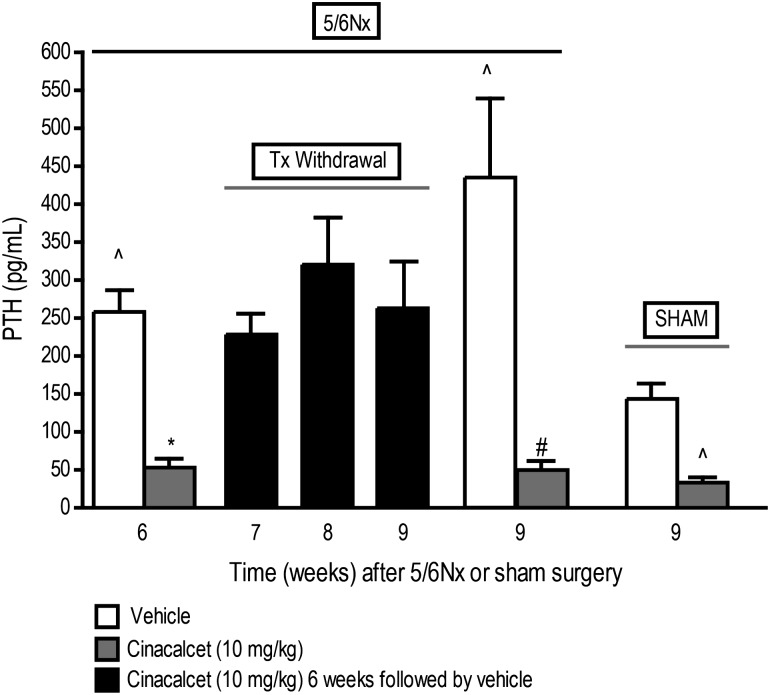
Serum PTH levels were significantly higher in 5/6 Nx animals treated with vehicle for 6 weeks (258 ± 29; n = 10) when compared to sham controls treated with vehicle for the same period (144 ± 22 pg/mL; n = 10), consistent with the pathophysiology of sHPT in rodents previously described [25] (Figure 1A). Cinacalcet (10 mg/kg/day, orally) treatment for 6 weeks resulted in a significant (P < 0.05) reduction in serum PTH (53 ± 12; n = 9) compared to vehicle-treated 5/6 Nx animals (Figure 2). The significant reduction in PTH did not persist following discontinuation of cinacalcet and within 1–3 weeks of treatment termination, serum PTH increased to levels comparable to those in vehicle-treated rats (Figure 2). This is in contrast to the significantly decreased PTH levels in 5/6 Nx rats treated continuously with cinacalcet for 9 weeks (Figure 2). Serum PTH was also significantly lower in cinacalcet-treated sham rats than in vehicle-treated sham animals (Figure 2).
Fig. 2.
Cinacalcet-mediated reductions in PTH are reversed upon discontinuation of treatment. 5/6 Nx and sham rats were treated with cinacalcet (10 mg/kg/day) or vehicle for 6 weeks followed by up to 3 weeks of vehicle in all groups. Values (mean ± SEM; n = 8–10 per group) shown are from blood samples collected 4 h post-treatment at the end of 6, 7, 8 or 9 weeks after 5/6 Nx or sham surgery. Data represent mean ± SEM (*P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 6 week; #P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 9 week; ∧P < 0.05 as compared to respective sham, vehicle; n = 8–10 per group).
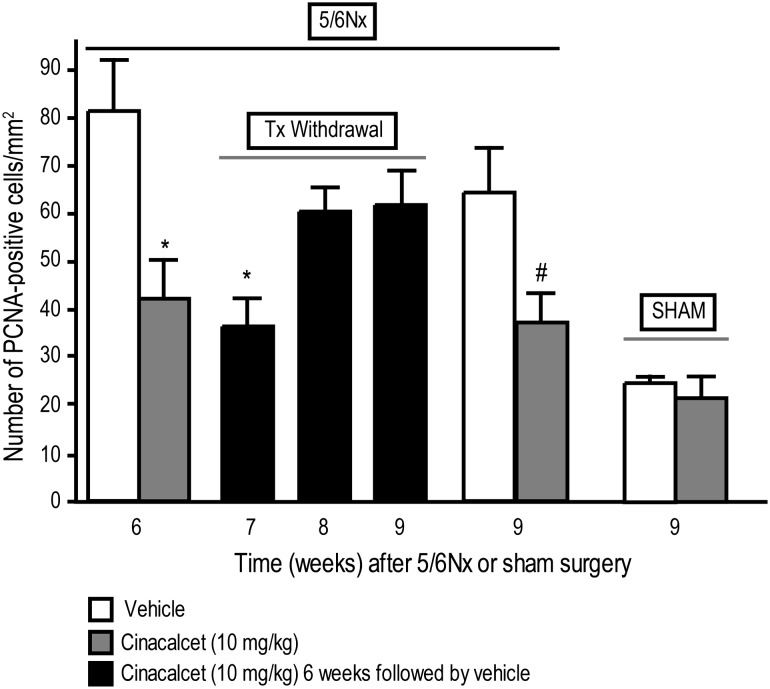
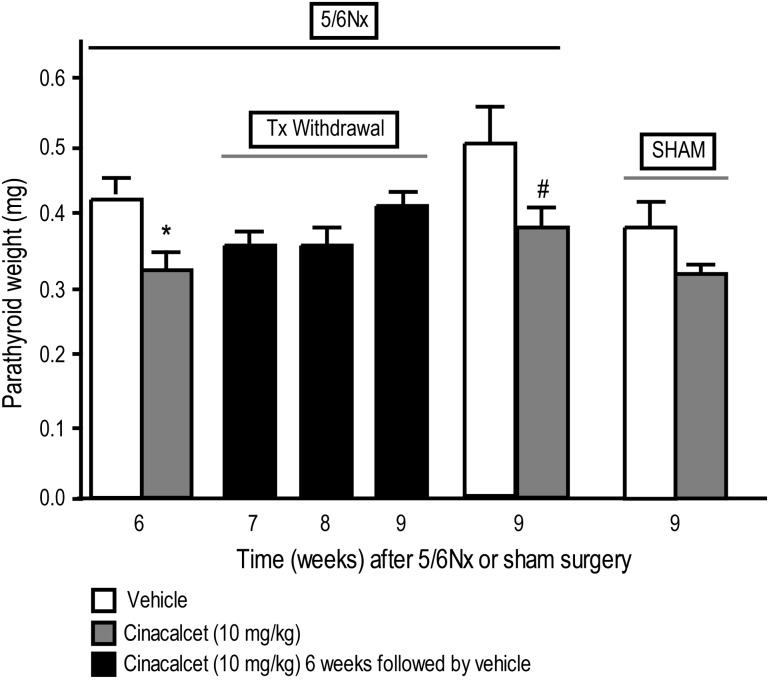
Cinacalcet treatment of 5/6 Nx rats for 6 weeks resulted in a significant reduction of parathyroid hyperplasia, as assessed by the number of PCNA-positive cells in the parathyroid glands (Figure 3) and by parathyroid weights (Figure 4). Cinacalcet-treated 5/6 Nx rats had ∼2-fold lower number of PCNA-positive cells than vehicle-treated 5/6 Nx rats, whose numbers were ∼3-fold higher than vehicle-treated sham rats (Figure 