Abstract
Solitary fibrous tumors (SFTs) are mesenchymal neoplasms of fibroblastic origin, most commonly found in the pleura. Numerous extrathoracic locations have been reported during the last 2 decades. Herein, we report the first case of an SFT in the round ligament of the liver. A 46-year-old Caucasian man presented with a 12-month history of abdominal pain. An ultrasonography-guided microbiopsy first revealed a desmoid tumor. After failure of first- and second-line medical treatments (celecoxib and tamoxifen, then imatinib), histological reexamination was suspicious for a low-grade sarcoma. MRI was also suspicious for a malignant process. Hence, surgery was decided. Laparotomy found a huge and well-limited tumor that, unexpectedly, was appended to the round ligament of the liver and free from any other intra-abdominal contact. The tumor was easily removed. Excision was monobloc and macroscopically complete. Histological analysis diagnosed an SFT arising from the round ligament of the liver. No adjuvant treatment was given. Ten months after surgery, the patient is alive without any signs or symptoms of relapse. This is the first report of SFT arising from the round ligament of the liver. It illustrates the difficulty in diagnosing such tumors. Whilst diagnosis of SFT is rare, it should be kept in mind to allow early diagnosis and complete surgical resection, which provide the best chance for recovery.
Key words: Solitary fibrous tumors, Liver, Round ligament
Introduction
Solitary fibrous tumors (SFTs) are rare mesenchymal neoplasms of fibroblastic origin, most commonly found in the pleura [1]. They were erroneously thought to be confined to the serosal surfaces. In fact, they are ubiquitous neoplasms [2], and non-serosal extrathoracic locations have been reported during the last 2 decades [3], including intra-abdominal cases [2]. Given the rarity of these tumors, misdiagnosis is frequent, and information regarding prognosis and treatment is lacking. Herein, we report the first case of SFT developing in the round ligament of the liver.
Case Report
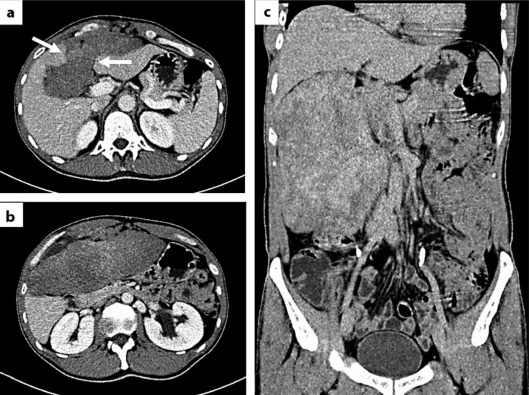
In August 2010, a 46-year-old Caucasian man presented with a 12-month history of abdominal pain. He had no specific medical history, personal or familial. The initial ultrasonography (US) scan, performed 1 year earlier, was interpreted as normal. On self-examination, the patient had recently palpated a mass of the right hypochondrium. The World Health Organization performance status (PS) was equal to 0. Hepatic, pancreatic and renal functions were normal. A CT scan showed a solid and inhomogeneous mass measuring 182 × 66 mm, located under the abdominal wall, causing displacement of the left and right hepatic lobes but distinct from them (fig. 1a–c). Iodine injection showed heterogeneous contrast uptake, with a fat interface between the tumor and intra- and retroperitoneal elements (fig. 1a, b).
Fig. 1.
CT of the tumor with (a, b) and without (c) iodine injection, in transversal (a, b) and coronal planes (c). The white arrows (a) indicate an infiltration of the umbilical fissure by the tumor.
