Abstract
Stroke is a major neurological disorder characterized by an increase in the Glu (glutamate) concentration resulting in excitotoxicity and eventually cellular damage and death in the brain. HIF-1 (hypoxia-inducible factor-1), a transcription factor, plays an important protective role in promoting cellular adaptation to hypoxic conditions. It is known that HIF-1α, the regulatable subunit of HIF-1, is expressed by astrocytes under severe ischaemia. However, the effect of HIF-1 on astrocytes following Glu toxicity during ischaemia has not been well studied. We investigated the role of HIF-1 in protecting ischaemic astrocytes against Glu toxicity. Immunostaining with GFAP (glial fibrillary acidic protein) confirmed the morphological modification of astrocytes in the presence of 1 mM Glu under normoxia. Interestingly, when the astrocytes were exposed to severe hypoxia (0.1% O2), the altered cell morphology was ameliorated with up-regulation of HIF-1α. To ascertain HIF-1's protective role, effects of two HIF-1α inhibitors, YC-1 [3-(50-hydroxymethyl-20-furyl)-1-benzylindazole] and 2Me2 (2-methoxyoestradiol), were tested. Both the inhibitors decreased the recovery in astrocyte morphology and increased cell death. Given that ischaemia increases ROS (reactive oxygen species), we examined the role of GSH (reduced glutathione) in the mechanism for this protection. GSH was increased under hypoxia, and this correlated with an increase in HIF-1α stabilization in the astrocytes. Furthermore, inhibition of GSH with BSO (l-butathione sulfoximine) decreased HIF-1α expression, suggesting its role in the stabilization of HIF-1α. Overall, our results indicate that the expression of HIF-1α under hypoxia has a protective effect on astrocytes in maintaining cell morphology and viability in response to Glu toxicity.
Keywords: brain ischaemia, cell viability, glutamate, glutathione, hypoxia-inducible factor 1, stroke
Abbreviations: BSO, l-butathione sulfoximine; CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; ENT-1, equilibrative nucleoside transporter 1; EPO, erythropoietin; FBS, fetal bovine serum; GFAP, glial fibrillary acidic protein; Glu, glutamate; GSH, reduced glutathione; HBSS, Hanks balanced salt solution; HIF-1, hypoxia-inducible factor-1; HO-1, haem oxygenase 1; HSP, heat-shock protein; LDH, lactate dehydrogenase; MCB, monochlorobimane; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; YC-1, 3-(50-hydroxymethyl-20-furyl)-1-benzylindazole; 2Me2, 2-methoxyoestradiol
INTRODUCTION
Brain ischaemia induces a cascade of events that involve a loss of glucose and oxygen, membrane depolarization, and Glu (glutamate) release, leading to excitotoxicity. This release of the neurotransmitter Glu and subsequent calcium influx is considered to be the most significant event in the pathogenesis of ischaemic brain damage. Astrocytes play an important role in maintaining extracellular Glu that is released from neurons below toxic levels. They do so by clearing up Glu from the synaptic region through excitatory amino acid transporters and converting the Glu into glutamine by glutamine synthetase. Glutamine is then shuttled back to neurons and is re-used for Glu synthesis (Lehmann et al., 2009). Astrocytes are also involved in the metabolic support to neurons and provide them with nutrients such as lactate to supplement energy requirements. In addition, astrocytes appear to be the main source of EPO (erythropoietin) and GSH (reduced glutathione) in the CNS (central nervous system), having a GSH concentration twice as high that in neurons (Bolanos et al., 1995). There is significant evidence of astrocytes providing GSH and EPO to neighbouring neurons against various stresses (Gabryel and Malecki, 2006). Previous studies have shown that, in ischaemic infarcts, neurons do not survive if neighbouring astrocytes are not viable (Takano et al., 2009). Therefore it is important to examine how ischaemia affects the function and viability of astrocytes.
Under ischaemic conditions, HIF-1 (hypoxia-inducible factor-1) is expressed to promote cell survival. HIF-1 is a heterodimeric protein formed by a continuously expressed subunit HIF-1β and an oxygen-regulated subunit HIF-1α that is stabilized under low oxygen levels. Activation of HIF-1 leads to the transcription of various genes that contribute to the cellular adaptation to these conditions. Some of the genes that play an important role in the protective effect of HIF-1 are those that are involved in angiogenesis such as VEGF (vascular endothelial growth factor), erythropoiesis such as EPO and genes involved in glucose metabolism such as glucose transporters (Siddiq et al., 2007). It has been shown that HIF-1 induces high levels of EPO expression in astrocytes (Masuda et al., 1994), making them the main source of EPO in the CNS. This demonstrates that HIF-1 plays an important role not only in neurons but also in astrocytes. In addition, Glu release during cerebral ischaemia causes the formation of ROS (reactive oxygen species) by the disruption of the mitochondrial electron transport chain and the activation of NAPDH oxidases (Brennan et al., 2009). Activation of HIF-1 has been shown to protect astrocytes against oxidative damage (Chu et al., 2010).
Since astrocytes play an important role in maintaining brain homoeostasis and providing neuroprotection, their response to Glu toxicity and ischaemic insult requires further understanding. The main objective of this study was to investigate the effects of Glu on the viability and morphology of astrocytes exposed to hypoxia and the role that HIF-1 plays under these conditions. Our results demonstrate that HIF-1 has a protective effect on primary rat cortical astrocytes in terms of increasing cell viability and maintaining cell morphology in response to Glu toxicity and severe oxygen deprivation. Furthermore, we show that GSH may contribute to this protection by providing optimal conditions for HIF-1 stabilization.
MATERIALS AND METHODS
Primary culture of astrocytes
All experiments were conducted with the approval of the University of Kansas Institutional Animal Care and Use Committee. Cortical tissue were dissected from the Sprague–Dawley rat (Charles River Laboratories) brains at postnatal day 0 (P0) to P4. The tissues were washed with HBSS (Hanks balanced salt solution) and trypsinized for 50 min at 37°C. The tissues were then dissociated using a fire polished glass pipette in a dissociation medium (HBSS, 0.1% BSA and 8 mM MgCl2), and centrifuged at 4000 g for 4 min at room temperature (22°C). The cells were transferred into and grown in 25 cm2 flasks with DMEM (Dulbecco's modified Eagle's medium) and 10% FBS (fetal bovine serum). After 3–4 weeks the flasks were shaken to purify the astrocytes by dislodging other cell layers. Following purification, astrocytes were plated on coverslips with DMEM and 10% FBS and used for experiments after 10–12 days.
In vitro hypoxia model
Hypoxia was induced by incubating the astrocytes in 0.1% O2/5% CO2 (balanced with N2) in a hypoxia chamber (COY Laboratories) for 3 h. To mimic the high levels of Glu release during ischaemia, astrocytes were treated with 0, 0.001, 0.01, 0.1 and 1 mM of Glu in serum-free medium (DMEM) at 37°C for 3 h. Control experiments were conducted at 21% O2/5% CO2.
