Abstract
Carcinoids are rare, slow-growing tumors originating from a variety of different neuroendocrine cell types. They are identified histologically by their affinity for silver salts and by positive reactions to neuroendocrine markers such as neuron-specific enolase, synaptophysin and chromogranin. They can present with various clinical symptoms and are difficult to diagnose. We present the case of a 43-year-old woman who was referred for evaluation of anemia. Upper endoscopy showed a duodenal bulb mass around 1 cm in size. Histopathological and immunohistochemistry staining were consistent with the diagnosis of a carcinoid tumor. Further imaging and endoscopic studies showed no other synchronous carcinoid lesions. Endoscopic ultrasound (EUS) revealed a 1 cm lesion confined to the mucosa and no local lymphadenopathy. Successful endoscopic mucosal resection of the mass was performed. Follow-up surveillance 6 months later with EUS and Octreoscan revealed no new lesions suggestive of recurrence. No consensus guidelines exist for the endoscopic management of duodenal carcinoid tumors. However, endoscopic resection is safe and preferred for tumors measuring 1 cm or less with no evidence of invasion of the muscularis layer.
Key Words: Duodenal carcinoid tumors, Neuroendocrine cell types, Endoscopic ultrasound, Endoscopic mucosal resection
Introduction
The first description of a carcinoid tumor was made by Otto Lubarsch in 1888 [1]. However it was Oberndorfer in 1907 who first used the term ‘Karzinoide’ to describe a carcinoma-like small bowel tumor that behaved in a benign fashion. The term ‘carcinoid’ represents a diverse group of neoplasms that arise from various neuroendocrine cell types and are characterized by variable biologic aggressiveness. Carcinoid tumors most commonly involve the lungs and gastrointestinal tract. Carcinoid tumors of the thymus, ovaries, testes, heart and middle ear have also been reported. Gastrointestinal carcinoids can secrete bioactive amines and peptides, including serotonin, 5-hydroxytryptophan, gastrin, calcitonin, somatostatin and glucagon. A common secretory product for all types of carcinoid tumors is the glycoprotein chromogranin A (CgA), the most important general tumor marker in these patients.
Little is known about the molecular genetic changes underlying tumorigenesis. Sporadic foregut tumors often display allelic losses at chromosome 11q13, and somatic MEN1 gene mutations have been reported in one third of sporadic foregut tumors. Deletions of chromosomes 18q and 18p have also been reported. Gene expression arrays for carcinoid tumors have demonstrated upregulation of the RET proto-oncogene, but no such mutations have been detected.
Primary duodenal carcinoids account for less than 2% of all gastrointestinal carcinoids [2]. The annual incidence of duodenal carcinoids is 0.07/100,000 [3]. It is higher in males than in females and higher in blacks than in whites. Gastrin cell tumors represent the most frequent histopathologic type, accounting for 50–60% of all duodenal carcinoids. They can be nonfunctioning or functioning as in Zollinger-Ellison syndrome (ZES) and may be associated with the MEN1 syndrome. Somatostatin cell tumors represent the second most common type (15–27%). Duodenal carcinoids are usually diagnosed on upper endoscopies performed for symptoms such as abdominal pain, upper gastrointestinal bleeding and anemia. A few patients with duodenal carcinoids (<10%) present with symptoms and/or signs of hormone overproduction. ZES occurs in approximately 10% of patients with duodenal carcinoids. Carcinoid syndrome may occur in 4%, and in rare cases Cushing syndrome and acromegaly may be seen. Diagnosis depends on histopathological examination. Morphologically, gastrin tumor cells, which are the most common type, are uniform with rounded nuclei and abundant cytoplasm. The growth pattern is usually trabecular or cribriform. Necroses are absent and the mitotic rate is low. Most of the tumor cells are positive for neuroendocrine markers such as CgA, synaptophysin, Leu7 and neuron-specific enolase. Multiple factors must be taken into account when considering treatment options. Well-differentiated, nonfunctioning duodenal carcinoids with no evidence of invasion of the muscularis layer and 1 cm or less in size can be endoscopically removed. These tumors carry a low risk for lymphatic or distant metastasis. Nowadays, endoscopic mucosal resection (EMR) is most widely performed. Until now no tumor recurrences have been observed which affected overall prognosis of the patient.
Here we present the case of a woman with a 1 cm duodenal carcinoid that was removed by EMR.