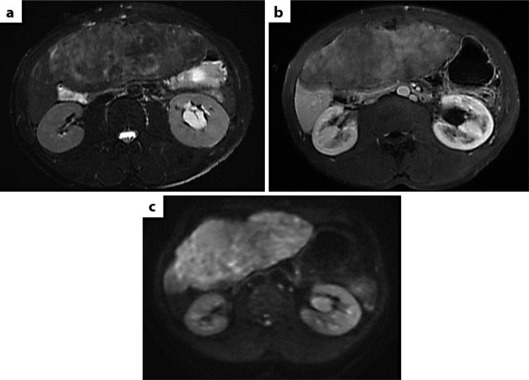
A US-guided microbiopsy was performed. Histological examination showed a proliferation of nonatypical round to spindle-shaped cells without mitoses, admixed with collagen. Immunohistochemistry (IHC) showed no expression of epithelial, smooth muscle or Schwann cell markers by tumor cells but solely expressed CD34. A diagnosis of gastrointestinal stromal tumor (GIST) was ruled out by IHC (no expression of CD117 or DOG1) and molecular biology (no KIT or PDGFRA mutation), and the diagnosis of desmoid tumor was made. There was no CTNNB1 mutation. First-line systemic treatment combining celecoxib and tamoxifen was decided upon. After a 3-month treatment, a CT scan showed no tumor response. Second-line treatment based on imatinib (400 mg daily) was started. Despite 3 months of treatment, the tumor grew slightly to 206 × 83 mm as seen on CT. The initial biopsy was reexamined and regarded as suspicious for low-grade sarcoma or SFT. MRI was performed: on T2-weighted sequences, the tumor had a higher signal than the muscle (fig. 2a); gadolinium injection was associated with gradual and heterogeneous contrast enhancement on T1-weighted sequences, evoking fibrosis (fig. 2b), but diffusion MRI showed important cellularity that was highly suspicious for a malignant process (fig. 2c). Surgery was therefore decided upon.
Fig. 2.
MRI of the tumor. a T2-weighted sequences. b T1-weighted sequences with gadolinium injection. c Diffusion sequences.
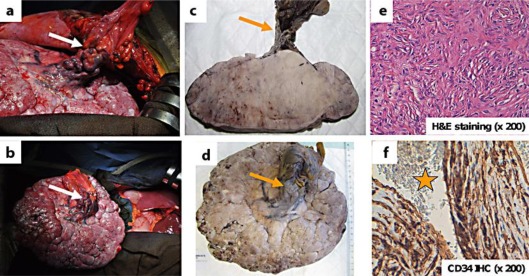
Laparotomy found a huge and well-limited tumor that, unexpectedly, was appended to the round ligament of the liver (fig. 3a, b) with a ‘caput medusae’ vascularization. The tumor was free from any other intra-abdominal contact, especially from the liver parenchyma, and was easily removed without any further dissection. Excision was monobloc and macroscopically complete. The tumor measured 210 × 70 mm and weighed 1,990 g. After formalin fixation, it showed a fleshy appearance with gray-white color on cut section and was centered on and appended to the round ligament (fig. 3c, d). Unlike the microbiopsy, the histological examination of the surgical specimen showed many more characteristic areas of small nonatypical spindle cells scattered in a fibrous stroma without any clear architecture (fig. 3e). IHC analysis confirmed strong expression of CD34 by tumor cells and, above all, highlighted very characteristic ramified vessels, so-called ‘staghorn’ vessels (fig. 3f). There was no expression of epithelial (pancytokeratin, EMA), smooth muscle (α-smooth actin), and Schwann cell (PS100) markers. There was no cellular atypia. The cellular density was moderate. Mitotic count was very low, less than 1 mitosis per 10 high-power fields (HPF). The retained diagnosis was SFT arising from the round ligament of the liver. The postoperative course was uneventful. No adjuvant treatment was given. A regular clinical and radiological monitoring was set up. At the 10-month follow-up, the patient was alive with excellent PS, without any signs or symptoms of relapse.
Fig. 3.
Intraoperative findings and histological aspects. a, b Intraoperative findings: the tumor is appended to the round ligament of the liver (white arrows) and free from any other intra-abdominal contact. c, d Macroscopic aspect on cut section after formalin fixation. Firm, gray-white tumor appended to the round ligament (orange arrows). e, f Microscopic aspect and IHC. In e, nonatypical small spindle cells scattered in a fibrous stroma (H&E staining, original magnification × 200). In f, tumor cells strongly express CD34. A large ramified vessel can be seen (orange star is in the lumen of the vessel; original magnification × 200).
