Abstract
Two desferrioxamine B-ciprofloxacin conjugates with “trimethyl-lock” based linkers that are designed to release the antibiotic after esterase or phosphatase-mediated hydrolysis were synthesized. The potential esterase-sensitive conjugate 13 displayed moderate to good antibacterial activities against selected ferrioxamine-utilizing bacteria, although the activities were lower than the parent drug ciprofloxacin. However, the potential phophatase-sensitive conjugate 23 was inactive against the same panel of organisms tested. These properties appeared to be related to the activating efficiency of the linker by the enzyme and to the outer membrane protein recognition of the chemically modified siderophore used in the conjugate.
I. Introduction
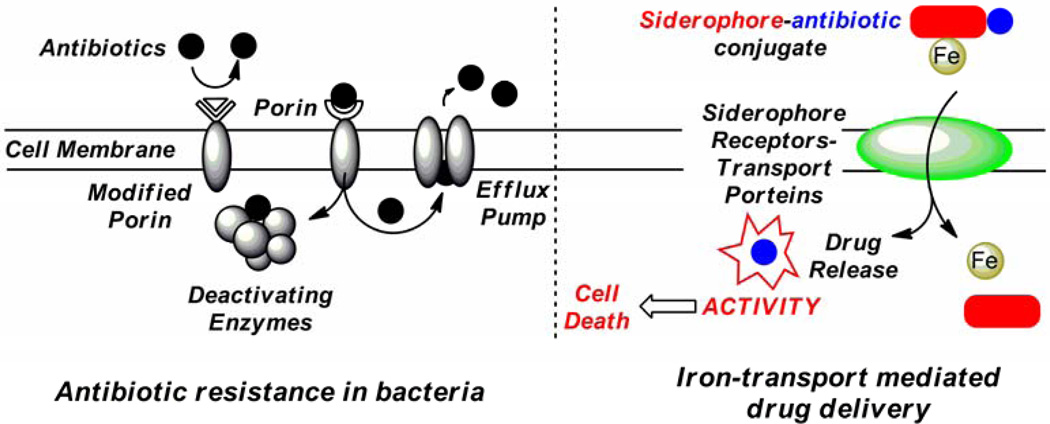
The emergence of pathogenic bacteria that are resistant to existing antibiotics has increased at an alarming rate.1–2 Due to the misuse and overuse of antibiotics, bacteria have developed various mechanisms for resistance, including 1) changing membrane permeability via porin modification; 2) decreasing intracellular drug concentration by efflux pump systems and 3) enzymatically deactivating the antibiotic.3 (Figure 1) Among these modes of resistance, the lack of cell wall permeability resulting from a limitation of passive diffusion through size restricted porins is especially significant.4 Therefore, the development of effective and efficient drug delivery processes for combating porin-mediated antibiotic resistance is of particular interest.
Figure 1.
Antibiotic resistance in bacteria and iron-transport mediated drug delivery (the “Trojan Horse” strategy).
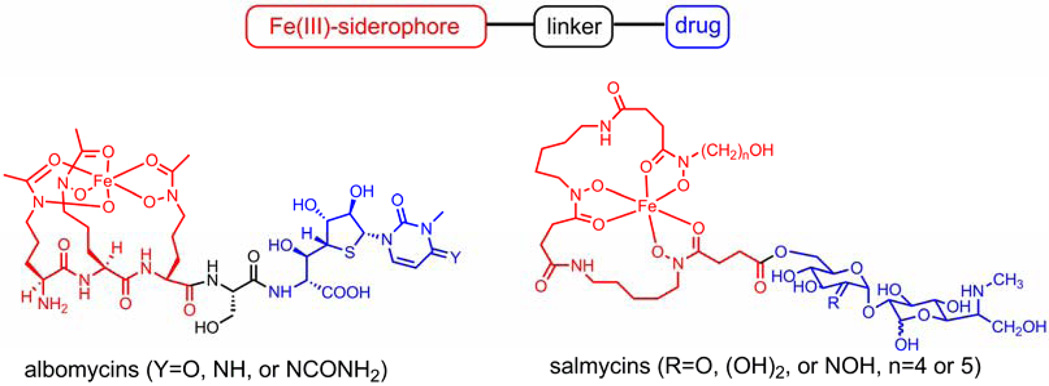
One attractive strategy for antibiotic delivery is to exploit the microbial iron acquisition system which is essential to all pathogenic bacteria. Iron is a crucial element for almost all forms of life. At physiological pH, however, the bioavailability of iron is restricted by its low solubility. To assimilate iron for survival, microorganisms synthesize and secrete low molecular weight iron chelators called siderophores which bind iron(III) effectively by forming high affinity complexes. The resulting siderophore-iron(III) complexes can be recognized by specific outer membrane proteins and then be actively transported into the cell via certain protein channels.5 The indispensable iron uptake system has been recognized as a suitable target for developing new antibiotic strategies. For example, using siderophore-drug conjugates as a “Trojan Horse” for the delivery of antibiotics can bypass the membrane-associated resistance mechanism as the conjugates are actively transported by the iron uptake system in the target microbe. This “Trojan Horse” approach has been adopted by certain bacteria during evolution to compete with other microorganisms for necessary nutrients. Naturally occurring siderophore-drug conjugates (sideromycins) such as albomycins6–8 and salmycins9–10 are found to be effective antibacterial agents and to enter bacterial cells via active iron uptake pathways. (Scheme 1)
Scheme 1.
General siderophore drug conjugates and two naturally occurring examples.
Structurally, a typical siderophore-drug conjugate consists of three parts: siderophore, linker and drug. (Scheme 1) For a long time, the linker was considered as a subunit of the drug. However, it actually is a key component of the conjugate. In some cases in which the drug must be cleaved from the siderophore moiety to for optimum potency, incorporation of a linker with a drug release function in the conjugate becomes crucial. In the aforementioned albomycins, after being internalized by non-producing strains of bacteria, the drug-moiety, namely the thionucleoside, is enzymatically cleaved by the action of a serine peptidase N. The drug-release process is essential for antibiotic activity of albomycins as microorganisms lacking serine peptidase N to cleave the drug are not albomycin-sensitive.6–8 Intracellular drug release has also been shown to be necessary for maximum activity for synthetic siderophore-drug conjugates. For example, fluoroquinolones have been conjugated to pseudomonal siderophores pyoverdin and pyochelin to target Pseudomonas aeruginosa.11–13 These conjugates displayed greatest activity with a hydrolysable linkage that releases the antibiotic, however, in a random manner. Therefore, novel linkers with drug release function controlled by a biochemical process need to be further explored.
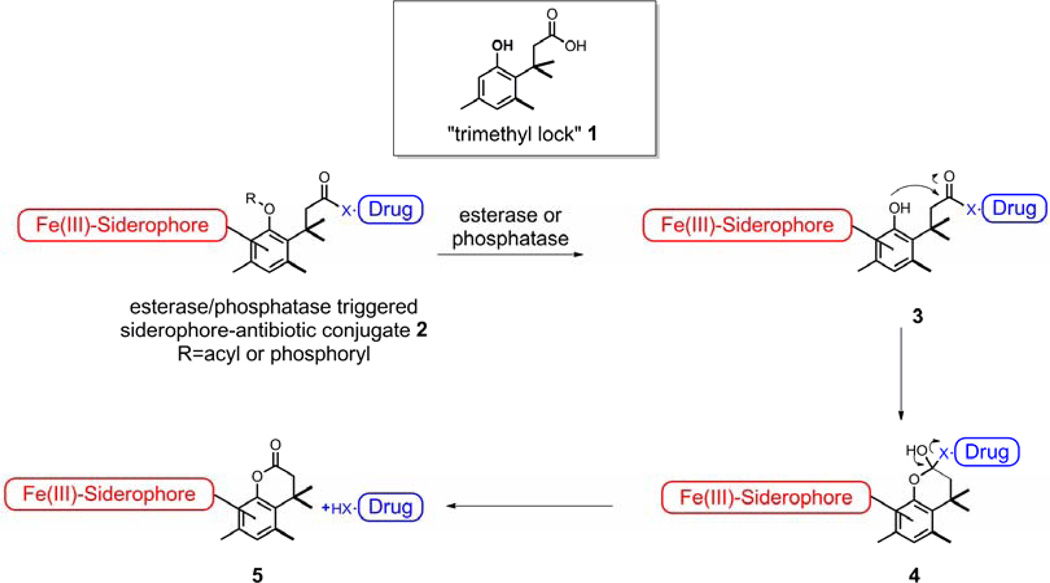
We reasoned that a “trimethyl lock” induced lactonization triggered by the action of enzymes such as esterases/phosphatases could be used for the drug release process. The chemical structure of the “trimethyl lock” 1 is an o-hydroxycinnamic acid derivative in which unfavorable steric interactions between the three methyl groups encourage rapid lactonization to form a hydrocoumarin.14–16 The rate enhancements resulting from the “trimethyl lock” are on the order of 105 relative to an unsubstituted dihydrocoumarin acid.14, 17–18 The “trimethyl lock” structure has been applied to several prodrug strategies, including those sensitive to esterases and phosphatases.19–27 In bacteria and fungi, esterases and phosphatases are known to not only be widely distributed, but also for often being substrate non-specific, which led to the hypothesis that they have evolved to enable access to carbon or phosphorus sources for uptake and use by the microbes.28–29 Therefore, linkers with a “trimethyl lock” structure triggered by potential esterases and phosphatases could be incorporated in a “Trojan Horse” approach where a siderophore is attached to an acyl or phosphoryl phenolic derivative of the “trimethyl lock” structure 1, and a drug is condensed with the carboxyl group as an ester or amide to give siderophore-antibiotic conjugate 2. Enzymatic hydrolysis of conjugate 2 with potential esterases or phosphatases could produce the transient “trimethyl lock” structure 3 which is anticipated to undergo rapid lactonization with concomitant release of the drug. (Scheme 2)
Scheme 2.