Drug treatments
YC-1 [3-(50-hydroxymethyl-20-furyl)-1-benzylindazole] and 2Me2 (2-methoxyoestradiol; Cayman Chemical Company) were used for HIF-1α inhibition studies. Prior to hypoxia exposure, the astrocytes were incubated with 0.1 mM of the inhibitors for 1 h. Preliminary experiments showed that these conditions were sufficient for HIF-1α inhibition during severe hypoxia, as shown in Figure 3. For GSH depletion, astrocytes were pre-incubated with 5 mM BSO (l-butathione sulfoximine; Sigma–Aldrich) for 12 h as described by Noda et al. (2001). The BSO was present for an additional 3 h during the hypoxia treatment to inhibit the re-synthesis of GSH.
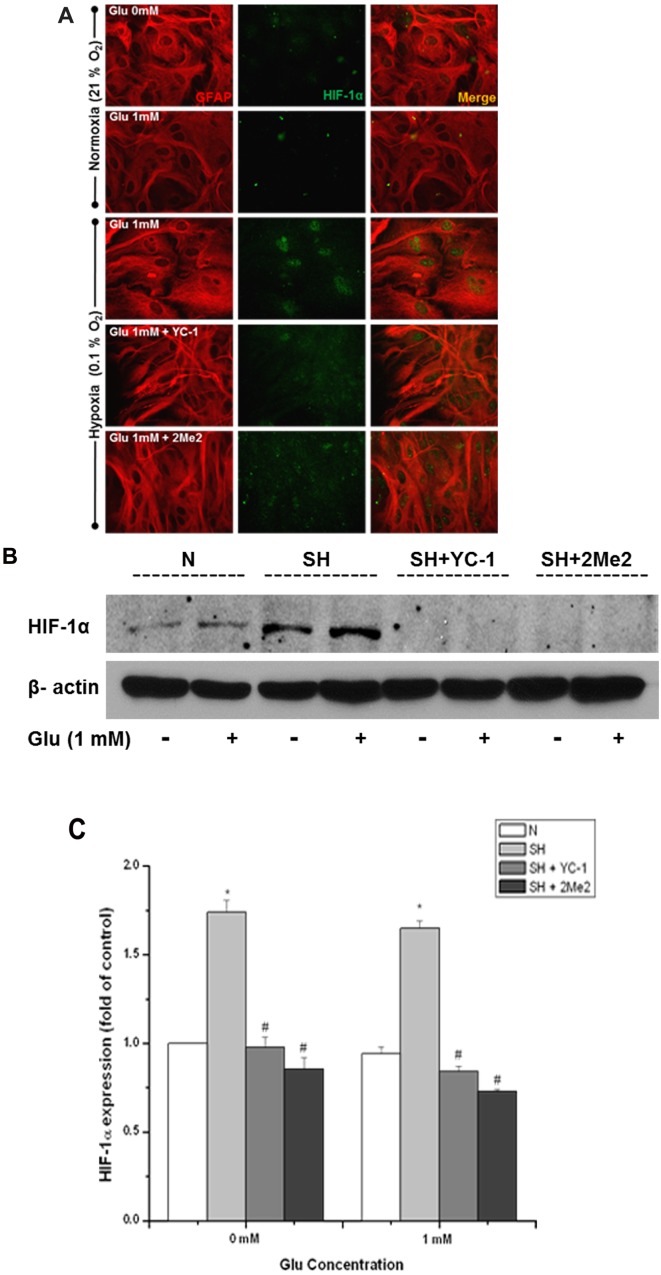
Figure 3. YC-1 and 2Me2 attenuated the protection provided by hypoxia in astrocytes.
(A) Representative immunofluorescent images demonstrating the effect of YC-1 and 2Me2 on HIF-1α (green) expression and astrocyte morphology (GFAP, red). Astrocytes were pre-treated with 0.1 mM YC-1 and 2Me2 followed by 1 mM Glu with exposure to N (normoxia) or SH (severe hypoxia) 3 h. (B) Protein stabilization of HIF-1α determined by Western-blot analysis. Equalization of protein loading was determined using β-actin as the housekeeping protein. *P<0.05 versus 0 mM Glu under normoxia, #P<0.05 versus 0 mM Glu under severe hypoxia (n = 3).
Immunocytochemistry
Following treatments, astrocytes were washed with PBS and fixed with 4% PFA (paraformaldehyde) for 20 min at room temperature. The cells were then permeabilized using 0.3% Triton X-100 for 15 min at room temperature and incubated in a blocking solution (0.05% Triton X-100 and 0.25% BSA dissolved in PBS) for 30 min at room temperature. Astrocytes were incubated with primary antibodies of GFAP (glial fibrillary acidic protein) (1–500, MAB3402, Millipore) and HIF-1α (1–100, sc-8711, Santa Cruz Biotechnology) overnight at 4°C. Cells were washed and incubated with the appropriate secondary antibodies [GFAP: donkey anti-mouse TRITC (tetramethylrhodamine β-isothiocyanate) (1–50; Jackson ImmunoResearch) and HIF-1α: goat anti-rabbit conjugated to Alexa Fluor® 488 (1–100; Molecular Probes)]. Coverslips were washed and mounted by using Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories). Images were acquired on a Leica DMI4000 microscope with a ×40 objective and a Leica DFC340 FX digital camera.
GSH measurement
The GSH level was measured by using the MCB (monochlorobimane) method (Chatterjee et al., 1999). Following the treatments, astrocytes were incubated with 0.1 mM of MCB for 30 min at 37°C. Fluorescence images were then taken immediately, directly from the culture dish. For co-localization studies, the astrocytes were fixed after the MCB treatment and double-stained for GFAP and HIF-1α using the immunocytochemistry procedure described above. The intensity of the fluorescent GSH conjugate (GSH–MCB) of single cells was measured from the images using ImageJ software. Readings of whole-cell intensity were taken from 15 cells from three different culture preparations.
Cytotoxicity assessment
Cell death was assessed by measuring the activity of LDH (lactate dehydrogenase) in the culture medium using an LDH cytotoxicity assay kit (Cayman) as described by Bonfoco et al. (1995). The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay kit (Invitrogen) was also used to assess cell survival and to quantify the Glu-mediated cytotoxicity as described by Mosmann (1983).
Immunoblot analysis
Astrocytes were lysed in 200 μl of RIPA buffer (Thermo Scientific) and the protease inhibitor cocktail kit (Thermo Scientific) and scraped with the aid of a cell lifter (Biologix Research Company). The lysates were centrifuged at 15300 g for 10 min at 4°C, and the protein concentration of the supernatants was determined using a protein assay kit (Bio-Rad). Proteins were separated by SDS/PAGE and the separated proteins were transferred to a nitrocellulose membrane (Bio-Rad). After being blocked with 5% (w/v) non-fat dried skimmed milk powder in TBST (Tris-buffered saline with Tween), the membrane was incubated with the primary antibody (HIF-1α: 1–1000; BD Transduction Laboratories) overnight at 4°C and the secondary antibody (1–3000; goat anti-mouse; Santa Cruz Biotechnology) for 1 h at room temperature. Immunoblots were quantified using ImageJ software and HIF-1α levels were normalized to β-actin.
Texture analysis
Changes in astrocyte texture were determined using CellProfiler cell image analysis software as described previously by Haralick et al. (1973) and Carpenter et al. (2006). Quantification of texture was done from fluorescence images from three different culture preparations. Five microscopic fields were obtained from each culture dish and readings from six to eight cells were taken for further analysis.
Statistical analysis
Data are presented as means±S.D. from a minimum of three independent experiments. One-way ANOVA and the Student's t test were used for overall significance. Differences of P<0.05 were considered statistically significant. Image-Pro Plus 5.1 (Media Cybernetics), ImageJ and Excel were used for data analyses.
