Abstract
Background
Massive bleeding and transfusion of packed red blood cells (PRBC), fresh frozen plasma (FFP) and platelets are associated with increased morbidity, mortality and costs.
Patients and Methods
We analysed the transfusion requirements after implementation of point-of-care (POC) coagulation management algorithms based on early, calculated, goal-directed therapy with fibrinogen concentrate and prothrombin complex concentrate (PCC) in different perioperative settings (trauma surgery, visceral and transplant surgery (VTS), cardiovascular surgery (CVS) and general and surgical intensive care medicine) at 3 different hospitals (AUVA Trauma Centre Salzburg, University Hospital Innsbruck and University Hospital Essen) in 2 different countries (Austria and Germany).
Results
In all institutions, the implementation of POC coagulation management algorithms was associated with a reduction in the transfusion requirements for FFP by about 90% (Salzburg 94%, Innsbruck 88% and Essen 93%). Furthermore, PRBC transfusion was reduced by 8.4–62%. The incidence of intraoperative massive transfusion (≥10 U PRBC) could be more than halved in VTS and CVS (2.56 vs. 0.88%; p < 0.0001 and 2.50 vs. 1.06%; p = 0.0007, respectively). Platelet transfusion could be reduced by 21–72%, except in CVS where it increased by 115% due to a 5-fold increase in patients with dual antiplatelet therapy (2.7 vs. 13.7%; p < 0.0001).
Conclusions
The implementation of perioperative POC coagulation management algorithms based on early, calculated, goal-directed therapy with fibrinogen concentrate and PCC is associated with a reduction in the transfusion requirements for FFP, PRBC and platelets as well as with a reduced incidence of massive transfusion. Thus, the limited blood resources can be used more efficiently.
Keywords: Thromboelastometry, Transfusion algorithms, Fibrinogen concentrate, Prothrombin complex concentrate, Transfusion-associated adverse events, Pharmacoeconomics
Abstract
Hintergrund
Sowohl schwere Blutungen als auch die Transfusion von Erythrozytenkonzentraten (EK), Frischplasma (FFP) und Thrombozytenkonzentraten (TK) sind mit einer Steigerung der Morbidität, der Mortalität und der Kosten assoziiert.
Patienten und Methoden
Wir analysierten den Einfluss der Implementierung von Point-of-Care(POC)-Algorithmen zum Gerinnungsmanagement, basierend auf der frühzeitigen, kalkulierten, zielgerichteten Therapie mit Fibrinogenkonzentrat und Prothrombin-Komplex-Konzentrat (PPSB), auf den Transfusionsbedarf in unterschiedlichen perioperativen Bereichen (Unfallchirurgie, Viszeral-und Transplantationschirurgie, kardiovaskuläre Chirurgie und allgemeine und chirurgische Intensivmedizin) in 3 verschiedenen Kliniken (AUVA Traumazentrum Salzburg, Universitätsklinikum Innsbruck und Universitätsklinikum Essen) in 2 verschiedenen Ländern (Österreich und Deutschland).
Ergebnisse
In allen Kliniken war die Implementierung der POC-Algorithmen zum Gerinnungsmanagement mit einer etwa 90%igen Reduktion des Transfusionsbedarfs an FFP assoziiert (Salzburg 94%, Innsbruck 88% und Essen 93%). Außerdem konnte die Zahl der transfundierten EK um 8,4–62% reduziert werden. Die Inzidenz von intraoperativen Massivtransfusionen (≥10 EK) konnte in der Viszeral-und Transplantationschirugie sowie in der kardiovaskulären Chirurgie mehr als halbiert werden (2,56 vs. 0,88%; p < 0,0001 bzw. 2,50 vs. 1,06%; p = 0,0007). Die Transfusion von TK konnte mit Ausnahme der kardiovaskulären Chirurgie um 21–72% reduziert werden. In Letzterer stieg der Verbrauch an TK aufgrund einer 5-fachen Zunahme der Patienten mit dualer antithrombozytärer Therapie (2,7 vs. 13,7%; p < 0,0001) um 115% an.
Schlussfolgerungen
Die Implementierung von POC-Algorithmen zum perioperativen Gerinnungsmanagement, basierend auf der frühzeitigen, kalkulierten, zielgerichteten Therapie mit Fibrinogenkonzentrat und PCC, ist mit einer Reduktion des Transfusionsbedarfs für EK, FFP und TK sowie mit einer Reduktion der Inzidenz von Massivtransfusionen assoziiert. Dadurch können die knappen Blutressourcen effektiver genutzt werden.
General Medical and Economic Implications of Massive Bleeding and Blood Transfusion
Massive bleeding and blood transfusion are associated with increased morbidity, mortality, and costs [1, 2, 3, 4]. This is not only true for the transfusion of packed red blood cells (PRBCs) but also of fresh frozen plasma (FFP) and platelets [5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. Therefore, it is most important to detect and treat the underlying haemostatic disturbance depending on the individual defects and as fast as possible in order to avoid unnecessary blood transfusions. This target can be achieved by combining point-of-care (POC) diagnostics such as rotation thromboelastometry (ROTEM®, Tem Innovations, Munich, Germany) and whole-blood impedance aggregometry (multiple-electrode aggregometry (MEA = Multiplate®, Roche AG, Grenzach, Germany)) with an early goal-directed therapy using coagulation factor concentrates (such as fibrinogen concentrate, prothrombin complex concentrate (PCC), factor XIII concentrate, and activated recombinant factor VII (rFVIIa)) and haemostatic drugs (such as tranexamic acid and 1-deamino-8-Darginine vasopressin (DDAVP, desmopressin)) [15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]. On the other hand, POC tests and the use of coagulation factor concentrates also generate considerable costs for the hospital. Since an economic approach is of increasing importance for hospitals, economic considerations are also increasingly relevant in medical decision-making, especially linked to the use of expensive drugs such as antibiotics, antimycotics and coagulation factor concentrates. Therefore, besides a risk-benefit analysis, also a cost-benefit analysis comes more and more into the pharmacoeconomic focus.
Therapeutic Strategies in Massive Bleeding and Perioperative Coagulopathy
Several authors reported that the implementation of transfusion and coagulation management algorithms was efficient in the reduction of perioperative blood loss and transfusion requirements in massively bleeding patients [29, 30, 31, 32, 33, 34, 35].
Three therapeutic strategies are mainly used in massively bleeding patients:
– formula-driven transfusion algorithms (e.g., the 1:1:1 concept),
– conventional laboratory test-driven transfusion and coagulation management algorithms,
– POC test-driven transfusion and coagulation management algorithms.
