Abstract
Background:
As a prelude to combination studies aimed at resistance reversal, this dose-escalation/dose-expansion study investigated the selective Src kinase inhibitor saracatinib (AZD0530) in combination with carboplatin and/or paclitaxel.
Methods:
Patients with advanced solid tumours received saracatinib once-daily oral tablets in combination with either carboplatin AUC 5 every 3 weeks (q3w), paclitaxel 175 mg m−2 q3w, paclitaxel 80 mg m−2 every 1 week (q1w), or carboplatin AUC 5 plus paclitaxel 175 mg m−2 q3w. The primary endpoint was safety/tolerability.
Results:
A total of 116 patients received saracatinib 125 (N=20), 175 (N=44), 225 (N=40), 250 (N=9), or 300 mg (N=3). There were no clear dose-related trends within each chemotherapy regimen group in number or severity of adverse events (AEs). However, combining all groups, the occurrence of grade ⩾3 asthenic AEs (all causality) was dose-related (125 mg, 10% 175 mg, 20% ⩾225 mg, 33%), and grade ⩾3 neutropenia occurred more commonly at doses ⩾225 mg. There was no evidence that saracatinib affected exposure to carboplatin or paclitaxel, or vice versa. Objective responses were seen in 5 out of 44 patients (11%) receiving carboplatin plus paclitaxel q3w, and 5 out of 24 (21%) receiving paclitaxel q1w.
Conclusion:
Saracatinib doses up to 175 mg with paclitaxel with/without carboplatin showed acceptable toxicity in most patients, and are suitable for further trials.
Keywords: carboplatin, combination chemotherapy, paclitaxel, saracatinib, Src
Src is a non-receptor tyrosine kinase involved in control of various cellular processes, including proliferation, adhesion, and motility (Wheeler et al, 2009). Src is overexpressed or activated in many cancers, and clinical evidence suggests that the degree of Src activity may correlate with cancer stage (Talamonti et al, 1993; Wiener et al, 2003; Zou et al, 2009). Moreover, in vitro studies have implicated Src in the development of resistance to cytotoxic cancer chemotherapy (Masumoto et al, 1999; Boudny and Nakano, 2002; Pengetnze et al, 2003; Peterson-Roth et al, 2009). Gene expression profiling has identified several genomic signatures associated with advanced and chemotherapy-resistant ovarian cancer (Gevaert et al, 2008; Cancer Genome Atlas Research Network, 2011), including the Src pathway (Dressman et al, 2007), although the predictive nature of a Src-related signature has yet to be conclusively established. Src activity also has an important role in tumour angiogenesis, and its inhibition has been demonstrated experimentally to have anti-tumour effects acting through this mechanism (Han et al, 2006).
Saracatinib (AZD0530) is a potent and selective inhibitor of Src kinase that has shown anticancer effects as well as synergy with other therapies, particularly taxanes, in a range of in vitro and in vivo preclinical models (Van Schaeybroeck et al, 2008; Chen et al, 2009; Green et al, 2009; Hawthorne et al, 2009; Lu et al, 2009). In a phase I study in patients with advanced solid tumours, saracatinib monotherapy was tolerated at doses up to 175 mg per day, and demonstrated inhibition of Src activity in tumour tissue at doses of 50 mg and above (Baselga et al, 2010). Saracatinib is suitable for once-daily oral administration and reaches stable plasma concentrations after 10–17 days in humans (Baselga et al, 2010).
These results indicate that saracatinib might have the potential to enhance the clinical anticancer effects of platinum- and/or taxane-based cytotoxic chemotherapies. The objectives of this phase I study (clincialtrials.gov identifier NCT00496028) were to investigate the safety, tolerability, and pharmacokinetics (PK) of saracatinib, in combination with carboplatin and/or paclitaxel in patients with advanced solid tumours, and to identify suitable doses of saracatinib for future chemotherapy combination studies.
Methods
Patients
Patients aged ⩾18 years with locally advanced or metastatic solid tumours suitable for treatment with either carboplatin or paclitaxel were eligible. Patients had to have a World Health Organization performance status of 0–2 and life expectancy of >12 weeks. Patients were excluded if they had haemoglobin ⩽9 g dl−1; inadequate haematopoietic function (absolute neutrophil count ⩽1.5 × 109 per liter, platelet count ⩽100 × 109 per liter); inadequate liver function (serum bilirubin ⩾2 times the upper limit of reference range (ULRR); aspartate (AST) or alanine aminotransferase (ALT) ⩾2.5 times ULRR (⩾5 times the ULRR in the presence of liver metastases)); measured glomerular filtration rate (GFR) ⩽50 ml min−1; last dose of systemic chemotherapy or radiotherapy within 4 weeks before first dose of study treatment; concomitant medication with potent inhibitors or inducers of CYP3A4; severe or uncontrolled systemic conditions (e.g., interstitial lung disease); significant immunodeficiency; risk of transmitting HIV, or hepatitis B or C virus; brain metastases or spinal cord compression unless treated ⩾4 weeks before study entry, and stable without steroid treatment for 1 week; surgery ⩽4 weeks before study entry (except placement of vascular access); concurrent anti-cancer therapy (except stable endocrine therapy); or if they were pregnant, breast feeding or not willing to practice barrier contraception.