“Trimethyl lock” derived siderophore-antibiotic conjugates.
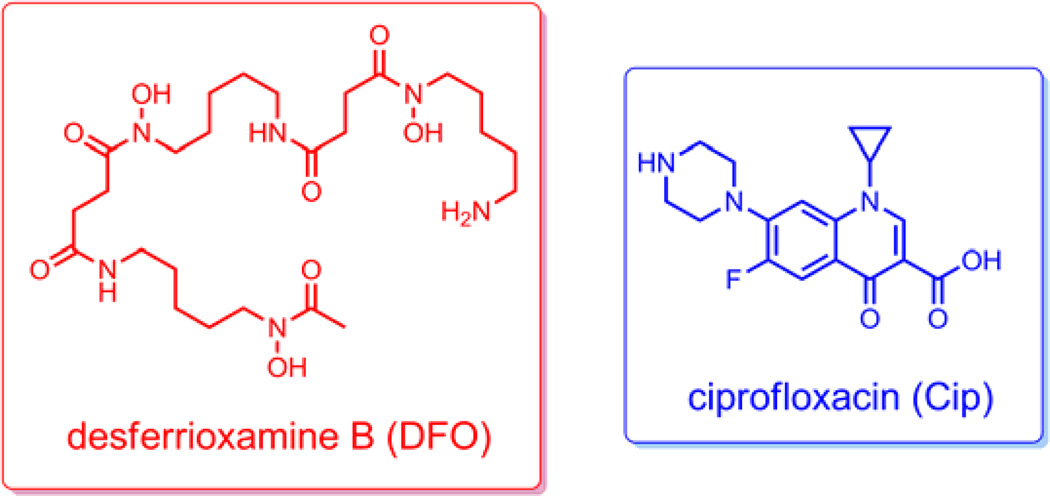
Our choice for drug attachment was ciprofloxacin (Scheme 3), a quinolone antibiotic used to treat a wide variety of both Gram-negative and Gram-positive bacteria infections.30 It targets bacterial DNA gyrase and DNA topoisomerase IV, both of which are required for cell growth and division. Previous studies showed that some siderophore-fluoroquinolone conjugates are up to 50 times less active than the parent drug in terms of gyrase inhibition activity, most likely due to decreased target recognition from steric hindrance caused by the presence of the siderophore in the conjugate.12 Therefore, an intracellular drug release process to ensure efficacy of the conjugates is highly preferred. Desferrioxamine B (Scheme 3) was selected as the siderohore in the conjugates. It is an important and well-characterized trihydroxamate siderophore produced by several species of Nocardia, Streptomyces, Micromonospora, Arthrobacter, Chromobacterium, and Pseudomonas, and is utilized by a variety of other bacteria and fungi.31 Herein we describe the syntheses of two desferrioxamine B-ciprofloxacin conjugates with potential esterase and phosphatase triggered “trimethyl lock” linkers, respectively, with the aim of exploring their antibacterial activities and possible drug release mechanisms.
Scheme 3.
Structures of desferrioxamine B (DFO) and Ciprofloxacin (Cip).
II. Results and Discussion
1. Synthesis of conjugates
In the design of the potential esterase triggered conjugate, the phenolic oxygen in the linker was masked with a succinyl group with the desferrioxamine attached. In the potential phosphatase triggered conjugate, the phenolic oxygen was masked with a phosphate group while the desferrioxamine was attached on an additional phenolic oxygen at the para position. We anticipated that both conjugates could restore the “trimethyl lock” structure after the masking acyl or phosphoryl groups were enzymatically cleaved to release the ciprofloxacin.
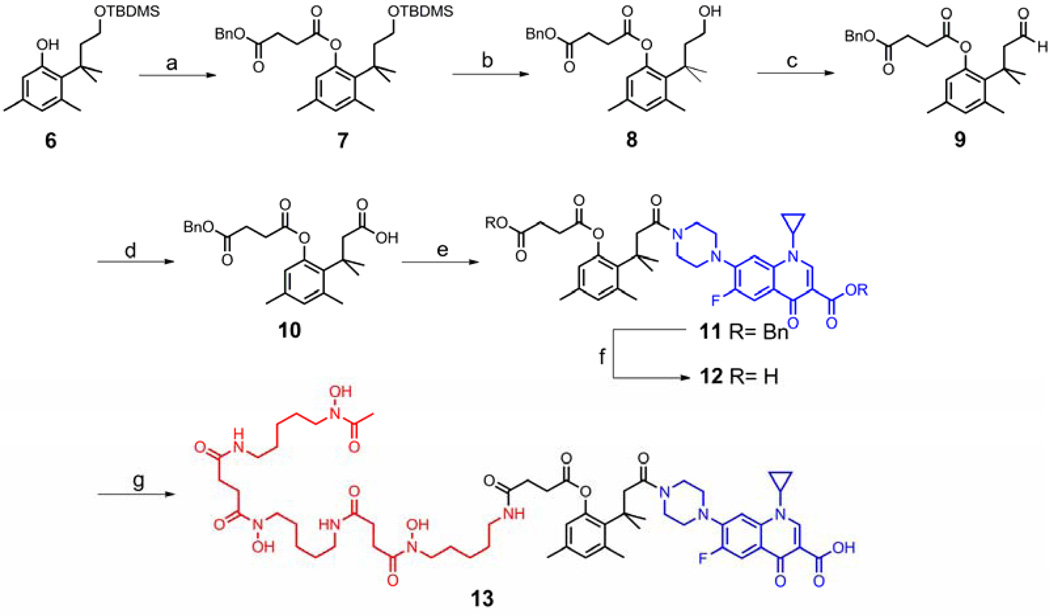
Due to the facile lactonization of the “trimethyl lock” system, the incorporation of the linker into the conjugates required derivatization of the phenolic hydroxyl group. Therefore, synthesis of the esterase triggered conjugate started with TBDMS-protected diol 6 which was prepared from 3,5-dimethylphenol according to the methods of Borchardt.21 DCC-mediated coupling of 6 with monobenzyl succinate provided the TBDMS ether 7 in 77% yield. Treatment of 7 with a mixture of acetic acid, THF and water removed the TBDMS group to give the primary alcohol 8. A two-step oxidation with PCC and sodium chlorite proceeded in excellent yield to convert alcohol 8 to acid 10 which was ready to couple with ciprofloxacin. To increase the coupling efficiency and simplify the purification, ciprofloxacin was converted to its benzyl ester. Coupling of acid 10 with O-benzyl ciprofloxacin using EDC and HOBt afforded ciprofloxacin derivative 11 in 70% yield. Both benzyl protecting groups in 11 were removed by hydrogenolysis to give acid 12, which was coupled with desferrioxamine to yield potential esterase triggered desferrioxamine-ciprofloxacin conjugate 13. (Scheme 4)
Scheme 4.
Synthesis of the potential esterase triggered desferrioxamine-ciprofloxacin conjugate 13. Reagents and conditions: (a) Monobenzyl succinate, DCC, DMAP, CH2Cl2, rt (ca. 23 °C), 77%; (b) AcOH:THF:H2O=3:3:1, rt, 97%; (c) PCC, CH2Cl2, rt, 82%; (d) NaClO2, H2O2, NaH2PO4, CH3CN/H2O, 0 °C-rt, 97%; (e) EDC, HOBt, O-benzyl ciprofloxacin, Et3N, DMAP, CH2Cl2, rt, 70%; (f) Pd/C, H2 balloon, rt, 86%; (g) EDC, NHS, DMF then DFO mesylate, Et3N, 47%.
