Abstract
Malignant hemorrhagic ascites may complicate the terminal evolution of digestive cancers with peritoneal carcinomatosis. It has a bad influence on prognosis and may severely impair patients’ quality of life. Palliative laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) has been proposed to treat debilitating malignant ascites. Two cases of peritoneal carcinomatosis causing hemorrhagic ascites and severe anemia that needed iterative blood transfusions are reported. These patients were treated by laparoscopic HIPEC (mitomycin C and cisplatin with an inflow temperature of 43°C), resulting in cessation of peritoneal bleeding. No postoperative complication or relapse of ascites occurred during the following months. No more blood transfusion was needed. Laparoscopic HIPEC might be an effective and safe therapeutic option to consider in patients with malignant hemorrhagic ascites.
Key Words: Ascites, Hemoperitoneum, Palliative surgery, Cancer, Hyperthermic intraperitoneal chemotherapy, Laparoscopy, Intraperitoneal drug delivery
Introduction
Malignant ascites may occur in the evolution of digestive or gynecologic cancers when disseminated to the intra-abdominal cavity [1, 2]. There are several factors participating to its constitution [1]. Disseminated intra-abdominal tumor induces peritoneal neovascularization and increases microvascular permeability and production of peritoneal fluid. Besides, intraperitoneal fluid accumulation is also related to intra-abdominal and diaphragmatic lymph vessel obstruction due to tumor invasion [1]. Hemoperitoneum is a rare but poor prognosis complication of peritoneal carcinomatosis. It can be due to vascular extension of peritoneal carcinomatosis or bleeding from the primary tumor. Patients’ quality of life is often severely impaired due to symptoms including chronic anemia, and even worsened by repeated paracentesis and blood transfusions.
Iterative paracentesis, diuretics and albumin perfusion are used to treat cirrhotic ascites and might be useful to treat malignant ascites at its beginning, but lose effectiveness over time because of different physiopathology. As peritoneal dissemination is a major poor prognosis factor in patients with intra-abdominal malignancy, it represents a classical contraindication for aggressive therapeutic strategies such as surgical resection. Palliative laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) has been proposed to treat debilitating malignant ascites [1, 3, 4, 5, 6, 7]. The goal of this procedure is palliative symptom relief with reduced invasiveness and morbidity. To our knowledge, no treatment has been reported to show effectiveness in carcinomatosis-associated hemoperitoneum, and patients are usually treated with iterative paracentesis, repeated blood transfusions and eventual palliative systemic chemotherapy. Two patients with peritoneal carcinomatosis causing malignant ascites and peritoneal bleeding requiring iterative blood transfusion treated by laparoscopic HIPEC are reported.
Case 1
A 48-year-old Caucasian man was referred for management of ascites. Endoscopic examination of the upper gastrointestinal tract showed a thickened and stiff appearance of the cardial region. Histological examination of gastric biopsies showed evidence of undifferentiated adenocarcinoma with independent cells. Abdominal computed tomography (CT) scan was suggestive of gastric linitis with synchronous peritoneal carcinomatosis. The patient was treated by chemotherapy in the AVAGAST randomized study (6 cycles of capecitabine and cisplatin +/– bevacizumab, then 14 cycles of capecitabine +/– bevacizumab). After 15 months of chemotherapy the disease was uncontrolled, as ascites relapsed more often and became hemorrhagic. A second-line chemotherapy using 5-fluorouracil and oxaliplatin did not have an effect on hemorrhagic ascites. Anemia was severe (mean hemoglobin 5.2–8.7 g/dl) and needed iterative blood transfusions (1 unit of blood every 7 days), erythropoietin and iron intravenous injection. No active bleeding could be observed on CT scan. As blood transfusions were more and more frequent, an explorative laparoscopy followed by HIPEC was proposed after discussion in multidisciplinary care team meeting.
Explorative laparoscopy confirmed hemorrhagic ascites in which hemoglobin was measured at 0.6 g/dl. The primary tumor was ill-bounded and completely sheathed the submesocolic region, and particularly the left lobe of the liver. The epiploon was impaired by carcinomatosis, but the small intestine and pelvis were not involved. The HIPEC procedure consisted in intraperitoneal perfusion of mitomycin C (75 mg) and cisplatin (250 mg) during 30 min with an inflow temperature of 43°C under laparoscopy. Operative time was 120 min. The postoperative course was unremarkable.