Study design and treatment
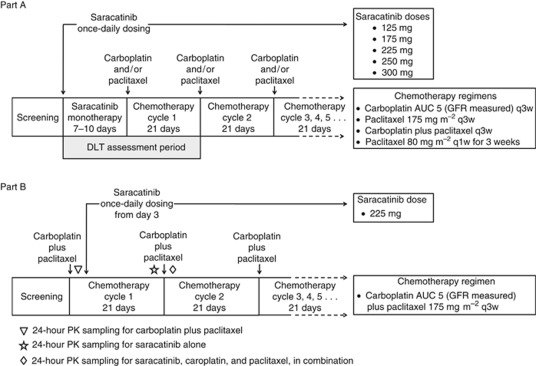
This was a two-part, phase I, multi-centre, open-label study (Figure 1). The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice. The protocol and consent forms were approved by appropriate independent review boards, and all patients gave written informed consent.
Figure 1.
Study design.
Part A was a dose-escalation phase to establish the maximum-tolerated doses of saracatinib in combination with carboplatin and/or paclitaxel chemotherapy. Four parallel escalation studies were initiated with saracatinib once-daily, in combination with: carboplatin every 3 weeks (q3w), carboplatin plus paclitaxel q3w, paclitaxel alone q3w, and paclitaxel alone q1w. Chemotherapy was initiated after a 7 to 10-day run-in period of once-daily saracatinib monotherapy to ensure that saracatinib had reached steady-state concentrations. Starting at 125 mg, saracatinib doses were assessed sequentially in cohorts of three patients, with dose escalation proceeding to the next dose cohort if no dose-limiting toxicity (DLT) was encountered. To maximise the potential for enhancement of chemotherapy, careful exploration of doses above the phase I monotherapy maximum-tolerated dose was permitted. If one patient experienced a DLT, the cohort was expanded to six, and dose escalation proceeded if no further patients encountered a DLT. If two or more of a maximum of six evaluable patients experienced a DLT within a cohort, that dose was considered not tolerated. Intrapatient dose escalation was not permitted. Patients were considered evaluable for toxicity if they experienced a DLT during the DLT-assessment period (Figure 1) or completed this period with ⩾75% of the planned doses of saracatinib. The following events were defined as DLTs if considered by the investigator to be of clinical significance, and to be related to saracatinib monotherapy or combination therapy: Common Terminology Criteria (CTC) grade 4 haematological toxicity, except neutropenia of ⩽6 days duration; grade ⩾3 neutropenia requiring hospitalisation or with fever ⩾38.5 °C; grade ⩾3 thrombocytopenia associated with non-traumatic bleeding (not applicable to patients on therapeutic anticoagulation); grade ⩾3 non-haematological toxicity not related to tumour progression, and despite adequate supportive care (excluding alopecia); grade ⩾3 vomiting for >24 h despite suitable anti-emetics; grade 4 ALT, AST, or alkaline phosphatase elevations in patients with liver involvement; and delay of ⩾2 weeks in administration of the second chemotherapy cycle. Dose-escalation decisions were made by the safety review committee.
Part B was a dose-expansion phase to further evaluate the tolerable saracatinib dose established in Part A, in combination with carboplatin plus paclitaxel. In Part B, saracatinib was started 3 days after the cycle-1 chemotherapy infusions to allow assessment of saracatinib and carboplatin-plus-paclitaxel PK individually and in combination (Figure 1).
Saracatinib was administered once daily as oral tablets (strengths 50 and 125 mg). Carboplatin AUC 5, based on measured GFR derived using 51Cr EDTA (edetic acid), was administered as an i.v. infusion over approximately 1 h. Paclitaxel was administered iv over 3 h for doses of 175 mg m−2 (q3w), and over 1 h for doses of 80 mg m−2 (every 1 week (q1w)). After the first 21-day cycle, chemotherapy doses could be reduced successively by one dose level if toxicities were encountered. Patients continued to receive chemotherapy in combination with saracatinib until occurrence of unacceptable toxicity or tumour progression. Patients who discontinued chemotherapy in the absence of disease progression continued on single-agent saracatinib at the same dose as they had been assigned in combination.
Assessments
Adverse events (AEs) were recorded throughout the study period and for 30 days after the last dose of study treatment, using CTC for AEs version 3.0. Serum creatinine levels were measured to investigate the possibility that saracatinib increases creatinine levels without affecting measured GFR; the results of this analysis will be presented separately.
Baseline radiological tumour assessment was carried out ⩽4 weeks before the start of treatment. Tumour response was assessed according to RECIST version 1.0, before dosing of cycle 3, and approximately every 9 weeks thereafter, until withdrawal from the study. All analyses were descriptive.