RESULTS
Severe hypoxia-protected astrocytes from Glu toxicity
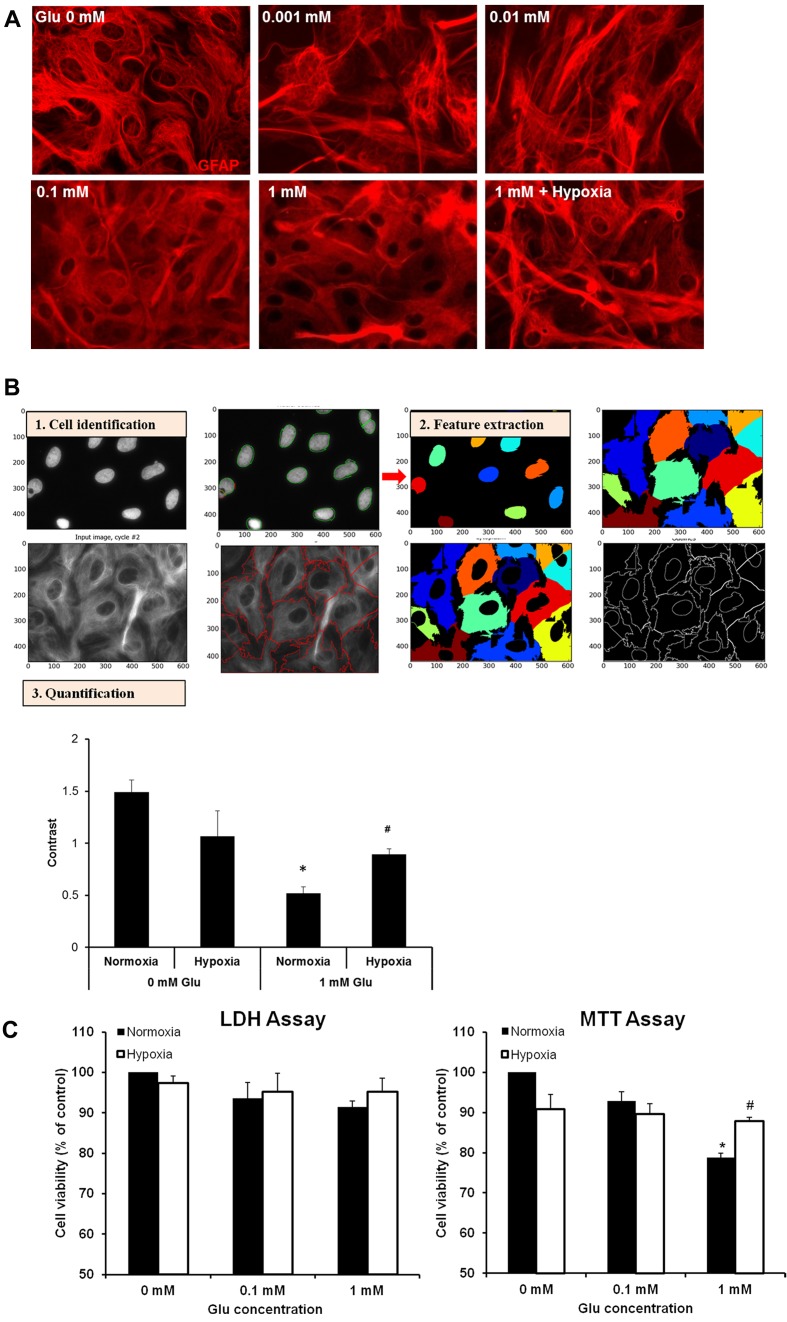
Excessive Glu accumulation is a major cause of neuronal death in the brain during ischaemia. Astrocytes are very important for the clearance of excessive Glu from the extracellular space; however, high concentrations of Glu also affect astrocytes and can lead to their death under normal conditions. Here, we studied the morphological changes in primary rat cortical astrocytes exposed to Glu at various concentrations (0, 0.001, 0.01, 0.1 and 1 mM) for 3 h. The morphology was assessed based on GFAP expression. Lower concentrations (0.001 and 0.01 mM) of Glu had no effect on the morphology. Increased concentrations (0.1 and 1 mM) caused changes in the structure of the astrocytes (Figure 1A). Under control conditions, astrocytes appeared fibrous. However, high Glu resulted in a disrupted or diffuse-like structure. To quantify the GFAP-based structural/morphological changes, we conducted texture analysis by using CellProfiler which measures the amount of local variation present (Carpenter et al., 2006). A higher value with more contrast suggests a more complex structure. The results demonstrate that Glu altered the astrocyte morphology under normoxia, compared with the control (no Glu). Interestingly, when astrocytes were exposed to severe hypoxia (0.1% O2) in the presence of 1 mM Glu, the astrocyte morphology was improved when compared with 1 mM Glu under normoxia. These data are evidence that hypoxia protects astrocytes against Glu toxicity. As the low concentrations of Glu had no effects on astrocyte morphology, the following studies were carried out with 0.1 and 1 mM Glu.
Figure 1. Hypoxia ameliorated astrocyte damage induced by Glu.
(A) Immunostaining characterization of cultured rat cortical astrocytes. Representative images depicting GFAP (red) in astrocytes treated with 0, 0.001, 0.1, 0.1 and 1 mM Glu under normoxia (21% O2) and 1 mM exposed to severe hypoxia (0.1% O2) for 3 h. (B) Morphological profiling of astrocytes stained for GFAP and DAPI (for nucleus staining). Individual cells were identified using CellProfiler software and divided with clear boundaries (1). Representation (3) of quantification of morphology using one of the 13 features computed from each cell to measure and compare texture (2). *P<0.05 versus 0 mM Glu under normoxia. (C) Astrocyte viability assessed using the LDH and MTT assay. *P<0.05 versus 0 mM Glu under normoxia, #P<0.05 versus 1 mM Glu under normoxia (n = 3).
To further examine the protective effect of hypoxia on astrocyte against Glu, we measured cell death with the LDH assay as shown in Figure 1(C). Under normoxia, there was a Glu-concentration-dependent decrease trend in astrocyte viability. Under severe hypoxia there was a sign of recovery. When cell viability was determined with the MTT assay, we observed a significant decrease in cell viability when astrocytes were exposed to 1 mM Glu under normoxia and a significant recovery in astrocyte survival under hypoxia. The difference in the cell viability may be due to the sensitivity of the two cytotoxicity assays. The LDH assay requires a more severe insult that causes damage to the cell membrane. The MTT assay on the other hand works by measuring the metabolic activity of the mitochondria. Nevertheless, results from both assays indicate that hypoxia reduces astrocyte damage caused by Glu.