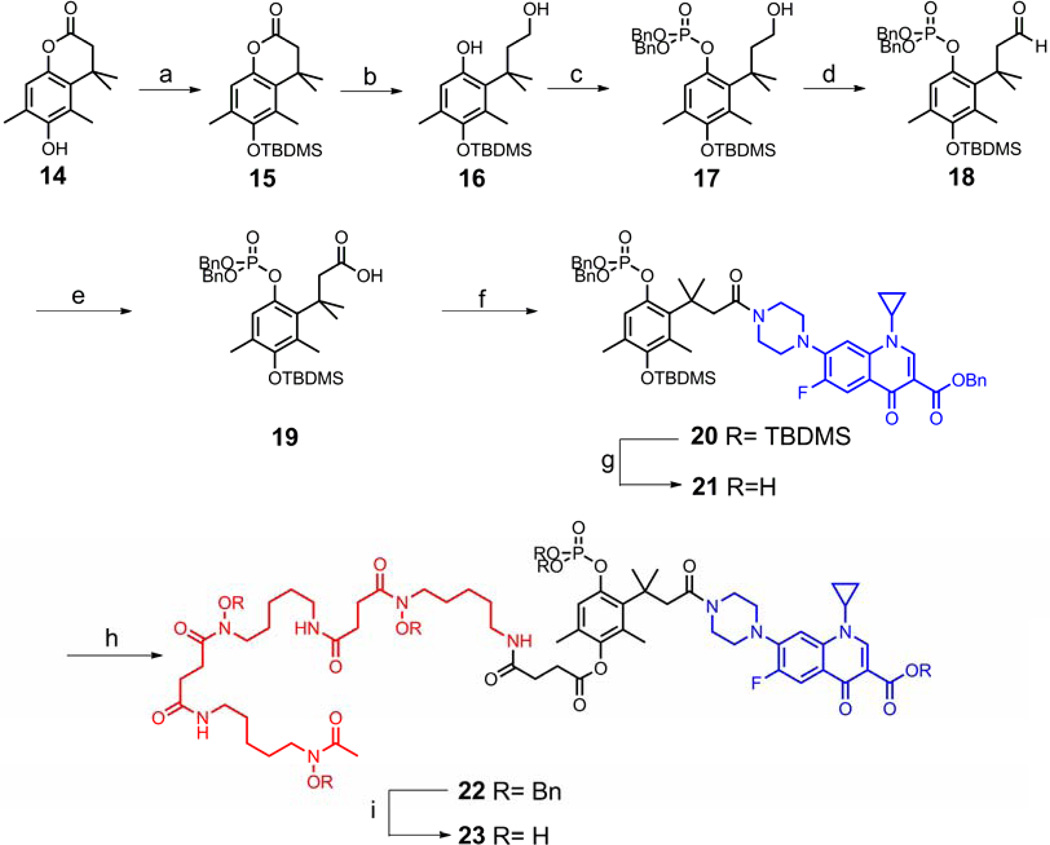
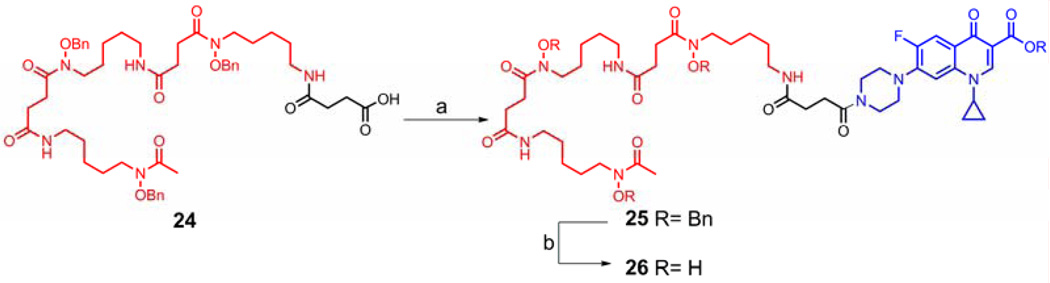
The synthesis of the intended phosphatase triggered conjugate started from lactone 14, which was prepared using chemistry developed by Carpino.32 After protection of the free phenolic hydroxyl group in 14 with a TBDMS group, reductive ring opening of the protected lactone 15 afforded alcohol 16 in moderate yield. Using NaH as the base, selective phosphorylation of the phenol in 16 was achieved with tetrabenzyl pyrophosphate33 to give alcohol 17. Similar to the process used to make the potential esterase triggered conjugate, treatment of 17 with PCC followed by sodium chlorite provided acid 19. EDC-mediated coupling of 19 with O-benzyl ciprofloxacin generated the ciprofloxacin derivative 20 in 59% yield. The silyl protecting group was removed with KF to expose the phenolic hydroxyl group in 21, which served as the coupling site with O-benzyl desferrioxamine succinic acid to yield the protected conjugate 22. Global deprotection of 22 by hydrogenolysis produced the potential phosphatase triggered desferrioxamine-ciprofloxacin conjugate 23. (Scheme 5) As a control compound, conjugate 26 with a succinyl linkage which is stable under physiological conditions was also synthesized and included in the biological studies. (Scheme 6)
Scheme 5.
Synthesis of the potential phosphatase triggered desferrioxamine-ciprofloxacin conjugate 23. Reagents and conditions: (a) TBDMSCl, DBU, CH2Cl2, 90%; (b) LiAlH4, THF, rt-reflux, 51%; (c) NaH, tetrabenzyl pyrophosphate, THF-DMF, 0 °C-rt, 53%; (d) PCC, CH2Cl2, rt, 78%; (e) NaClO2, H2O2, NaH2PO4, CH3CN/H2O, 0°C-rt, 99%; (f) EDC, HOBt, O-benzyl ciprofloxacin, Et3N, DMAP, CH2Cl2, rt, 59%; (g) KF, DMF-H2O, rt, 74%; (h) EDC, O-benzyl desferri- oxamine succinic acid 24, DMF, rt, 45%; (i) Pd/C, H2 balloon, THF-H2O, 35%.
Scheme 6.
Synthesis of the desferrioxamine-ciprofloxacin conjugate 26 with a stable linker. Reagents and conditions: (a) EDC, HOBt, O-benzyl ciprofloxacin, Et3N, DMAP, CH2Cl2, rt, 75%; (b) Pd/C, H2 balloon, MeOH, 82%.
2. Biological studies
The desferrioxamine B-ciprofloxacin conjugates 13 and 23 were evaluated for their ability to inhibit the growth of bacteria using the agar well diffusion test. Representative Gram-positive strains included Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus. The Gram-negative strains studied included a wild type (K799/wt) and a penetration mutant strain (K799/61) of Pseudomonas aeruginosa and an Escherichia coli test strain. Previous studies showed that desferrioxamine B is a growth promoter of all the strains used in the assay except E. coli. The parent drug ciprofloxacin and conjugate 26 with a stable succinyl linker were also included in the assay as controls.
The results of assays for antibacterial activity (Table 1) showed that all three conjugates displayed moderate activities against the E. coli strain, indicating that they can reach their cellular target regardless of the drug being released or not. Conjugate 26 with a stable succinyl linker has moderate activity against both B. subtilis and S. aureus but showed virtually no activity against M. luteus, against which ciprofloxacin itself is only weakly active. A similar activity profile was observed in the structurally related desferridanoxamine-ciprofloxacin conjugate.34 Conjugate 13 with the potential esterase triggered linker was almost equipotent relative to conjugate 26 against Gram-positive strains and the P. aeruginosa penetration mutant strain. However, in the case of wild type P. aeruginosa, conjugate 13 displayed higher activity (larger inhibition zone) than conjugate 26 which is completely inactive, suggesting that the drug was partially released, although the possibility of hydrolysis caused by some extracellular esterase cannot be ruled out.35 In all the strains tested, conjugate 13 was less active than the parent drug ciprofloxacin, implying that 13 may be a poor substrate for microbial esterases. Conjugate 23 with the phosphatase triggered linker was not active against all the strains in the panel tested except the E. coli penetration mutant, suggesting that this conjugate was no longer recognized by siderophore outer membrane receptors thus could not enter bacterial cells via the iron uptake pathway.
Table 1.
Diameter of growth inhibition zones (mm) in the agar diffusion antibacterial susceptibility assay.
| Gram-positive bacteria | Gram-negative bacteria | |||||
|---|---|---|---|---|---|---|
| Compds |
Bacillus subtilis |
Staphylococcus aureus |
Micrococcus luteus |
Pseudomonas aeruginosa |
Escherichia coli |
|
| ATCC 6633 |
SG 511 | ATCC 10240 | K799/wt | K799/61 | X580 | |
| Ciprofloxacin | 28b | 28a | 12Pa | 21b | 24/32pb | 31c |
| 26 | 21 | 18 | 0 | 0 | 26P | 30/33p |
| 13 | 26 | 16 | 0 | 26 | 27/37p | 29 |
| 23 | 0 | 0 | 0 | 0 | 0 | 21 |
p, partially clear inhibition zone/colonies in the inhibition zone
P, unclear inhibition zone/many colonies in the inhibition zone
Exactly 50 L of a 0.2 mM solution of each compound dissolved in 1:9 DMSO/MeOH was filled in 9 mm wells in agar media (Standard I Nutrient Agar, Serva or Mueller Hinton II Agar, Becton, Dickinson and Company). Inhibition zones read after incubation at 37 °C for 24 h.
Ciprofloxacin was tested at (a) 5 g/mL, (b) 1.66 g/mL, (c) 0.33 g/mL in H2O.
Conjugate 13 was also tested for antibacterial activity against a panel of ESKAPEE bacteria2 by determining their minimum inhibitory concentrations (MIC90) using the broth microdilution assay (Table 2). Conjugate 13 displayed moderate to good antibacterial activity (1–32 M) against all the strains except E. faecium (>28 M). In all the strains tested, conjugate 13 showed reduced activities compared to the free drug ciprofloxacin, which is consistent with the trend observed in the agar diffusion assay, further suggesting that conjugate 13 might be a poor substrate for the esterases needed to release the drug.