Formula-Driven Transfusion Algorithms
In the USA, the 1:1:1 concept is favoured for the treatment of military trauma patients. Here, in patients with (expected) massive transfusion of PRBCs, FFP and platelet concentrates (PCs) were transfused at a fixed ratio of 1:1:1, independently of laboratory results. In many hospitals, the blood bank hands out the blood products in form of packages, e.g. with 5 units of PRBCs, 5 units of FFP, and 1 pooled PC (which corresponds to 4–5 single-donor PCs). The advantage of this strategy is that no laboratory analysis is necessary. However, this concept is only effective if coagulopathy can be avoided by prophylactic administration of FFP and PCs. According to the German cross-sectional guidelines for therapy with blood components and plasma derivatives published by the board of the German Medical Association on the recommendation of the scientific advisory board in 2009, prophylactic transfusion of FFP and PCs should be avoided based on a recommendation with an evidence level of 1A [36].
Furthermore, Gonzales et al. [37] demonstrated in a study performed in a big US American trauma centre that, using the 1:1 concept, it took on average 14.8 h to correct a coagulopathy (to reduce the international normalised ratio (INR) ad admission of 1.8 ± 0.2 below a level of 1.5). Therefore, Gonzalez et al. came to the conclusion that pre-intensive care unit (ICU) interventions to correct coagulopathy should be more aggressive in order to stop the bleeding, reduce the transfusion requirements and improve the patients’ outcome. Furthermore, they recommended the development and implementation of standardised transfusion and coagulation management protocols, both empirical and data-driven ones.
Since FFP has to be thawed before it can be transfused, in a study published by Snyder et al. [38] in 2009 the first unit of FFP was transfused after a median time of 93 min, whereas the first unit of PRBCs was already transfused after 19 min. In consideration of the median time to death of 293 min in this study, this resulted in a survival bias and the authors therefore concluded that the non-survivors in their study did not die because they got a lower FFP/PRBC ratio but rather that they got a lower ratio because they died [38].
In order to shorten the ‘time-to-treat’ in their bleeding emergency cases, several trauma centres are using pre-thawed AB plasma as a universal donor plasma. However, data from 2 large retrospective studies from the USA and Scandinavia demonstrated that exposure to more than 6 units of AB0-compatible non-identical plasma transfusions increased the overall complication rate of acute lung injury and sepsis by up to 70% and increased mortality as well [39, 40]. Again, this limits the efficacy and safety of the formula-driven treatment concepts of massively bleeding patients [41, 42, 43, 44, 45].
Conventional Laboratory Test-Driven Transfusion and Coagulation Management Algorithms
There is emerging consensus that plasma-based conventional laboratory tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) measurements are inadequate to define and address the bleeding origin in massively bleeding patients and to guide therapy [20, 22, 23, 46, 47]. This may be due to the fact that they are not able to assess the interaction between the cellular and plasma components of clot formation, which plays a central role in our current understanding of haemostasis according to the new cell-based model [48]. Furthermore, the turnaround time of conventional coagulation tests in the central laboratory results in a delay of goal-directed therapeutic interventions of about 30 to 60 min, compared to POC testing [49, 50].
POC Test-Driven Transfusion and Coagulation Management Algorithms
POC diagnostics based on thromboelastography/-metry and whole-blood impedance aggregometry (Multiplate) enable a contemporary detection of several haemostatic disorders within 15 min in patients with massive bleeding [15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]. Furthermore, this allows for an early goal-directed therapy of these haemostatic disorders on a rational and calculated basis with specific coagulation factor concentrates and/or haemostatic drugs [15, 18, 19, 21, 26, 27, 28, 51]. This concept enables a ‘bleed-to-treat-time’ of 20 to 30 min. However, POC diagnostics and early goal-directed therapy should be connected by corresponding coagulation management algorithms adapted to specific patient populations and clinical settings [18, 19, 20, 21, 26, 28, 51, 52, 53, 54, 55, 56].
Calculated, Goal-Directed Use of Coagulation Factor Concentrates and FFP in Bleeding Patients According to the POC-Based Coagulation Management Algorithms
Fibrinogen Concentrate
As the final substrate of coagulation and the ligand of the platelet GPIIb/IIIa receptors, fibrinogen plays a key role in clot formation. Fibrinogen represents 85–90% of the whole amount by weight of plasmatic coagulation factors and is the first to fall below a critical value during bleeding and haemodilution. The reference value for fibrinogen is 1.5–3.0 g/l plasma, which is increased to 4–6 g/l at the end of pregnancy. Even in inflammation and sepsis, the plasma fibrinogen concentration increases up to 10 g/l as an acute-phase protein. Since there are no reserves for fibrinogen outside of the plasma, the overall stock of fibrinogen within the body amounts to about 10 g (per 80 kg body weight). In massive bleeding, fibrinogen should be substituted if the plasma concentration falls below 1.5–2.0 g/l [36, 57]. However, the targeted fibrinogen plasma concentration seems to be dependent on the clinical setting and/or the severity of the trauma. Based on thromboelastometry measurements, a maximum clot firmness (MCF) in FibTEM® below 8–15 mm (corresponding to an amplitude after 10 min (A10) in FibTEM < 6–12 mm) can be used as a trigger value for fibrinogen substitution in bleeding patients [18, 19, 21, 25, 26, 28, 52, 54, 55, 58, 59]. In this setting, the use of fibrinogen concentrate enables a fast and predictable increase in plasma fibrinogen concentration, even above a level of 2.5 g/l [60, 61].
The required fibrinogen dosage can be calculated based on the targeted increase in MCF or A10 in FibTEM and the body weight. The following formula and the values in table 1 are based on the experience of the University Hospital Essen and the Medical School Hannover in the management of massively bleeding patients, and the corresponding results differ by less than 10% [52, 60, 61, 62].
Table 1.
Required fibrinogen dosage
| Body weight, kg | Fibrinogen, g | |||
|---|---|---|---|---|
| Targeted increase in MCF (A10) in FibTEM® | ||||
| 4 mm | 8 mm | 12 mm | 16 mm | |
| 20 | 0.5 | 1 | 1.5 | 2 |
| 40 | 1 | 2 | 3 | 4 |
| 60 | 1.5 | 3 | 4.5 | 6 |
| 80 | 2 | 4 | 6 | 8 |
| (1) |
A clinically relevant increase in the plasma fibrinogen concentration can only be achieved by transfusion of high amounts of FFP (15–30 ml per kg body weight) [5, 15, 36, 63, 64]. However, this may result in a transfusion-associated circulatory overload (TACO) syndrome and a transfusion-relevant decrease in haemoglobin concentration. In principle, plasma fibrinogen concentrations above 2.5 g/l cannot be achieved by FFP transfusion.