Case Report
A 43-year-old woman of Hispanic origin was referred to the gastroenterology clinic for evaluation of anemia. She complained of occasional upper abdominal pain, burning in nature, 7/10 in intensity, intermittent, nonradiating, worse with food, but no relieving factors. The patient denied any nausea, vomiting, fever, chills, changes in bowel habits, dysphagia, odynophagia, hematochezia, melena or weight loss. Her past medical history was significant for hypertension and peptic ulcer disease with an upper endoscopy 1 year before revealing a gastric ulcer. Her medications included lisinopril, ferrous sulfate and multivitamin tablets. Her menstrual periods were regular but occasionally heavy. She denied any history of surgeries or use of NSAIDs, tobacco, alcohol or illicit drugs. Her family history was not significant either. Vital signs were unremarkable. Pertinent physical examination revealed a normal build with mild conjunctival pallor. The respiratory and cardiovascular system were unremarkable. The abdomen was soft, nontender, nondistended, with no organomegaly and with normoactive bowel sounds. There were no icterus, cyanosis, clubbing, rash, joint swelling or deformities noted. Complete blood count showed hemoglobin and hematocrit to be 7.4 g/dl and 23.9%, with a MCV of 63 fl and a RDW of 18.5%. WBC, platelet count and comprehensive metabolic panel were within normal limits. Iron panel showed evidence of iron deficiency anemia with iron 18 µg/dl, total iron binding capacity 482 µg/dl, ferritin 7 ng/ml and transferrin saturation 4%. Upper endoscopy revealed a duodenal bulb mass with heaped-up edges (fig. 1) and a small healing ulcer at the prepyloric area with heaped-up mucosa. Histopathologic examination of the duodenal bulb mass biopsy was suggestive of a duodenal carcinoid tumor with a solid and trabecular pattern and no necrosis. Less than 1/50 HPF mitotic activity was seen. Immunohistochemistry staining was positive for both chromogranin and synaptophysin, confirming the diagnosis of a carcinoid tumor. CgA, serotonin and 24 h urine 5-HIAA levels were within normal limits. A small bowel series and colonoscopy were performed and essentially unremarkable. CT of the abdomen and pelvis showed evidence of fatty liver but was otherwise normal. Octreotide scan showed a prominent focal area of increased radiotracer uptake in the proximal duodenum correlating with the area of biopsy. Examination with a 20 MHz high-resolution endoscopic ultrasound (EUS) probe revealed the duodenal bulb mass to be confined to the mucosa. The mass measured approximately 10 mm in its largest diameter (fig. 2). Subsequently EMR of the mass was performed. Postresection margins were smooth without evidence of residual polypoid tissue. Multiple clips were used to close the mucosal defect and prevent delayed bleeding (fig. 3). The resection margins were free of tumor on histopathological examination. The patient tolerated the procedure well. Six-monthly surveillance EUS revealed no evidence of new lesions or adjacent lymphadenopathy suggestive of any recurrence. Repeat Octreoscan failed to show the increased radiotracer uptake in the duodenal bulb seen earlier. CgA level was within normal limits.
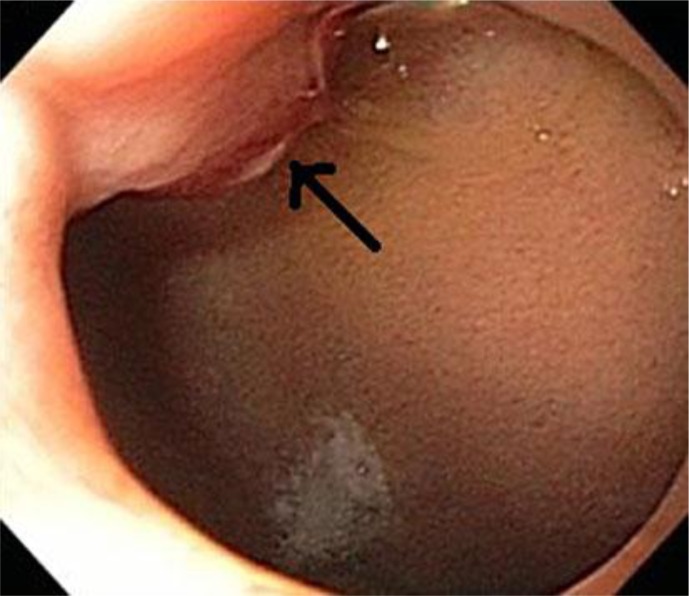
Fig. 1.