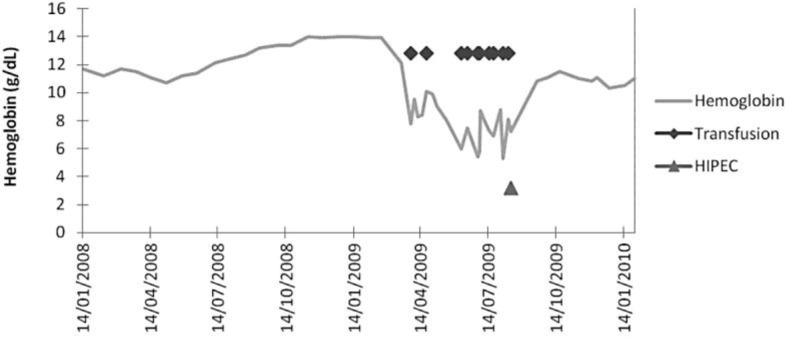
During the following months the ascites did not relapse and no carcinomatous nodule was palpable. Hemoglobin became stable (11.2 g/dl) and no more transfusion support was needed. Fig. 1 shows the evolution of hemoglobin over time. A simple surveillance was then decided. Five months after laparoscopy, the ascites relapsed, and scanography showed progression of the disease. The patient then received everolimus or placebo in the GRANIT study, but died 2 months later, 7 months after surgery and 25 months after diagnosis.
Fig. 1.
Evolution of hemoglobin and transfusion support in case 1. After laparoscopic HIPEC, hemoglobin became stable and no more transfusions were needed.
Case 2
A 78-year-old woman presented with ascites. CT scan showed peritoneal carcinomatosis with multiple abdominal and pelvic nodes. A diagnostic laparoscopy was performed and found a pelvic infiltrative tumor with diffuse infracentimetric peritoneal carcinomatosis nodes. Pathological examination of the preoperative samples showed a poorly differentiated adenocarcinoma. No primary tumor could be clearly identified, although ovarian origin was strongly suspected. Despite systemic chemotherapy with cisplatin- (8 cycles) and paclitaxel-based regimen (2 cycles), the patient suffered from abundant bloody ascites with severe anemia (mean hemoglobin 6.1–9.5 g/dl) that required iterative paracenteses (one paracentesis every 4 days) and transfusion support.
After discussion in multidisciplinary care team meeting, laparoscopic HIPEC was proposed. Preoperative exploration confirmed the ill-bounded pelvic bulk, with retro- and subperitoneal development. Peritoneal carcinomatosis consisted in a diffuse involvement of all quadrants. The procedure consisted in intraperitoneal perfusion of mitomycin C (75 mg) and cisplatin (184 mg) for 20 min with an inflow temperature of 43°C. Operative time was 120 min. No postoperative complication occurred. This therapeutic procedure resulted in regression of ascites and complete resolution of hemoperitoneum, leading to cessation of blood transfusions and improvement of quality of life.
Three months later, as the patient complained of asthenia and weight loss. A CT scan was performed and showed resurgence of ascites and progression of the abdominal lesions. Best supportive care was decided, and the patient died 3 weeks later.
Discussion
Malignant ascites may complicate digestive or gynecologic cancers in their terminal phase, especially ovarian or gastric cancer. The prognosis is poor as median survival rarely exceeds 6 months [1]. In their retrospective analysis of 209 patients with malignant ascites, Ayantunde and Parsons [2] reported a median survival time of 5.7 months after diagnosis of ascites.
Malignant ascites may be complicated by hemorrhage, leading to chronic anemia. This can be due to vascular extension of peritoneal carcinomatosis or bleeding from the primary tumor. Hemorrhagic malignant ascites might be an even poorer prognostic factor due to anemia and repeated blood transfusions. No treatment has shown its effectiveness on peritoneal bleeding associated to malignant ascites. As malignant ascites is a poor prognostic factor, the aim of therapy is palliation and improvement of quality of life [3]. Therapeutics used in cirrhotic ascites are usually ineffective since the physiopathology is different. Iterative paracentesis and intravenous chemotherapy may be initially effective, but classically lose their effectiveness over time.