Furthermore, the evidence is growing that, apart from haemostatic drugs such as DDAVP, tranexamic acid and rFVIIa, high fibrinogen plasma concentrations can compensate for low platelet counts or platelet dysfunction [65, 66, 67, 68, 69, 70, 71, 72]. However, high amounts of fibrinogen concentrate have to be administered in order to increase the MCF in FibTEM to the high normal values of 18–25 mm that seem to be necessary to achieve this goal, and further studies are required to prove this concept [21, 55, 60, 61, 68, 71, 73].
Prothrombin Complex Concentrate
A clinically relevant deficiency in vitamin K-dependent coagulation factors results in a prolongation of the coagulation time (CT) in ExTEM®. A therapeutic intervention is recommended in bleeding patients with a CT in ExTEM > 80 s [18, 19, 21, 26, 28, 51, 52, 55, 62].
PCC is approved in the European Union (EU) for the treatment and perioperative prophylaxis of bleeding in congenital and acquired deficiency of prothrombin complex coagulation factors (factors II, VII, IX and X). This applies to patients with prolonged PT (increased INR or decreased Quick value) due to vitamin K deficiency, treatment with vitamin K antagonists/oral anticoagulation (phenprocoumarin, warfarin), liver cirrhosis, severe blood loss and haemodilution, when rapid correction of the deficiency is required, and in congenital deficiency of any of the vitamin K-dependent coagulation factors, when purified specific coagulation factor concentrates are not available [74, 75, 76, 77, 78]. Apart from the coagulation factors II, VII, IX and X, balanced four-factor PCCs used in the EU contain also the vitamin K-dependent coagulation inhibition proteins C, S, and Z, as well as small amounts of antithrombin and heparin [79]. The application of 1 IU PCC per kg body weight usually results in an increase of the activity of the corresponding coagulation factors (and the Quick value) by 0.6–1% [80, 81].
In case of a clinically relevant diffuse bleeding and a prolongation of the CT in ExTEM > 80 s, a primary dose of about 25 IU PCC per kg body weight (corresponding to 1 ml PCC per kg body weight) should be administered [18, 19, 26, 51, 52, 55]. The PCC dosage can be enhanced up to 40 IU per kg body weight in case of massive bleeding and a prolongation of the CT in ExTEM > 100 s.
| (2) |
Fresh Frozen Plasma
In most perioperative clinical settings, a substitution of fibrinogen and prothrombin complex coagulation factors seems to be sufficient to stabilise clot formation in bleeding patients. Although an isolated decreased activity of coagulation factors V and XI to 10–20% does not compromise thrombin generation, the endogenous thrombin potential increases proportionally to the plasma prothrombin concentration [82]. Furthermore, von Willebrand factor and factor VIII are released very quickly from the endothelium during trauma and surgery, which results in a factor VIII activity of about 150% already 2 h after cardiac surgery [83]. However, in ongoing massive bleeding, the activity of coagulation factor V, VIII or XI may limit thrombin generation in patients who have been treated exclusively with fibrinogen and PCC. Therefore, the indication for FFP transfusion according to our POC-based coagulation management algorithm is an expected or detected coagulation factor deficiency (V, VIII, or XI) that cannot be treated by specific coagulation factor concentrates or if these products are currently not available. FFP should be transfused in a dose of 15–30 ml per kg body weight in these cases [36, 52, 55, 62, 64].
Factor XIII Concentrate
The clinical relevance of factor XIII deficiency in perioperative settings is discussed controversially. In particular, factor XIII administration should be considered in neurosurgical and tumour patients with an activity of factor XIII below 60%, in bleeding patients with increased clot lysis in ROTEM analysis not responding to antifibrinolytics but to in vitro spiking with factor XIII, in ongoing bleeding without pathological findings in ROTEM and Multiplate analysis, and in repeated postoperative diffuse bleeding without detectable surgical source of bleeding [15, 18, 19, 36, 51, 52, 55, 62, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93].
Recombinant Activated Factor VII
Although rFVIIa plays a definite role in the management of patients with acquired haemophilia with inhibitors, its effectiveness and safety as a more general haemostatic drug, either prophylactically or therapeutically, remains uncertain. Therefore, the use of rFVIIa outside its currently licensed indications should be limited to prospective randomised trials and ‘ultima ratio’ interventions [21, 36, 52, 55, 57, 62, 94, 95, 96, 97, 98].
Impact of POC-Based Transfusion and Coagulation Management Algorithms on Transfusion Requirements in Massive Bleeding
Using algorithms based on POC diagnostics and early, calculated, goal-directed therapy with specific coagulation factor concentrates such as fibrinogen concentrate and PCC, the requirements for FFP could be reduced dramatically in several Austrian and German hospitals in different clinical settings.
Department of Anaesthesiology and Intensive Care Medicine, AUVA Trauma Centre Salzburg, Austria
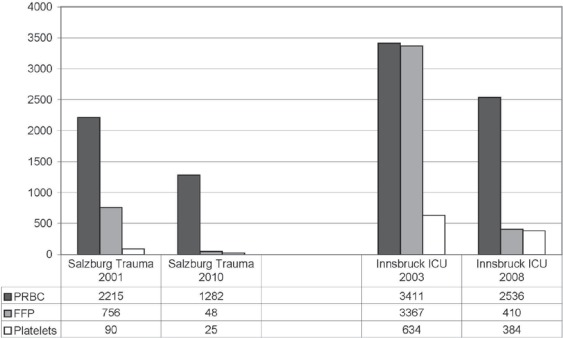
After implementation of thromboelastometry and goal-directed therapy with fibrinogen concentrate and PCC in 2002, the transfusion requirements for FFP could be reduced by 94% (756 units transfused to 63 patients vs. 48 units transfused to 4 patients) within 9 years (from 2001 to 2010) at the Department of Anaesthesiology and Intensive Care Medicine at the AUVA Trauma Centre Salzburg in Austria. Furthermore, the transfusion requirements for PRBCs and PCs could be reduced by 42% (2,215 vs. 1,282 units) and 72% (90 vs. 25 units), respectively (fig. 1). The incidence of massive transfusion (≥10 U PRBCs transfused intraoperatively) decreased from 20 to 16 patients per year. At the same time, the administration of fibrinogen concentrate increased 8-fold (43 vs. 390 g per year), while the administration of PCC decreased by 36% (185,500 vs. 118,200 IU per year).
Fig. 1.
Reduction of transfusion requirements for PRBCs, FFP and PCs at the Department of Anaesthesiology and Intensive Care Medicine, AUVA Trauma Centre Salzburg, Austria, and at the Department of General and Surgical Critical Care Medicine (ICU) at the Medical University Innsbruck, Austria, after implementation of POC coagulation management with early, calculated, goal-directed therapy with coagulation factor concentrates.