Blood samples for determination of PK parameters in Part B were taken over a 24-h period, starting immediately pre-infusion and/or pre-dose on day 1 of cycles 1 and 2, and pre-dose on day 21 of cycle 1 (Figure 1), and subsequently at 2, 4, 6, 8, and 24 h for saracatinib assessment; and at 3, 3.5, 4, 5, 8, 10, and 24 h for carboplatin and paclitaxel assessment. Carboplatin in heparinised human plasma was determined as platinum equivalents using a validated inductively coupled plasma atomic emission spectroscopy method at Eurofins Medinet B.V., Breda, The Netherlands. Concentrations of paclitaxel in human plasma were determined using a validated liquid–liquid extraction followed by HPLC-MS/MS at PRA International, Assen, The Netherlands. Concentrations of saracatinib in human plasma were determined using a validated solid-phase extraction followed by HPLC-MS/MS at PRA International.
Results
Patient characteristics and disposition
A total of 116 patients were treated with saracatinib: 96 in Part A and 20 in Part B. Patients had various primary tumour types (Table 1). Thirteen patients discontinued study treatment during the saracatinib monotherapy run-in period in Part A, and did not receive chemotherapy; the main reasons being AEs (N=6) and disease progression (N=5). Saracatinib doses of 125, 175, 225, 250, and 300 mg were evaluated in Part A (Table 2), and a dose of 225 mg was evaluated in Part B. The most common reasons for discontinuation from the study were disease progression (52%), followed by AEs (37%).
Table 1. Patient characteristics.
|
Part A
|
Part B | Total | ||||
|---|---|---|---|---|---|---|
| Saracatinib with C+P q3w ( N =24) | Saracatinib with C q3w ( N =21) | Saracatinib with P q3w ( N =27) | Saracatinib with P q1w ( N =24) | Saracatinib with C+P q3w ( N =20) | All patients ( N =116) | |
| Mean age, years (range) | 59 (41–77) | 55 (26–71) | 61 (41–75) | 59 (38–71) | 59 (34–73) | 59 (26–77) |
| Sex female/male, N | 10/14 | 14/7 | 14/13 | 15/9 | 6/14 | 59/57 |
| Primary tumour, N (%) | ||||||
| Ovary | 4 (17) | 5 (24) | 2 (7) | 5 (21) | 2 (10) | 18 (16) |
| Oesophagus | 2 (8) | 0 | 3 (11) | 4 (17) | 3 (15) | 12 (10) |
| Colorectal carcinoma | 4 (17) | 4 (19) | 1 (4) | 1 (4) | 1 (5) | 11 (9) |
| Skin/soft tissue | 2 (8) | 1 (5) | 3 (11) | 0 | 5 (25) | 11 (9) |
| Lung | 2 (8) | 2 (10) | 2 (7) | 2 (8) | 0 | 8 (7) |
| Stomach | 0 | 1 (5) | 3 (11) | 3 (13) | 1 (5) | 8 (7) |
| Breast | 1 (4) | 1 (5) | 2 (7) | 1 (4) | 2 (10) | 7 (6) |
| Prostate | 2 (8) | 0 | 0 | 0 | 3 (15) | 5 (4) |
| Pancreas | 1 (4) | 0 | 1 (4) | 1 (4) | 1 (5) | 4 (3) |
| Other | 6 (25) | 7 (33) | 10 (37) | 7 (29) | 2 (10) | 32 (28) |
| WHO performance status, N (%) | ||||||
| 0 | 7 (29) | 7 (33) | 10 (37) | 10 (42) | 8 (40) | 42 (36) |
| 1 | 15 (63) | 13 (62) | 14 (52) | 12 (50) | 12 (60) | 66 (57) |
| 2 | 2 (8) | 1 (5) | 3 (11) | 2 (8) | 0 | 8 (7) |
| Number of previous chemotherapy regimens | ||||||
| Mean±s.d. | 3.2±1.9 | 5.0±3.8 | 3.2±2.6 | 2.7±2.2 | 3.6±2.9 | 3.5±2.8 |
| Median (range) | 3 (1–8) | 4 (1–15) | 2 (1–9) | 2 (1–9) | 3 (1–12) | 3 (1–15) |
| Previous treatment, N (%) | ||||||
| Surgery | 20 (83) | 18 (86) | 21 (78) | 18 (75) | 18 (90) | 95 (82) |
| Chemotherapy | 22 (92) | 21 (100) | 26 (96) | 24 (100) | 19 (95) | 112 (97) |
| Radiotherapy | 12 (50) | 4 (19) | 14 (52) | 10 (42) | 10 (50) | 50 (43) |
| Immunotherapy/hormone therapy | 6 (25) | 7 (33) | 4 (15) | 5 (21) | 7 (35) | 29 (25) |
| Other therapy | 7 (29) | 8 (38) | 3 (11) | 2 (8) | 5 (25) | 25 (22) |
Abbreviations: C=carboplatin; P=paclitaxel.