HIF-1α is highly expressed in astrocytes under severe hypoxia
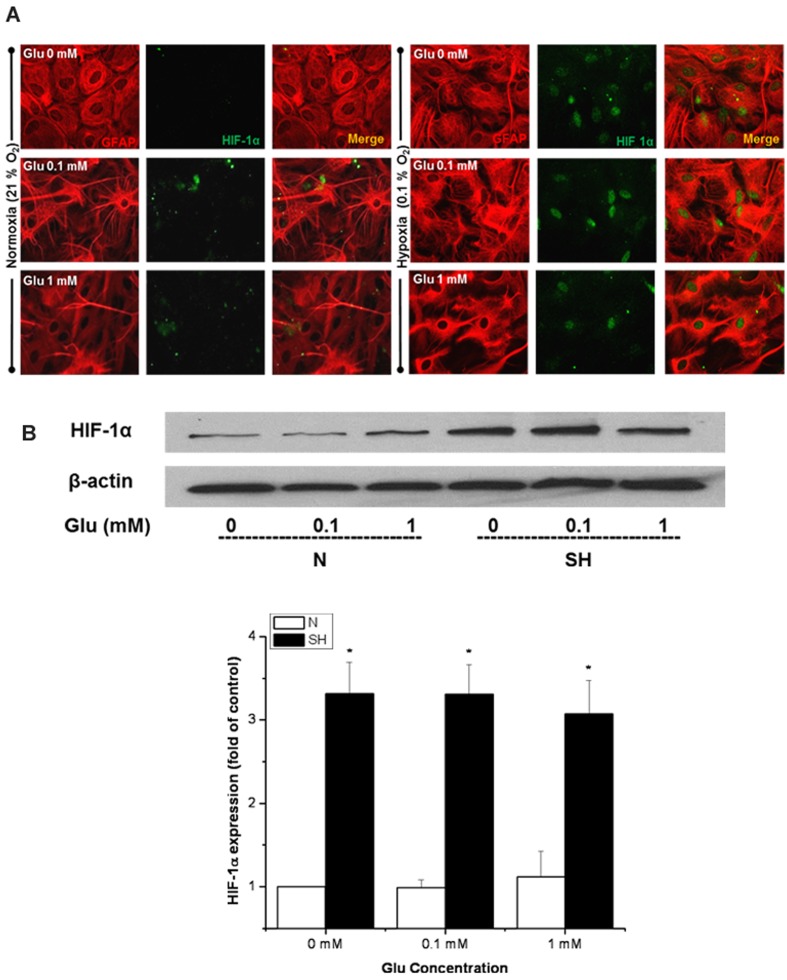
Next, we aimed to address the mechanism by which the astrocytes recovered from the Glu toxicity during severe hypoxia. It is known that HIF-1α is stabilized under low oxygen levels and can contribute to cellular protection under these conditions. HIF-1α expression was first analysed by immunostaining. As expected, there was no HIF-1α expression under normoxia, while treatments with severe hypoxia increased HIF-1 expression in the nuclei of the astrocytes (Figure 2A). HIF-1α was also expressed in the nucleus of the astrocytes following severe hypoxia treatment in the presence of Glu. For further confirmation, Western-blot analysis demonstrated a significant increase in HIF-1α protein levels under severe hypoxia, both with and without Glu treatments (Figure 2B). However, with 1 mM Glu under severe hypoxia there was a slight decrease in the HIF-1α protein levels. This could be due to increased proteasomal degradation induced by an increase in ROS (see the Discussion for further explanation).
Figure 2. HIF-1α expression was increased in astrocytes exposed to severe hypoxia and Glu.
(A) Representative immunofluorescent images showing GFAP (red) and HIF-1α (green) labelling in astrocytes treated with 0, 0.1 and 1 mM Glu under N (normoxia) or SH (severe hypoxia) for 3 h. (B) Protein stabilization of HIF-1α determined by Western-blot analysis. Equalization of protein loading was determined using β-actin as the housekeeping protein. *P<0.05 versus 0 mM Glu under normoxia (n = 3).
HIF-1α inhibition attenuates the recovery of astrocytes from Glu toxicity under severe hypoxia
To confirm whether the recovery of the cell morphology and cell viability under severe hypoxia was in fact due to the expression of HIF-1α, the effect of two HIF-1α inhibitors, YC-1 and 2Me2, were examined on astrocyte damage. Although the mechanism of HIF-1α inhibition by YC-1 and 2Me2 is not fully understood, it appears as though YC-1 acts at a post-translational level and inhibits HIF-1α activation (Li et al., 2008), while 2Me2 inhibits HIF-1α at the level of translation (Mabjeesh et al., 2003). Astrocytes were pre-treated with a 0.1 mM concentration of the inhibitors for 1 h and then subjected to severe hypoxia with the addition of 1 mM Glu for an additional 3 h. Astrocytes treated with YC-1 or 2Me2 showed much less HIF-1α expression in the nuclei of astrocytes under severe hypoxia in the presence of 0 and 1 mM Glu (Figure 3A). The immunostaining also reveals that the addition of either YC-1 or 2Me2 resulted in an attenuation of the recovery of the cell morphology when compared with the controls. Western blotting confirmed that the inhibitors did decrease the HIF-1α protein level (Figure 3B).
Table 1 shows the effects of the two HIF-1 inhibitors on cell morphology quantified by the texture analysis and cell viability assays. Both YC-1 and 2Me2 treatments resulted in a significant change in cell morphology and significant increase in cell death. Astrocytes treated with the HIF-1α inhibitors under normoxia showed no significant effect (Table 1). The increase in cell death was consistent with no recovery in texture with either YC-1 or 2Me2 treatment under severe hypoxia. This suggests that the recovery in astrocyte morphology is caused by the stabilization of HIF-1α under low oxygen levels.
Table 1. Effect of HIF-1α inhibitors on astrocytes exposed to Glu and hypoxia.
Results are presented as means±S.D. (n = 3). N, normoxia; SH, severe hypoxia. *P<0.05 versus normoxia. **P<0.05 versus normoxia 1 mM. #P<0.05 versus severe hypoxia 0 mM.