Department of General and Surgical Critical Care Medicine, Medical University Innsbruck, Austria
The Department for General and Surgical Critical Care Medicine of the Medical University Innsbruck, Austria (1 trauma ICU including 11 beds and 1 surgical ICU including 12 beds of the highest category) uses POC monitoring since 2000. By using a POC-based algorithm including a goal-directed coagulation management, the need for FFP transfusion became more and more redundant [19]. From 2003 to 2008, transfusion of FFP was reduced by 88% (3367 vs. 410 units). Simultaneously, the transfusion requirements for PRBCs and PCs decreased by 26% (3,411 vs. 2,536 units) and 39% (634 vs. 384 units), respectively (fig. 1). While the need for PCC was also reduced by 89% within that period, fibrinogen concentrate was administered significantly more, which resulted in an increase of 58% in the costs for coagulation factor concentrates from 2003 to 2008. Nevertheless, the overall cost reduction for blood products including coagulation factor concentrates from 2005 to 2008 compared to 2003 was about EUR 200,000 per year. For this cost analysis, only the institutional costs, not the real costs for FFP, RBCs and PCs, were taken into account. Nevertheless, the simplified acute physiology score II (SAPS II) at admission at the ICU increased during that time.
Since 2008, this positive cost-benefit ratio was not achieved any more in Innsbruck because of the following 2 reasons. First of all, the price for fibrinogen concentrate was increased by about 40% by the industry and, secondly, because of a widespread use especially of fibrinogen concentrate in Innsbruck for more and more prophylactic indications rather than in acutely bleeding patients.
Department of Anaesthesiology and Intensive Care Medicine, University Hospital Essen, Germany
Thromboelastometry (ROTEM) was implemented for POC coagulation management in visceral surgery and liver transplantation (LTX) in 2000 at the Department of Anaesthesiology and Intensive Care Medicine at the University Hospital Essen in Germany. In 2003, POC coagulation management was also implemented in trauma surgery (TS). Based on our experience in perioperative POC coagulation management from 2000 to 2003, we developed algorithms for ROTEM-based coagulation management in LTX and trauma patients in 2004, which were published for the first time in 2006 [52, 99, 100]. In 2005, POC coagulation management was also implemented in cardiovascular surgery (CVS) based on thromboelastometry and whole-blood impedance aggregometry (Multiplate). Our POC algorithm for perioperative coagulation management in CVS was developed in collaboration with colleagues at the University Hospital Frankfurt and the Medical School Hannover, and was published for the first time in 2007 [21, 101]. All 3 algorithms are based on POC diagnostics combined with first-line, calculated, goal-directed therapy with specific coagulation factor concentrates such as fibrinogen and PCC and were refined continuously during the last 5 years (fig. 2) [28, 54, 55, 102, 103].
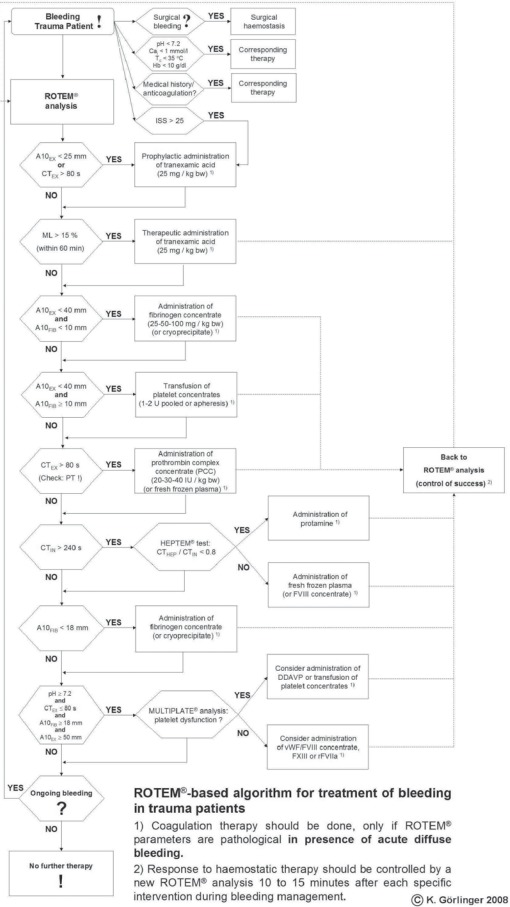
Fig. 2.
Algorithm for POC coagulation management in trauma surgery at the Department of Anaesthesiology and Intensive Care Medicine, University Hospital Essen, Germany. Thromboelastometric variables and assays: CT = clotting time; A10 = amplitude of clot firmness after 10 min; ML = maximum lysis. Assays: EX = ExTEM®; FIB = FibTEM®; IN = InTEM®; HEP = HepTEM®. Other abbreviations: Tc = core temperature; Cai = ionised calcium; Hb = haemoglobin; ISS = injury severity score; PT = prothrombin time; bw = body weight; PCC = prothrombin complex concentrate; FFP = fresh frozen plasma; DDAVP = desmopressin; F VIII/vWF concentrate = factor VIII/von Willebrand factor concentrate; F XIII = factor XIII concentrate; rFVIIa = activated recombinant factor VII.
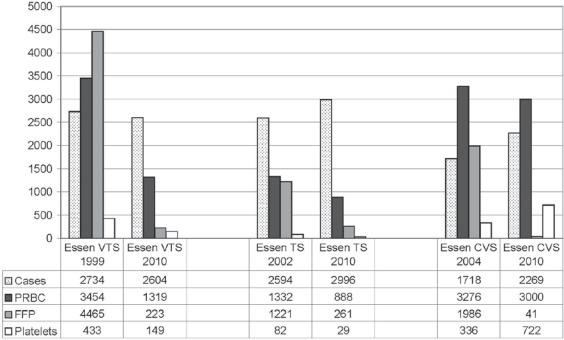
Transfusion requirements for FFP could be reduced in visceral surgery and LTX (VTS) by 95% (4465 units in 1999 vs. 223 unit in 2010), in TS by 79% (1221 in 2002 units vs. 261 units in 2010) and in CVS by 98% (1986 units in 2004 vs. 41 units in 2010) (fig. 3). Overall, this resulted in a reduction in transfusion requirements for FFP by 93% (7,672 units per year before and 525 units per year after implementation of POC coagulation management algorithms).
Fig. 3.
Reduction of intraoperative transfusion requirements for PRBCs, FFP and platelets in VTS, TS, and CVS at the University Hospital Essen, Germany, after implementation of POC coagulation management algorithms with early, calculated, goal-directed therapy with coagulation factor concentrates.
At the same time, the number of cases in VTS decreased by 4.8% (2,734 vs. 2,604 cases) while the number of LTX increased by 66% (97 vs. 161 LTX). LTX were responsible for 43% of all allogeneic blood products transfused in 2009 and for 52% of all massive transfusions (≥10 units of PRBCs intraoperatively transfused) in VTS. The number of cases in TS and CVS increased by 15.5% (2594 vs. 2996 cases) and 32.1% (1717 vs. 2269 cases), respectively.