Table 2. Dose escalation and dose-limiting toxicities (first cycle of therapy).
| Saracatinib dose, mg | Saracatinib with C+P q3w ( N =24) | Saracatinib with C q3w ( N =21) | Saracatinib with P q3w ( N =27) | Saracatinib with P q1w ( N =24) |
|---|---|---|---|---|
| 125 | 5/7 | 6/7 | 3/3 | 3/3 |
| 175 | 6/11 | 8/14 • Grade 3 fatigue • Grade 3 colitis ulcerativea | 6/6 • Grade 5 neutropenic sepsis | 10/13 |
| 225 | 5/6 • Grade 3 hyponatraemia | — | 4/7 | 6/8 • Grade 3 neutropenia • Grade 4 febrile neutropenia |
| 250 | — | — | 6/8 • Grade 3 febrile neutropenia | — |
| 300 | — | — | 0/3 | — |
Abbreviations: C=carboplatin; P=paclitaxel. Number of evaluable patients/total patients per cohort; bullet points indicate dose-limiting toxicities. aExacerbation of pre-existing condition.
Dose escalation and dose-limiting toxicities
Of the 83 patients who received saracatinib in combination with chemotherapy in Part A, 68 were evaluable for DLTs (Table 2). Fifteen patients were non-evaluable for DLTs, because of discontinuation due to AEs, considered by the investigator to be unrelated to saracatinib (N=7), disease progression (N=6), or voluntary discontinuation (N=2). At the 175-mg dose, the safety review committee decided to expand two cohorts (carboplatin q3w and paclitaxel q1w) to at least six evaluable patients, to further evaluate the safety and tolerability of these dose combinations.
Overall, seven patients experienced a DLT in the first cycle (Table 2), comprising grade 3 fatigue (1 case), grade 3 hyponatraemia (1 case), grade 3 neutropenia (1 case), grade 3/4 febrile neutropenia (2 cases), grade 5 neutropenic sepsis (1 case), and grade 3 colitis (1 case—involving pre-existing disease).
Duration of treatment
The chemotherapy regimen associated with most 3-weekly cycles in combination with saracatinib was paclitaxel q1w (Supplementary Figure 1). Across chemotherapy regimens, the number of chemotherapy cycles and the mean actual saracatinib exposure declined continuously with increasing saracatinib dose (Supplementary Table 1).
Tolerability
Irrespective of causality, dose, and combination, the most common AEs were nausea and fatigue, which were reported by more than half the patients overall (Table 3). Although there were no clear saracatinib dose–response effects within each chemotherapy arm for number or severity of AEs, there were some trends overall. Asthenic AEs of CTC grade ⩾3 (comprising asthenia, fatigue, lethargy, malaise, mobility decreased, muscular weakness, and somnolence) occurred more commonly at saracatinib doses ⩾225 mg than at saracatinib doses ⩽175 mg (33 vs 17%, respectively). Adverse events of neutropenia of CTC grade ⩾3 (comprising neutropenia, febrile neutropenia, and neutropenic sepsis) occurred more commonly at saracatinib doses ⩾225 mg than at saracatinib doses ⩽175 mg (37% vs 16%, respectively). Febrile neutropenia/neutropenic sepsis was reported in a total of six patients (5%), all of whom received paclitaxel-containing chemotherapy, and five of whom received saracatinib ⩾225 mg. The proportion of patients with febrile neutropenia/neutropenic sepsis was higher in Part B (15%) than in Part A (3%).
Table 3. All-cause adverse events.
|
Part A
|
Part B | Total | ||||
|---|---|---|---|---|---|---|
| Number of patients (%) | Saracatinib with C+P q3w ( N =24) | Saracatinib with C q3w ( N =21) | Saracatinib with P q3w ( N =27) | Saracatinib with P q1w ( N =24) | Saracatinib with C+P q3w ( N =20) | All patients ( N =116) |
| AEs occurring in ⩾15% of patients overall | ||||||
| Nausea | 10 (42) | 16 (76) | 10 (37) | 15 (63) | 16 (80) | 67 (58) |
| Fatigue | 15 (63) | 11 (52) | 12 (44) | 13 (54) | 9 (45) | 60 (52) |
| Decreased appetite | 12 (50) | 7 (33) | 10 (37) | 10 (42) | 8 (40) | 47 (41) |
| Diarrhoea | 10 (42) | 6 (29) | 7 (26) | 11 (46) | 9 (45) | 43 (37) |
| Vomiting | 8 (33) | 9 (43) | 7 (26) | 8 (33) | 9 (45) | 41 (35) |
| Alopecia | 10 (42) | 2 (10) | 9 (33) | 6 (25) | 13 (65) | 40 (34) |