Use of palliative laparoscopic HIPEC has been proposed to treat debilitating malignant ascites [1, 4, 5, 6, 7, 8]. By destroying the peritoneal carcinomatosis, HIPEC may induce hemostasis and progressive fibrosis of the peritoneum, preventing ascites and bleeding [4, 5]. However, HIPEC is linked to important morbidity and mortality, mostly determined by the extent of cytoreduction and peritoneal resection, the number of digestive anastomoses, the dose of chemotherapy and the operative time [9]. In patients with malignant ascites and eventually related hemoperitoneum, the therapeutic goal is not increased survival but palliative symptom relief. In this way, no extended resection is required that might result in a relative decrease of the morbidity impact related to HIPEC. The laparoscopic approach has been considered in the last decade in an attempt to reduce the invasiveness of the procedure in patients undergoing HIPEC with a palliative intent [5, 9]. Indeed, laparoscopic HIPEC may integrate the benefits of a definitive palliation of ascites with a minimal invasion, leading to fewer postoperative complications. Retrospective studies related complete clinical regression of ascites in most patients, resulting in symptom relief, and significant improvement in quality of life [4, 5, 6, 7, 8]. In the larger studies, Valle et al. [7] reported a definitive regression of ascites in 94% of 52 patients.
Although laparoscopic HIPEC has been evaluated in patients with malignant ascites, we have presented here the originality of its efficacy in malignant hemoperitoneum [4, 7]. We have reported two cases of peritoneal carcinomatosis causing peritoneal bleeding requiring iterative blood transfusion, treated by laparoscopic HIPEC, resulting in the reduction of debilitating ascites and cessation of peritoneal bleeding without postoperative complication. This laparoscopic procedure could be considered as a palliative success as far as it improved quality of life and reduced symptoms, especially anemia. Ba et al. [8] reported that 2 of their 16 patients presented bloody ascites that turned clear after laparoscopic HIPEC, but there is no information about the severity of peritoneal bleeding in these patients. However, this supports that laparoscopic HIPEC might be effective to dry up carcinomatosis-associated peritoneal bleeding. Although there are not enough reported cases of laparoscopic HIPEC to treat malignant bleeding ascites, we may consider it as a therapeutic option.
Recent studies reported the potential benefit of using new targeted agents by intraperitoneal route in malignant ascites. Heiss et al. [10] demonstrated clinically relevant benefits of catumaxomab in patients with recurrent malignant ascites due to carcinomas of different origin, with frequent but acceptable chemo-induced toxicity. Additionally, bevacizumab is currently under evaluation in clinical studies to treat and prevent malignant ascites [11]. Combination of laparoscopic HIPEC with these targeted drugs might be an option to consider, and its efficacy and toxicity should be evaluated.
In conclusion, laparoscopic HIPEC might be an interesting therapeutic option in selected patients with debilitating malignant ascites associated with hemoperitoneum. This technique appears to be interesting because of improvement of quality of life with a minimally invasive approach.
Disclosure Statement
None of the authors declares any conflict of interest.
References
- 1.Adam RA, Adam YG. Malignant ascites: past, present, and future. J Am Coll Surg. 2004;198:999–1011. doi: 10.1016/j.jamcollsurg.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–949. doi: 10.1093/annonc/mdl499. [DOI] [PubMed] [Google Scholar]
- 3.Becker G, Galandi D, Blum H. Malignant ascites: systematic review and guideline for treatment. Eur J Cancer. 2006;42:589–597. doi: 10.1016/j.ejca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Garofalo A, Valle M, Garcia J, Sugarbaker PH. Laparoscopic intraperitoneal hyperthermic chemotherapy for palliation of debilitating malignant ascites. Eur J Surg Oncol. 2006;32:682–685. doi: 10.1016/j.ejso.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol. 2008;34:154–158. doi: 10.1016/j.ejso.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 6.McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 7.Valle M, van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: a multi-institutional retrospective analysis in 52 patients. J Surg Oncol. 2009;100:331–334. doi: 10.1002/jso.21321. [DOI] [PubMed] [Google Scholar]
- 8.Ba MC, Cui SZ, Lin SQ, et al. Chemotherapy with laparoscope-assisted continuous circulatory hyperthermic intraperitoneal perfusion for malignant ascites. World J Gastroenterol. 2010;16:1901–1907. doi: 10.3748/wjg.v16.i15.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roviello F, Caruso S, Marrelli D, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol. 2011;20:38–54. doi: 10.1016/j.suronc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Shami K, Elsaid A, El-Kerm Y. Open-label safety and efficacy pilot trial of intraperitoneal bevacizumab as palliative treatment in refractory malignant ascites. J Clin Oncol. 2007;25(suppl 18) abstract 9043. [Google Scholar]