3). Discontinuation of cinacalcet at the end of a 6-week treatment resulted in gradual increases in parathyroid hyperplasia [number of PCNA-positive cells in the parathyroid (Figure 3) and parathyroid weights (Figure 4)] over the next 3 weeks in contrast to the significantly decreased parathyroid hyperplasia in 5/6 Nx rats treated continuously with cinacalcet for the entire 9-week period (Figures 3 and 4). Cinacalcet treatment had no significant effect on the number of parathyroid PCNA-positive cells (Figure 3) or parathyroid weight (Figure 4) in sham animals compared to vehicle-treated sham animals.
Fig. 3.
Parathyroid hyperplasia returns after cessation of cinacalcet treatment. Parathyroid hyperplasia was assessed by measuring PCNA-positive cells in parathyroid glands from cinacalcet- or vehicle-treated rats at the end of 6 or 9 weeks of treatment and 1–3 weeks after termination of treatment. 5/6 Nx and sham rats were treated with cinacalcet (10 mg/kg/day) or vehicle for 6 weeks followed by up to 3 weeks of vehicle in all groups. Rats were sacrificed at 6, 7, 8 or 9 weeks, and parathyroid sections were stained for PCNA as a marker of cell proliferation. Data represent mean ± SEM (*P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 6 week; #P < 0.05 as compared to 5/6 Nx vehicle, sacrifice 9 week. n = 11–17 parathyroid gland sections stained for PCNA per group).
Fig. 4.
Continued cinacalcet treatment is required to prevent increases in parathyroid glandular weight. Parathyroid tissue weights from cinacalcet- or vehicle-treated rats at the end of 6 or 9 weeks of treatment and 1–3 weeks after termination of treatment. 5/6 Nx and sham rats were treated with cinacalcet (10 mg/kg/day) or vehicle for 6 weeks followed by up to 3 weeks of vehicle in all groups. Rats were sacrificed at 6, 7, 8 or 9 weeks, and parathyroid weights were determined. Data represent mean ± SEM (*P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 6 week; #P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 9 week; n = 13–17 parathyroid glands per group).
Cinacalcet affects p21 expression the parathyroid glands of uremic but not normal rats.