| Anaemia | 10 (42) | 7 (33) | 4 (15) | 7 (29) | 11 (55) | 39 (34) |
| Constipation | 8 (33) | 5 (24) | 8 (30) | 7 (29) | 8 (40) | 36 (31) |
| Pyrexia | 8 (33) | 7 (33) | 5 (19) | 4 (17) | 9 (45) | 33 (28) |
| Neutropenia | 9 (38) | 0 | 0 | 5 (21) | 12 (60) | 26 (22) |
| Rash | 6 (25) | 2 (10) | 3 (11) | 9 (38) | 5 (25) | 25 (22) |
| Dysgeusia | 6 (25) | 1 (5) | 4 (15) | 4 (17) | 6 (30) | 21 (18) |
| Abdominal pain | 2 (8) | 5 (24) | 5 (19) | 5 (21) | 2 (10) | 19 (16) |
| Arthralgia | 5 (21) | 0 | 5 (19) | 4 (17) | 5 (25) | 19 (16) |
| Cough | 4 (17) | 0 | 3 (11) | 10 (42) | 1 (5) | 18 (16) |
| Lethargy | 2 (8) | 2 (10) | 5 (19) | 5 (21) | 4 (20) | 18 (16) |
| Weight decreased | 5 (21) | 3 (14) | 3 (11) | 3 (13) | 4 (20) | 18 (16) |
| AEs of CTC grade ⩾3 occurring in ⩾5% of patients overall | ||||||
| Neutropenia | 8 (33) | 0 | 0 | 4 (17) | 10 (50) | 22 (19) |
| Fatigue | 4 (17) | 3 (14) | 3 (11) | 1 (4) | 5 (25) | 16 (14) |
| Anaemia | 3 (13) | 2 (10) | 3 (11) | 2 (8) | 2 (10) | 12 (10) |
| Hyponatraemia | 3 (13) | 5 (24) | 0 | 0 | 1 (5)a | 9 (8) |
| Leucopenia | 2 (8) | 0 | 0 | 0 | 7 (35) | 9 (8) |
| Asthenia | 1 (4) | 2 (10) | 3 (11) | 0 | 2 (10) | 8 (7) |
| Dyspnoea | 1 (4) | 2 (10) | 1 (4) | 2 (8) | 0 | 6 (5) |
| Hypokalaemia | 1 (4) | 2 (10) | 0 | 2 (8) | 1 (5) | 6 (5) |
| Thrombocytopenia | 0 | 3 (14) | 0 | 0 | 3 (15) | 6 (5) |
Abbreviations: C=carboplatin; P=paclitaxel.
In Part B, sodium ⩽135 mmol l−1 at enrolment was an exclusion criterion.
Adverse events of CTC grade ⩾3 hyponatraemia were reported in 9 of 65 patients (14%) in carboplatin-treatment groups, compared with none of 51 patients in non-carboplatin-treatment groups. The incidence of hyponatraemia did not appear to be related to the dose of saracatinib. An interim review of laboratory values showed that most patients with hyponatraemia during the study also had low sodium at baseline. A protocol amendment was introduced for Part B to exclude enrolment of patients with sodium <135 mmol l−1, and the incidence of hyponatraemia declined substantially (Table 3). Hypokalaemia of CTC grade ⩾3 occurred in six patients overall, and was commonly associated with vomiting or diarrhoea.
Forty patients (34%) experienced an AE leading to discontinuation of saracatinib. No single event dominated as a reason for discontinuation. There were 15 deaths on study; 14 attributed by the investigator to disease, and 1 attributed to an AE of neutropenic sepsis considered related to saracatinib and paclitaxel. In 2 of the 14 deaths attributed to disease, a contribution from an AE considered related to saracatinib was reported as follows: respiratory failure as part of a multi-organ failure of inflammatory aetiology, secondary to tumour dissemination; and pneumonitis for which a causal relationship with saracatinib could not be ruled out, although other factors for interstitial change were present. Other possible pneumonitis-like AEs of CTC grade ⩾3 for which the investigators could not rule out a relationship with saracatinib were pneumonia (n=1) and lower respiratory tract infection (n=2).
Response assessment
Objective responses occurred in 5 of the 44 patients (11%) who received carboplatin plus paclitaxel q3w (primary tumours were skin (N=2), ovarian (N=2), and breast (N=1)), and 5 of the 24 patients (21%) who received paclitaxel q1w (primary tumours were ovarian, oesophageal, lung, testes, and cervical). All responses were partial, and median duration of response was 129 days (range 94–336). The two longest response durations were seen in patients with ovarian cancer: 336 days (saracatinib 125 mg with paclitaxel q1w) and 245 days (saracatinib 225 mg with carboplatin plus paclitaxel q3w). No objective responses were seen in the carboplatin q3w group or the paclitaxel q3w group.
Stable disease of ⩾16 weeks was recorded in 6 of the 44 patients (14%) who received carboplatin plus paclitaxel q3w, 3 of 21 (14%) who received carboplatin q3w, 4 of 27 (15%) who received paclitaxel q3w, and 3 of 24 (13%) who received paclitaxel q1w.