The transfusion requirements for PRBCs decreased in VTS by 62% (3,454 vs. 1,319 units), in TS by 33% (1,332 vs. 888 units) and in CVS by 8.4% (3,276 vs. 3,000 units). The transfusion requirements for PCs decreased in VTS by 66% (433 vs. 149 units) and in TS by 65% (82 vs. 29 units). In contrast, the transfusion requirements for PCs increased in CVS by 115% (336 vs. 722 units). This isolated increase in platelet transfusion in CVS was most likely due to a 5-fold increase in the incidence of patients with dual antiplatelet therapy from 2004 to 2009 (2.7 vs. 13.7%; p < 0.0001) [103].
In VTS and CVS, the incidence of massive transfusion (≥10 units of PRBC transfused intraoperatively) could be reduced from 2.56 to 0.88% (p < 0.0001) and from 2.50 to 1.06% (p = 0.0007), respectively. In the whole 2010 patient population (VTS, TS and CVS; n = 7,869 patients), the incidence of massive transfusion was 0.8% (66/7,869) and the incidence of FFP transfusion was 1.2% (92/7,869). The PRBC-to-FFP ratio decreased from 1:0.95 (8062/7,672) before implementation of POC-based coagulation management to 1:0.1 (5,207/525) in 2010.
The usage of fibrinogen concentrate increased in VTS from 68 to 745 g per year (from 1999 to 2009) and in CVS from 179 to 702 g per year (from 2004 to 2009). PCC administration increased in VTS from 65,500 to 238,500 IU per year and in CVS from 162,000 to 388,000 IU per year, whilst antithrombin administration decreased in VTS and CVS from 150,500 to 32,500 IU and from 223,000 to 170,000 IU, respectively. Since the implementation of POC coagulation management, there was no case of ‘off-label’ use of rFVIIa.
Cost savings in VTS accounted for EUR 270,000 (-36% overall cost in 2009 compared to 1999) and in CVS for EUR 50,000 (-6.5% cost savings per patient in 2009 compared to 2004) [102]. The calculation of costs was based on the internal transfer prices of blood components and coagulation factor concentrates in 2009 for the University Hospital Essen (1 U PRBCs EUR 85, 1 U FFP EUR 65, 1 pooled PC EUR 250, 1 g fibrinogen concentrate EUR 288, 500 IU PCC EUR 126, 500 IU antithrombin EUR 44, 1250 IU factor XIII EUR 527 und 4.8 mg rFVIIa EUR 3,203). Costs for blood products (PRBCs, FFP, and PCs) are about 20–40% higher in Austria compared to Germany. Therefore, the cost savings after the implementation of perioperative POC coagulation management with early, calculated, goal-directed therapy should be higher in Austria.
Notably, this cost analysis matches only the primary, intraoperative costs for blood products and coagulation factor concentrates, without consideration of the activity-based costs of blood transfusions or the secondary costs of transfusion-associated adverse events such as transfusion-related acute lung injury (TRALI), TACO and sepsis [104, 105, 106]. However, the reduction of the secondary costs by reducing the incidence of transfusion-associated adverse events can account for a reduction of the overall hospital costs far beyond the reduction of the primary costs of blood transfusion [2, 4, 107, 108, 109].
Conclusions and Practical Recommendations
Perioperative POC coagulation management with early, calculated, goal-directed therapy was effective in the reduction of FFP transfusion requirements by about 90% in different perioperative settings (TS, visceral surgery, LTX, CVS, and intensive care medicine), in 3 different hospitals (Salzburg, Innsbruck, and Essen) and in 2 different countries (Austria and Germany). Furthermore, the transfusion requirements for PRBCs and PCs could be reduced up to 62 and 72%, respectively, dependent on the clinical setting and the patient population. Thus, the limited blood resources can be used more efficiently. In addition, cost savings can be realised if reasonable transfusion and coagulation management algorithms are implemented and used accordingly.
A cost-effective perioperative transfusion and coagulation management algorithm should be based on the following keystones:
– medical and bleeding history,
– contemporary and reasonable POC diagnostics (e.g., by using ROTEM and Multiplate),
– quickly available, effective, and calculable goal-directed haemostatic interventions with a good risk-benefit and cost-benefit ratio (e.g., by using tranexamic acid and specific coagulation factor concentrates such as fibrinogen and PCC),
– connection of diagnostics and therapy by the implementation of evidence- or at least experience-based algorithms for perioperative transfusion and coagulation management.
Here, the following principles have to be considered:
– The indication to carry out a haemostatic intervention should be based on the detection of a clinically relevant diffuse bleeding!
– POC diagnostics can be helpful to identify the haemostatic intervention with the best chance to stop the diffuse bleeding (without inducing life-threatening adverse events).
– In the absence of clinically relevant bleeding, pathological laboratory results (irrespective of whether they are assessed by POC tests in the operation room or by conventional tests in the central laboratory) are not an indication for a haemostatic intervention (Don't treat numbers!).
– Haemostatic drugs such as tranexamic acid, DDAVP and coagulation factor concentrates have to be basically (national approval) and locally (storage in the operation room or at the ICU) available. Decision-making should be based on POC diagnostics according to algorithms, by an experienced anaesthetist or intensivist.
– Every haemostatic intervention should be based on a rational indication described in the transfusion and coagulation management algorithm.
– Prophylactic or unspecific transfusion of FFP should be avoided if a goal-directed therapy with specific coagulation factor concentrates is performed. FFP has to be transfused if specific coagulation factor concentrates are not available to treat the coagulopathy.
– A restrictive transfusion strategy for PRBCs, FFP and platelets should be preferred in order to avoid unnecessary blood transfusions and corresponding transfusion-associated adverse events.
Disclosure Statement
The authors point out that they received fees and honoraria from CSL Behring GmbH and TEM international GmbH for consulting and scientific lectures. The content of this manuscript was not influenced by that.