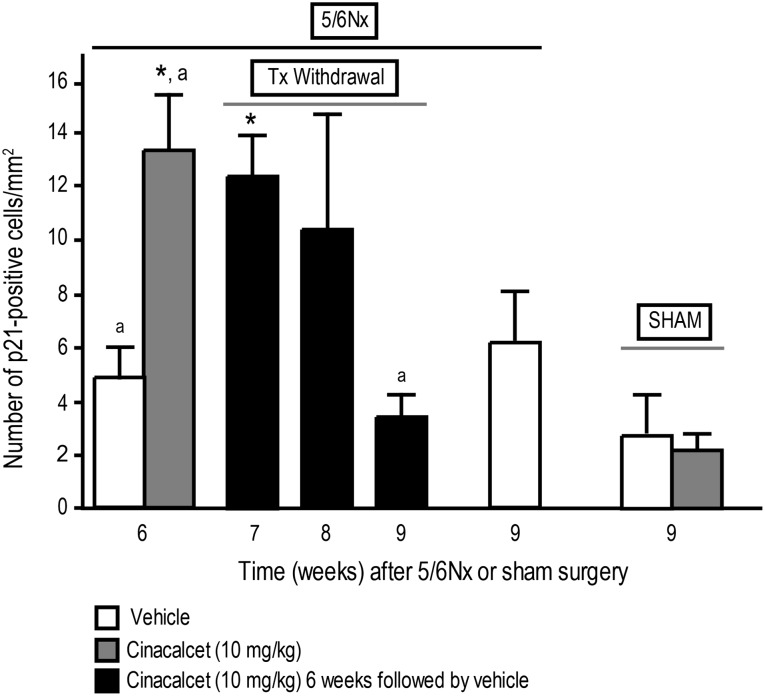
As previously reported [1], 6 weeks of cinacalcet administration to 5/6 Nx rats resulted in increased p21 expression, an effect that did not persist at 3 weeks following discontinuation of treatment. We expanded on these studies to determine the time course for changes in p21 in uremic rats 1 and 2 weeks after discontinuation of cinacalcet (in addition to the 3-week discontinuation previously reported) and to control against the expression of p21 in uremic rats treated with vehicle for 9 weeks and in normal, sham-operated rats treated with cinacalcet or vehicle. Expression of p21 in parathyroid gland sections (p21-positive cells/mm2) from uremic rats 1 week after discontinuation of cinacalcet was ∼2.5-fold higher than in 5/6 Nx rats treated with vehicle at the end of 6 weeks of treatment (Figure 5; P < 0.05), similar to the ∼2.5-fold increase at the end of 6 weeks of cinacalcet previously reported by Drueke et al. [1]. There was a trend for expression of p21 to be elevated 2 weeks after discontinuation of cinacalcet (Figure 5); however, the overall effect was not significant. The expression of p21 in uremic rats treated with vehicle for 9 weeks was not significantly different from rats treated with vehicle for 6 weeks or in rats 3 weeks after discontinuation of cinacalcet (Figure 5). Cinacalcet had no effect on the number of p21-positive parathyroid cells in control sham-operated rats compared with sham-operated vehicle-treated rats (Figure 5).
Fig. 5.
Continued cinacalcet treatment is required to maintain increases in p21 expression in parathyroid glands. Number of p21-positive cells from cinacalcet- or vehicle-treated rats at the end of 6 or 9 weeks of treatment and 1–3 weeks after termination of treatment. 5/6 Nx and sham rats were treated with cinacalcet (10 mg/kg/day) or vehicle for 6 weeks followed by up to 3 weeks of vehicle in all groups. Rats were sacrificed at 6, 7, 8 or 9 weeks, and numbers of p21-positive cells were determined. Data represent mean ± SEM (*P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 6 week; adata previously reported [1]; n = 13–17 parathyroid gland sections stained for p21 per group).
Cinacalcet effects on serum BUN, creatinine, calcium and phosphorous
Uremic induction following 5/6 Nx in rats resulted in a significant (P < 0.05) elevation in BUN and creatinine indicative of CKD (Table 1). Treatment (cinacalcet or vehicle) had no effect on these biomarkers of uremia in either sham or 5/6 Nx rats (Table 1).
Table 1.
Effect of preventative cinacalcet treatment on BUN and creatinine levels in CKD rats (Experiment 1)a
| Experiment 1 | ||||
| 5/6 Nx |
Sham |
|||
| Vehicle | Cinacalcet | Vehicle | Cinacalcet | |
| BUN (mg/dL) | ||||
| Baseline (prior to surgery) | 23 ± 1 | 21 ± 0 | 22 ± 1 | 22 ± 1 |
| 6 week (6 weeks of treatment) | 52 ± 3* | 58 ± 9* | 22 ± 1 | 21 ± 1 |
| Baseline (prior to surgery) | 21 ± 1 | 21 ± 1 | 22 ± 1 | 22 ± 1 |
| 9 week (9 weeks of treatment) | 61 ± 15* | 42 ± 2* | 22 ± 1 | 19 ± 1 |
| Creatinine (mmol/L) | ||||
| Baseline (prior to surgery) | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 |
| 6 week (6 weeks of treatment) | 0.9 ± 0.0* | 1.1 ± 0.2* | 0.4 ± 0.0 | 0.4 ± 0.0 |
| Baseline (prior to surgery) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 |
| 9 week (9 weeks of treatment) | 1.2 ± 0.3* | 0.8 ± 0.0* | 0.4 ± 0.0 | 0.4 ± 0.0 |
Values are mean ± SEM from serum collected 4 h after last treatment, n = 8–10 animals per group. In 5/6 Nx animals, values were measured from the same group of animals at baseline and 6 weeks or a different group of animals at baseline and 9 weeks. In sham animals, values were measured from the same group of animals at baseline, 6 weeks and 9 weeks.