| Cell viability (%) | ||||
|---|---|---|---|---|
| Glu concentration | Treatment | Texture analysis | LDH assay | MTT assay |
| 0 mM | N | 1.49±0.12 | 100 | 100 |
| N+YC-1 | 1.19±0.04 | 99.12±3.09 | 98.22±1.74 | |
| N+2Me2 | 1.13±0.03 | 99.47±2.52 | 97.95±2.57 | |
| SH | 1.07±0.24 | 97.35±1.79 | 90.82±2.93 | |
| SH+YC-1 | 0.97±0.31 | 66.15±6.42# | 62.74±2.20# | |
| SH+2Me2 | 0.89±0.28 | 66.34±1.96# | 64.41±3.09# | |
| 1 mM | N | 0.52±0.06* | 94.09±1.49 | 82.84±1.77* |
| N+YC-1 | 0.44±1.08* | 98.29±1.51 | 80.42±5.03* | |
| N+2Me2 | 0.48±1.11* | 97.30±3.24 | 81.03±4.12* | |
| SH | 0.89±0.05** | 95.17±3.42 | 89.86±4.02 | |
| SH+YC-1 | 0.55±0.11* | 59.44±3.59# | 55.91±5.24# | |
| SH+2Me2 | 0.51±0.05* | 65.56±5.14# | 58.62±3.37# | |
GSH stabilizes HIF-1α expression
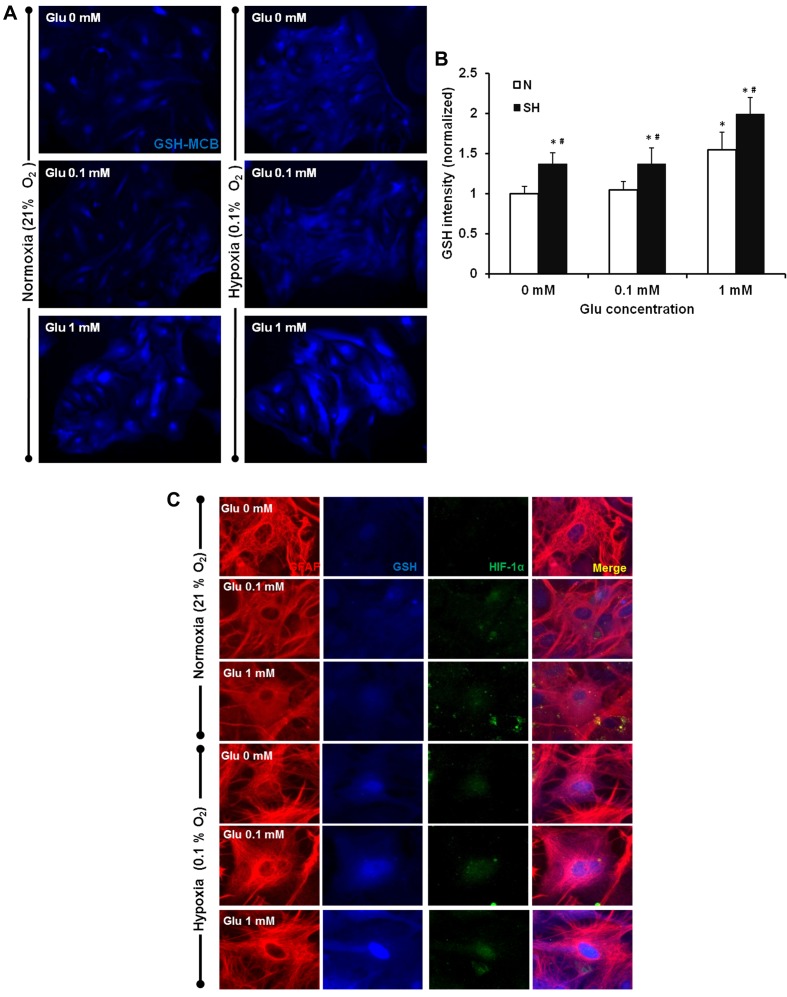
GSH is the most abundant small molecule anti-oxidant that suppresses free radical levels and protects cells against various stress conditions. Previous data from our laboratory has shown that GSH increases HIF-1α expression during oxygen deprivation (Guo et al., 2008). To determine whether GSH plays a role in the HIF-1α stability and protective effect in astrocytes, we compared GSH levels between the different treatments. The cellular GSH level was measured using the MCB method. Fluorescence intensities of GSH-MCB were quantified to differentiate between the levels of GSH present in astrocytes treated with 0.1 and 1 mM Glu under normoxia versus severe hypoxia. The results showed no change in GSH levels in the presence of Glu lower than 0.1 mM under normoxia; however, 1 mM Glu caused a significant increase in GSH (Figures 4A and 4B). Hypoxia increased the GSH levels in astrocytes. The presence of Glu further increased the GSH level in astrocytes exposed to hypoxia.
Figure 4. Astrocyte GSH levels were increased with Glu and severe hypoxia treatments.
Astrocytes were treated with 0.1 mM or 1 mM Glu with exposure to N (normoxia) or SH (severe hypoxia) for 3 h. Astrocytes were loaded with 0.1 mM MCB following treatments. (A) Representatives of GSH-MCB labelling in astrocytes exposed to various conditions. (B) Quantification of the cytosolic GSH intensity in astrocytes exposed to various conditions. *P<0.05 versus 0 mM Glu under normoxia, #P<0.05, normoxia versus severe hypoxia for each Glu concentration. (C) Co-localization of GFAP (red), HIF-1α (green) and GSH (blue) in astrocytes treated with 0, 0.1 and 1 mM Glu under normoxia or severe hypoxia (n = 3).
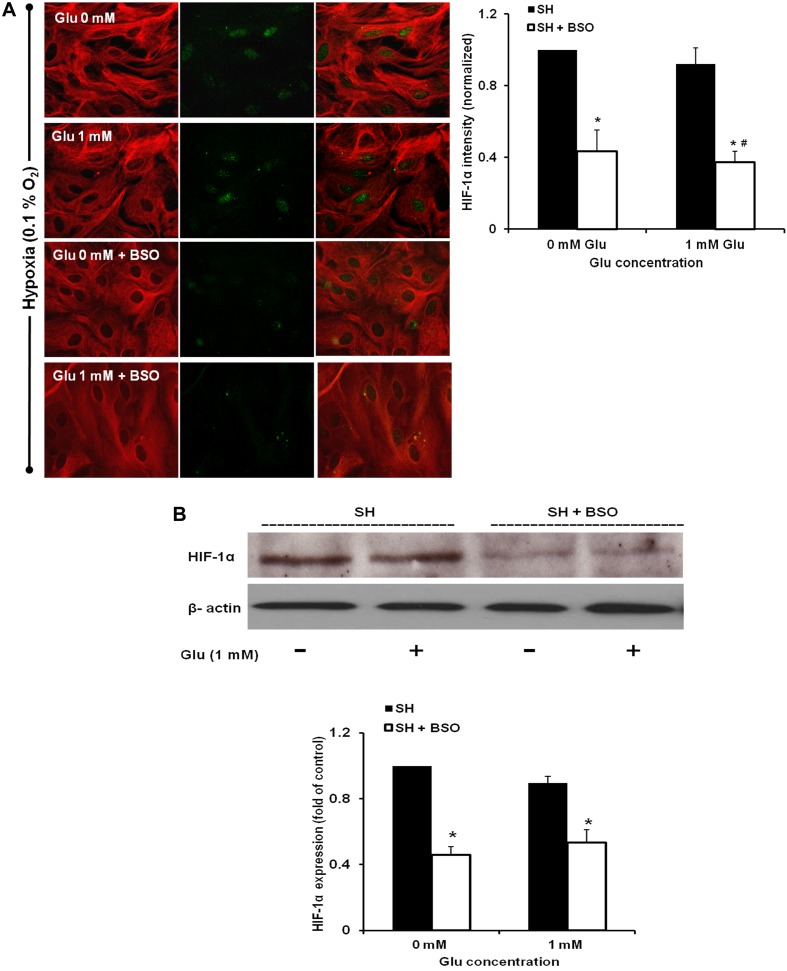
To determine whether an increase in GSH levels contributed to the up-regulated HIF-1α expression, we fixed the cells after MCB treatment with or without severe hypoxia in the presence of Glu and then co-stained for GFAP and HIF-1α. The results showed a co-localization of increased GSH with HIF-1α expression in astrocytes treated with both 0.1 and 1 mM Glu (Figure 4C). This suggests that GSH might help stabilize HIF-1α expression in the astrocytes. To confirm the role of GSH in the HIF-1α up-regulation, astrocytes were pre-treated with 5 mM BSO, which decreases cellular GSH levels, for 12 h and then exposed to Glu and hypoxia. This resulted in a significant attenuation of HIF-1α stabilization (Figure 5). Furthermore, inhibition of GSH and consequently HIF-1α, decreased astrocyte survival and abolished the morphological recovery under hypoxia (Table 2). These results are consistent with the results of HIF-1α inhibition using YC-1 and 2Me2; indicating that HIF-1 provides protection against Glu in hypoxic astrocytes.
