Abstract
Background:
There is a need to clarify the usefulness of and problems associated with cylindrical costal osteochondral autograft for reconstruction of large defects of the capitellum due to osteochondritis dissecans.
Methods:
Twenty-six patients with advanced osteochondritis dissecans of the humeral capitellum were treated with use of cylindrical costal osteochondral autograft. All were males with elbow pain and full-thickness articular cartilage lesions of ≥15 mm in diameter. Clinical, radiographic, and magnetic resonance imaging outcomes were evaluated at a mean follow-up of thirty-six months (range, twenty-four to fifty-one months).
Results:
All patients had rapid functional improvement after treatment with costal osteochondral autograft and returned to their former activities, including sports. Five patients needed additional minor surgical procedures, including screw removal, loose body removal, and shaving of protruded articular cartilage. Mean elbow function, assessed with use of the clinical rating system of Timmerman and Andrews, was 111 points preoperatively and improved to 180 points at the time of follow-up and to 190 points after the five patients underwent the additional operations. Mean elbow motion was 126° of flexion with 16° of extension loss preoperatively and improved to 133° of flexion with 3° of extension loss at the time of follow-up. Osseous union of the graft on radiographs was obtained within three months in all patients. Revascularization of the graft depicted on T1-weighted magnetic resonance imaging and congruity of the reconstructed articular surface depicted on T2-weighted or short tau inversion recovery imaging were assessed at twelve and twenty-four months postoperatively. Functional recovery was good, and all patients were satisfied with the final outcomes.
Conclusions:
Cylindrical costal osteochondral autograft was useful for the treatment of advanced osteochondritis dissecans of the humeral capitellum. Functional recovery was rapid after surgery. Additional operations were performed for five of the twenty-six patients, whereas the remaining patients showed essentially full recovery within a year. All patients were satisfied with the results at the time of short-term follow-up.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Osteochondritis dissecans of the humeral capitellum is a common sports-related disorder in young athletes, especially baseball players and gymnasts. With severe osteochondritis dissecans, the damaged articular cartilage detaches from the capitellum and there is a full-thickness articular cartilage defect and radiocapitellar incongruity. Treatment for early-stage osteochondritis dissecans is principally nonoperative. When the lesion progresses to a large, full-thickness articular cartilage defect, treatment remains challenging. In the elbow, arthroscopic debridement has been widely applied with good results1-6. However, studies have shown that a large articular defect should be repaired or reconstructed as this is a risk factor for subsequent degenerative changes3,7-9.
Results of multiple cylindrical osteochondral plug grafts for capitellar osteochondritis dissecans with use of a non-weight-bearing site from the knee (“mosaicplasty”) have been encouraging. Shimada et al. reported that postoperative remodeling of the graft was observed for two to three years, and findings from a second look at the reconstructed lesion, including histological examination, were excellent10. Several studies have followed this approach, and most patients returned to their former sports activity without progression of degenerative changes11-14. However, unavoidable donor-site problems remain a disadvantage. One report showed no adverse effects on the donor site, whereas another described unfavorable donor-site problems after mosaicplasty15,16. Another disadvantage of this procedure is the fragility of the graft when placed in the lateral defect of the capitellum10. These are considered potential risk factors for poor results when the osteochondritis dissecans lesion is large and involves the lateral aspect of the capitellum11.
The ribs represent another potential donor site for osteochondral grafts comprising combined hyaline cartilage and bone. Several authors have described costal osteochondral autograft transplantation for upper limb arthroplasty, reporting favorable clinical results with minimum donor-site morbidity17-19. For the elbow, Oka and Ikeda reported the case of a patient with a large osteochondritis dissecans lesion that was reconstructed by osteochondral autografting with use of costal tissue, Sato et al. described several patients treated in the same manner, and Mihara et al. followed this procedure with good results20-23. Costal osteochondral autograft provides a larger graft than each osteochondral plug in mosaicplasty and can cover a large part of the defect, including the lateral aspect of the capitellum, although the procedure is technically difficult22. To standardize the procedure, we modified the costal osteochondral autograft by using a cylindrical graft on the basis of our experiences with mosaicplasty. We applied this procedure for severely damaged full-thickness articular lesions of ≥15 mm in diameter, as a key advantage of this technique is the applicability to lesions larger than the diameter of rib cartilage, which is approximately 15 mm in Japanese males. The goal of this operation is to obtain a stable and congruent articulation, allowing restoration of a painless elbow with good function. This report describes our retrospective evaluation in a case series of costal osteochondral autograft for elbows with advanced osteochondritis dissecans.
Materials and Methods
We received institutional review board approval for this clinical research work.
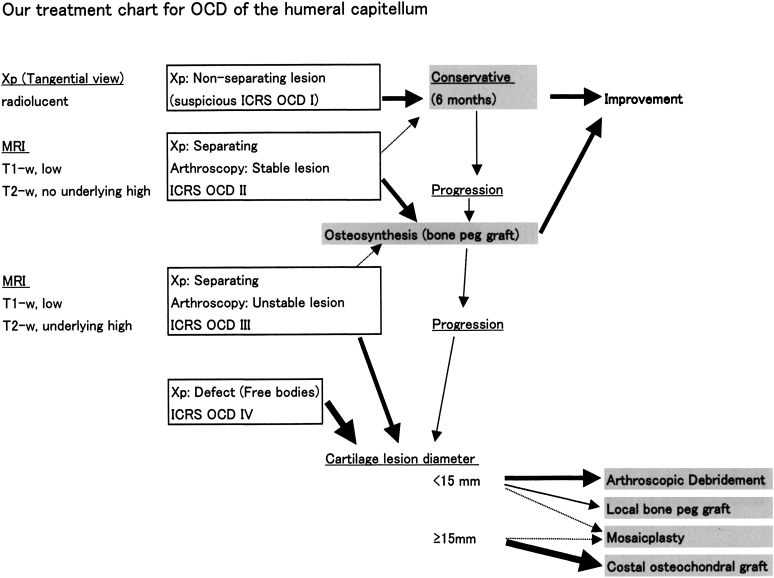
When a patient is diagnosed with elbow osteochondritis dissecans, grading is performed on the basis of the results of radiography and magnetic resonance imaging (MRI), and the treatment is selected according to the treatment chart (Fig. 1). The surgical indication for cylindrical costal osteochondral autograft is an International Cartilage Repair Society (ICRS) osteochondritis dissecans (OCD) III or IV lesion with a diameter of ≥15 mm24.