Upper endoscopy revealing the presence of a duodenal bulb mass with headed-up edges (arrow). Histopathological examination of the mass was suggestive of a carcinoid.
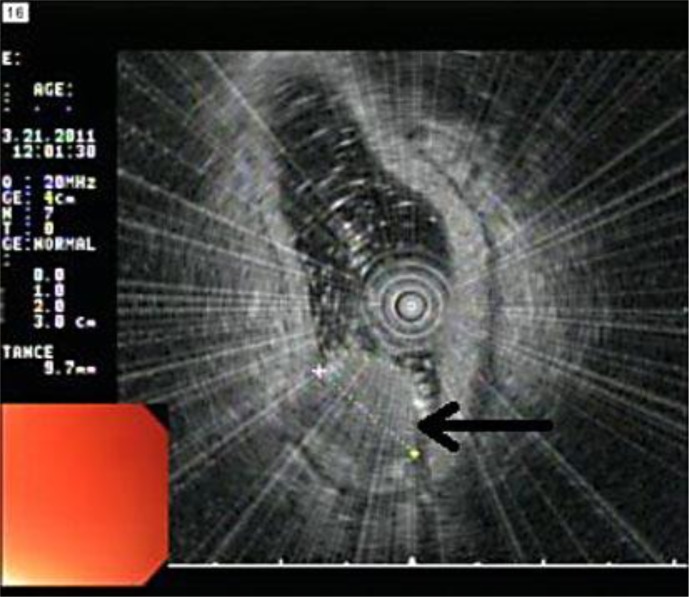
Fig. 2.
EUS view with a high-resolution 20 MHz probe showing the mass (arrow). The mass was confined to the mucosa and measured 10 mm in its largest diameter.
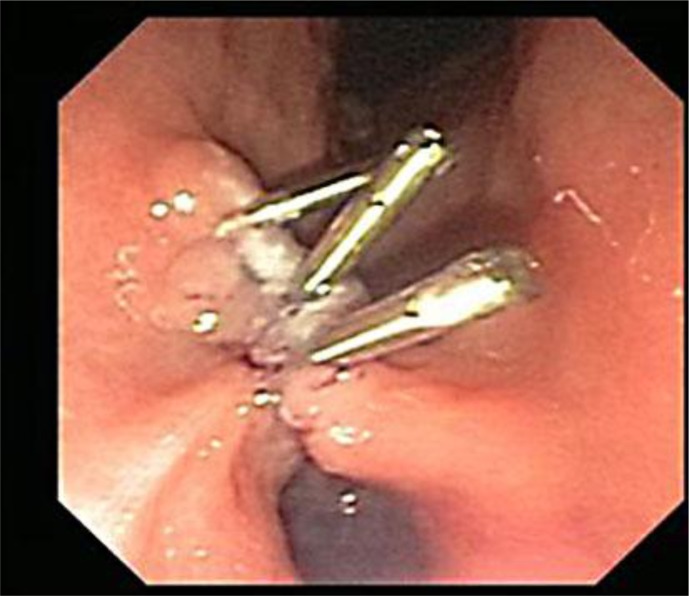
Fig. 3.
EMR of the mass was performed. Multiple surgical clips (shown in the figure) were used to close the mucosal defect.
Discussion
To date eight different neuroendocrine cell types have been identified ultrastructurally: ECL-, EC-, G-, D-, D1-, A-, P- and X-. Each cell type has characteristic neurosecretory granules, some with identified secretory products: enterochromaffin cells (EC) secrete serotonin, EC like cells (ECL) secrete histamine, A- cells secrete glucagon, D- cells secrete somatostatin, and G- cells secrete gastrin. They are identified histologically by their affinity for silver salts and by their positive reactions to neuroendocrine markers such as neuron-specific enolase, synaptophysin and chromogranin.
The WHO classification of gastrointestinal endocrine tumors recognizes four main tumor categories: (1) well-differentiated endocrine tumor (WDET, carcinoid), (2) well-differentiated endocrine carcinoma (WDEC, malignant carcinoid), (3) poorly differentiated endocrine (small cell) carcinoma (PDEC), and (4) mixed exocrine-endocrine tumors. WDET differs from WDEC in the demonstration of distant metastases or extensive local growth. The latest figures from the US Surveillance Epidemiology and End Results (SEER) Register show that the number of neuroendocrine tumors of the small intestine has increased by 300–500% in the past 35 years [4, 5]. This has been mainly attributed to the increasing use of endoscopy and immunohistochemistry. Other risk factors for small intestinal carcinoids include alcohol, female gender, and positive family history [6]. Small intestinal carcinoids also occur with an increased frequency in a few inherited syndromes such as MEN1.