Figure 5. GSH inhibition reduces HIF-1α expression.
(A) Representative immunofluorescence images demonstrating the effect of BSO on HIF-1α (green) expression and astrocyte morphology. Astrocytes were pre-treated with 5 mM BSO followed by 1 mM Glu with exposure to SH (severe hypoxia) and compared with astrocytes treated with 0 and 1 mM Glu under severe hypoxia only. HIF-1α intensity was measured and normalized to the control. *P<0.05 versus 0 mM Glu under normoxia, #P<0.05 versus 1 mM Glu under severe hypoxia. (B) Immunoblotting showing HIF-1α protein levels. Equalization of protein loading was determined using β-actin as the housekeeping protein. Quantitative results for Western blot data. *P<0.05 versus 0 mM Glu under normoxia. (n = 3).
Table 2. Effect of BSO on HIF-1α-induced protection of astrocytes.
Results were presented as means±S.D. (n = 3). N, normoxia; SH, severe hypoxia. *P<0.05 versus severe hypoxia. #P<0.05 versus severe hypoxia 1 mM.
| Cell viability (%) | ||||
|---|---|---|---|---|
| Glu concentration | Treatment | Texture analysis | LDH assay | MTT assay |
| 0 mM | SH | 1.10±0.13 | 100 | 100 |
| SH+BSO | 0.59±0.18* | 63.70±3.29* | 57.36±4.69* | |
| 1 mM | SH | 0.96±0.13* | 96.14±1.17 | 97.06±2.37 |
| SH+BSO | 0.54±0.17*# | 60.68±2.79*# | 54.35±5.26*# | |
DISCUSSION
To date, there are no effective neuroprotectants for human stroke, and the development of neuroprotective strategies is considered extremely challenging. Although many agents have been tested for the treatment of ischaemic stroke, such as anti-oxidative, anti-apoptotic, anti-excitotoxic and anti-inflammatory drugs, they have all proved unsuccessful. One of the reasons for this may be our incomplete understanding of the mechanisms that are responsible for cellular death. Furthermore, the study of cell death following cerebral ischaemia has been primarily focused on neurons. In addition to neurons, ischaemia also causes damage to astrocytes (Martin et al., 1997; Yu et al., 2001; Lukaszevicz et al., 2002; Giffard and Swanson, 2005), which are critical in maintaining neuronal viability and functions under ischaemic conditions. Therefore targeting astrocytes can be an important strategy to enhance neuronal survival.
Recently, HIF-1 is being focused on as a potential target for stroke therapy as it appears to have many beneficial roles in the ischaemic brain (Baranova et al., 2007). Although HIF-1 has been shown to have a protective effect specifically in astrocytes in neurological diseases such as Alzheimer's disease (Schubert et al., 2009), few studies have been done to investigate the effect of HIF-1α expression on astrocytes during stroke.
Given the increase in Glu in the ischaemic brain and the scavenging function of astrocytes on Glu, we examined the effects of Glu on the viability and morphology of astrocytes exposed to hypoxia. Compared with neurons, astrocytes are very resistant to hypoxia due to several factors. First, astrocytes have large glycogen stores (Phelps, 1972) that can be metabolized to glucose and lactate and supply energy during ischaemia and glucose deprivation (Swanson and Choi, 1993). Secondly, astrocytes have a low energy demand, unlike neurons, which have a high density of ion channels and require more ATP to maintain ionic gradients. Thus ionic deregulation occurs slowly in astrocytes (Silver et al., 1997). Thirdly, astrocytes have a higher level of GSH, an important antioxidant, than neurons and provide GSH or substrates for GSH synthesis to neurons (Makar et al., 1994, Chen and Swanson 2003). In our experiments, astrocytes were exposed to severe hypoxia (0.1% O2) because a milder hypoxic exposure (1% O2) was not sufficient to cause any significant changes in HIF-1α expression (data not shown). As the results demonstrated, Glu caused a concentration-dependent change in astrocyte morphology, which resembled a more diffuse-like structure from a fibrous one, under normoxia. This was correlated with a decrease in cell viability determined by LDH and MTT assays. Interestingly, when the astrocytes were treated with the same Glu concentrations under severe hypoxia there was a recovery in cell morphology and cell viability. Our observation is consistent with a previous study that demonstrated that pre-treatment with hypoxia reduced astrocyte damage by 45–55% (Chen et al., 2000).
One of the remarkable observations in our study is that increased GSH is not sufficient to protect astrocytes from damage induced by Glu under normoxia. As shown in Figures 4(A) and 4(B), Glu at 1 mM significantly elevated the GSH level in astrocyte under normoxia and induced significant cell damage detected by cellular texture and viability (Figure 1). In contrast, under hypoxic conditions the increase of GSH was accompanied by improved cellular texture and astrocyte viability (Figures 1 and 4). This protective effect provided in the hypoxic conditions is largely ascribed to the expression of HIF-1, since inhibiting HIF-1 abolished the protection (Figure 3 and Table 1). Furthermore, stabilizing HIF-1α expression under normoxia with cobalt chloride appeared to have an even more significant effect in the recovery of cell morphology (Supplementary Figure S1B available at http://www.asnneuro.org/an/004/an004e090add.htm). Taken together, these findings strongly support that the activation of HIF-1 is responsible for promoting the astrocytic survival and protection against Glu toxicity under hypoxia.
Many factors may contribute to HIF-1-mediated protection in hypoxic astrocytes. First, HIF-1 up-regulates EPO (Semenza et al., 1997), which provides cellular protection under different stresses. Many studies have examined the effect of EPO on astrocytes, since they are the main source of EPO in the brain. For example, EPO has been shown to protect astrocytes from damage in response to oxidative stress (Liu et al., 2006) and other agents that induce apoptosis (Diaz et al., 2005). Secondly, HIF-1 leads to the induction of VEGF in astrocytes (Sinor et al., 1998). VEGF has been shown to play an important role in cellular protection in hypoxic preconditioning (Wick et al., 2002) by promoting angiogenesis (Jin et al., 2000). A study by Mani et al. (2005) has revealed that exogenous VEGF induces astrocyte proliferation. In addition, HIF-1 may promote the production of adenosine that offers neuroprotective properties (Heurteaux et al., 1995; Wardas 2002; Lin et al., 2008). Adenosine binds to the presynaptic A1 receptor and may lead to a decrease in Ca2+ influx. This further decreases the release of Glu and excitation of the NMDA (N-methyl-D-aspartate) receptors, thus preventing cellular damage caused by the subsequent increases in Ca2+ influx (Monopoli et al., 1998; Wardas 2002). A study by Batti et al. (2010) has shown that the stabilization of HIF-1α through prolyl hydroxylase inhibition protected against Glu-induced damage in the hippocampus of the rat ischaemic brain mainly through adenosine accumulation in response to hypoxia. A review article by Vangeison and Rempe (2009) clearly describes how hypoxia and HIF-1 can regulate various proteins, including connexin 43, CD73 and the ENT-1 (equilibrative nucleoside transporter 1), which ultimately leads to enhanced adenosine levels. Of these, both CD73 and ENT-1 have been shown to be regulated by HIF-1 in intestinal epithelia (Synnestvedt el al. 2002) and endothelial cells (Eltzschig et al., 2005) respectively. CD73 and ENT-1 are expressed in astrocytes (Vangeison and Rempe 2009); therefore it is possible that HIF-1 can regulate their activity and increase adenosine in astrocytes. In fact, it has been shown that adenosine has a direct protective effect on rat primary astrocytes by reducing death induced by glucose deprivation (Shin et al., 2002) and in reducing damage in human astroglioma D384 cells following oxygen deprivation through the preservation of ATP levels (Bjorklund et al., 2008).