Fig. 1.
Treatment chart for osteochondritis dissecans (OCD) of the humeral capitellum. Patients with elbow osteochondritis dissecans in the early stage (suspected ICRS OCD I) are treated conservatively. Patients showing a more advanced stage and/or those for whom conservative treatment for six months has failed are treated surgically. In patients with ICRS-II lesions, we proceed to stabilizing the lesion (usually with bone peg grafts). For ICRS-III or IV lesions, we measure the diameter. When the lesion is <15 mm in diameter, we chose a less invasive procedure such as arthroscopic debridement. When the lesion is ≥15 mm in diameter, we choose a more aggressive reconstructive procedure—initially this was mosaicplasty, but currently we use osteochondral autograft with costal cartilage. OCD = osteochondritis dissecans, Xp = radiographs, MRI = magnetic resonance imaging, ICRS OCD = osteochondritis dissecans classification system of the International Cartilage Research Society24, and T1-w and T2-w = T1-weighted and T2-weighted.
Patients
A total of forty-three patients had undergone this procedure since 2006, with all surgical procedures performed by one senior surgeon (K.S.). Twenty-six patients had more than two years of follow-up postoperatively (Table I). Most of these twenty-six patients were teenage male athletes, with the exception of a forty-three-year-old man (Case 5) who developed severe elbow pain due to dislodgement of an old osteochondritis dissecans fragment. The osteochondritis dissecans lesion was located in the central capitellum in eight patients and in a central-lateral part including the lateral cortex in eighteen patients. The mean age at the time of surgery was sixteen years (range, twelve to forty-three years). The stage at the time of surgery was ICRS OCD III in twelve patients and ICRS OCD IV in fourteen patients. The smallest lesion was 11 × 16 mm, and the largest lesion was 20 × 18 mm. The mean lesion diameter was 16 mm (range, 11 to 21 mm). The mean defect size according to the method described by Takahara et al. (the ratio of the defect diameter to the capitellum diameter) was 72% (range, 52% to 92%)7. The mean duration of follow-up was thirty-six months (range, twenty-four to fifty-one months).
TABLE I.
Data on the Patients
| Location and Size of the Lesion |
|||||||||
| Diameter (mm) |
|||||||||
| Case | Age at Surgery (yr) | ICRS Classification* | Location† | Transverse | Vertical | Size‡ (%) | Donor Site | Wedge Bone Graft | Duration of Follow-up (mo) |
| 1 | 16 | IV | LC | 20 | 18 | 90 | 5th rib | – | 51 |
| 2 | 13 | III | LC | 14 | 18 | 73 | 6th rib | – | 50 |
| 3 | 13 | IV | C | 12 | 17 | 71 | 6th rib | – | 48 |
| 4 | 16 | IV | LC | 20 | 15 | 80 | 6th rib | + | 24 |
| 5 | 43 | IV | LC | 17 | 13 | 83 | 5th rib | + | 48 |
| 6 | 13 | IV | LC | 17 | 21 | 95 | 5th rib | + | 48 |
| 7 | 14 | III | C | 16 | 12 | 69 | 6th rib | – | 42 |
| 8 | 16 | IV | LC | 15 | 12 | 67 | 6th rib | – | 31 |
| 9 | 13 | IV | LC | 18 | 11 | 71 | 6th rib | + | 40 |
| 10 | 14 | III | C | 15 | 21 | 85 | 6th rib | + | 36 |
| 11 | 16 | IV | C | 11 | 16 | 71 | 5th rib | – | 32 |
| 12 | 13 | III | LC | 18 | 15 | 64 | 4th and partial 5th ribs | + | 42 |
| 13 | 16 | IV | LC | 19 | 17 | 92 | 5th rib | + | 36 |
| 14 | 14 | III | LC | 16 | 18 | 89 | 6th rib | + | 34 |
| 15 | 15 | III | LC | 13 | 16 | 67 | 6th rib | – | 34 |
| 16 | 12 | IV | LC | 15 | 21 | 71 | 6th rib | + | 32 |
| 17 | 14 | III | LC | 11 | 16 | 52 | 5th rib | – | 24 |
| 18 | 14 | III | LC | 16 | 14 | 64 | 6th rib | + | 36 |
| 19 | 14 | III | C | 13 | 16 | 85 | 6th rib | + | 36 |
| 20 | 14 | III | LC | 13 | 15 | 53 | 6th rib | + | 36 |
| 21 | 16 | IV | LC | 16 | 16 | 71 | 6th rib | – | 24 |
| 22 | 14 | III | C | 19 | 17 | 70 | 6th rib | + | 36 |
| 23 | 14 | III | LC | 18 | 19 | 71 | 6th rib | + | 24 |
| 24 | 16 | IV | LC | 16 | 12 | 65 | 6th rib | + | 24 |
| 25 | 16 | IV | C | 13 | 16 | 58 | 6th rib | – | 33 |
| 26 | 15 | IV | C | 12 | 17 | 57 | 6th rib | – | 31 |
| Mean | 16 | 16 | 16 | 72 | 36 | ||||
ICRS = International Cartilage Repair Society.
LC = lateral aspect of the capitellum, and C = capitellum.
Defect size means the percentage of the articular lesion in the capitellum, as assessed on preoperative radiographs according to the method described by Takahara et al.7.
Surgical Procedure
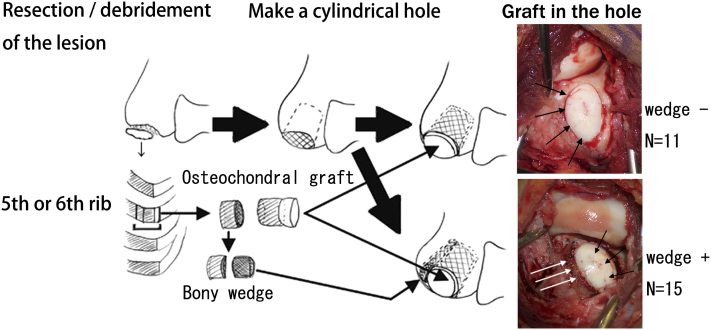
The surgical procedure consists of five steps (Fig. 2)25.
Fig. 2.