*P < 0.05 versus sham-vehicle or sham-cinacalcet (10 mg/kg).
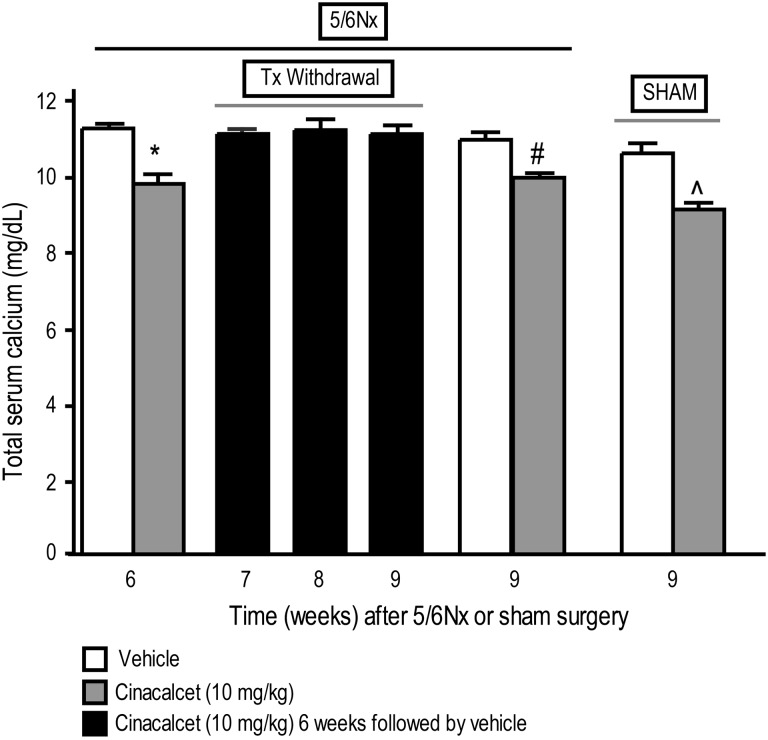
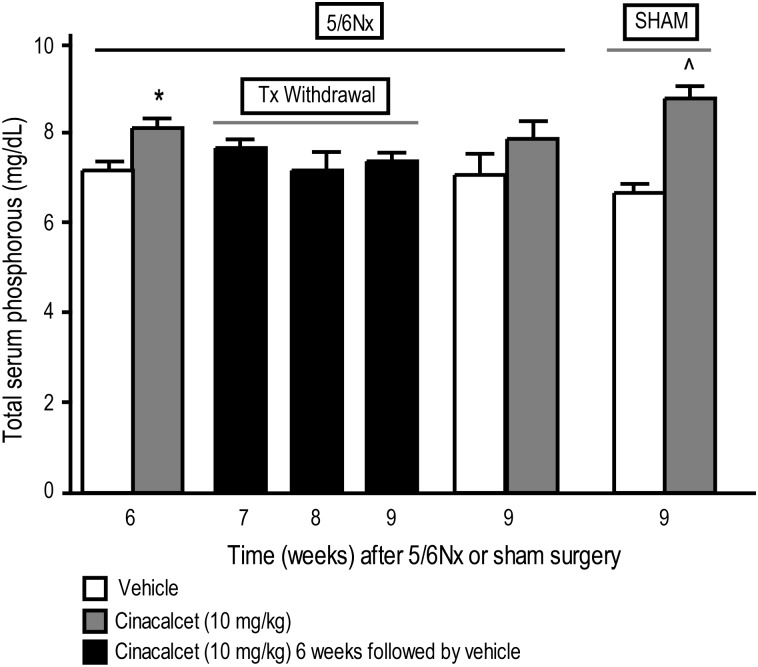
Cinacalcet significantly decreased serum calcium (Figure 6) and increased serum phosphorous (Figure 7) in 5/6 Nx rats treated daily for 6 weeks compared with vehicle-treated 5/6 Nx animals. This effect did not persist after discontinuation of cinacalcet treatment; serum calcium and phosphorous returned toward vehicle-treated control levels by Weeks 7, 8 and 9 corresponding to 1, 2 and 3 weeks, respectively, after treatment termination (Figures 6 and 7). This is in contrast to the persisting effect in 5/6 Nx rats treated continuously with cinacalcet for the whole 9-week period (Figures 6 and 7). In sham-operated rats, 9-week cinacalcet treatment significantly decreased serum calcium and increased serum phosphorous compared with sham animals treated with vehicle for the same duration (Figures 6 and 7).
Fig. 6.