References
- 1.Aronson D, Dann EJ, Bonstein L, Blich M, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H. Impact of red blood cell transfusion on clinical outcomes in patients with acute myocardial infarction. Am J Cardiol. 2008;102:115–119. doi: 10.1016/j.amjcard.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Christensen MC, Krapf S, Kempel A, von Heymann C. Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg. 2009;138:687–693. doi: 10.1016/j.jtcvs.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 4.Murphy GV, Reeves BC, Rogers CA, Rizvi SIA, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 5.Dara SI, Rana R, Afessa B, Moore SB, Gajic O. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Crit Care Med. 2005;33:2667–2671. doi: 10.1097/01.ccm.0000186745.53059.f0. [DOI] [PubMed] [Google Scholar]
- 6.Däubener W, Funk M, Heiden M, Hoch J, Hornei B, Wirsing von König CH, Walther-Wenke G. Reduktion des Septikämierisikos bei der Anwendung von Thrombozytenkonzentraten. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2008;51:1485–1487. [Google Scholar]
- 7.Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34(suppl 5):S109–S113. doi: 10.1097/01.CCM.0000214311.56231.23. [DOI] [PubMed] [Google Scholar]
- 8.Keller-Stanislawski B, Reil A, Günay S, Funk MB. Frequency and severity of transfusion-related acute lung injury – German haemovigilance data (2006–2007) Vox Sang. 2010;98:70–77. doi: 10.1111/j.1423-0410.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 9.Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB, Hubmayr RD, Gajic O. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 10.Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083–1091. doi: 10.1213/ane.0b013e3181948a59. [DOI] [PubMed] [Google Scholar]
- 11.Sarani B, Dunkman WJ, Dean L, Sonnad S, Rohrbach JI, Gracias VH. Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med. 2008;36:1114–1118. doi: 10.1097/CCM.0b013e318168f89d. [DOI] [PubMed] [Google Scholar]
- 12.Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, Murkin J, Nadel A. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor C (Ed.), Cohen H, Mold D, Jones H, et al.; on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group: The 2009 Annual SHOT Report. www.shotuk.org/wp-content/uploads/2010/07/SHOT2009.pdf, 2010.
- 14.Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, Cuschieri J, Maier RV, Billiar TR, Peitzman AB, Inflammation and Host Response to Injury Investigators Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–230. doi: 10.1097/TA.0b013e3181ad5957. [DOI] [PubMed] [Google Scholar]
- 15.Bolliger D, Görlinger K, Tanaka KA. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology. 2010;113:1205–1219. doi: 10.1097/ALN.0b013e3181f22b5a. [DOI] [PubMed] [Google Scholar]
- 16.Despotis G, Avidan M, Eby C. Prediction and management of bleeding in cardiac surgery. J Thromb Haemost. 2009;7(suppl 1):111–117. doi: 10.1111/j.1538-7836.2009.03412.x. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth. 2009;103(suppl 1):i14–i22. doi: 10.1093/bja/aep318. [DOI] [PubMed] [Google Scholar]
- 18.Fries D, Innerhofer P, Perger P, Gütl M, Heil S, Hofmann N, Kneifel W, Neuner L, Pernerstorfer T, Pfanner G, Schöchl H, Ziegler B, Kölblinger C, Kozek-Langenecker S. Gerinnungsmanagement bei traumatisch bedingter Massivblutung – Empfehlungen der Arbeitsgruppe für perioperative Gerinnung der ÖGARI. Anästhesiol Intensivmed Notfallmed Schmerzther. 2010;45:552–561. doi: 10.1055/s-0030-1265746. [DOI] [PubMed] [Google Scholar]
- 19.Fries D, Innerhofer P, Schobersberger W. Time for changing coagulation management in trauma-related massive bleeding. Curr Opin Anaesthesiol. 2009;22:267–274. doi: 10.1097/ACO.0b013e32832678d9. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36:723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Görlinger K, Jambor C, Hanke AA, Dirkmann D, Adamzik M, Hartmann M, Rahe-Meyer N. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transfus Med Hemother. 2007;34:396–411. [Google Scholar]
- 22.Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and thromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, Barnett C, Stahel P, Sillman CC, Sauaia A, Banerjee A. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 24.Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, David JS. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100:792–797. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 25.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 26.Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schöchl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waydhas C, Görlinger K. Gerinnungsmanagement beim Polytrauma. Unfallchirurg. 2009;112:942–950. doi: 10.1007/s00113-009-1681-3. [DOI] [PubMed] [Google Scholar]
- 29.Ak K, Isbir CS, Tetik S, Atalan N, Tekeli A, Aljodi M, Civelek A, Arsan S. Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24:404–410. doi: 10.1111/j.1540-8191.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 30.Anderson L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Eur J Cardiothorac Surg. 2007;31:1052–1057. doi: 10.1111/j.1365-3148.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 31.Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, Hunt BJ. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178–186. doi: 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 32.Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion. 2008;48(suppl 1):2S–30S. doi: 10.1111/j.1537-2995.2007.01573.x. [DOI] [PubMed] [Google Scholar]
- 33.Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, Ereth MH. Efficacy of a simple intraoperative transfusion algorithm for non-erythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–781. doi: 10.1097/00000542-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Shore-Lesserson L, Manspreizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusion in complex cardiac surgery. Anesth Analg. 1999;88:312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastography (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18:277–288. doi: 10.1016/j.hlc.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 36.The Board of the German Medical Association on the Recommendation of the Scientific Advisory Board Cross-sectional guidelines for therapy with blood components and plasma derivatives, 4th ed. Transfus Med Hemother. 2009;36:351–482. [Google Scholar]
- 37.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–129. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 38.Snyder CW, Weinberg JA, McGwin G, Jr, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW, 3rd, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–364. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 39.Inaba K, Branco BC, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, Nelson J, Demetriades D. Impact of ABO-identical vs ABO-compatible non-identical plasma transfusion in trauma patients. Arch Surg. 2010;145:899–906. doi: 10.1001/archsurg.2010.175. [DOI] [PubMed] [Google Scholar]