Schematic showing the procedure. Detached or unstable articular lesions were completely debrided. Sclerotic subchondral bone was perforated, and the area of the cartilage defect was deepened cylindrically. The osteochondral junction of the fifth or sixth rib was shaped like a cone and was pressed into the prepared hole in the recipient site. The cartilage surface was shaped and contoured to fit the radial head with use of a scalpel (black arrows in the intraoperative photograph). In fifteen patients, residual rib bone was sliced like an osseous wedge and packed into the space between the osteochondral graft and the posterolateral cortex of the recipient humerus (white arrows in the intraoperative photograph).
1. Excision or debridement of the unstable lesion. Any loose bodies identified, particularly in the anterior part of the elbow, are removed arthroscopically.
2. Creation of a cylindrical hole 15 mm deep with use of a high-speed surgical burr.
3. Elevation of the costal osteochondral junction from the fifth or sixth rib.
4. Preparation of a conical graft approximately 18 mm in height, including a 5-mm-high cartilage cap and osseous wedges for wedge graft.
5. Packing of graft into the hole, with insertion of a residual osseous wedge into the cleft between the graft and donor cortex, if necessary. The graft surface is then shaved to fit the radial head.
One and a half ribs were harvested and grafted in the manner of mosaicplasty for a patient with a very large osteochondritis dissecans lesion (Case 12). In the initial two patients (Cases 1 and 2), we added internal fixation using a compression screw or a headless screw, and the first patient (Case 1) underwent a second operation for removal of the internal fixation. Since establishing this “wedge technique,” we have not needed to use any internal fixation because the graft is stable after the procedure.
Postoperative Rehabilitation and Guidance
After two weeks of immobilization of the arm, active exercise is started. The patient is not allowed to play sports for three months and is allowed to make a step-by-step return to sporting activities with some limitations once complete graft union (trabecular continuity) has been achieved. We advise the patient to return to usual activities without limitation at six months postoperatively, and allow baseball pitchers to start pitching while limiting the number of pitches per day and per week until twelve months postoperatively. The patient is subsequently seen for follow-up evaluation every three or six months in the outpatient clinic.
Assessment
Clinical Assessment
We assessed all patients preoperatively and every three or six months after surgery (Table II). Subjective findings (pain at rest and during sports or heavy activity, swelling, catching sensation, and ability to perform activities of daily living) were obtained, and elbow motion was measured with use of a goniometer (MMI-goniometer; Muranaka Medical Instruments, Osaka, Japan). Elbow function was assessed according to the clinical rating system described by Timmerman and Andrews (see Appendix)26.
TABLE II.
Clinical, Radiographic, and Magnetic Resonance Imaging Data on the Twenty-six Elbows
| Timmerman and Andrews Score* |
Elbow Range of Flexion-Extension (deg) |
||||||||||||||||||||
| Preop. |
At Follow-up |
After Revision |
Preop. |
Follow-up |
After Revision |
MRI at 9 to 12 Months† |
MRI at 24 Months or Before Revision† |
||||||||||||||
| Case | Total | Pain | Overall | Total | Pain | Overall | Total | Pain | Overall | Flex. | Ext. | Flex. | Ext. | Flex. | Ext. | Osseous Union | Marrow | Congruity | Marrow | Congruity | Additional Surgery |
| 1 | 70 | 5 | P | (130) | (20) | F | 160 | 20 | G | 120 | −45 | (125) | (−30) | 135 | −25 | + | (A)‡ | (N)‡ | − | − | At 24 mo postop, removal of screw and shaving of osteoarthritis |
| 2 | 125 | 10 | F | 175 | 25 | G | 175 | 25 | 130 | −10 | 125 | −15 | 125 | −15 | + | (A)‡ | (N)‡ | − | − | ||
| 3 | 110 | 10 | P | 195 | 25 | E | 195 | 25 | 125 | −15 | 130 | 0 | 130 | 0 | + | N | N | N | N | ||
| 4 | 120 | 10 | F | 200 | 25 | E | 200 | 25 | 115 | 0 | 125 | 10 | 125 | 10 | + | A | N | N | N | ||
| 5 | 135 | 10 | F | 200 | 25 | E | 200 | 25 | 120 | 0 | 135 | 10 | 135 | 10 | + | A | A | − | − | ||
| 6 | 50 | 5 | P | 140 | 25 | F | 140 | 25 | 110 | −40 | 125 | −5 | 125 | −5 | + | (A)‡ | (A)‡ | N | A | ||
| 7 | 125 | 10 | F | 200 | 25 | E | 200 | 25 | 120 | −5 | 140 | 0 | 140 | 0 | + | A | N | N | N | ||
| 8 | 110 | 5 | P | (95) | (10) | P | 200 | 25 | E | 125 | −10 | (125) | (−30) | 135 | 5 | + | A | A | N | A | At 18 mo, removal of free body (partial) |
| 9 | 115 | 5 | P | 200 | 25 | E | 200 | 25 | 135 | −5 | 140 | 10 | 140 | 10 | + | A | N | − | − | ||
| 10 | 145 | 10 | F | (170) | (20) | G | 200 | 25 | E | 130 | −5 | (140) | (5) | 140 | 0 | + | A | N | N | A | At 21 mo, shaving of protruding cartilage |
| 11 | 145 | 10 | F | 200 | 25 | E | 200 | 25 | 145 | −5 | 135 | 0 | 135 | 0 | + | N | N | N | N | ||
| 12 | 120 | 5 | F | (170) | (20) | G | 200 | 25 | E | 130 | −5 | (130) | (0) | 135 | 15 | + | N | A | N | A | At 20 mo, shaving of protruding cartilage |
| 13 | 65 | 10 | P | 200 | 25 | E | 200 | 25 | 125 | −45 | 140 | 0 | 140 | 0 | + | A | N | N | N | ||
| 14 | 85 | 5 | P | (130) | (10) | F | 180 | 25 | E | 130 | −30 | (135) | (−20) | 130 | −5 | + | A | N | N | A | At 24 mo, removal of unstable cartilage tissue |
| 15 | 140 | 10 | F | 200 | 25 | E | 200 | 25 | 130 | 0 | 140 | 5 | 140 | 5 | + | N | N | N | N | ||
| 16 | 45 | 5 | P | 180 | 25 | E | 180 | 25 | 110 | −65 | 135 | −5 | 135 | −5 | + | N | N | N | A | ||
| 17 | 110 | 5 | P | 195 | 25 | E | 195 | 25 | 125 | −25 | 140 | −5 | 140 | −5 | + | N | N | N | N | ||
| 18 | 130 | 5 | F | 200 | 25 | E | 200 | 25 | 135 | −5 | 140 | 5 | 140 | 5 | + | A | A | A | A | ||