Total serum calcium in cinacalcet- or vehicle-treated rats at the end of 6 or 9 weeks of treatment and 1–3 weeks after termination of treatment. 5/6 Nx and sham rats were treated with cinacalcet (10 mg/kg/day) or vehicle for 6 weeks followed by up to 3 weeks of vehicle in all groups. Values (mean ± SEM; n = 8–10 per group) shown are from blood samples collected 4 h post-treatment at the end of 6, 7, 8 or 9 weeks after 5/6 Nx or sham surgery. Data represent mean ± SEM (*P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 6 week; #P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 9 week; ^P < 0.05 as compared to sham, vehicle, sacrifice 9 week; n = 8–10 per group).
Fig. 7.
Total serum phosphorus in cinacalcet- or vehicle-treated rats at the end of 6 or 9 weeks of treatment and 1–3 weeks after termination of treatment. 5/6 Nx and sham rats were treated with cinacalcet (10 mg/kg/day) or vehicle for 6 weeks followed by up to 3 weeks of vehicle in all groups. Values (mean ± SEM; n = 8–10 per group) shown are from blood samples collected 4 h post-treatment at the end of 6, 7, 8 or 9 weeks after 5/6 Nx or sham surgery. Data represent mean ± SEM (*P < 0.05 as compared to 5/6 Nx, vehicle, sacrifice 6 week; n = 8–10 per group).
Experiment 2—therapeutic cinacalcet regimen: reduction of parathyroid gland hyperplasia in response to therapeutic administration of cinacalcet in rats with established CKD
Cinacalcet reverses established PTH elevations and parathyroid hyperplasia.
Serum PTH (pg/mL) measured 6 weeks after 5/6 Nx and before treatment with cinacalcet (329 ± 51; n = 10) or vehicle (298 ± 50; n = 10) was significantly higher (P < 0.05) than PTH in sham-operated rats (115 ± 13; n = 10) (Figure 1B). Administration of cinacalcet to 5/6 Nx animals for 5 weeks starting 6 weeks after surgery resulted in significantly reduced serum PTH (25 ± 5 pg/mL, n = 9; P < 0.001) compared to 5/6 Nx rats treated with vehicle (398 ± 81 pg/mL) or sham-operated rats treated with vehicle (162 ± 27 pg/mL; P < 0.05) for the same period.
Administration of cinacalcet to 5/6 Nx animals for 5 weeks starting 6 weeks after surgery resulted in significantly reduced parathyroid hyperplasia (41 ± 8 PCNA-positive cells/mm2 and 0.396 ± 0.031 mg parathyroid gland weight) as compared to 5/6 Nx rats treated with vehicle [83 ± 8 PCNA-positive cells/mm2 (P < 0.001) and 0.566 ± 0.038 mg parathyroid gland weight (P < 0.05)]. Parathyroid hyperplasia in vehicle-treated 5/6 Nx rats (11 weeks post-5/6 Nx) was not increased over that of a separate group of vehicle-treated 5/6 Nx rats sacrificed 6 weeks after surgery (78 ± 9 PCNA-positive cells/mm2 and 0.607 ± 0.045 mg parathyroid gland weight) but was significantly increased compared to vehicle-treated sham rats [27 ± 5 PCNA-positive cells/mm2 (P < 0.001) and 0.362 ± 0.030 mg parathyroid gland weight (P < 0.05)], indicating significant parathyroid hyperplasia in the uremic animals at 6 and 11 weeks after surgery.
Cinacalcet effects on serum BUN, creatinine, calcium and phosphorous.
Serum markers of uremia including BUN and creatinine were significantly increased in 5/6 Nx rats treated with vehicle for 5 weeks starting 6 weeks after surgery (11 weeks total post-5/6 Nx) compared to baseline pre-surgical levels (Table 2; P < 0.01) or vehicle-treated sham rats (Table 2; P < 0.01), indicating persistence of CKD. There was no significant effect of cinacalcet treatment in 5/6 Nx rats on these serum markers of uremia (Table 2).
Table 2.
Effect of therapeutic cinacalcet treatment on BUN, creatinine, calcium and phosphorous levels in CKD rats (Experiment 2)a
| Experiment 2 | |||
| 5/6 Nx |
Sham | ||
| Vehicle | Cinacalcet | Vehicle | |
| BUN (mg/dL) | |||
| Baseline (prior to surgery) | 18 ± 1 | 19 ± 1 | 19 ± 1 |
| 11 week (6 weeks vehicle + 5 weeks of treatment) | 54 ± 4** | 48 ± 7** | 21 ± 1 |
| Creatinine (mmol/L) | |||
| Baseline (prior to surgery) | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 |
| 11 week (6 weeks vehicle + 5 weeks of treatment) | 1.1 ± 0.2** | 0.8 ± 0.1** | 0.4 ± 0.0 |
| Calcium (mg/dL) | |||
| 11 week (6 weeks vehicle + 5 weeks of treatment) | 11.4 ± 0.3 | 10.1 ± 0.2* | 11.2 ± 0.3 |
| Phosphorous (mg/dL) | |||
| 11 week (6 weeks vehicle + 5 weeks of treatment) | 6.4 ± 0.4 | 8.0 ± 0.4* | 5.9 ± 0.3 |
Values are mean ± SEM, n = 10 animals per group.
*P < 0.05 versus vehicle-treated 5/6 Nx.