Discussion
In the past 2 decades, SFTs have been identified in the abdominopelvic cavity [2, 3]. Regarding the liver, fewer than 30 cases have been reported in the English literature [4, 5, 6]. To our knowledge, our case represents the first case of SFT arising from the round ligament of the liver.
The round ligament, or ligamentum teres, is a degenerative string of tissue stemming from the umbilical vein involution and that anatomically divides the left lobe of the liver from the medial and lateral sections. Tumors in the round ligament are rare. Most of them are metastases of gastrointestinal cancers (in particular, mucinous appendiceal neoplasm), peritoneal mesothelioma [7], and more rarely of breast cancer and hepatocarcinoma [8, 9]. Cases of primary mesenchymal tumors have also been reported, including sarcomas (malignant fibrous histiocytoma, leiomyosarcoma) [10], and benign tumors such as lipomas, lymphangiomas, stromal tumors [11] and PEComas [12]. SFTs of the round ligament of the liver have not been reported so far.
Given their rarity, misdiagnosis of intra-abdominal SFTs is frequent. They often manifest as large, asymptomatic, slow-growing tumors [2], and their clinical presentation has no specificity (pain, abdominal mass). In our case, it is likely that the tumor had been evolving slowly for several years before the onset of abdominal pain, and that the US scan was falsely negative because of similar echogenicity between the tumor and the perihepatic tissues. Radiological signs of SFTs are not specific. These tumors demonstrate remarkable radiological heterogeneity, with variable degrees of enhancement, necrosis, or hemorrhage. CT is the initial investigative modality of choice for the detection of SFTs, and is better than a US scan [2], as exemplified in our case. Typically, the tumor appears as a well-circumscribed hypervascular mass that may displace or exert pressure effects on neighboring organs. At MRI, it typically displays intermediate signal intensity on T1-weighted images, heterogeneous low-signal intensity with flow voids on T2-weighted images, and intense enhancement after administration of gadolinium [2]. In our case, these features existed, but diffusion MRI was falsely alarming. On presurgical imaging, the large size of the tumor did not allow for the definition of its precise origin or the prediction of a simple surgical removal. The origin in the round ligament was obvious during the laparotomy only; however, a postoperative re-reading of the initial CT and MRI scans showed that the tumor had infiltrated the umbilical fissure.
The positive diagnosis of SFTs is histological, while keeping in mind the frequent difficulty to precisely characterize a mesenchymal neoplasm on small biopsies, as illustrated by our case. Analysis shows a proliferation of rounded to fusiform cells arranged without any well-defined architecture (so-called ‘patternless pattern’) in a fibrous stroma. Appearance can be very different from one area to another depending on the relative proportion of cells and stroma. This can explain some of the difficulties on small biopsy samples. Branching staghorn vessels are characteristically seen. IHC shows strong expression of CD34 by tumor cells and importantly helps to rule out numerous differential diagnoses, namely smooth muscle tumors, desmoid tumors, tumors of peripheral nerves, GIST, spindle cell sarcomas, notably dedifferentiated liposarcomas and synovial sarcomas. As with our patient's tumor, molecular biology can also help to rule out some differential diagnoses, mainly GISTs [2].
Regarding their prognosis, extrapleural SFTs have a classically more indolent behavior than pleural SFTs and are most always defined as benign. However, their clinical outcome is unpredictable, and some of them can display histological aggressiveness criteria (size >5 cm, mitotic count >4 per 10 HPF, high cellularity, necrosis, hemorrhage, cytological atypia and infiltrative growth pattern) or present local invasiveness or local or metastatic relapses [3, 13]. Like in pleural locations, the presence of aggressive features does not always forecast an unfavorable clinical outcome, and half of cases with a malignant lesion (defined by the presence of high cellularity and/or high mitotic count and/or pleomorphism) are cured by simple excision [14]. For many authors, the complete resection of the tumor is the most important prognostic factor [14]. In our case, and thanks to its ‘fortunate’ origin in the round ligament, the tumor was easily and completely removed, and the only pejorative criterion was its large size. Surgical excision is the treatment of choice for SFTs, with a 5-year survival rate close to 100% after complete excision. Systemic chemotherapy and radiotherapy have been tried, but with variable success rates. In unresectable SFTs, antiangiogenic therapy is associated with promising results [15]. As the evolution of extrapleural SFTs is unpredictable, long-term follow-up is mandatory [2].