Table 2.
MIC90 values of conjugate 13 and ciprofloxacin against ESKAPEE panel of bacteria.
| MIC90(M) Against ESKAPEE Test Organismsa, b | |||||||
|---|---|---|---|---|---|---|---|
| Compds |
E. faecium |
S. aureus |
K. pneunonia |
A. baumanii |
P. aeruginosa |
E. aerogenes |
E. coli |
| NCTC 7171 |
ATCC 29213 |
ATCC 700603 |
ATCC 17961 |
ATCC 27853 |
ATCC 35029 |
ATCC 25922 |
|
| 13 | >128 | 32 | 16 | 8 | 8 | 1 | 1 |
| Ciprofloxacin | 8 | NT | 0.25 | 0.25 | 0.25 | <0.015 | <0.015 |
MIC90 values (µM) were determined using the broth microdilution method in Mueller-Hinton broth No.2 (MHII) with visual end point analysis according to the CLSI guidelines. 36
Each compound was tested in triplicate.
NT, not tested.
III. Conclusion
Two desferrioxamine B-ciprofloxacin conjugates with potential esterase and phosphatase triggered linkers were synthesized. The linkers, with a “trimethyl-lock” structure, were designed to release the antibiotic by a fast and spontaneous lactonization process after enzymatic hydrolysis of the conjugates. Thus, the conjugates had the potential to use active iron transport processes to deliver the antibiotic to bacteria cells and release the drug intracellularly to negate potential steric hindrance due to the presence of the siderophore-linker moiety. The potential esterase triggered conjugate 13 had moderate to good antibiotic activity against several bacterial strains, but the activity was weaker than that of the parent drug ciprofloxacin, most likely because 13 is a poor substrate for the esterase. The potential phosphatase triggered conjugate 23 was inactive in the same panel of bacteria tested, probably due to the failure of using iron uptake pathway to enter bacterial cells. To further develop siderophore-drug conjugates with microbial triggered linkers, an appropriate choice of siderophore to ensure active transport after chemical modification is essential. Meanwhile, linkers that are truly inert to the extracellular environment of bacterial growth cultures but only selectively activated inside microbial cells are required. Taking both factors into consideration, new conjugates based on the “trimethyl lock” concept are being synthesized and their biological studies will be reported in due course.
IV. Experimental section
General
All reactions were carried out under argon by using standard techniques. All solvents and reagents were obtained from commercial sources and used without further purification unless otherwise stated. NMR spectra were recorded on a Varian Unityplus 300 MHz spectrometer, Varian Inova 500 MHz spectrometer, or Varian 600 MHz spectrometer at ambient temperature. Silica gel column chromatography was performed using Sorbent Technologies silica gel 60 (32–63 µm). Reverse Phase C18 Silica Gel was a generous gift from Eli Lilly and Co. The following compounds were prepared according to published procedures: TBDMS-protected diol 6,21 lactone 14,32 tetrabenzyl pyrophosphate,33 O-benzyl ciprofloxacin,37 O-benzyl desferrioxamine succinic acid 24.38
Benzyl 2-(4-(tert-butyldimethylsilyloxy)-2-methylbutan-2-yl)-3,5-dimethylphenyl succinate (7)
Monobenzyl succinate (4.35 g, 20.9 mmol) was dissolved in 200 mL of anhydrous dichloromethane. The solution was cooled to 0 °C and DCC (4.70 g, 23.0 mmol) was added in three portions. The solution was kept at 0 °C for 10 min before 6 (8.09 g, 25.1 mmol) and DMAP (25 mg, 0.21 mmol) were added. The reaction mixture was warmed to room temperature (ca. 23 °C). After stirring at room temperature for 16 h, the solution was filtered to remove the precipitated DCU. The filtrate was washed sequentially with sat. NaHCO3, 5% HCl, brine, dried over Na2SO4 and filtered. The solvent was removed under vacuum to give the crude product which was purified by chromatography on a silica gel column (10% ethyl acetate in hexanes) to give 7 (8.68 g, 77%) as a colorless oil: IR (thin film) 2953, 2927, 2855, 1740, 1472 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.36–7.47 (m, 5H), 6.81 (s, 1H), 6.54 (s, 1H), 5.17 (s, 2H), 3.48 (t, J = 7.5 Hz, 2H), 2.78–2.91 (m, 4H), 2.53 (s, 3H), 2.22 (s, 3H), 2.04 (t, J = 7.5 Hz, 2H), 1.58 (s, 6H), 0.85 (s, 9H), −0.02 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 172.2, 171.6, 149.9, 138.6, 136.2, 135.9, 134.2, 132.6, 128.8, 128.5, 128.4, 123.2, 66.9, 61.0, 46.2, 39.2, 32.0, 30.1, 29.3, 26.2, 25.5, 20.4, 18.4, −5.1; HRMS (ESI) calcd. for C30H45O5Si (M+H)+: 513.3031, found 513.3015.
Benzyl 2-(4-hydroxy-2-methylbutan-2-yl)-3,5-dimethylphenyl succinate (8)
Silyl ether 7 (8.50 g, 16.6 mmol) was dissolved in a mixture of 50 mL of THF, 50 mL of H2O and 150 mL of AcOH. The solution was vigorously stirred at room temperature for 3 h. After removal of the solvents under vacuum, the residue was dissolved in 100 mL of EtOAc and washed sequentially with water, sat. NaHCO3, brine, dried over Na2SO4 and filtered. The combined organic layers were evaporated to give alcohol 8 (6.40 g, 97%) as a colorless oil: IR (thin film) 2925, 1736, 1615, 1473 cm−1; 1H NMR (300 MHz, CD3OD) δ 7.29–7.37 (m, 5H), 6.84 (s, 1H), 6.52 (s, 1H), 5.16 (s, 2H), 3.37 (t, J = 6.9 Hz, 2H), 2.86–2.90 (m, 2H), 2.75–2.79 (m, 2H), 2.52 (s, 3H), 2.19 (s, 3H), 2.05 (t, J = 6.9 Hz, 2H), 1.46 (s, 6H); 13C NMR (75 MHz, CD3OD) δ 173.9, 173.7, 151.4, 139.6, 137.6, 137.5, 135.1, 133.4, 129.7, 129.3, 124.4, 67.7, 60.7, 40.1, 32.6, 31.0, 30.0, 25.6, 20.4; HRMS (ESI) calcd. for C24H30NaO5 (M+Na)+: 421.1985, found 421.1981.
Benzyl 3,5-dimethyl-2-(2-methyl-4-oxobutan-2-yl)phenyl succinate (9)
Alcohol 8 (6.33 g, 15.9 mmol) was dissolved in 200 mL of dichloromethane at room temperature under argon. PCC (6.87 g, 31.8 mmol) was added in three portions. The dark slurry was stirred at room temperature for 3 h. After removal of the solvent, the residue was suspended in 30 mL of dichloromethane and filtered through a short silica gel column. The column was further washed with 20% ethyl acetate in hexanes. The solvents were removed under reduced pressure to give aldehyde 9 (5.17 g, 82%) as a yellow oil: IR (thin film) 2922, 2851, 1736, 1352 cm−1; 1H NMR (300 MHz, CDCl3) δ 9.54 (t, J = 2.4 Hz, 1H), 7.32–7.37 (m, 5H), 6.86 (s, 1H), 6.60 (s, 1H), 5.17 (s, 2H), 2.87–2.92 (m, 2H), 2.83 (d, J = 2.4 Hz, 2H), 2.78–2.83 (m, 2H), 2.54 (s, 3H), 2.23 (s, 3H), 1.57 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 203.1, 172.1, 171.6, 149.5, 138.0, 137.1, 135.8, 133.0, 132.8, 128.8, 128.5, 128.4, 123.4, 66.9, 56.8, 38.3, 31,8, 30.1, 29.1, 26.1, 25.6, 20.4; HRMS (ESI) calcd. for C24H28NaO5 (M+Na)+: 419.1815, found 419.1829.
3-(2-(4-(Benzyloxy)-4-oxobutanoyloxy)-4,6-dimethylphenyl)-3-methylbutanoic acid (10)
To a solution of aldehyde 9 (4.76 g, 12.0 mmol) in 12 mL of CH3CN and 6 mL of H2O, were added sodium dihydrogen phosphate (450 mg, 3.26 mmol) and 30% H2O2 (1 mL). The mixture was cooled to 0 °C and a solution of sodium chlorite (2.00 g, 22.2 mmol) in 18 mL of H2O was added slowly. The reaction was kept at 0 °C for 1 h and warmed to room temperature. Then 30 mL of sat. Na2S2O3 was added slowly to destroy excess H2O2 and sodium chlorite. The solution was acidified with 3 N HCl to pH=1–2 and extracted with EtOAc (200 mL×3). The combined organic layers were washed sequentially with H2O, brine, dried over Na2SO4 and evaporated to give acid 10 (4.82g, 97%) as a light yellow oil: IR (thin film) 2926, 2850, 1772, 1739, 1647, 1473 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.33–7.38 (m, 5H), 6.82 (s, 1H), 6.58 (s, 1H), 5.18 (s, 2H), 2.89–2.94 (m, 2H), 2.84 (s, 2H), 2.78–2.83 (m, 2H), 2.54 (s, 3H), 2.22 (s, 3H), 1.58 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 177.4, 172.2, 171.6, 149.5, 138.2, 136.5, 135.9, 133.5, 132.7, 128.8, 128.5, 128.4, 123.1, 66.9, 47.7, 38.8, 31.4, 30.0, 29.2, 25.4, 20.4; HRMS (ESI) calcd. for C24H28NaO6 (M+Na)+: 435.1778, found 435.1760.