The results presented in this paper clearly demonstrate that GSH increases the HIF-1α level in astrocytes exposed to hypoxia. As shown in Figure 4, there was an increase in GSH following the 3 h Glu and severe hypoxia treatment. In addition, BSO, which inhibits GSH synthesis, decreased HIF-1α expression. Our results are consistent with previous studies that have shown that altering redox status can effect HIF-1α expression in other types of cells. Inhibition of GSH synthesis by BSO reduced HIF-1α expression in lung epithelial cells (Haddad and Land, 2000) and in hepatic cells (Jin et al., 2011). Treatment with N-acetylcysteine, the GSH precursor, increased HIF-1α expression in lung epithelial cells (Haddad et al., 2000) and in hepatic cells (Sommani et al 2007; Jin et al., 2011). Our previous results have also demonstrated that HIF-1α stability favours a reducing environment in neurons (Guo et al., 2008). Recently, Tajima et al. (2009) showed that the induction of HIF under hypoxia is regulated by the redox state of GSH in HSC-2 (human oral squamous cells). They suggest that GSH can regulate the activation of HIF by directly binding to the thiol groups of regulatory cellular proteins. Since HIF-1α is sensitive to redox status and can be degraded by increased ROS (Liu et al., 2004; Wellman et al., 2004), it is reasonable to consider that the mechanism by which GSH increases HIF-1α is through the clearance of excessive ROS and by promoting a suitable reducing environment that prolongs its stabilization. Our immunoblot results showed that, under normoxia, there was an increase in HIF-1α levels when astrocytes were treated with 1 mM Glu. Even though this increase was not significant, it did correlate with increased GSH levels. It seems as though GSH is able to stabilize HIF-1α and reduce its degradation when it is normally expressed during hypoxia. However, during normoxia, the oxygen levels are sufficient to maintain prolyl hydroxylase activity and target HIF-1α for ubiquitination.
Meanwhile, HIF-1 may increase the level of GSH in hypoxic astrocytes, as shown in our results. Others have also reported that HIF-1 is able to maintain GSH levels in brains of rats exposed to hypoxia (Shrivastava et al, 2008). In addition, previous studies have shown that HIF-1 can protect astrocytes from ROS-induced injury (Chu et al., 2010) and that GSH depletion induces astrocytic death in response to ROS (Im et al., 2006). All these results indicate that maintaining GSH levels and reducing ROS toxicity is part of HIF-1-mediated neuroprotection. Based on our results and others, we postulate the GSH–HIF-1 crosstalk in hypoxic astrocytes (Figure 6). Hypoxia induces accumulation of HIF-1, which subsequently switches on the expression of genes that promote cell survival such as glucose transporters and glycolytic enzymes, EPO, VEGF, HSPs (heat-shock proteins) (Baird et al., 2006), HO-1 (haem oxygenase 1) (Shrivastava et al., 2008), etc. Some of the genes such as EPO and VEGF provide direct cell protection against hypoxic stress. Others, such as those of glucose metabolism, HSPs and HO-1, may increase the level of GSH, contributing to HIF-1 stabilization.
Figure 6. Schematic diagram of GSH–HIF-1 cross-talk in promoting cell survival during hypoxia.
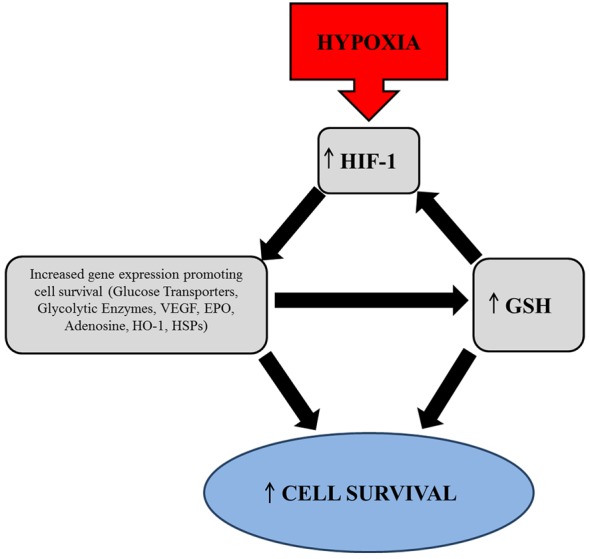
Hypoxia leads to HIF-1 stabilization which subsequently activates the expression of various pro-survival genes. These include EPO, VEGF, HSPs, HO-1 and proteins that increase glucose metabolism such as glucose transporters and glycolytic enzymes. Genes such as those of glucose metabolism, HSPs and HO-1 may increase the level of GSH causing it to exert its anti-oxidant effect and promote survival. In addition, the reducing environment created by GSH can in turn contribute to HIF-1 stabilization.
In conclusion, there are still large gaps that exist in our understanding of how astrocytes are affected during stroke. Given that the release of the Glu is considered to be the leading cause of brain damage following stroke, we determined the effect of HIF-1α expression and stabilization on how astrocytes respond to Glu toxicity during stroke. Our study has shown that HIF-1α expression protects astrocytes from Glu-induced damage. We also provide evidence that GSH plays a role in HIF-1α stabilization and promotes its protective effect. Taking this fact into consideration will lead to a better understanding of the protective mechanisms of astrocytes and provide a more effective approach not only to stroke therapy but also other pathological conditions that cause, or are exacerbated by, excitotoxicity.
Online data
ACKNOWLEDGEMENT
We thank Heather E. Shinogle for assistance with CellProfiler analysis.
Footnotes
This work was supported in part by the National Institutes of Health [grant number R01NS058807] and a startup fund from the University of Kansas Center for Research.