Pharmacokinetics
Geometric mean steady-state PK parameters of saracatinib in Part B were similar at the end of cycle 1 (saracatinib alone) and at the beginning of cycle 2 (in combination with carboplatin plus paclitaxel; Table 4). Geometric mean AUC and Cmax for carboplatin and paclitaxel in Part B were similar between cycle 1 (chemotherapy alone) and cycle 2 (in combination with saracatinib; Table 4).
Table 4. Assessment of potential pharmacokinetic drug interactions.
| Dosing regime | N | Cmax/Cmax, ss (ng ml−1) | AUC/AUCss (ng.h ml−1) |
|---|---|---|---|
| Saracatinib alone | 9 | 590 (38.4) | 10600 (43.8) |
| Saracatinib+carboplatin+paclitaxel | 9 | 545 (42.2) | 9990 (35.5) |
| Ratio of saracatinib in combination/alone | 9 | 0.923 (0.675–1.25) | 0.940 (0.774–1.23) |
| Carboplatina without saracatinib | 4 | 33600 (20.4) | 64933 (24.8) |
| Carboplatina in combination with saracatinib | 4 | 36500 (15.5) | 73867 (16.0) |
| Ratio of carboplatina with/without saracatinib | 4 | 1.087 (0.898–1.53) | 1.14 (0.921–1.67) |
| Paclitaxelb without saracatinib | 6 | 2970 (35.4) | 9850 (22.8) |
| Paclitaxelb in combination with saracatinib | 6 | 2840 (22.2) | 10100 (20.9) |
| Ratio of paclitaxelb with/without saracatinib | 6 | 0.957 (0.682–1.82) | 1.02 (0.871–1.51) |
Abbreviations: AUCss=area under the plasma concentration–time curve during any dosing interval at steady state (for saracatinib); AUC=area under the plasma concentration–time curve (for carboplatin and paclitaxel); Cmax, ss=maximum plasma concentration at steady state (for saracatinib); Cmax=maximum plasma concentration (for carboplatin and paclitaxel); N=number subjects used to calculate parameters. Parameters are expressed as geometric mean (coefficient of variation, %); ratios are geometric mean (range).
In combination with paclitaxel.
In combination with carboplatin.
Discussion
This was the first clinical study of the Src inhibitor saracatinib in combination with carboplatin and/or paclitaxel. The trial was designed to assess the safety, tolerability, and PK of saracatinib, in combination with carboplatin and/or paclitaxel, to identify regimens suitable for further evaluation in phase II/III trials. Overall, the general tolerability profile was acceptable for this heavily pre-treated population, in which the effects of previous chemotherapy might contribute to the incidence of AEs such as myelosuppression. Nevertheless, saracatinib at doses ⩾225 mg was associated with increased incidences of CTC grade ⩾3 AEs of asthenia and neutropenia, compared with doses ⩽175 mg. These effects contributed to a general overall burden of toxicity at saracatinib doses ⩾225 mg, even though in some chemotherapy combinations, such doses were deemed tolerable according to the specific DLT and dose-escalation criteria. The increased general toxicity at higher saracatinib doses was reflected by an apparent inverse relationship between dose and duration of treatment, with the caveat that duration of therapy may also be dependent on the biology of the cancer being treated. In addition, dose-limiting toxicities such as grade 3 fatigue, although only noted in one case during the formal DLT period (first cycle), were seen in a number of cases in subsequent cycles. This highlights the challenges inherent in phase I trials of molecularly targeted agents, for which various forms of toxicity may become more apparent after the first cycle (Postel-Vinay et al, 2011).
Febrile neutropenia/neutropenic sepsis was reported in 5% of patients overall, all of whom received paclitaxel-containing chemotherapy. These events were reported in a considerably higher proportion of patients in Part B (15%) than in Part A (3%). Our Part B findings are not dissimilar from the reporting rate of febrile neutropenia in a randomised phase II study of saracatinib in combination with carboplatin and paclitaxel: 23% of patients in the saracatinib arm vs 3% in the placebo arm (Poole et al, 2010). Most reports of febrile neutropenia in the randomised study occurred in cycle 1, and the outcome was uncomplicated: events were not generally associated with infection and did not have fatal outcomes or complications of organ failure, and repeated episodes of febrile neutropenia rarely occurred in the same patient (data on file, AstraZeneca). In our study, the Part B treatment schedule was similar to that of the randomised study (Poole et al, 2010); saracatinib treatment started on the same day as, or shortly after the first chemotherapy infusion, whereas in Part A of our study, saracatinib treatment started 7–10 days before the first chemotherapy infusion. A temporally coincident occurrence of paclitaxel-induced neutropenia and the events of pyrexia attributable to saracatinib treatment (which occur in the second week of treatment) might explain the reporting rate of febrile neutropenia. According to this hypothesis, the saracatinib run-in period may temporally separate these pyrexic episodes from the paclitaxel-induced neutropenia, which would otherwise contribute to a coincidentally exaggerated incidence of febrile neutropenia. A schedule with a 1-week saracatinib run-in before paclitaxel chemotherapy is being investigated in an ongoing randomised placebo-controlled trial in patients with relapsed platinum-resistant ovarian cancer (clinicaltrials.gov identifier NCT01196741).