| 19 | 135 | 5 | F | 175 | 25 | G | 175 | 25 | 130 | −15 | 125 | −10 | 125 | −10 | + | N | N | N | N | ||
| 20 | 145 | 5 | F | 200 | 25 | E | 200 | 25 | 135 | 0 | 135 | 10 | 135 | 10 | + | A | N | N | N | ||
| 21 | 120 | 5 | F | 200 | 25 | E | 200 | 25 | 135 | −5 | 140 | 5 | 140 | 5 | + | N | N | N | N | ||
| 22 | 145 | 10 | F | 200 | 25 | E | 200 | 25 | 125 | −5 | 130 | 10 | 130 | 10 | + | N | N | N | N | ||
| 23 | 115 | 10 | P | 170 | 20 | G | 170 | 20 | 120 | −20 | 135 | −10 | 135 | −10 | + | A | N | A | A | ||
| 24 | 115 | 10 | P | 175 | 25 | G | 175 | 25 | 115 | −5 | 120 | −15 | 120 | −15 | + | A | A | N | A | ||
| 25 | 80 | 5 | P | 200 | 25 | E | 200 | 25 | 115 | −20 | 140 | 0 | 140 | 0 | + | A | N | N | N | ||
| 26 | 90 | 5 | P | 185 | 25 | E | 185 | 25 | 135 | −30 | 140 | −10 | 140 | −10 | + | N | N | N | N | ||
| Mean# | 111 | 7 | 180§ | 23 | 190§ | 25 | 126 | −16 | 133§ | −3§ | 134§ | −1§ | + | ||||||||
According to the criteria of Timmerman and Andrews26, an overall score of E indicated excellent (180-200); G, good (160-179); F, fair (120-159); and P, poor (<120). Final follow-up status of these patients was the status after revision surgery.
Marrow, or revascularization, was assessed with T1-weighted MRI scans, N indicates nearly normal marrow intensity, and A indicates abnormal low intensity. Congruity was assessed with T2-weighted short tau inversion recovery scans, with N indicating round spherical articular surface and A indicating abnormal contour such as protrusion or irregular articular surface.
MRI was performed at six months postoperatively in Cases 1, 2, and 6, as indicated by parentheses. Dates are shown in parentheses. MRI was performed for the other twenty-three patients at nine to twelve months postoperatively. MRI was performed a second time at approximately twenty-four months postoperatively or before revision surgery.
A significant increase was detected compared with the preoperative status (p < 0.05) for the Timmerman and Andrews total score at follow-up (p = 0.000009 [or 0.0000084]) and after revision (p = 0.000008 [or 0.0000083]) and for elbow flexion (p = 0.000141) and extension (p = 0.000377) at follow-up and after revision (p = 0.000041 and p = 0.000015, respectively).
The Timmerman and Andrews overall score was F for thirteen patients and P for thirteen preoperatively; E for sixteen, G for six, F for three, and P for one patient at the time of follow-up; and E for twenty, G for five, and F for one after revision. The average arc of motion of the elbow was 110° preoperatively, 131° at follow-up, and 133° after revision. All twenty-six patients had osseous union. At nine to twelve months, MRI demonstrated a total of ten elbows with normal findings and sixteen with abnormal findings for marrow and twenty elbows with normal findings and six with abnormal findings for congruity. At twenty-four months or before revision, twenty elbows had normal findings and two had abnormal findings for marrow and thirteen had normal findings and nine had abnormal findings for congruity. Imaging was not available for four elbows.
Radiography
Postoperative osseous union of the graft and subchondral osseous remodeling were assessed on radiography every three to six months. Bridging or continuity of the trabeculae was defined as osseous union. Three radiographic views, including anteroposterior, lateral, and a tangential view in 45° of flexion to assess the anteroinferior area of the capitellum, were made.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed on a 1.5-T scanner (MAGNETOM Symphony; Siemens, Erlangen, Germany) at six to twelve months postoperatively for all patients (three patients at six months and twenty-three patients at twelve months), with follow-up imaging at eighteen to twenty-four months postoperatively for twenty-two patients. Conventional MRI, including T1-weighted, T2-weighted, and short tau inversion recovery (STIR) imaging was performed with a section thickness of 3.0 mm, in-plane resolution of 0.8 × 0.6 mm, and field of view of 15 cm. The T1-weighted imaging used a repetition time of 572 ms, an echo time of 12 ms, and signal acquisition of one. The T2-weighted imaging used a repetition time of 3500 ms, an echo time of 71 ms, and signal acquisition of one. STIR imaging used a repetition time of 4360 ms, an echo time of 82 ms, inversion time of 140 ms, and signal acquisition of one.
Assessment was performed according to the scoring system of Roberts et al.27 (see Appendix). Articular cartilage signal and thickness were not assessed in this study because those findings were basically different from the surrounding tissue in this operation. Revascularization of the graft was assessed as changes in underlying bone. The assessment was considered as nearly normal when most of the graft had regained normal high intensity on T1-weighted imaging. Postoperative surface integrity and contour of the grafted cartilage were assessed on T2-weighted or STIR imaging. The assessment was considered nearly normal when most of the graft surface showed a spherical contour.
Findings at Second-Look Surgery
Additional surgery was performed in five patients at eighteen to twenty-four months postoperatively because of minor symptoms or for removal of instrumentation. We visually assessed the lesion and the reconstructed articular surface in those five patients.
Statistical comparisons of the status preoperatively, postoperatively, and at the time of the final follow-up were performed. The Timmerman and Andrews score was assessed with use of Wilcoxon t test because the dates were nonparametric. Range of motion was assessed with use of the paired t test because the dates were parametric. P values of <0.05 were considered significant.
Source of Funding
No external funding source played any role in the investigation in this study.