REFERENCES
- Baird NA, Turnbull DW, Johnson EA. Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J Biol Chem. 2006;281:38675–38681. doi: 10.1074/jbc.M608013200. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batti L, Taylor C, O'Connor J. Hydroxylase inhibition reduces synaptic transmission and protects against a glutamate-induced ischemia in the CA1 region of the rat hippocampus. Neuroscience. 2010;167:1014–1024. doi: 10.1016/j.neuroscience.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Bjorklund O, Shang M, Tonazzini I, Dare E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurons and astrocytes in primary culture. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Noack H, Possel H, Keilhoff G, Wolf G. Glutathione levels in primary glial cultures: monochlorobimane provides evidence of cell type-specific distribution. Glia. 1999;27:152–161. [PubMed] [Google Scholar]
- Chen CJ, Liao SL, Kuo JS. Gliotoxic action of glutamate on cultured astrocytes. J Neurochem. 2000;75:1557–1565. doi: 10.1046/j.1471-4159.2000.0751557.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Chu PWY, Beart PM, Jones NM. Preconditioning protects against oxidative injury involving hypoxia-inducible factor-1 and vascular endothelial growth factor in cultured astrocytes. Eur J Pharmacol. 2010;633:24–32. doi: 10.1016/j.ejphar.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Diaz Z, Assaraf MI, Miller WH Jr, Schipper HM. Astroglial cytoprotection by erythropoietin pre conditioning: implications for ischemic and degenerative CNS disorders. J Neurochem. 2005;93:392–402. doi: 10.1111/j.1471-4159.2005.03038.x. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryel B, Malecki A. Ebselen attenuates oxidative stress in ischemic astrocytes depleted of glutathione. Comparison with glutathione precursors. Pharmacol Rep. 2006;58:381–392. [PubMed] [Google Scholar]
- Giffard RG, Swanson RA. Ischemia-induced programmed cell death in astrocytes. Glia. 2005;50:299–306. doi: 10.1002/glia.20167. [DOI] [PubMed] [Google Scholar]
- Guo S, Bragina O, Xu Y, Cao Z, Chen H, Zhou B, Morgan M, Lin Y, Jiang BH, Liu KJ. Glucose upregulates HIF-1α expression in primary cortical neurons in response to hypoxia through maintaining cellular redox status. J Neurochem. 2008;105:1849–1860. doi: 10.1111/j.1471-4159.2008.05287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJE, Land SC. O2-evoked regulation of HIF-1α and NF-κB in perinatal lung epithelium requires glutathione biosynthesis. Am J Physiol Lung Cell Mol Physiol. 2000;278:L492–L503. doi: 10.1152/ajplung.2000.278.3.L492. [DOI] [PubMed] [Google Scholar]
- Haddad JJE, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1 and NF-κB redox sensitivity. J Biol Chem. 2000;275:21130. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cyber. 1973;3:610–621. [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JY, Paik SG, Han PL. Cadmium-induced astroglial death proceeds via glutathione depletion. J Neurosci Res. 2006;83:301–308. doi: 10.1002/jnr.20722. [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA. Induction of vascular endothelial growth factor receptors and phosphatidylinositol 3′-kinase/Akt signaling by global cerebral ischemia in the rat. Neuroscience. 2000;100:713–717. doi: 10.1016/s0306-4522(00)00331-6. [DOI] [PubMed] [Google Scholar]
- Jin W, Kong Z, Shen Z, Jin Y, Zhang W, Chen G. Regulation of hypoxia inducible factor-1α expression by the alteration of redox status in HepG2 cells. J Exp Clin Cancer Res. 2011;30:61. doi: 10.1186/1756-9966-30-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Bette S, Engele J. High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res. 2009;1297:1–8. doi: 10.1016/j.brainres.2009.08.070. [DOI] [PubMed] [Google Scholar]
- Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1. Mol Cancer Ther. 2008;7:3729. doi: 10.1158/1535-7163.MCT-08-0074. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Narasimhan P, Song YS, Nishi T, Yu F, Lee YS, Chan PH. Epo protects SOD2 deficient mouse astrocytes from damage by oxidative stress. Glia. 2006;53:360–365. doi: 10.1002/glia.20289. [DOI] [PubMed] [Google Scholar]
- Liu Q, Berchner-Pfannschmidt U, Moller U, Brecht M, Wotzlaw C, Acker H, Jungermann K, Kietzmann T. A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc Natl Acad Sci USA. 2004;101:4302. doi: 10.1073/pnas.0400265101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszevicz AC, Sampaio N, Guegan C, Benchoua A, Couriaud C, Chevalier E, Sola B, Lacombe P, Onteniente B. High sensitivity of protoplasmic cortical astroglia to focal ischemia. J Cereb Blood Flow Metab. 2002;22:289–298. doi: 10.1097/00004647-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJL. Vitamin E, ascorbate, glutathione, glutathicne disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem. 1994;62:45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- Mani N, Khaibullina A, Krum JM, Rosenstein JM. Astrocyte growth effects of vascular endothelial growth factor (VEGF) application to perinatal neocortical explants: receptor mediation and signal transduction pathways. Exp Neurol. 2005;192:394–406. doi: 10.1016/j.expneurol.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269:19488. [PubMed] [Google Scholar]
- Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischemia in rats. Neuroreport. 1998;9:3955. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Noda T, Iwakirir R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001;15:2131–2139. doi: 10.1096/fj.01-0131com. [DOI] [PubMed] [Google Scholar]
- Phelps CH. Barbiturate-induced glycogen accumulation in brain: an electron microscopic study. Brain Res. 1972;39:225–234. doi: 10.1016/0006-8993(72)90797-4. [DOI] [PubMed] [Google Scholar]
- Schubert D, Soucek T, Blouw B. The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur J Neurosci. 2009;29:1323. doi: 10.1111/j.1460-9568.2009.06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- Shin CY, Jang ES, Choi JW, Ryu JR, Kim WK, Kim HC, Choi CR, Ko KH. Adenosine and purine nucleosides protect rat primary astrocytes from peroxynitrite-potentiated, glucose deprivation-induced death: preservation of intracellular ATP level. Exp Neurol. 2002;176:175–182. doi: 10.1006/exnr.2002.7913. [DOI] [PubMed] [Google Scholar]
- Shrivastava K, Shukla D, Bansal A, Sairam M, Banerjee P, Ilavazhagan G. Neuroprotective effect of cobalt chloride on hypobaric hypoxia-induced oxidative stress. Neurochem Int. 2008;52:368–375. doi: 10.1016/j.neuint.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Ratan RR. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res. 2007;32:931–46. doi: 10.1007/s11064-006-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Deas J, Erecińska M. Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. J Neurosci. 1997;78:589–602. doi: 10.1016/s0306-4522(96)00600-8. [DOI] [PubMed] [Google Scholar]
- Sinor AD, Irvin SM, Cobbs CS, Chen J, Graham SH, Greenberg DA. Hypoxic induction of vascular endothelial growth factor (VEGF) protein in astroglial cultures. Brain Res. 1998;812:289–291. doi: 10.1016/s0006-8993(98)00976-7. [DOI] [PubMed] [Google Scholar]
- Sommani P, Yamashita K, Miyoshi T, Tsunemine H, Kodaki T, Mori H, Hirota K, Arai T, Sasada M, Makino K. Inhibitory effect of 6-formylpterin on HIF-1α protein accumulation. Biol Pharm Bull. 2007;30:2181–2184. doi: 10.1248/bpb.30.2181. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Choi DW. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13:162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M, Kurashima Y, Sugiyama K, Ogura T, Sakagami H. The redox state of glutathione regulates the hypoxic induction of HIF-1. Eur J Pharmacol. 2009;606:45–9. doi: 10.1016/j.ejphar.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40((3 Suppl)):S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangeison G, Rempe DA. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist. 2009;15:579. doi: 10.1177/1073858409332405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardas J. Neuroprotective role of adenosine in the CNS. Polish J Pharmacol. 2002;54:313–326. [PubMed] [Google Scholar]
- Wellman TL, Jenkins J, Penar PL, Tranmer B, Zahr R, Lounsbury KM. Nitric oxide and reactive oxygen species exert opposing effects on the stability of hypoxia-inducible factor-1α (HIF-1α) in explants of human pial arteries. FASEB J. 2004;18:379–381. doi: 10.1096/fj.03-0143fje. [DOI] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–7. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ACH, Wong HK, Yung HW, Lau LT. Ischemia-induced apoptosis in primary cultures of astrocytes. Glia. 2001;35:121–130. doi: 10.1002/glia.1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.