An increased incidence of hyponatraemia was observed in patients receiving saracatinib plus carboplatin, compared with patients receiving saracatinib plus paclitaxel only. However, the incidence of hyponatraemia declined substantially after a protocol amendment was introduced to exclude patients with sodium ⩽135 mmol l−1 at enrolment. Although platinum-based chemotherapies are known to be associated with hyponatraemia, the possibility that saracatinib may potentiate the hyponatraemic effects of carboplatin cannot be ruled out.
The opportunity was taken in Part B of the study to undertake a limited evaluation of the possibility of drug–drug interactions. On theoretical grounds, such interactions were not predicted, as the major clearance pathways of saracatinib, carboplatin and paclitaxel are different. Saracatinib is thought to be cleared mainly through metabolism by CYP3A4 (AstraZeneca data on file), whereas carboplatin is cleared predominantly via the kidney without metabolism (Elferink et al, 1987). Paclitaxel is cleared by metabolism, but the major enzyme involved is CYP2C8 (Cresteil et al, 1994). Furthermore, it has been shown previously that paclitaxel clearance is not modulated even by the presence of the very potent CYP3A4 inhibitor ketoconazole (Jamis-Dow et al, 1997). The PK of saracatinib described here are similar to the PK of saracatinib monotherapy in a previously reported study (Baselga et al, 2010). Moreover, saracatinib had no apparent effect on the PK of carboplatin or paclitaxel. Although the numbers are small, there is no evidence in this study that the exposure of carboplatin or paclitaxel in patients with solid tumours is affected by the co-administration of saracatinib. These results support the prediction and indicate that there is no PK interaction between saracatinib and carboplatin or paclitaxel.
This phase I study was not designed to assess efficacy, and in the absence of a control arm, comparisons with other trials and data sets are problematic. The relatively modest response to carboplatin/paclitaxel in this study might be attributable to the advanced state of disease in the study population, as evidenced by the large number of prior chemotherapies. A more thorough assessment of the efficacy of saracatinib in combination with carboplatin plus paclitaxel has been made in a recent randomised placebo-controlled phase II trial in patients with recurrent platinum-sensitive epithelial ovarian cancer (Poole et al, 2010). The preliminary analysis indicated no difference in objective response rate between saracatinib and placebo in combination with carboplatin plus paclitaxel. However, clinical data suggest that the impact of saracatinib on chemosensitivity may best be seen in the context of platinum resistance (Dressman et al, 2007), although these data are yet to be replicated in large numbers. With the caveat that the numbers involved are small, the most significant efficacy in the present study was indeed observed in patients with platinum-resistant ovarian cancer, including one patient with ovarian cancer treated with saracatinib and paclitaxel q1w, who had a partial response lasting 336 days. She had previously been treated with carboplatin/paclitaxel, doxorubicin HCl liposome, carboplatin/gemcitabine, and carboplatin/capecitabine, and her disease was platinum-refractory. That the longest responses were seen in patients treated with paclitaxel is consistent with in vitro evidence of potential synergy between Src pathway inhibition and taxane treatment (Halder et al, 2005; Konecny et al, 2009; Teoh et al, 2011). The combination of saracatinib with paclitaxel q1w is currently being examined in a randomised phase II trial in patients with platinum-resistant ovarian cancer (clinicaltrials.gov identifier NCT01196741).
In conclusion, our data indicated that saracatinib doses up to 175 mg once daily can be given to most patients with acceptable toxicity in combination with paclitaxel (q1w or q3w) with or without carboplatin. There was no evidence that the presence of saracatinib affected exposure to carboplatin or paclitaxel chemotherapy, or vice versa.
Acknowledgments
This work was supported by AstraZeneca, Macclesfield, UK. SBK acknowledges support to the Drug Development Unit through Experimental Cancer Medicine Centre (ECMC) and NIHR Biomedical Research Centre grants by Cancer Research UK and the Department of Health to the Institute of Cancer Research and the Royal Marsden Hospital NHS Foundation Trust. We thank Matt Lewis, PhD of Lucid Medical Writing for medical writing assistance funded by AstraZeneca.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
MS, DP, WB, and UE are employees of AstraZeneca, and MS holds stock in AstraZeneca. SK, GK, RJ, and EP-L have received remuneration for consulting and/or advisory board attendance from AstraZeneca. RJ has received research support from AstraZeneca. MN has received an honorarium for lecturing from Bristol Myers Squibb, and EB has received research funding from Roche. SA, GF, EdV, JB, SS, DT, VH, BK, RR, and SB have no potential conflicts of interest to declare.