Results
Clinical Findings
All patients had substantial elbow pain during daily life or sports activity before the operation. Pain disappeared or was markedly reduced at the time of follow-up. All patients returned to previous daily activities without pain within four to six weeks postoperatively, and all achieved a successful return to former sporting activities by six months postoperatively. Ten baseball pitchers started a pitching program at six months, returning to full pitching activity within nine to twelve months. After returning to full sports and/or daily activity, seven patients (including five pitchers) showed catching symptoms or mild pain during heavy elbow activity with deteriorated findings on MRI. Five of the twenty-six patients received additional minor surgery at eighteen to twenty-four months postoperatively. The reconstructed articular surface was identified in those patients. Two patients had catching (Case 8) or motion pain (Case 14) at eighteen and twenty-four months, respectively, after the osteochondral autografting procedure and showed recurrence of a loose body or unstable cartilaginous flap lesion representing part of the cartilage that had peeled off from the reconstructed capitellum. These lesions were removed and the rough articular surface was debrided. The involved lesions were smaller than the original defect as part of the graft survived. Two patients (Cases 10 and 12) showed a rough surface protrusion on the lateral corner of the graft area on follow-up MRI. This protrusion represented hypertrophy of the reconstructed cartilage and was arthroscopically identified and shaved (Figs. 3-A and 3-B). Another patient (Case 1) had already shown osteoarthritic changes before surgery. He was the first patient in the series, and graft fixation was augmented with use of a compression screw. The screw was removed with additional osteophyte debridement as a scheduled operation at twenty-four months postoperatively. All of these patients returned to former activities soon after the second surgery, without any limitation.
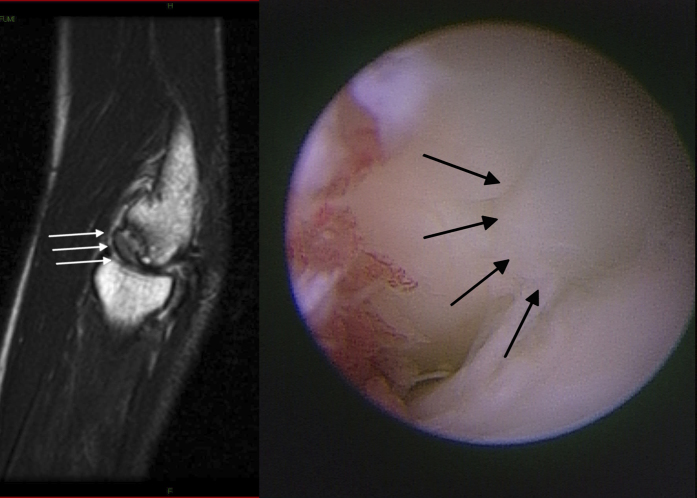
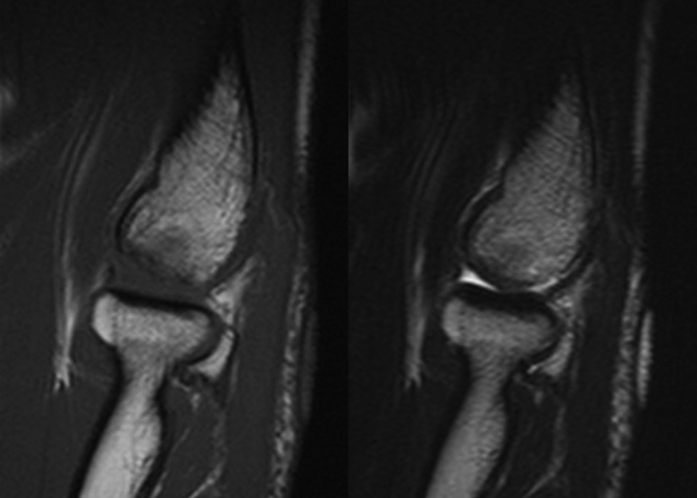
Fig. 3.
Case 10. A patient who returned to activity as a baseball pitcher nine months postoperatively, but had mild pain recur at twenty-one months postoperatively after overuse. A T2-weighted image (left) shows a spherical but protruding articular surface in the grafted area (white arrows). This protruding articular surface (right) was identified arthroscopically (black arrows), and the protruding area was shaved. The patient quickly returned to sporting activity after the second operation.
The mean arc of motion for elbows was 110° preoperatively (126° of flexion to –16° of extension), improving to 130° (133° of flexion to –3° of extension) postoperatively and to 133° (134° of flexion to –1° of extension) at the time of final follow-up after all second surgical procedures. Elbow motion improved significantly both in flexion and in extension from the preoperative status to the postoperative status (p = 0.000141 for flexion and p = 0.000377 for extension) and to the status at the time of the final follow-up (p = 0.000041 for flexion and p = 0.000015 for extension). Elbow function, as assessed with use of the clinical rating system of Timmerman and Andrews, was initially 111 (including thirteen elbows with fair results and thirteen with poor results), improving to 180 (including sixteen elbows with excellent results, six with good, three with fair, and one with poor results), and to 190 (including twenty elbows with excellent results, five with good, and one with fair results) at the time of the final follow-up. This improvement from the preoperative status was significant compared with the postoperative status (p = 0.000009) and with the status at the time of final follow-up (p = 0.000008). All patients returned to their previous level of activity, including sports or heavy manual labor, and were satisfied with the final results.
Image Findings
Osseous union of the graft was obtained within three months in all patients. Grafted cartilage was usually thicker than the cartilage of the recipient area and continued to be identifiable as an osseous defect on radiography, but five patients showed new bone formation compensating for the defect (Figs. 4-A and 4-B). The defect was not completely buried, but bone remodeling progressed to match the capitellum in these patients. One patient (Case 6) showed slight sinking of the reconstructed capitellum followed by hypertrophic remodeling of the radial head. Clinical assessment in this patient was rated as fair because of limitation of motion, but he returned to playing baseball as an infielder. No other patients showed degenerative changes or progression of osteoarthritic changes after surgery.