O-Bn ciprofloxacin conjugate (11)
To a solution of acid 10 (500 mg, 1.21 mmol) in 20 mL anhydrous of CH2Cl2 cooled to 0°C were added EDC (470 mg, 2.45 mmol) and HOBt (331 mg, 2.45 mmol). The mixture was stirred at that temperature for 5 min before O-benzyl ciprofloxacin (530 mg, 1.26 mmol), Et3N (0.67 mL, 4.80 mmol) and DMAP (5 mg) were added. The reaction mixture was warmed to room temperature and stirred for 18 h. The mixture was diluted with 30 mL of CH2Cl2 and the organic layer was washed sequentially with water, sat. NaHCO3, brine, dried over Na2SO4 and filtered. The solvent was removed under vacuum and the residue was purified by chromatography on a silica gel column (5% methanol in CH2Cl2) to give 11 (690 mg, 70%) as a light yellow oil: 1H NMR (600 MHz, CDCl3) 8.52 (s, 1H), 8.03 (d, J = 13.20 Hz, 1H), 7.29–7.54 (m, 10H), 7.19 (d, J = 7.04 Hz, 1H), 6.82 (d, J = 2.05 Hz, 1H), 6.57 (d, J = 2.05 Hz, 1H), 5.38 (s, 2H), 5.15 (s, 2H), 3.76 (br. s., 2H), 3.49 (br. s., 2H), 3.38 (m, 1H), 3.12 (br. s., 2H), 3.04 (br. s., 2H), 2.85–2.93 (m, 4H), 2.78–2.84 (m, 2H), 2.57 (s, 3H), 2.20 (s, 3H), 1.62 (s, 6H), 1.28 (m, 2H), 1.09 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 173.1, 172.0, 171.3, 170.0, 165.4, 154.1, 152.4, 149.5, 148.4, 144.1, 138.2, 137.9, 136.4, 136.2, 135.5, 134.2, 132.5, 128.6, 128.5, 128.3, 128.1, 128.0, 127.9, 123.3, 122.9, 113.4, 110.1, 104.9, 66.7, 66.4, 50.3, 49.4, 45.8, 45.0, 41.2, 39.5, 34.5, 32.0, 30.0, 28.9, 25.6, 22.6, 20.2, 8.1; HRMS (ESI) calcd. for C48H51FN3O8 (M+H)+: 816.3655, found 816.3627.
Ciprofloxacin conjugate (12)
Conjugate 11 (122 mg, 0.17 mmol) was dissolved in 5 mL of 10% H2O in THF under argon. 10% Pd/C (10 mg) was added and the flask was flushed with H2 gas and left to stir under a hydrogen atmosphere (balloon) at room temperature for 3 h. After purging with argon, the reaction mixture was filtered over Celite and the solvent was evaporated to give acid 12 (92 mg, 86%) as a light yellow solid: mp=128–129 °C (dec.); IR (thin film) 2922, 2853, 1729, 2626, 1466 cm−1; 1H NMR (300 MHz, CD3OD) δ 8.78 (s, 1H), 7.89 (d, J = 13.2 Hz), 7.53 (bs, 1H), 6.84 (s, 1H), 6.63 (s, 1H), 3.66–3.79 (m, 3H), 3.58 (s, 2H), 3.26 (s, 2H), 3.13 (s, 2H), 2.96 (s, 2H), 2.86–2.90 (m, 2H), 2.70–2.74 (m, 2H), 2.57 (s, 3H), 2.18 (s, 3H), 1.63 (s, 6H), 1.42 (s, 2H), 1.21 (s, 2H)); 13C NMR (150 MHz, DMSO-d6) δ 176.4, 173.9, 169.7, 168.0, 165.9, 153.8, 152.1, 151.1, 148.1, 145.0, 144.9, 139.2, 136.9, 136.0, 129.0, 126.8, 118.8, 115.4, 111.1, 110.9, 106.8, 106.7, 49.6, 49.2, 44.6, 44.3, 40.8, 35.9, 34.5, 28.9, 28.7, 28.5, 27.4, 27.2, 22.6, 20.1, 7.6; HRMS (ESI) calcd. for C34H39FN3O8 (M+H)+: 636.2716, found 636.2723.
Ciprofloxacin conjugate (13)
To a solution of acid 12 (80 mg, 0.12 mmol) in anhydrous CH2Cl2 (5 mL) were added EDC (46 mg, 0.24 mmol) and N-hydroxysuccinimide (28 mg, 0.24 mmol). The reaction mixture was stirred at room temperature for 18 h and diluted with 20 mL of CH2Cl2. The solution was sequentially washed with sat. NaHCO3, water, brine, dried over Na2SO4 and filtered. Removal of the solvent in vacuo to give the crude NHS ester as a yellow foam which was used directly in the next step. Desferrioxamine B mesylate (40 mg, 0.06 mmol) and triethylamine (34 L, 0.24 mmol) were dissolved in 10 mL anhydrous DMF (gentle heating was required to generate a homogeneous solution), and the NHS ester of 12 was added in one portion. The solution was stirred at room temperature for 18 h and the solvent was removed under reduced pressure. The residue was purified by chromatography on a reverse-phase silica gel column (C18, CH3CN:H2O=1:1) to give conjugate 13 (33 mg, 47%) as a white solid: mp=173–175 °C; IR (thin film) 2927, 2852, 1754, 1615, 1472, 1265 cm−1; 1H NMR (600 MHz, CD3OD) δ 8.76 (s, 1H), 7.87 (d, J = 13.2 Hz, 1H), 7.51 (d, J = 6.75 Hz, 1H), 6.84 (s, 1H), 6.64 (s, 1H), 4.59 (s, 4H), 3.75 (s, 1H), 3.70 (s, 2H), 3.53–3.60 (m, 6H), 3.27 (s, 2H), 3.15 (m, 6H), 2.96 (s, 2H), 2.89 (t, J = 6.75 Hz, 2H), 2.72–2.77 (m, 4H), 2.62 (t, J = 6.75 Hz, 2H), 2.57 (s, 3H), 2.42–2.45 (m, 4H), 2.19 (s, 3H), 2.09 (s, 3H), 1.56–1.65 (m, 6H), 1.61 (s, 6H), 1.48–1.54 (m, 6H), 1.39–1.43 (m, 2H), 1.29–1.35 (m, 6H), 1.20–1.23 (m, 2H); 13C NMR (150 MHz, CD3OD) δ 178.4, 175.0, 174.6, 174.0, 173.8, 173.7, 172.6, 169.8, 155.9, 154.3, 151.4, 149.5, 146.9, 140.9, 139.8, 137.5, 135.7, 133.3, 124.3, 112.8, 112.6, 107.4, 51.1, 50.6, 47.1, 46.4, 42.6, 40.8, 40.5, 40.4, 37.1, 33.0, 31.7, 31.5, 31.3, 30.1, 29.1, 29.0, 27.5, 26.1, 25.0, 20.4, 8.7; HRMS (ESI) calcd. for C59H85FN9O15 (M+H)+: 1178.6144, found 1178.6152.
6-((tert-Butyldimethylsilyl)oxy)-4,4,5,7-tetramethylchroman-2-one (15)
Lactone 14 (3.0 g, 13.6 mmol) and TBDMSCl (3.5 g, 22.8 mmol) were dissolved in 100 mL of CH2Cl2. To this solution was added DBU (3.4 mL, 22.8 mmol). After 1 h, the reaction was quenched with 100 mL of sat. NH4Cl solution. The organic phase was separated and washed with brine, dried over Na2SO4, filtered and the solvent was removed under reduced pressure. The residue was purified by chromatography on a silica gel column (hexanes:EtOAc=6:1) to give 15 as a waxy solid (4.1 g, 90%): 1H NMR (600 MHz, CDCl3) 6.72 (s, 1H), 2.57 (s, 2H), 2.31 (s, 3H), 2.18 (s, 3H), 1.43 (s, 6H), 1.04 (s, 9H), 0.18 (s, 6H); 13C NMR (150 MHz, CDCl3) 168.7, 149.0, 145.4, 128.3, 128.2, 127.1, 117.1 46.0, 35.4, 27.5, 26.0, 18.7, 17.6, 16.6, −3.1; HRMS (ESI) calcd. for C19H31O3Si (M+H)+: 335.2037, found 335.2065.