Conclusion
This is the first report of SFT arising from the round ligament of the liver described in the literature. It illustrates the difficulty of diagnosis of such tumors. Even though rare, this diagnosis should be kept in mind to allow early diagnosis and complete surgical resection, which provide the best chance for recovery.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
Our work is supported by Institut Paoli-Calmettes and Aix-Marseille University. We thank our patient who kindly gave his consent for this publication.
References
- 1.Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006;13:264–269. doi: 10.1177/107327480601300403. [DOI] [PubMed] [Google Scholar]
- 2.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross-sectional imaging spectrum. Radiographics. 2011;31:393–408. doi: 10.1148/rg.312105080. [DOI] [PubMed] [Google Scholar]
- 3.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501–1511. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Korkolis DP, Apostolaki K, Aggeli C, Plataniotis G, Gontikakis E, Volanaki D, Sebastiadou M, Dimitroulopoulos D, Xinopoulos D, Zografos GN, Vassilopoulos PP. Solitary fibrous tumor of the liver expressing CD34 and vimentin: a case report. World J Gastroenterol. 2008;14:6261–6264. doi: 10.3748/wjg.14.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HS, Kim YK, Cho BH, Moon WS. Pedunculated hepatic mass. Liver Int. 2011;31:541. doi: 10.1111/j.1478-3231.2010.02318.x. [DOI] [PubMed] [Google Scholar]
- 6.Perini MV, Herman P, D'Albuquerque LA, Saad WA. Solitary fibrous tumor of the liver: report of a rare case and review of the literature. Int J Surg. 2008;6:396–399. doi: 10.1016/j.ijsu.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH, Bijelic L. The porta hepatis as a site of recurrence of mucinous appendiceal neoplasms treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Tumori. 2008;94:694–700. doi: 10.1177/030089160809400509. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Lim JH. Extension of hepatoma to the rectus abdominis muscle via ligamentum teres hepatis. Gastrointest Radiol. 1985;10:119–121. doi: 10.1007/BF01893084. [DOI] [PubMed] [Google Scholar]
- 9.Prete FP, Di Giorgio A, Alfieri S, Doglietto GB. Breast-cancer metastasis in the round ligament of the liver. Lancet Oncol. 2006;7:354. doi: 10.1016/S1470-2045(06)70663-5. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi J, Azuma T, Fujioka H, Tanaka K, Furui J, Tomioka T, Kanematsu T. Leiomyosarcoma occurring in the ligamentum teres of the liver: a case report and a review of seven reported cases. Hepatogastroenterology. 1996;43:1051–1056. [PubMed] [Google Scholar]
- 11.Poilblanc M, Coquaz S, Welschbillig K, Arnaud JP, Hamy A. A case report of stromal tumor in the ligamentum teres hepatis (in French) Gastroenterol Clin Biol. 2006;30:469–470. doi: 10.1016/s0399-8320(06)73204-3. [DOI] [PubMed] [Google Scholar]
- 12.Sotiropoulos GC, Moskalenko V, Lang H. Clinical challenges and images in GI. Image 2. Perivascular epithelioid cell (PEC) tumor of the ligamentum teres. Gastroenterology. 2009;136:2065. doi: 10.1053/j.gastro.2009.01.045. 2416. [DOI] [PubMed] [Google Scholar]
- 13.Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol. 1995;26:440–449. doi: 10.1016/0046-8177(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 14.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Park MS, Araujo DM. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol. 2009;21:327–331. doi: 10.1097/CCO.0b013e32832c9532. [DOI] [PubMed] [Google Scholar]