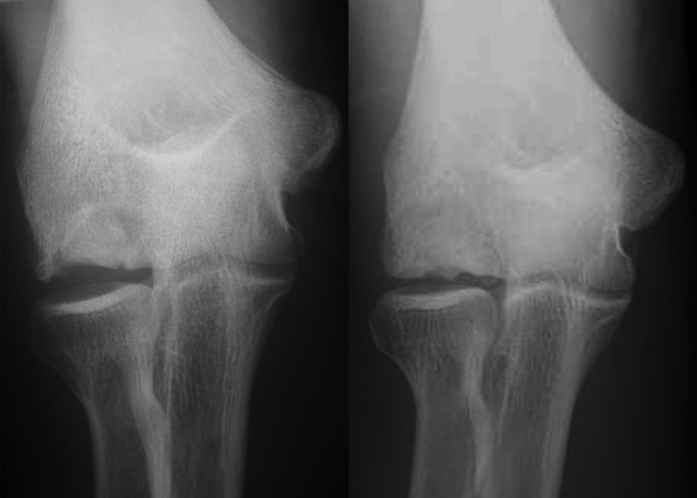
Fig. 4.
Case 3. Anteroposterior elbow radiograph made immediately postoperatively (left) and anteroposterior elbow radiograph made forty-eight months postoperatively (right). These images indicate subchondral remodeling of the graft with no progression of degeneration.
MRI scans at six months postoperatively did not show homogeneous signal hyperintensity of the bone part of the graft on T1-weighted imaging in three patients, which may indicate poor revascularization. Ten (43%) of twenty-three patients examined at twelve months postoperatively showed homogeneous signal hyperintensity on T1-weighted imaging, and the remaining thirteen patients (57%) showed a combination of high and low-signal intensity, indicative of possible remodeling. Ten of the patients with low-signal intensity showed progression of revascularization at eighteen to twenty-four months. Twenty (77%) of the twenty-six patients showed congruous joint contours on T2-weighted or STIR images at six to twelve months postoperatively. Thirteen (59%) of the twenty-two patients examined at eighteen to twenty-four months postoperatively showed congruous joint contours. Those with good patterns on both T1-weighted and T2-weighted imaging generally experienced a good clinical course (Figs. 5-A and 5-B). Of the patients with an incongruent articular surface on MRI, two had recurrence of loose or unstable cartilaginous lesions and another two showed a slight hypertrophied protrusion (Figs. 3-A and 3-B). All four of these patients were baseball pitchers who returned to high-level activity within a year. Three of these patients showed abnormal intensity on T1-weighted imaging at twelve months postoperatively. The other patients with abnormal findings on MRI scans at twenty-four months postoperatively had no symptoms at the time of follow-up, but continued to be followed.
Fig. 5.
Case 11. MRI scans made twelve months postoperatively. A T1-weighted sagittal image (left) shows well-revascularized subchondral bone in the graft area, and congruent articular contours are apparent on a T2-weighted sagittal image (right). The patient returned to sporting activity as a Judo athlete within one year.
Complications
No patient had infection or difficulties with wound-healing. One patient had postoperative pneumothorax because of damage to the costal pleura during the harvesting of the costal cartilage. It was treated with insertion of a chest tube and resolved in one day. All patients reported donor-site pain for a few days postoperatively, but no complaints were made after that time. Some patients showed new subperiosteal bone formation at the donor site on chest radiography without any symptoms at two years postoperatively.
Discussion
Treatment for early-stage osteochondritis dissecans is principally conservative, but many patients continue to play sports, resulting in lesion progression. Unfortunately, most of our patients with elbow osteochondritis dissecans are in the progressive stage, showing a large full-thickness articular lesion. The elbow is not a weight-bearing area, but it receives very high load, pressure, and shearing stress during heavy daily and sports activity. Patients are typically eager to return to their original activities, including sports, but insufficient treatment or inadequate guidance can lead to irreversible progressive osteoarthritis, resulting in not only an end to the sporting activity but also to disturbance of daily life. Numerous treatment options are available for elbow osteochondritis dissecans, such as open or arthroscopic debridement with or without abrasion by microfracture or drilling1-9,28; fixation of the osteochondral fragment with osseous pegs or fixation materials such as wires, screws, staples, and biodegradable pins with additional bone graft29-36; and closed-wedge osteotomy of the distal end of the humerus37,38. However, consensus has been lacking with regard to dislodgment of loose fragments or very unstable lesions such as those classified as ICRS OCD III and IV39. This is attributable to the poor outcomes seen in patients who have undergone resection and/or debridement arthroplasty, particularly for large lesions involving >70% of the capitellum, and to the uncertain fixation of fragmented unstable osteochondral lesions in competitive athletes1,3,7-9,33.
Osteochondral autograft is one of the most reliable reconstructive procedures for the treatment of full-thickness articular defects currently available. This method allows simultaneous reconstruction of both the cartilage surface and the subchondral osseous support. Non-weight-bearing or minimal weight-bearing parts of the femoral condyle represent popular donor sites in the surgical treatment of osteochondral defects in the knee. Matsusue et al. first described the transfer of multiple cylindrical osteochondral plugs, and studies by Bobić and by Hangody et al. popularized this transfer with the use of sophisticated instruments40-42. These instruments facilitated firm fixation of the graft, and osteochondral autograft procedures have thus gained popularity and increased application as so-called mosaicplasty in other joints, including the elbow42-44. Shimada et al. reported the usefulness of osteochondral autograft from the knee for elbow osteochondritis dissecans in the first English-language report of a case series10. Numerous case series have followed, and the results have generally been excellent11-14. In a recent review article, mosaicplasty was considered for the treatment of extended lesions in elbow osteochondritis dissecans45. However, disadvantages such as donor-site problems and surface irregularity are unavoidable because of the “mosaic fashion” of the reconstructed articular surface10. For example, lesions of ≥15 mm in diameter usually need four cylindrical osteochondral plugs. This means that four deep cylindrical holes must be created in the patient's knee. One report on capitellar osteochondritis dissecans showed no adverse effects on the donor site; however, another study on talar osteochondritis dissecans found unfavorable donor-site problems after mosaicplasty15,16. Donor-site problems for large lesions must therefore be considered. Large lesions located in the lateral capitellum are still technically difficult, even in mosaicplasty, as perpendicular insertion of the osteochondral plugs is difficult, and oblique insertion of the plugs results in an incongruent articular surface. Excessively lateral insertion results in exposure of the fragile cancellous bone of the grafted plug following breakage10,11. The optimal reconstructive technique for large osteochondritis dissecans defects would provide stable, congruent articular reconstruction with minimal donor-site morbidity.