- 40.Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, Melbye M, Nyrén O, Reilly M. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 200;96:316–323. doi: 10.1111/j.1423-0410.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 41.Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PG, Shulman I, Nelson J, Demetriades D. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg. 2010;210:957–965. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 42.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–271. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 43.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50:1370–1383. doi: 10.1111/j.1537-2995.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 44.Nascimento B, Callum J, Rubenfeld G, Neto JB, Lin Y, Rizoli S. Clinical review: Fresh frozen plasma in massive bleedings – more questions than answers. Crit Care. 2010;14:202. doi: 10.1186/cc8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster KM, Davis KA, Lui FY, Maerz LL, Kaplan LJ. The status of massive transfusion protocols in United States trauma centers: massive transfusion or massive confusion? Transfusion. 2010;50:1545–1551. doi: 10.1111/j.1537-2995.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 46.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65:535–543. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 47.Steiner ME, Despotis GJ. Transfusion algorithms and how they apply to blood conservation: the high-risk cardiac surgical patient. Hematol Oncol Clin North Am. 2007;21:177–184. doi: 10.1016/j.hoc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–965. [PubMed] [Google Scholar]
- 49.Görlinger K. Time is life in trauma induced coagulopathy. Shock. 2011;35 submitted. [Google Scholar]
- 50.Toulon P, Ozier Y, Ankri A, Fléron MH, Leroux G, Samama CM. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost. 2009;101:394–401. [PubMed] [Google Scholar]
- 51.Gerlach R, Krause M, Seifert V, Goerlinger K. Hemostatic and hemorrhagic problems in neurosurgical patients. Acta Neurochir (Wien) 2009;151:873–900. doi: 10.1007/s00701-009-0409-z. [DOI] [PubMed] [Google Scholar]
- 52.Görlinger K. Coagulation management during liver transplantation. Hämostaseologie. 2006;26(3 suppl 1):S64–S76. [PubMed] [Google Scholar]
- 53.Görlinger K. Ist der Einsatz von Point-of-Care zur Gerinnungsdiagnostik effizient? In: Kuhlen R, Putensen C, Quintel M, editors. Interdisziplinäre Intensivmedizin Aktuell, Bd 1. Nürnberg: MEPS Medical Event & Publisher Services; 2008. pp. 1–19. [Google Scholar]
- 54.Görlinger K, Bergmann L, Hartmann M, Marggraf G, Kamler M, Müller-Beißenhirtz H. Reduction of blood transfusion rate by thrombelastometry and impedance aggregometry based point-of-care coagulation management in thoracic and cardiovascular surgery. Appl Cardiopulmon Pathophysiol. 2009;13:174–177. [Google Scholar]
- 55.Görlinger K, Dirkmann D, Weber CF, Rahe-Meyer N, Hanke AA. Algorithms for transfusion and coagulation management in massive haemorrhage. Anasth Intensivmed. 2011;52:145–159. [Google Scholar]
- 56.Hanke AA, Herold U, Jakob H, Görlinger K. A pilot study on transfusion rate, hospitalisation and economics after implementation of thrombelastometry for point-of-care coagulation management in aortic arch replacement after acute type A dissection. Appl Cardiopulmon Pathophysiol. 2009;13:179–184. [Google Scholar]
- 57.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Stahel PF, Vincent JL, Spahn DR, Task Force for Advanced Bleeding Care in Trauma Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinhöfer M, Brauer M, Franke U, Barz D, Marx G, Lösche W. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2008;19:212–219. doi: 10.1097/MBC.0b013e3282f3f9d4. [DOI] [PubMed] [Google Scholar]
- 59.Roullet S, Pillot J, Freyburger G, Biais M, Quinart A, Rault A, Revel P, Sztark F. Rotation thromboelastometry detects thrombocytopenia and hypofibrinogenaemia during orthotopic liver transplantation. Br J Anaesth A. 2010;104:422–428. doi: 10.1093/bja/aeq022. [DOI] [PubMed] [Google Scholar]
- 60.Rahe-Meyer N, Pichlmaier M, Haverich A, Solomon C, Winterhalter M, Piepenbrock S, Tanaka KA. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102:785–792. doi: 10.1093/bja/aep089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon C, Pichlmaier U, Schoechl H, Hagl C, Raymondos K, Scheinichen D, Koppert W, Rahe-Meyer N. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth. 2010;104:555–562. doi: 10.1093/bja/aeq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Görlinger K. Differenzierte Therapie komplexer Gerinnungsstörungen. J Anasth Intensivbeh. 2005;12:120–124. [Google Scholar]
- 63.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279–1285. doi: 10.1111/j.1537-2995.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 64.Chowdhury P, Saayman AG, Paulus U, Findlay GP, Collins PW. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 65.Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Crit Care. 2008;12:R105. doi: 10.1186/cc6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ang L, Palakodeti V, Khalid A, Tsimikas S, Idrees Z, Tran P, Clopton P, Zafar N, Bromberg-Marin G, Keramati S, Mahmud E. Elevated plasma fibrinogen and diabetes mellitus are associated with lower inhibition of platelet reactivity with clopidogrel. J Am Coll Cardiol. 2008;52:1052–1059. doi: 10.1016/j.jacc.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 67.Fenger-Eriksen C, Ingerslev J, Sørensen B. Fibrinogen concentrate – a potential universal hemostatic agent. Expert Opin Biol Ther. 2009;9:1325–1333. doi: 10.1517/14712590903193051. [DOI] [PubMed] [Google Scholar]
- 68.Görlinger K, Oprea G, Peters J, Hartmann M. Fibrinogen reverses the eptifibatide-induced decrease of maximum clot firmness but not impaired platelet aggregation. J Cardiothor Vasc Anesth. 2010;24(suppl 3):S35. [Google Scholar]
- 69.Mahmud E, Cavendish JJ, Tsimikas S, Ang L, Nguyen C, Bromberg-Marin G, Schnyder G, Keramati S, Palakodeti V, Penny WF, DeMaria AN. Elevated plasma fibrinogen level predicts suboptimal response to therapy with both single- and double-bolus eptifibatide during percutaneous coronary intervention. J Am Coll Cardiol. 2007;49:2163–2171. doi: 10.1016/j.jacc.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Rahe-Meyer N, Sørensen B. Fibrinogen concentrate for management of bleeding. J Thromb Haemost. 2011;9:1–5. doi: 10.1111/j.1538-7836.2010.04099.x. [DOI] [PubMed] [Google Scholar]
- 71.Velik-Salchner C, Haas T, Innerhofer P, Streif W, Nussbaumer W, Klingler A, Klima G, Martinowitz U, Fries D. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5:1019–1025. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 72.Weber CF, Schneider AC, Kirschning T, Hofstetter C, Zacharowski K, Görlinger K. Therapieoptionen der perioperativ erworbenen Thrombozytopathie. Anaesthesist. 2009;58:931–940. doi: 10.1007/s00101-009-1599-8. [DOI] [PubMed] [Google Scholar]
- 73.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301–310. doi: 10.1097/01.mbc.0000169225.31173.19. [DOI] [PubMed] [Google Scholar]
- 74.Levy JH, Tanaka KA, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology. 2008;109:918–926. doi: 10.1097/ALN.0b013e3181895bd8. [DOI] [PubMed] [Google Scholar]