Five major types of neuroendocrine tumors can be seen in the duodenum. (1) Gastrinomas (type I) are most common and are usually seen in the proximal duodenum. One third are associated with ZES and MEN1. (2) Second in frequency are somatostatinomas (type II), which often have a periampullary location. They may be associated with von Recklinghausen disease. (3) Gangliocytic paragangliomas (type III) are benign tumors found at the ampulla or in the periampullary region. (4) Type IV is rare and contains tumors that produce serotonin and calcitonin. (5) PDEC (type V) is extremely rare and highly malignant and is usually located at the ampulla of Vater [7].
Burke et al. [8] reported 99 cases of duodenal carcinoid tumors, consisting of 59 men and 40 women with an average of 59 years. The mean tumor diameter was 18 mm (range 2–50 mm). With regard to the lesion site, the duodenal bulb accounted for 43% of cases while 52% were found in the second portion of the duodenum. Regarding invasion depth, 49% showed submucosal lesions and 51% invasion of the muscularis mucosa. Metastasis to the lymph nodes, liver and other sites were observed in 21% [9].
Most duodenal carcinoids are asymptomatic and detected during upper gastrointestinal endoscopy for unrelated symptoms such as abdominal pain (37%), upper gastrointestinal bleeding (21%), anemia (21%) and jaundice (18%). In contrast, ampullary carcinoids more frequently cause symptoms with up to 60% presenting with jaundice. Duodenal neuroendocrine tumors are usually hormonally silent (60–98%). However some may develop ZES (up to 10%) or Cushing syndrome (4%), and rarely patients have a duodenal neuroendocrine tumor that is an insulinoma or a glucagonoma or even a growth hormone factor-releasing tumor that manifests as acromegaly. Very few patients develop carcinoid syndrome characterized by diarrhea, flushing, carcinoid heart disease, bronchial constriction and abdominal pain. A life-threatening exacerbation of carcinoid syndrome called carcinoid crisis can be triggered by anesthesia, drugs or surgery.
Upper gastrointestinal endoscopy and histological examination form the cornerstone for diagnosis. EUS should follow and allows the determination of the size and depth of infiltration and also assesses locoregional lymph nodes. It can also accurately determine the layer of origin and internal echo pattern. Serum and urine neuroendocrine markers (such as CgA and 5-HIAA) may have diagnostic value. CgA may also have prognostic significance and can be used during the follow-up of patients. Serum gastrin should also be determined, and if elevated in combination with a hyperacidic stomach a secretin stimulation test should be done to establish ZES. Conventional imaging methods (CT, MRI) can assist with tumor staging and localization. Burke et al. [8] identified three pathologic features for the primary tumor as independent risk factors for metastasis: invasion of the muscularis propria, size >2 cm and presence of mitotic figures. A large variety of carcinoid tumors and their metastases can express a high density of specific somatostatin receptors for which octreotide, a somatostatin analogue, has high affinity. Octreoscan (using 111In-labeled octreotide) not only helps in detection, but also predicts therapeutic effect of somatostatin analogues in these tumors [2]. 68Ga-DOTATOC-PET/CT is a new, very sensitive method for visualizing metastatic intestinal carcinoids [5, 10]. Due to frequent carcinoid heart disease (30–60% of patients with carcinoid syndrome), every patient with carcinoid syndrome should have cardiac imaging.
A TNM staging of foregut neuroendocrine tumors was recently approved by the European Neuroendocrine Tumor Society (table 1). Tumors limited to the lamina propria or submucosa and <1 cm are assigned Tx through T1. Tumors >1 cm or invading the muscularis propria are categorized T2 or greater [11].
Table 1.