**P < 0.01 versus sham-vehicle.
Cinacalcet treatment of 5/6 Nx animals for 5 weeks starting 6 weeks after surgery (11 weeks post-5/6 Nx) resulted in significantly decreased serum calcium and increased serum phosphorous compared to vehicle-treated 5/6 Nx animals at the end of 5 weeks of treatment (Table 2). Serum calcium and phosphorous levels in vehicle-treated 5/6 Nx animals were not significantly different than vehicle-treated sham animals (Table 2).
Discussion
These studies demonstrate that prophylactic treatment with cinacalcet, an allosteric modulator of the CaSR, reduced parathyroid cell proliferation and PTH in rats with sHPT, consistent with previous preventative studies utilizing calcimimetics [23–25]. Additionally, we demonstrated that discontinuation of calcimimetic treatment released the inhibition of parathyroid proliferation resulting in the development of parathyroid hyperplasia and return of PTH to levels observed in vehicle-treated rats. More importantly, we also demonstrated for the first time that a therapeutic paradigm of cinacalcet administration in rats with established uremia and sHPT can mediate regression of parathyroid cell proliferation and prevent increases in parathyroid gland weight. In addition to attenuating established parathyroid hyperplasia, cinacalcet caused a rapid decrease in serum PTH and calcium in 5/6 Nx rats and is in agreement with previous observations in normal and uremic rats and CKD patients on dialysis [20, 22, 23]. The present data, coupled with previous findings with another calcimimetic, R-568, offer encouraging support for efficient targeting of the CaSR with cinacalcet in sHPT.
The studies described herein show that parathyroid cell proliferation was reduced to control levels by cinacalcet treatment using either a prophylactic or therapeutic dosing regimen. These findings are in agreement with preventative studies with cinacalcet or the calcimimetic R-568 that showed inhibition of parathyroid cell proliferation in the 5/6 Nx rats [23, 24]. Furthermore, these data serve to support clinical findings demonstrating reduced parathyroid glandular volume and regression of parathyroid gland swelling in patients treated with cinacalcet as visualized by ultrasound [26, 27, 29]. This cinacalcet-induced reduction in parathyroid gland volume has subsequently been shown to be associated with decreased serum PTH [29]. It is important to note that the experimental model of sHPT that was used in the present studies, namely the 5/6 Nx, has limitations in that renal failure is not as extensive as it is in end-stage renal disease (ESRD) patients. This is exemplified by the differences in calcimimetic effects on serum phosphorous. Cinacalcet lowers serum phosphorous in ESRD patients; however, in the 5/6 Nx model, serum phosphorous is elevated. The effects on serum phosphorus observed in this study are to be expected in the presence of a partially functioning kidney (1/6th kidney function remaining) based on the known physiological effect of PTH on urinary phosphorous excretion and is consistent with the phosphatemic effect of cinacalcet observed in a phase II trial in patients with CKD not on dialysis (Stage 3/4) [32]. However, this is in stark contrast to the phosphate lowering effect in dialysis patients with very little no residual kidney function (CKD Stage 5) for whom cinacalcet is indicated [22]. Cinacalcet effects on PTH and calcium observed are in agreement with effects in patients.
Parathyroid hyperplasia involves a complex cascade of events, in which decreases in circulating calcium and 1,25-dihydroxyvitamin D and retention of phosphate have been implicated in disease progression; thus, decreased signaling through the CaSR is thought to be a major pathway promoting the progression of parathyroid hyperplasia. This hypothesis is supported by the fact that parathyroid hyperplasia is found in neonatal severe hyperparathyroidism, a disease attributed to loss-of-function mutations in the CaSR [33]. Furthermore, mice that lack CaSR develop parathyroid gland hyperplasia, suggesting that CaSR regulates chief cell proliferation in the absence of uremia [34].
The principal regulator of PTH secretion is serum calcium, which acts on CaSR in the parathyroid gland and has previously been shown to decrease parathyroid hyperplasia [35]. In the CKD setting, there is a reduced CaSR expression as parathyroid glands enlarge and undergo hyperplasia [36, 37]. The consequences of CaSR reductions could lead to abnormal calcium-regulated PTH secretion. Downregulation of CaSR is associated with high proliferative activity of parathyroid glands in patients with sHPT [38] and in uremic rats [39]. The reduction in CaSR and VDR, and enlargement of the parathyroid glands that causes enhanced capacity for PTH production, might explain why parathyroid glands become increasingly resistant to regulation by calcium and 1,25-dihydroxyvitamin D. Clinically, patients treated with cinacalcet have been shown to exhibit reduced parathyroid glandular volume [26] and regression of parathyroid gland swelling [27]. The efficacy of cinacalcet in reduction of gland volume in the Meola et al. [26] study appeared to be dependent upon the baseline gland volume (<500 mm3) supporting the notion that if disease progression is too advanced, the parathyroids may become increasingly resistant to therapy. However, in a 52-week, multicenter open-label study of patients receiving hemodialysis with moderate to severe sHPT, cinacalcet decreased serum PTH and reduced parathyroid gland volume in patients with marked parathyroid hyperplasia (i.e. regardless of baseline parathyroid size) [29].