- 75.Lorenz R, Kienast J, Otto U, Egger K, Kiehl M, Schreiter D, Kwasny H, Haertel S, Barthels M. Efficacy and safety of a prothrombin complex concentrate with two virus-inactivation steps in patients with severe liver damage. Eur J Gastroenterol Hepatol. 2003;15:15–20. doi: 10.1097/00042737-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Samama CM. Prothrombin complex concentrates: a brief review. Eur J Anaesthesiol. 2008;25:784–789. doi: 10.1017/S0265021508004675. [DOI] [PubMed] [Google Scholar]
- 77.Schick KS, Fertmann JM, Jauch KW, Hoffmann JN. Prothrombin complex concentrate in surgical patients: retrospective evaluation of vitamin K antagonist reversal and treatment of severe bleeding. Crit Care. 2009;13:R191. doi: 10.1186/cc8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scott LJ. Prothrombin complex concentrate (Beriplex P/N) Drugs. 2009;69:1977–1984. doi: 10.2165/11203690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Kalina U, Bickhard H, Schulte S. Biochemical comparison of seven commercially available prothrombin complex concentrates. Int J Clin Pract. 2008;62:1614–1622. doi: 10.1111/j.1742-1241.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 80.Scherer R, Gille A, Erhard J, Paar D, Kox WJ. Substitutionseffekt von AT III-und PPSB-Konzentraten bei Patienten mit terminaler Leberinsuffizienz. Anaesthesist. 1994;43:178–182. doi: 10.1007/s001010050046. [DOI] [PubMed] [Google Scholar]
- 81.Vigué B. Bench-to-bedside review: Optimising emergency reversal of vitamin K antagonists in severe haemorrhage – from theory to practice. Crit Care. 2009;13:209. doi: 10.1186/cc7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, Béguin S, Hemker HC. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. 2002;88:576–582. [PubMed] [Google Scholar]
- 83.Ternström L, Radulovic V, Karlsson M, Baghaei F, Hyllner M, Bylock A, Hansson KM, Jeppsson A. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res. 2010;126:e128–e133. doi: 10.1016/j.thromres.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 84.Gerlach R, Tölle F, Raabe A, Zimmermann M, Siegemund A, Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke. 2002;33:1618–1623. doi: 10.1161/01.str.0000017219.83330.ff. [DOI] [PubMed] [Google Scholar]
- 85.Gödje O, Gallmeier U, Schelian M, Grünewald M, Mair H. Coagulation factor XIII reduces postoperative bleeding after coronary surgery with extracorporeal circulation. Thorac Cardiovasc Surg. 2006;54:26–33. doi: 10.1055/s-2005-872853. [DOI] [PubMed] [Google Scholar]
- 86.Haas T, Fries D, Velik-Salchner C, Reif C, Klingler A, Innerhofer P. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106:1360–1365. doi: 10.1213/01.ane.0b013e3181684339. [DOI] [PubMed] [Google Scholar]
- 87.Korte W. Fibrin monomer and factor XIII: a new concept for unexplained intraoperative coagulopathy. Hämostaseologie. 2006;26(3 suppl 1):S30–S35. [PubMed] [Google Scholar]
- 88.Korte W. F. XIII in perioperative coagulation management. Best Pract Res Clin Anaesthesiol. 2010;24:85–93. doi: 10.1016/j.bpa.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Korte WC, Szadkowski C, Gähler A, Gabi K, Kownacki E, Eder M, Degiacomi P, Zoller N, Devay J, Lange J, Schnider T. Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology. 2009;110:239–245. doi: 10.1097/ALN.0b013e318194b21e. [DOI] [PubMed] [Google Scholar]
- 90.Spahn DR, Asmis LM. Excessive perioperative bleeding: Are fibrin monomers and factor XIII the missing link? Anesthesiology. 2009;110:212–213. doi: 10.1097/ALN.0b013e3181942c65. [DOI] [PubMed] [Google Scholar]
- 91.Tacke F, Fiedler K, von Depka M, Luedde T, Hecker H, Manns MP, Ganser A, Trautwein C. Clinical and prognostic role of plasma coagulation factor XIII activity for bleeding disorders and 6-year survival in patients with chronic liver disease. Liver Int. 2006;26:173–181. doi: 10.1111/j.1478-3231.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 92.Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmness in rotation thromboelastometry (ROTEM) Thromb Haemost. 2010;104:385–391. doi: 10.1160/TH09-12-0858. [DOI] [PubMed] [Google Scholar]
- 93.Weber CF, Jambor C, Marquardt M, Görlinger K, Zwissler B. Erfassung eines Faktor-XIII-Mangels mit der Thrombelastometrie. Anaesthesist. 2008;57:487–490. doi: 10.1007/s00101-008-1360-8. [DOI] [PubMed] [Google Scholar]
- 94.Gill R, Herbertson M, Vuylsteke A, Olsen PS, von Heymann C, Mythen M, Sellke F, Booth F, Schmidt TA. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–27. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 95.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ, Dimsits J, Bouillon B, CONTROL Study Group Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 96.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 97.Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2011;CD005011 doi: 10.1002/14651858.CD005011.pub3. [DOI] [PubMed] [Google Scholar]
- 98.O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295:293–298. doi: 10.1001/jama.295.3.293. [DOI] [PubMed] [Google Scholar]
- 99.Goerlinger K, Dirkmann D, Kiss G, Dusse F, Hanke A, Arvieux CC, Peters J. ROTEM-based management for diagnosis and treatment of acute haemorrhage during liver transplantation. Eur J Anaesth. 2006;23(suppl 37):85. [Google Scholar]
- 100.Goerlinger K, Kiss G, Dirkmann D, Dusse F, Hanke A, Arvieux CC, Peters J. ROTEM-based algorithm for management of acute haemorrhage and coagulation disorders in trauma patients. Eur J Anaesth. 2006;23(suppl 37):84–85. [Google Scholar]
- 101.Görlinger K, Jambor C, Hanke A, Adamzik M, Hartmann M, Rahe-Meyer N. Thrombelastometry and impedance aggregometry based algorithm for coagulation management in cardiac surgery. Intensive Care Med. 2007;33(suppl 2):S196. [Google Scholar]
- 102.Görlinger K, Dirkmann D, Müller-Beißenhirtz H, Hartmann M, Saner F. Thromboelastometrybased perioperative coagulation management in visceral surgery and liver transplantation: experience of 10 years and 1105 LTX. Liver Transplant. 2010;16(suppl 1):S86. [Google Scholar]
- 103.Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, Jakob H, Peters J. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–1191. doi: 10.1097/ALN.0b013e31823497dd. [DOI] [PubMed] [Google Scholar]
- 104.Shander A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery. 2007;142(suppl 4):S20–S25. doi: 10.1016/j.surg.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 105.Shander A, Hofmann A, Gombotz H, Theusinger OM, Spahn DR. Estimating the cost of blood: past, present, and future directions. Best Pract Res Clin Anaesthesiol. 2007;21:271–289. doi: 10.1016/j.bpa.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 107.Glenngård AH, Persson U, Söderman C. Costs associated with blood transfusions in Sweden – the societal cost of autologous, allogeneic and perioperative RBC transfusion. Transfus Med. 2005;15:295–306. doi: 10.1111/j.0958-7578.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 108.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 109.Kaushal R, Bates DW, Franz C, Soukup JR, Rothschild JM. Costs of adverse events in intensive care units. Crit Care Med. 2007;35:2479–2483. doi: 10.1097/01.CCM.0000284510.04248.66. [DOI] [PubMed] [Google Scholar]