4-((tert-Butyldimethylsilyl)oxy)-2-(4-hydroxy-2-methylbutan-2-yl)-3,5-dimethylphenol (16)
Lactone 15 (3.5 g, 10.4 mmol) was dissolved in 20 mL of anhydrous THF. The solution was added dropwise to a suspension of LiAlH4 (1.0 g, 26.3 mmol) in 100 mL of anhydrous THF cooled to 0°C. The mixture was warmed to room temperature and heated to reflux for 16 h. The excess LiAlH4 was quenched with 1 mL of cold water. After some MgSO4 was added, the mixture was allowed to stir for an additional 30 min and then filtered to remove the salt. The solvent was removed under reduced pressure to give the crude product which was purified by chromatography on a silica gel column (hexanes:EtOAc=2:1) to give 16 (1.8 g, 51%) as a light yellow solid: 1H NMR (600 MHz, CDCl3) 6.34 (s, 1H), 5.46 (bs, 1H), 3.64 (t, J = 6.7 Hz, 2H), 2.30 (s, 3H), 2.19 (t, J = 6.7 Hz, 2H), 2.11 (s, 3H), 1.56 (s, 6H), 1.03 (s, 9H), 0.14 (s, 6H); 13C NMR (150 MHz, CDCl3) 149.3, 146.8, 130.6, 129.6, 127.1, 117.7, 61.8, 45.1, 40.0, 32.5, 26.3, 18.9, 18.6, 17.6, −3.0; HRMS (ESI) calcd. for C19H35O3Si (M+H)+: 339.2350, found 339.2326.
Dibenzyl-(4-((tert-butyldimethylsilyl)oxy)-2-(4-hydroxy-2-methylbutan-2-yl)-3,5-dimethy lphenyl) phosphate (17)
To a solution of 16 (1.63 g, 4.81 mmol) in anhydrous THF:DMF=4:1 (20 mL) at 0 °C was added NaH (60% dispersion in mineral oil, 0.21 g, 5.25 mmol). The mixture was kept at that temperature for 30 min before tetrabenzyl pyrophosphate (3.88g, 7.21 mmol) was added in one portion. The mixture was warmed to room temperature and stirred for additional 8 h. The reaction was quenched by adding sat. NH4Cl (100 mL). The aqueous layer was extracted with EtOAc (50 mL×3). The combined organic layers were washed with brine, dried over Na2SO4, filtered and the solvent was removed under reduced pressure. The crude oil was purified by chromatography on a silica gel column (Hexanes:EtOAc=2:1) to yield 17 as a clear oil (1.54 g, 53%): 1H NMR (600 MHz, CDCl3) 7.32–7.37 (m, 10H), 7.00 (s, 1H), 5.07–5.14 (m, 4H), 3.52 (t, J = 7.6 Hz, 2H), 2.31 (s, 3H), 2.12 (t, J = 7.6 Hz, 2H), 2.07 (s, 3H), 1.50 (s, 6H), 1.02 (s, 9H), 0.15 (s, 6H); 13C NMR (150 MHz, CDCl3) 149.9, 144.0, 135.6, 134.6, 130.1, 128.6, 128.0, 127.0, 120.2, 69.8, 60.6, 46.1, 39.8, 32.2, 26.1, 18.7, 18.5, 17.5, −3.2; HRMS (ESI) calcd. for C33H47O6PSi (M+H)+: 599.2952, found 599.2942.
Dibenzyl (4-((tert-butyldimethylsilyl)oxy)-3,5-dimethyl-2-(2-methyl-4-oxobutan-2-yl) phenyl) phosphate (18)
Alcohol 17 (492 mg, 0.82 mmol) was dissolved in 20 mL of dichloromethane at room temperature under argon. PCC (264 mg, 1.23 mmol) was added in three portions. The dark slurry was stirred at room temperature for 3 h. After filtered through a Celite pad to remove the insoluble matters, the solvent was removed and the crude oil was purified by chromatography on a silica gel column (hexanes:EtOAc=3:1) to yield aldehyde 18 as a clear oil (370 mg, 78%): 1H NMR (600 MHz, CDCl3) 9.46 (t, J = 2.3 Hz, 1H), 7.29–7.34 (m, 10H), 7.05 (s, 1H), 5.06–5.12 (m, 4H), 2.87 (d, J = 2.3 Hz, 2H), 2.31 (s, 3H), 2.09 (s, 3H), 1.56 (s, 6H), 1.02 (s, 9H), 0.15 (s, 6H); 13C NMR (150 MHz, CDCl3) 203.4, 150.3, 143.7, 135.7, 133.4, 129.7, 128.8, 128.7, 128.2, 127.9, 120.5, 70.1, 57.2, 38.8, 31.7, 26.3, 18.9, 18.8, 17.8, −2.9; HRMS (ESI) calcd. for C33H46O6PSi (M+H)+: 597.2796, found 597.2774.
3-(6-((bis(Benzyloxy)phosphoryl)oxy)-3-((tert-butyldimethylsilyl)oxy)-2,4-dimethylpheny l)-3-methylbutanoic acid (19)
To a solution of 18 (341 mg, 0.57 mmol) in 6 mL of CH3CN and 3 mL of H2O were added sodium dihydrogen phosphate (230 mg, 1.65 mmol) and 30% H2O2 (0.2 mL). The mixture was cooled to 0 °C and a solution of sodium chlorite (92 mg, 1.03 mmol) in 1 mL of H2O was added slowly. The reaction was kept at 0 °C for 1 h and warmed to room temperature. Sat. Na2S2O3 (2 mL) was added slowly to destroy excess H2O2 and NaClO2. The solution was acidified with 3 N HCl to pH=1–2 and the mixture was extracted with EtOAc (20 mL×3). The combined organic layers were washed sequentially with H2O, brine, dried over Na2SO4, filtered and the solvent was evaporated to give acid 19 (345 mg, 99%) as a colorless oil: 1H NMR (600 MHz, CDCl3) 7.30–7.35 (m, 10H), 6.94 (s, 1H), 5.07–5.13 (m, 4H), 2.86 (s, 2H), 2.32 (s, 3H), 2.05 (s, 3H), 1.60 (s, 6H), 1.01 (s, 9H), 0.13 (s, 6H); 13C NMR (150 MHz, CDCl3) 150.0, 143.7, 135.4, 134.0, 130.2, 128.7, 128.6, 128.0, 127.3, 120.1, 70.0, 47.4, 39.6, 31.7, 26.1, 18.7, 18.4, 17.5, −3.2; HRMS (ESI) calcd. for C33H45NaO7PSi (M+Na)+: 635.2564, found 635.2577.
O-Bn-O’-TBS ciprofloxacin conjugate (20)
To a solution of acid 19 (143 mg, 0.23 mmol) in 5 mL of anhydrous CH2Cl2 cooled to 0 °C were added EDC (88mg, 0.46 mmol) and HOBt (62 mg, 0.46 mmol). The mixture was stirred at that temperature for 5 min before O-benzyl ciprofloxacin (106 mg, 0.25 mmol), Et3N (130 µL, 0.92 mmol), DMAP (2 mg) were added. The reaction mixture was warmed to room temperature and stirred for 18 h. The mixture was diluted with 30 mL of CH2Cl2 and the organic layer was washed sequentially with water, sat. NaHCO3, brine, dried over Na2SO4 and filtered. The solvent was removed in vacuo and the crude product was purified by chromatography on a silica gel column (5% methanol in CH2Cl2) to yield 20 (138 mg, 59%) as a light yellow oil: 1H NMR (500 MHz, CDCl3) 8.54 (s, 1H), 8.08 (d, J = 13.2 Hz, 1H), 7.52–7.54 (m, 2H), 7.37–7.40 (m, 2H), 7.26–7.33 (m, 11H), 7.15 (d, J = 7.0 Hz, 1H), 6.98 (s, 1H), 5.40 (s, 2H), 5.04–5.13 (m, 4H), 3.68–3.70 (m, 2H), 3.43–3.45 (m, 2H), 3.31–3.36 (m, 1H), 3.05–3.07 (m, 2H), 2.94–2.96 (m, 2H), 2.95 (s, 2H), 2.38 (s, 3H), 2.06 (s, 3H), 1.63 (s, 6H), 1.25–1.29 (m, 2H), 1.06–1.09 (m, 2H), 1.02 (s, 9H), 0.15 (s, 6H); 13C NMR (125 MHz, CDCl3) 170.5, 165.9, 150.1, 148.6, 144.0, 136.7, 135.7, 130.4, 129.0, 128.9, 128.8, 128.3, 128.2, 128.1, 127.2, 119.8, 113.8, 113.6, 110.5, 105.2, 70.1, 66.7, 45.8, 45.5, 41.2, 40.1, 34.7, 32.3, 26.3, 19.0, 18.9, 17.8, 8.4, −2.9; HRMS (ESI) calcd. for C57H67FN3NaO9PSi (M+Na)+: 1038.4260, found 1038.4257.