The ribs represent another potential donor site for reconstruction of articular defects with osteochondral autograft. This procedure has already been introduced for the treatment of defects involving the mandible, fingers, carpal bones, and elbow17-23,46. Good clinical results have been demonstrated, although the cases of relatively few patients have been reported and the procedure has not been firmly established. Costal cartilage is hyaline cartilage with similar biomechanical properties to articular cartilage, and the composition of the osteochondral junction resembles the subchondral area of the synovial joint. The technical difficulties and restrictions to surgical indications for costal osteochondral grafts have been described20. Matching the shape of the graft with the articular defect is difficult and requires a very precise technique22. In the present study, we introduced a new technique using costal tissue on the basis of our experience with cylindrical osteochondral autograft from the knee. We created a cylindrical hole in the elbow, press-fitted the graft, and shaved the cartilage surface using a scalpel to obtain a congruent surface (Fig. 2)25. Measurement of the depth of the hole and preparation of the graft height enabled replacement of the subchondral component. In the case of very large lesions, a cleft exists between the graft and the posterolateral wall of the recipient capitellum. We shaped the remnant of the rib into a wedge that was packed into this cleft to stabilize the graft. This wedge technique was used in fifteen patients (58%), and the results were similar to those in patients who had osteochondral autograft alone. Donor-site morbidity was low. Careful stripping of the rib periosteum is important when harvesting the graft, as accidental laceration of the costal pleura can cause pneumothorax. New bone formation in the harvested area and hard scar tissue around the donor site were observed within two years postoperatively. No patient in this series had any chest and/or rib discomfort or deformity at the time of follow-up.
We encountered two patients with recurrent loose bodies and two patients with impingement of hypertrophied fibrous tissue over the graft. These patients were baseball pitchers, all of whom had returned to sports activities after six to twelve months postoperatively. T1-weighted MRI scans from three of those patients showed immature remodeling of the underlying bone at twelve months postoperatively. These symptoms occurred at around eighteen months postoperatively and could have resulted from excessive shearing stress on the immature graft and surrounding tissue. Baseball pitching at six to twelve months postoperatively may be excessively strenuous for the elbow with insufficient graft maturity. Stress on the grafted cartilage too early is likely to risk poor results. For athletic patients, timing of the return to activities should be carefully planned, particularly for those who do not show adequate remodeling of the graft on MRI. We currently allow such patients to increase participation in sporting activities in a step-by-step manner after six months postoperatively. Full activity is allowed for patients showing homogeneous signal intensity on T1-weighted imaging and a congruous articular surface on T2-weighted or STIR imaging at twelve months postoperatively.
Overall results of cylindrical costal osteochondral autograft were comparable with those for previously described procedures such as arthroscopic debridement or mosaicplasty. Clinical findings were good, with minimal donor-site morbidity at a mean follow-up of thirty-six months. MRI is useful for postoperative assessment of the lesion. Patients were quickly able to return to previous activities and were satisfied with the results, although proper postoperative guidelines for athletes must be considered. While long-term follow-up is clearly necessary, the approach described in the present study could represent a new surgical option for articular reconstruction of elbows with full-thickness articular cartilage lesions, such as osteochondritis dissecans of the capitellum.
Appendix
Tables showing the clinical rating system described by Timmerman and Andrews26 and the magnetic resonance image scoring system of Roberts et al.27 are available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors thank Dr. Eiji Sogo, Dr. Nobuyuki Kubo, Dr. Hiroyuki Nakamura, and Dr. Yukitoshi Kaisawa for their assistance and contribution to the preparation of this manuscript and for operative assistance and collection of patients’ clinical and imaging data.
Footnotes
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Jackson DW, Silvino N, Reiman P. Osteochondritis in the female gymnast's elbow. Arthroscopy. 1989;5:129-36 [DOI] [PubMed] [Google Scholar]
- 2.Baumgarten TE, Andrews JR, Satterwhite YE. The arthroscopic classification and treatment of osteochondritis dissecans of the capitellum. Am J Sports Med. 1998;26:520-3 [DOI] [PubMed] [Google Scholar]
- 3.Ruch DS, Cory JW, Poehling GG. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 1998;14:797-803 [DOI] [PubMed] [Google Scholar]
- 4.Byrd JW, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30:474-8 [DOI] [PubMed] [Google Scholar]
- 5.Rahusen FT, Brinkman JM, Eygendaal D. Results of arthroscopic debridement for osteochondritis dissecans of the elbow. Br J Sports Med. 2006;40:966-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlow HC, O'Connor-Read LM, Perko M. Arthroscopic treatment of osteochondritis dissecans of the capitellum. Knee Surg Sports Traumatol Arthrosc. 2006;14:198-202 [DOI] [PubMed] [Google Scholar]
- 7.Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363:108-15 [PubMed] [Google Scholar]
- 8.Shimada K, Akita S, Hamada M, Nakata K, Yoshida T. [Sports-related disorders of the elbow]. Rinshou Seikeigeka Clin Orthop. 2000;35:1217-26 Japanese [Google Scholar]
- 9.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89:1205-14 [DOI] [PubMed] [Google Scholar]
- 10.Shimada K, Yoshida T, Nakata K, Hamada M, Akita S. Reconstruction with an osteochondral autograft for advanced osteochondritis dissecans of the elbow. Clin Orthop Relat Res. 2005;435:140-7 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34:714-20 [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki N, Kato H, Ishikawa J, Saitoh S, Minami A. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34:1233-9 [DOI] [PubMed] [Google Scholar]