A potential mechanism underlying the ability of calcimimetics to prevent parathyroid cell proliferation in uremic rats is through increased expression of the cell cycle inhibitor p21 [1, 30], which controls cell entry into the actively dividing S phase through inhibition of the activity of a number of CDKs [40]. In the present study, cinacalcet obviated the effects of uremia on parathyroid cell proliferation after 6 weeks of treatment and at this time, p21 expression was reportedly increased [1]. Interestingly, 1 week after discontinuation of cinacalcet, p21 expression remained significantly elevated (Figure 5) and the number of proliferating cells (PCNA-positive cells) in the parathyroid gland was still significantly decreased (Figure 3). Thus, cinacalcet-mediated increases in p21 expression in the parathyroid glands from uremic rats may keep cells under normal cell cycle control and prevent them from entering a proliferative hyperplastic state. While the underlying mechanism of calcimimetic-induced increases in p21 in uremic rats has not been completely elucidated, the observation that calcimimetics increase this endogenous inhibitor of cell cycle CDK activity may explain why parathyroid cell proliferation is decreased in calcimimetic-treated compared to vehicle-treated rats. However, it is also possible that instead of being causative, changes in p21 may be a response to the changes in cell proliferation. It is noteworthy that 3 weeks following discontinuation of cinacalcet, p21 was no longer increased in uremic rats and parathyroid cell proliferation, parathyroid gland weights and PTH were increased to levels observed in vehicle-treated uremic rats. This reversibility of the effects of cinacalcet is in contrast to parathyroidectomy as an alternative therapeutic option for parathyroid hyperplasia, which by nature has irreversible effects. Each treatment has benefits and drawbacks that need to be weighed by the physician and patient to determine the proper clinical decision. Reversibility of cinacalcet effects on parathyroid gland proliferation after discontinuation of treatment suggests that treatment with cinacalcet must be maintained in order to keep parathyroid hyperplasia suppressed. Whether CaSR signaling directly regulates p21 expression remains to be determined.
In contrast to the increased p21 expression and decreased parathyroid hyperplasia in cinacalcet-treated uremic rats, parathyroid hyperplasia and p21 expression were unaffected in vehicle-treated uremic rats and expression remained at low levels in these animals. This finding confirms those previously reported indicating that p21 does not increase in uremic rats or humans with diffuse hyperplasia [7]. However, when disease progresses (e.g. severe nodular parathyroids), expression of p21 has been reported to decrease, suggesting that loss of p21 may lead to cell cycle deregulation and promote disease progression to a more severe state [7]. Factor(s) that prevent upregulation of p21 in uremic animals to keep parathyroid cells from entering the proliferative state or that downregulate p21 in severe disease states have not been identified.
Taken together, these findings demonstrate that the effects of cinacalcet are not limited to decreasing serum PTH levels but extend to modifying disease progression by reducing parathyroid cell proliferation and gland weight in CKD animals. While these findings in an animal model of CKD are not directly applicable to parathyroid hyperplasia in CKD patients, recent clinical findings demonstrating that cinacalcet appears to attenuate progression of parathyroid hyperplasia supports the notion that cinacalcet could arrest the development of parathyroid hyperplasia in CKD patients. However, further research with refined imaging capabilities in larger patient populations is warranted to determine at what stage of hyperplasia and gland size can therapeutic intervention be effective, and if cinacalcet can reverse parathyroid hyperplasia in CKD patients, as observed in this animal model of CKD. The present preclinical data demonstrating that discontinuation of cinacalcet results in loss of PTH control and return of parathyroid hyperplasia suggests that continued therapy is likely warranted to keep the disease under control in patients.
Acknowledgments
Funding for this study and the preparation of this manuscript was provided by Amgen, Inc. Writing support was provided by Jon Nilsen, PhD (an employee of Amgen, Inc.).
Conflict of interest statement. J.D., E.S. and C.M.H. are employees of and stockholders in Amgen, Inc. G.M., M.C. and D.M. were formally affiliated with Amgen, Inc. G.M. is a stockholder of Amgen, Inc.