O-Bn ciprofloxacin conjugate (21)
Compound 20 (101 mg, 97 µmol) was dissolved in a DMF:H2O=5:1 solution (2 mL). Argon was bubbled through the solution for 5 min before KF (12 mg, 0.21 mmol) was added and the mixture was vigorously stirred for 30 min. The reaction was quenched by adding 10 mL of sat. NH4Cl and extracted with chloroform (5 mL×3). The organic layers were combined and washed sequentially with brine, dried over Na2SO4 and filtered. After removal of the solvent under vacuum, the residue was applied to a preparative silica gel TLC plate, eluted with 5% MeOH in CHCl3 to afford 21 (65 mg, 74%) as a clear oil: 1H NMR (600 MHz, CD3OD) 8.60 (s, 1H), 7.82 (d, J=13.2 Hz, 1H), 7.47–7.48 (m, 2H), 7.36–7.38 (m, 2H), 7.28–7.33 (m, 12H), 6.84 (s, 1H), 5.31 (s, 2H), 5.06–5.18 (m, 4H), 3.61–3.62 (m, 2H), 3.50–3.54 (m, 1H), 3.46–3.47 (m, 2H), 3.11–3.12 (m, 2H), 2.98–2.99 (m, 2H), 2.98 (s, 2H), 2.41 (s, 3H), 2.06 (s, 3H), 1.60 (s, 6H), 1.23–1.28 (m, 2H), 1.06–1.09 (m, 2H); 13C NMR (150 MHz, CD3OD) 172.4, 155.5, 153.9, 152.4, 152.3, 150.1, 145.8, 145.8, 143.9, 143.9, 139.7, 137.9, 136.9, 136.8, 136.2, 136.1, 129.9, 129.7, 129.7, 129.6, 129.6, 129.4, 129.3, 129.2, 129.2, 128.5, 128.4, 127.8, 127.7, 124.5, 120.6, 120.6, 113.2, 113.0, 107.2, 71.4, 71.4, 71.2, 71.2, 67.2, 51.0, 50.5, 47.0, 46.8, 42.3, 40.7, 36.3, 32.5, 31.9, 17.3, 17.2, 16.6, 16.5, 8.5; HRMS (ESI) calcd. for C51H54FN3O9P (M+H)+: 902.3576, found 902.3599.
O-Bn desferrioxamine-ciprofloxacin conjugate (22)
To a solution of 21 (33 mg, 37 µmol) and O-benzyl desferrioxamine succinic acid (70 mg, 75 µmol) in 1 mL anhydrous DMF, was added EDC (30 mg, 156 µmol) in one portion. After stirring at room temperature for 24 h, the reaction was diluted with 30 mL of CHCl3. The solution was washed sequentially with sat. NaHCO3, brine, dried over Na2SO4 and filtered. After removal of the solvent under vacuum, the residue was applied to a preparative silica gel TLC plate and eluted with 10% MeOH in CHCl3 to afford 22 (30 mg, 45%) as a white foam: 1H NMR (600 MHz, CDCl3) 8.53 (s, 1H), 8.08 (d, J = 13.2 Hz, 1H), 7.24–7.52 (m, 30H), 7.16 (d, J = 7.0 Hz, 1 H), 7.06 (s, 1H), 6.40 (br. s., 3H), 5.40 (s, 2H), 5.07 (m, 4H), 4.77–4.89 (m, 6H), 3.56 – 3.71 (m, 8H), 3.40 (m, 2H), 3.35 (m, 1H), 3.12–3.24 (m, 6H), 3.05 (m, 2H), 2.94 (m, 4H), 2.80 (m, 4H), 2.57–2.67 (m, 4H), 2.42–2.53 (m, 4H), 2.30 (s, 3H), 2.06–2.12 (m, 3H), 1.98 (s, 3H), 1.55–1.68 (m, 12H), 1.42–1.54 (m, 6H), 1.21–1.37 (m, 8H), 1.05–1.08 (m, 2H); 13C NMR (150 MHz, CDCl3) 174.3, 173.9, 173.3, 172.4, 171.2, 171.2, 170.3, 165.8, 154.3, 152.7, 148.5, 147.1, 146.3, 144.4, 138.2, 136.6, 135.7, 135.5, 135.4, 134.6, 131.8, 129.4, 129.4, 129.4, 129.2, 129.1, 129.0, 128.9, 128.9, 128.9, 128.7, 128.3, 128.2, 128.1, 123.5, 120.0, 110.4, 105.2, 76.9, 76.5, 76.5, 70.3, 70.2, 66.6, 49.6, 45.7, 45.5, 45.1, 41.2, 40.1, 39.6, 35.0, 34.7, 32.2, 32.1, 31.1, 30.8, 30.8, 29.9, 29.9, 29.8, 29.8, 29.7, 29.6, 29.5, 29.4, 29.4, 29.2, 29.0, 28.8, 28.6, 28.3, 28.2, 28.0, 27.5, 27.2, 26.7, 26.6, 26.1, 24.1, 24.0, 23.9, 23.8, 22.9, 20.7, 16.9, 16.5, 14.3, 8.3; HRMS (ESI) calcd. for C101H121FN9NaO19P (M+Na)+: 1836.8393, found 1836.8407.
Desferrioxamine-ciprofloxacin conjugate (23)
Compound 22 (17.5 mg, 9.6 µmol) was dissolved in 4 mL of THF:H2O=10:1 solution under argon. To this solution was added 10% Pd/C (5 mg) and the flask was flushed with H2 gas and left to stir under a hydrogen atmosphere (balloon) at room temperature for 6 h. After removal of the catalyst by filtration, the solvent was evaporated under reduced pressure. The resulting yellow oil was dissolved in 0.5 mL of CH3CN:H2O=1:1 solution and further purified by chromatography on a reverse-phase silica gel column (C18, 100% H2O to CH3CN:H2O=1:1) to afford 23 (4.3 mg, 35%) as a yellow foam: 1H NMR (600 MHz, CD3OD) 8.82 (s, 1H), 7.92 (d, J = 13.2 Hz, 2H), 7.52 (d, J = 7.34 Hz, 1H), 7.40 (s, 1H), 3.72–3.79 (m, 2H), 3.57–3.62 (m, 6H), 3.52–3.54 (m, 2H), 3.13–3.22 (m, 8H), 3.06–3.13 (m, 4H), 2.87 (m, 2H), 2.71–2.80 (m, 4H), 2.52 (m, 2H), 2.41–2.50 (m, 4H), 2.23 (s, 3H), 2.09 (s, 3H), 2.02 (s, 3H), 1.74 (s, 6H), 1.20–1.64 (m, 22H); 13C NMR (150 MHz, CD3OD) 177.1, 173.5, 173.0, 173.0, 172.4, 172.2, 172.1, 171.1, 168.3, 154.5, 152.9, 150.1, 147.9, 145.6, 145.6, 144.0, 139.4, 134.0, 133.9, 130.2, 128.2, 119.3, 119.2, 119.2, 111.0, 110.8, 106.6, 106.3, 105.0, 49.2, 49.0, 47.2, 45.8, 43.6, 41.2, 40.5, 38.9, 38.8, 35.6, 30.1, 30.0, 29.8, 28.6, 28.5, 28.5, 27.5, 27.5, 25.9, 23.5, 23.5, 18.8, 15.6, 15.3, 7.2; HRMS (ESI) calcd. for C59H86FN9O19P (M+H)+: 1274.5756, found 1274.5799.
Succinyl O-Bn desferrioxamine-ciprofloxacin conjugate (25)
To a solution of O-benzyl desferrioxamine succinic acid 24 (84 mg, 0.09 mmol) in 3 mL of anhydrous CH2Cl2 cooled to 0 °C were added EDC (35 mg, 0.18 mmol) and HOBt (24 mg, 0.18 mmol). The mixture was stirred at that temperature for 5 min before O-benzyl ciprofloxacin (50 mg, 0.12 mmol), Et3N (50 µL, 0.36 mmol) and DMAP (2 mg) were added. The reaction mixture was warmed to room temperature and stirred for 18 h. The mixture was diluted with 20 mL of CH2Cl2 and the organic layer was washed sequentially with water, sat. NaHCO3, brine, dried over Na2SO4 and filtered. The solvent was removed in vacuo and the crude oil was purified by chromatography on a silica gel column (5% methanol in CH2Cl2) to give 25 (90 mg, 75%) as a light yellow oil: 1H NMR (600 MHz, CDCl3) 8.55 (s, 1H), 8.10 (d, J = 12.91 Hz, 1H), 7.29–7.53 (m, 20H), 7.26 (d, J = 7.5 Hz, 1H), 6.51 – 6.62 (m, 2H), 6.37 (br. s., 1H), 5.40 (s, 2H), 4.85 (s, 4H), 4.81 (s, 2H), 3.82 (m, 2H), 3.73 (m, 2H), 3.64 (br. s., 6H), 3.41 (m, 1H), 3.27 (m, 2H), 3.20 (m, 8H), 2.82 (m, 4H), 2.73 (t, J = 6.90 Hz, 2H), 2.58 (t, J = 7.04 Hz, 2H), 2.49 (m, 4H), 2.09 (s, 3H), 1.60–1.66 (m, 6H), 1.49–1.53 (m, 6H), 1.26–1.31 (m, 8H), 1.08–1.15 (m, 2H); 13C NMR (150 MHz, CDCl3) 173.2, 172.3, 170.9, 165.8, 154.4, 152.7, 148.6, 144.3, 144.2, 138.2, 136.6, 129.4, 129.4, 129.2, 128.9, 128.7, 128.2, 128.1, 123.9, 113.9, 113.8, 110.5, 105.3, 76.6, 76.5, 66.6, 50.4, 50.0, 45.5, 41.7, 39.6, 34.7, 31.4, 30.8, 30.7, 29.3, 28.7, 26.8, 26.6, 24.2, 23.9, 23.8, 8.4; HRMS (ESI) calcd. for C74H93FN9O13 (M+H)+: 1334.6871, found 1334.6874.