- 13.Ansah P, Vogt S, Ueblacker P, Martinek V, Woertler K, Imhoff AB. Osteochondral transplantation to treat osteochondral lesions in the elbow. J Bone Joint Surg Am. 2007;89:2188-94 [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki N, Kato H, Ishikawa J, Masuko T, Funakoshi T, Minami A. Autologous osteochondral mosaicplasty for osteochondritis dissecans of the elbow in teenage athletes. J Bone Joint Surg Am. 2009;91:2359-66 [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki N, Kato H, Kamishima T, Suenaga N, Minami A. Donor site evaluation after autologous osteochondral mosaicplasty for cartilaginous lesions of the elbow joint. Am J Sports Med. 2007;35:2096-100 [DOI] [PubMed] [Google Scholar]
- 16.Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80-5 [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa T, Yamano Y. Arthroplasty of the proximal interphalangeal joint using costal cartilage grafts. J Hand Surg Br. 1992;17:583-5 [DOI] [PubMed] [Google Scholar]
- 18.Sandow MJ. Proximal scaphoid costo-osteochondral replacement arthroplasty. J Hand Surg Br. 1998;23:201-8 [DOI] [PubMed] [Google Scholar]
- 19.Glard Y, Gay A, Valenti D, Berwald C, Guinard D, Legré R. Costochondral autograft as a salvage procedure after failed trapeziectomy in trapeziometacarpal osteoarthritis. J Hand Surg Am. 2006;31:1461-7 [DOI] [PubMed] [Google Scholar]
- 20.Oka Y, Ikeda M. Treatment of severe osteochondritis dissecans of the elbow using osteochondral grafts from a rib. J Bone Joint Surg Br. 2001;83:738-9 [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Mio F, Hosoya T, Ito Y. Two cases with osteochondritis dissecans of the capitulum humeri treated with costal osteochondral graft transplantation. J Shoulder Elbow Surg. 2003;12:403-7 [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Nakamura T, Toyama Y, Ikegami H. Costal osteochondral grafts for osteochondritis dissecans of the capitulum humeri. Tech Hand Up Extrem Surg. 2008;12:85-91 [DOI] [PubMed] [Google Scholar]
- 23.Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19:31-7 [DOI] [PubMed] [Google Scholar]
- 24.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85 Suppl 2:58-69 [DOI] [PubMed] [Google Scholar]
- 25.Shimada K, Tanaka H, Matsumoto T, Miyake J, Higuchi H, Gamo K, Fuji T. Cylindrical costal osteochondral autograft for reconstruction of large defects of the capitellum due to osteochondritis dissecans. Essential Surgical Techniques. [In press] [DOI] [PMC free article] [PubMed]
- 26.Timmerman LA, Andrews JR. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22:230-5 [DOI] [PubMed] [Google Scholar]
- 27.Roberts S, McCall IW, Darby AJ, Menage J, Evans H, Harrison PE, Richardson JB. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodward AH, Bianco AJ., Jr Osteochondritis dissecans of the elbow. Clin Orthop Relat Res. 1975;110:35-41 [DOI] [PubMed] [Google Scholar]
- 29.Oka Y, Ohta K, Fukuda H. Bone-peg grafting for osteochondritis dissecans of the elbow. Int Orthop. 1999;23:53-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh Y. [Sports injuries of the elbow]. Nippon Seikeigeka Gakkai Zasshi (J Jpn Orthop Assoc.) 2008;82:45-58 Japanese [Google Scholar]
- 31.Kondo M, Asoh K. [The treatment of osteochondritis dissecans of the elbow. –pull out wire method-]. Kansetsugeka (J Joint Surg). 1992;11:630-6 Japanese [Google Scholar]
- 32.Takeda H, Watarai K, Matsushita T, Saito T, Terashima Y. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent baseball players. Am J Sports Med. 2002;30:713-7 [DOI] [PubMed] [Google Scholar]
- 33.Nobuta S, Ogawa K, Sato K, Nakagawa T, Hatori M, Itoi E. Clinical outcome of fragment fixation for osteochondritis dissecans of the elbow. Ups J Med Sci. 2008;113:201-8 [DOI] [PubMed] [Google Scholar]
- 34.Kuwahata Y, Inoue G. Osteochondritis dissecans of the elbow managed by Herbert screw fixation. Orthopedics. 1998;21:449-51 [DOI] [PubMed] [Google Scholar]
- 35.Harada M, Ogino T, Takahara M, Ishigaki D, Kashiwa H, Kanauchi Y. Fragment fixation with a bone graft and dynamic staples for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2002;11:368-72 [DOI] [PubMed] [Google Scholar]
- 36.Takeba J, Takahashi T, Hino K, Watanabe S, Imai H, Yamamoto H. Arthroscopic technique for fragment fixation using absorbable pins for osteochondritis dissecans of the humeral capitellum: a report of 4 cases. Knee Surg Sports Traumatol Arthrosc. 2010;18:831-5 [DOI] [PubMed] [Google Scholar]
- 37.Yoshizu T. [Wedge osteotomy of the lateral humeral condyle for the treatment of osteochondritis dissecans of the capitellum in baseball elbows]. Shujutsu. (Operation). 1986;40:131-6 Japanese [Google Scholar]
- 38.Kiyoshige Y, Takagi M, Yuasa K, Hamasaki M. Closed-Wedge osteotomy for osteochondritis dissecans of the capitellum. A 7- to 12-year follow-up. Am J Sports Med. 2000;28:534-7 [DOI] [PubMed] [Google Scholar]
- 39.Bradley JP, Petrie RS. Osteochondritis dissecans of the humeral capitellum. Diagnosis and treatment. Clin Sports Med. 2001;20:565-90 [DOI] [PubMed] [Google Scholar]
- 40.Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318-21 [DOI] [PubMed] [Google Scholar]
- 41.Bobić V. Arthroscopic osteochondral autograft transplantation in anterior cruciate ligament reconstruction: a preliminary clinical study. Knee Surg Sports Traumatol Arthrosc. 1996;3:262-4 [DOI] [PubMed] [Google Scholar]
- 42.Hangody L, Kish G, Kárpáti Z, Szerb I, Eberhardt R. Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique—a preliminary report. Foot Ankle Int. 1997;18:628-34 [DOI] [PubMed] [Google Scholar]
- 43.Hayashi K, Ochi M, Uchio Y, Takao M, Kawasaki K, Yamagami N. A new surgical technique for treating bilateral Freiberg disease. Arthroscopy. 2002;18:660-4 [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa Y, Matsusue Y, Ikeda N, Asada Y, Nakamura T. Osteochondral grafting and arthroplasty for end-stage osteochondritis dissecans of the capitellum. A case report and review of the literature. Am J Sports Med. 2001;29:650-5 [DOI] [PubMed] [Google Scholar]
- 45.Baker CL, 3rd, Romeo AA, Baker CL., Jr Osteochondritis dissecans of the capitellum. Am J Sports Med. 2010;38:1917-28 [DOI] [PubMed] [Google Scholar]
- 46.MacIntosh RB, Henny FA. A spectrum of application of autogenous costochondral grafts. J Maxillofac Surg. 1977;5:257-67 [DOI] [PubMed] [Google Scholar]