References
- 1.Drueke T, Martin D, Rodriguez M. Can calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studies. Nephrol Dial Transplant. 2007;22:1828–1839. doi: 10.1093/ndt/gfm177. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 3.Drueke TB. The pathogenesis of parathyroid gland hyperplasia in chronic renal failure. Kidney Int. 1995;48:259–272. doi: 10.1038/ki.1995.292. [DOI] [PubMed] [Google Scholar]
- 4.Kaczmarek E, Lacka K, Majewski P, et al. Selected markers of proliferation and apoptosis in the parathyroid lesions: a spatial visualization and quantification. J Mol Histol. 2008;39:509–517. doi: 10.1007/s10735-008-9190-1. [DOI] [PubMed] [Google Scholar]
- 5.Niculescu AB, 3rd, Chen X, Smeets M, et al. Effects of p.21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 7.Tokumoto M, Tsuruya K, Fukuda K, et al. Reduced p.21, p.27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62:1196–1207. doi: 10.1111/j.1523-1755.2002.kid585.x. [DOI] [PubMed] [Google Scholar]
- 8.Dusso AS, Pavlopoulos T, Naumovich L, et al. p.21WAF1 and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth1. Kidney Int. 2001;59:855–865. doi: 10.1046/j.1523-1755.2001.059003855.x. [DOI] [PubMed] [Google Scholar]
- 9.Canalejo A, Canalejo R, Rodriguez ME, et al. Development of parathyroid gland hyperplasia without uremia: role of dietary calcium and phosphate. Nephrol Dial Transplant. 2010;25:1087–1097. doi: 10.1093/ndt/gfp616. [DOI] [PubMed] [Google Scholar]
- 10.Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2009;77:232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 11.Galitzer H, Ben-Dov IZ, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2009;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 12.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meir T, Levi R, Lieben L, et al. Deletion of the vitamin D receptor specifically in the parathyroid demonstrates a limited role for the receptor in parathyroid physiology. Am J Physiol Renal Physiol. 2009;297:F1192–F1198. doi: 10.1152/ajprenal.00360.2009. [DOI] [PubMed] [Google Scholar]
- 14.Li YC, Amling M, Pirro AE, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 15.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 18.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 19.Henley C, Davis J, Miller G, et al. The calcimimetic AMG 641 abrogates parathyroid hyperplasia, bone and vascular calcification abnormalities in uremic rats. Eur J Pharmacol. 2009;616:306–313. doi: 10.1016/j.ejphar.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeth EF, Heaton WH, Miller M, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth EF, Steffey ME, Hammerland LG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 23.Colloton M, Shatzen E, Miller G, et al. Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int. 2005;67:467–476. doi: 10.1111/j.1523-1755.2005.67103.x. [DOI] [PubMed] [Google Scholar]
- 24.Chin J, Miller SC, Wada M, et al. Activation of the calcium receptor by a calcimimetic compound halts the progression of secondary hyperparathyroidism in uremic rats. J Am Soc Nephrol. 2000;11:903–911. doi: 10.1681/ASN.V115903. [DOI] [PubMed] [Google Scholar]
- 25.Wada M, Furuya Y, Sakiyama J, et al. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest. 1997;100:2977–2983. doi: 10.1172/JCI119851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meola M, Petrucci I, Barsotti G. Long-term treatment with cinacalcet and conventional therapy reduces parathyroid hyperplasia in severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2009;24:982–989. doi: 10.1093/ndt/gfn654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terawaki H, Nakano H, Takeguchi F, et al. Regression of parathyroid gland swelling by treatment with cinacalcet. Nephrol Dial Transplant. 2009;24:690–691. doi: 10.1093/ndt/gfn579. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 28.Komaba H, Fukagawa M. Regression of parathyroid hyperplasia by calcimimetics—fact or illusion? Nephrol Dial Transplant. 2009;24:707–709. doi: 10.1093/ndt/gfn717. [DOI] [PubMed] [Google Scholar]
- 29.Komaba H, Nakanishi S, Fujimori A, et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5:2305–2314. doi: 10.2215/CJN.02110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J, Davis J, Van G, et al. Inhibition of parathyroid gland hyperplasia and increased expression of p.21 in the parathyroid are reversed upon discontinuation of cinacalcet HCl treatment (abstract SP045 ERA-EDTA, Glasgow 2006) Nephrol Dial Transplant. 2006;21(Suppl 4):iv30. [Google Scholar]
- 31.WHO Collaborating Centre for Drug Statistics Methodology. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD) 2010. http://www.whocc.no/atc_ddd_index/?code=H05BX1 (29 April 2011, date last accessed) [Google Scholar]
- 32.Charytan C, Coburn JW, Chonchol M, et al. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005;46:58–67. doi: 10.1053/j.ajkd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Chattopadhyay N, Mithal A, Brown EM. The calcium-sensing receptor: a window into the physiology and pathophysiology of mineral ion metabolism. Endocr Rev. 1996;17:289–307. doi: 10.1210/edrv-17-4-289. [DOI] [PubMed] [Google Scholar]
- 34.Ho C, Conner DA, Pollak MR, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2005;288:F253–F264. doi: 10.1152/ajprenal.00302.2004. [DOI] [PubMed] [Google Scholar]
- 36.Gogusev J, Duchambon P, Hory B, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 37.Kifor O, Moore FD, Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1598–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 38.Yano S, Sugimoto T, Tsukamoto T, et al. Association of decreased calcium-sensing receptor expression with proliferation of parathyroid cells in secondary hyperparathyroidism. Kidney Int. 2000;58:1980–1986. doi: 10.1111/j.1523-1755.2000.00370.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown AJ, Ritter CS, Finch JL, et al. Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int. 1999;55:1284–1292. doi: 10.1046/j.1523-1755.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 40.Cozzolino M, Brancaccio D, Gallieni M, et al. Pathogenesis of parathyroid hyperplasia in renal failure. J Nephrol. 2005;18:5–8. [PubMed] [Google Scholar]