Succinyl desferrioxamine-ciprofloxacin conjugate (26)
Compound 25 (40 mg, 0.03 mmol) was dissolved in 5 mL of MeOH under argon. 10% Pd/C (4 mg) was added and the flask was flushed with H2 gas and left to stir under a hydrogen atmosphere (balloon) for 6 h. After purging with argon, the mixture was filtered and concentrated in vacuo to give compound 26 (24 mg, 82%) as a light yellow foam: 1H NMR (600 MHz, DMSO-d6) 9.67 (br. s., 1H), 9.62 (br. s., 2H), 8.67 (s, 1H), 7.94 (d, J = 13.2 Hz, 1H), 7.74–7.84 (m, 3H), 7.58 (d, J = 7.6 Hz, 1H), 3.82 (m, 2H), 3.68 (m, 4H), 3.45 (m, 6H), 3.25–3.31 (m, 2H), 2.94–3.05 (m, 6H), 2.53–2.64 (m, 6H), 2.34 (t, J = 6.9 Hz, 2H), 2.26 (t, J = 7.0 Hz, 4H), 1.96 (s, 3H), 1.49 (br. s., 6H), 1.28–1.43 (m, 8H), 1.13–1.27 (m, 8H); 13C NMR (151 MHz, DMSO-d6) 176.4, 172.0, 171.2, 170.1, 166.0, 148.1, 139.2, 106.8, 47.1, 46.8, 38.5, 35.9, 30.4, 29.9, 28.8, 27.8, 27.6, 26.0, 23.5, 20.4, 7.6; HRMS (ESI) calcd. for C46H68FN9NaO13 (M+Na)+: 996.4813, found 996.4848.
Acknowledgments
This research was supported by grant AI054193 from the NIH. We gratefully acknowledge the use of the NMR facilities provided by the Lizzadro Magnetic Resonance Research Center at the University of Notre Dame (UND) under the direction of Dr. Jaroslav Zajicek and the mass spectrometry services provided by The UND Mass Spectrometry & Proteomics Facility (Mrs. N. Sevova, Dr. W. Boggess, and Dr. M. V. Joyce; supported by the National Science Foundation under CHE-0741793). We gratefully thank Mrs. Patricia A. Miller (UND) for antibacterial susceptibility testing and the group of Prof. Shahriar Mobashery for the ESKAPEE assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J. Clin. Infect. Dis. 2008;46:155. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Clin. Infect. Dis. 2009;48:1. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Walsh C. Antibiotics: Actions, Origins, Resistance. Washington, DC: American Society for Microbiology (ASM) Press; 2003. [Google Scholar]
- 4.Pages JM, James CE, Winterhalter M. Nat. Rev. Microbiol. 2008;6:893. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 5.Roosenberg JM, Lin YM, Lu Y, Miller MJ. Curr. Med. Chem. 2000;7:159. doi: 10.2174/0929867003375353. [DOI] [PubMed] [Google Scholar]
- 6.Braun V, Pramanik A, Gwinner T, Koberle M, Bohn E. Biometals. 2009;22:3. doi: 10.1007/s10534-008-9199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun V, Gunthner K, Hantke K, Zimmermann L. J. Bacteriol. 1983;156:308. doi: 10.1128/jb.156.1.308-315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann A, Fiedler HP, Braun V. Eur. J. Biochem. 1979;99:517. doi: 10.1111/j.1432-1033.1979.tb13283.x. [DOI] [PubMed] [Google Scholar]
- 9.Möllmann U, Dong L, Vertesy L, Miller MJ. The 2nd international Biometals symposium; Garmisch-Partenkirchen.2004. [Google Scholar]
- 10.Vertesy L, Aretz W, Fehlhaber HW, Kogler H. Helv. Chim. Acta. 1995;78:46. [Google Scholar]
- 11.Rivault F, Liebert C, Burger A, Hoegy F, Abdallah MA, Schalk IJ, Mislin GLA. Bioorg. Med. Chem. Lett. 2007;17:640. doi: 10.1016/j.bmcl.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Hennard C, Truong QC, Desnottes JF, Paris JM, Moreau NJ, Abdallah MA. J. Med. Chem. 2001;44:2139. doi: 10.1021/jm990508g. [DOI] [PubMed] [Google Scholar]
- 13.Noel S, Gasser V, Pesset B, Hoegy F, Rognan D, Schalk IJ, Mislin GLA. Org. Biomol. Chem. 2011;9:8288. doi: 10.1039/c1ob06250f. [DOI] [PubMed] [Google Scholar]
- 14.Milstien S, Cohen LA. J. Am. Chem. Soc. 1972;94:9158. doi: 10.1021/ja00781a029. [DOI] [PubMed] [Google Scholar]
- 15.Borchard. Rt, Cohen LA. J. Am. Chem. Soc. 1972;94:9166. doi: 10.1021/ja00781a030. [DOI] [PubMed] [Google Scholar]
- 16.Milstien S, Cohen LA. Proc. Natl. Acad. Sci. U.S.A. 1970;67:1143. doi: 10.1073/pnas.67.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King MM, Cohen LA. J. Am. Chem. Soc. 1983;105:2752. [Google Scholar]
- 18.Caswell M, Schmir GL. J. Am. Chem. Soc. 1980;102:4815. [Google Scholar]
- 19.Houghton TJ, Tanaka KSE, Kang T, Dietrich E, Lafontaine Y, Delorme D, Ferreira SS, Viens F, Arhin FF, Sarmiento I, Lehoux D, Fadhil I, Laquerre K, Liu J, Ostiguy V, Poirier H, Moeck G, Parr TR, Far AR. J. Med. Chem. 2008;51:6955. doi: 10.1021/jm801007z. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald RB, Choe YH, Conover CD, Shum K, Wu DC, Royzen M. J. Med. Chem. 2000;43:475. doi: 10.1021/jm990498j. [DOI] [PubMed] [Google Scholar]
- 21.Wang BH, Gangwar S, Pauletti GM, Siahaan TJ, Borchardt RT. J. Org. Chem. 1997;62:1363. [Google Scholar]
- 22.Pauletti GM, Gangwar S, Wang BH, Borchardt RT. Pharm. Res. 1997;14:11. doi: 10.1023/a:1012091014242. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaou MG, Yuan CS, Borchardt RT. J. Org. Chem. 1996;61:8636. doi: 10.1021/jo961069l. [DOI] [PubMed] [Google Scholar]
- 24.Wang BH, Zhang HJ, Wang W. Bioorg. Med. Chem. Lett. 1996;6:945. [Google Scholar]
- 25.Wang BH, Wang W, Zhang HJ, Shan DX, Smith TD. Bioorg. Med. Chem. Lett. 1996;6:2823. [Google Scholar]
- 26.Amsberry KL, Gerstenberger AE, Borchardt RT. Pharm. Res. 1991;8:455. doi: 10.1023/a:1015890809507. [DOI] [PubMed] [Google Scholar]
- 27.Amsberry KL, Borchardt RT. Pharm. Res. 1991;8:323. doi: 10.1023/a:1015885213625. [DOI] [PubMed] [Google Scholar]
- 28.Bornscheuer UT. FEMS Microbiol. Rev. 2002;26:73. doi: 10.1111/j.1574-6976.2002.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 29.Beacham IR. Int. J. Biochem. 1979;10:877. doi: 10.1016/0020-711x(79)90117-4. [DOI] [PubMed] [Google Scholar]
- 30.Mitscher LA. Chem. Rev. 2005;105:559. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- 31.Muller G, Raymond KN. J. Bacteriol. 1984;160:304. doi: 10.1128/jb.160.1.304-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpino LA, Triolo SA, Berglund RA. J. Org. Chem. 1989;54:3303. [Google Scholar]
- 33.Nelson TDRJD, Bhupathy M, McNamara J, Sowa MJ, Rush C, Crocker LS. Org. Synth. 2003;80 [Google Scholar]
- 34.Wencewicz TA, Möllmann U, Long TE, Miller MJ. Biometals. 2009;22:633. doi: 10.1007/s10534-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelm S, Tommassen J, Jaeger KE. J. Bacteriol. 1999;181:6977. doi: 10.1128/jb.181.22.6977-6986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 8th ed. Villanova, PA, USA: Clinical and Laboratory Standards Institute (CLSI); 2009. approved standard document M07-A7. [Google Scholar]
- 37.Wencewicz T. Ph. D. Dissertation. Notre Dame, IN: University of Notre Dame; 2011. [Google Scholar]
- 38.Roosenberg JM., II . Dissertation. Notre Dame, IN: University of Notre Dame; 2000. [Google Scholar]