TNM staging of foregut neuroendocrine tumors
| T: primary tumor | |
| Tx | primary tumor cannot be assessed |
| T0 | no evidence of primary tumor |
| T1 | tumor invades the lamina propria or submucosa and size ≤1 cm |
| T2 | tumor invades the muscularis propria or size>1 cm |
| T3 | tumor invades the pancreas or retroperitoneum |
| T4 | tumor invades the peritoneum or other organs |
| N: regional lymph nodes | |
| Nx | regional lymph nodes cannot be assessed |
| N0 | no regional lymph node metastasis |
| N1 | regional lymph node metastasis |
| M: distant metastasis | |
| Mx | distant metastasis cannot be assessed |
| M0 | no distant metastasis |
| M1 | distant metastasis |
Multiple factors such as differentiation, tumor localization, tumor stage and patient-related factors must be taken into account when considering treatment options. Endoscopic resection has been reported to be a safe, less invasive and effective procedure for tumors measuring <1 cm in diameter that are located outside the periampullary region, with no EUS signs of invasion of the muscularis layer. The appropriate treatment for duodenal carcinoid tumors >1 cm is still controversial. Some authors suggest that for tumors 1–2 cm in diameter, operative full-thickness excision is preferred. However there has been a case report of a 12 mm duodenal carcinoid tumor that was removed via EMR. Nonetheless it may be difficult to remove completely endoscopically tumors >1 cm, and they should be evaluated with EUS before an attempt at endoscopic resection owing to their potential for invasion beyond the submucosa. Endoscopic excision of a primary duodenal carcinoid tumor seems most appropriate for tumors ≤1 cm [12].
There are no controlled studies on the optimal management of ampullary neuroendocrine tumors. Endoscopic papillectomy and surgical tumor resection can be curative in early disease whereas patients with ampullary carcinoids >2 cm may benefit from Whipple procedure [13].
Disclosure Statement
The authors have no funding or conflicts of interest to disclose.
References
- 1.Oberg K. Neuroendocrine gastrointestinal and lung tumors (carcinoid tumors), carcinoid syndrome, and related disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. ed 12. Philadelphia: Elsevier/Saunders; 2011. pp. 1809–1828. [Google Scholar]
- 2.Nikou GC, Toubanakis C, Moulakakis KG, Pavlatos S, Kosmidis C, Mallas E, Safioleas P, Sakorafas GH, Safioleas MC. Carcinoid tumors of the duodenum and ampulla of Vater: current diagnostic and therapeutic approach in a series of 8 patients. Case series. Int J Surg. 2011;9:248–253. doi: 10.1016/j.ijsu.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Oberg K. Gastrointestinal carcinoid tumors (gastrointestinal neuroendocrine tumors) and the carcinoid syndrome. In: Feldman M, Friedman L, Brandt L, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. ed 9. Philadelphia: Elsevier/Saunders; 2010. pp. 475–490. [Google Scholar]
- 4.World Health Organization . Histological Typing of Endocrine Tumors. Geneva: WHO; 2000. International Classification of Tumors. [Google Scholar]
- 5.Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Neuroendocrine tumors of the small bowels are on the rise: early aspects and management. World J Gastrointest Endosc. 2010;2:325–334. doi: 10.4253/wjge.v2.i10.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Risk factors associated with neuroendocrine tumors: a U.S.-based case-control study. Int J Cancer. 2008;123:867–873. doi: 10.1002/ijc.23529. [DOI] [PubMed] [Google Scholar]
- 7.Kölby L, Nilsson O, Ahlman H. Gastroduodenal endocrine tumors. Scand J Surg. 2004;93:317–323. doi: 10.1177/145749690409300411. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Sobin LH, Federspiel BH, et al. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med. 1990;114:700–704. [PubMed] [Google Scholar]
- 9.Matsumoto S, Miyatani H, Yoshida Y, Nokubi M. Duodenal carcinoid tumors: 5 cases treated by endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1152–1156. doi: 10.1016/j.gie.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Baum RP, Prasad V, Hommann M, Hörsch D. Receptor PET/CT imaging of neuroendocrine tumors. Recent Results Cancer Res. 2008;170:225–242. doi: 10.1007/978-3-540-31203-1_18. [DOI] [PubMed] [Google Scholar]
- 11.ENETS guidelines TNM staging and standards of care. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai WP, Yue H. Endoscopic mucosa resection of a duodenum carcinoid tumor of 1.2 cm diameter: a case report. Med Oncol. 2009;26:319–321. doi: 10.1007/s12032-008-9123-6. [DOI] [PubMed] [Google Scholar]
- 13.Hartel M, Wente MN, Sido B, Friess H, Büchler MW. Carcinoid of the ampulla of Vater. J Gastroenterol Hepatol. 2005;20:676–681. doi: 10.1111/j.1440-1746.2005.03744.x. [DOI] [PubMed] [Google Scholar]