Supplementary Material
References
- Baselga J, Cervantes A, Martinelli E, Chirivella I, Hoekman K, Hurwitz HI, Jodrell DI, Hamberg P, Casado E, Elvin P, Swaisland A, Iacona R, Tabernero J (2010) Phase I safety, pharmacokinetics, and inhibition of Src activity study of saracatinib in patients with solid tumors. Clin Cancer Res 16: 4876–4883 [DOI] [PubMed] [Google Scholar]
- Boudny V, Nakano S (2002) Src tyrosine kinase augments taxotere-induced apoptosis through enhanced expression and phosphorylation of Bcl-2. Br J Cancer 86: 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Guggisberg N, Jorda M, Gonzalez-Angulo A, Hennessy B, Mills GB, Tan CK, Slingerland JM (2009) Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin Cancer Res 15: 3396–3405 [DOI] [PubMed] [Google Scholar]
- Cresteil T, Monsarrat B, Alvinerie P, Tréluyer JM, Vieira I, Wright M (1994) Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res 54: 386–392 [PubMed] [Google Scholar]
- Dressman HK, Berchuck A, Chan G, Zhai J, Bild A, Sayer R, Cragun J, Clarke J, Whitaker RS, Li L, Gray J, Marks J, Ginsburg GS, Potti A, West M, Nevins JR, Lancaster JM (2007) An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol 25: 517–525 [DOI] [PubMed] [Google Scholar]
- Elferink F, Van der Vijgh W, Klein I, Vermorken J, Gall H, Pinedo H (1987) Pharmacokinetics of carboplatin after iv administration. Cancer Treat Rep 71: 1231–1237 [PubMed] [Google Scholar]
- Gevaert O, De Smet F, Van Gorp T, Pochet N, Engelen K, Amant F, De Moor B, Timmerman D, Vergote I (2008) Expression profiling to predict the clinical behaviour of ovarian cancer fails independent evaluation. BMC Cancer 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW, Elvin P, Boyer B, Carragher N, Plé PA, Bermingham A, Holdgate GA, Ward WH, Hennequin LF, Davies BR, Costello GF (2009) Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol 3: 248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder J, Landen CN, Lutgendorf SK, Li Y, Jennings NB, Fan D, Nelkin GM, Schmandt R, Schaller MD, Sood AK (2005) Focal adhesion kinase silencing augments docetaxel-mediated apoptosis in ovarian cancer cells. Clin Cancer Res 11: 8829–8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LY, Landen CN, Trevino JG, Halder J, Lin YG, Kamat AA, Kim TJ, Merritt WM, Coleman RL, Gershenson DM, Shakespeare WC, Wang Y, Sundaramoorth R, Metcalf CA, Dalgarno DC, Sawyer TK, Gallick GE, Sood AK (2006) Antiangiogenic and antitumor effects of Src inhibition in ovarian carcinoma. Cancer Res 66: 8633–8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne VS, Huang WC, Neal CL, Tseng LM, Hung MC, Yu D (2009) ErbB2-mediated Src and signal transducer and activator of transcription 3 activation leads to transcriptional up-regulation of p21Cip1 and chemoresistance in breast cancer cells. Mol Cancer Res 7: 592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamis-Dow CA, Pearl ML, Watkins PB, Blake DS, Klecker RW, Collins JM (1997) Predicting drug interactions in vivo from experiments in vitro: human studies with paclitaxel and ketoconazole. Am J Clin Oncol 20: 592–599 [DOI] [PubMed] [Google Scholar]
- Konecny G, Glas R, Dering J, Manivong K, Qi J, Finn R, Yang G, Hong K, Ginther C, Winterhoff B (2009) Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer 101: 1699–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Tan M, Huang WC, Li P, Guo H, Tseng LM, Su X, Yang WT, Treekitkarnmongkol W, Andreeff M (2009) Mitotic deregulation by survivin in ErbB2-overexpressing breast cancer cells contributes to Taxol resistance. Clin Cancer Res 15: 1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto N, Nakano S, Fujishima H, Kohno K, Niho Y (1999) v-src induces cisplatin resistance by increasing the repair of cisplatin--DNA interstrand cross-links in human gallbladder adenocarcinoma cells. Int J Cancer 80: 731–737 [DOI] [PubMed] [Google Scholar]
- Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC (2003) Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun 309: 377–383 [DOI] [PubMed] [Google Scholar]
- Peterson-Roth E, Brdlik CM, Glazer PM (2009) Src-induced cisplatin resistance mediated by cell-to-cell communication. Cancer Res 69: 3619–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C, Lisyanskaya A, Rodenhuis S, Kristensen G, Pujade-Lauraine E, Cantarini M, Emeribe U, Stuart M, Ray-Coquard I (2010) A randomized phase II clinical trial of the Src inhibitor saracatinib (AZD0530) and carboplatin+paclitaxel (C+P) versus C+P in patients (pts) with advanced platinum-sensitive epithelial ovarian cancer (EOC). Ann Oncol 21(Suppl 8): viii304 (abstract 972O) [Google Scholar]
- Postel-Vinay S, Gomez-Roca C, Molife LR, Anghan B, Levy A, Judson I, De Bono J, Soria JC, Kaye S, Paoletti X (2011) Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities? J Clin Oncol 29: 1728–1735 [DOI] [PubMed] [Google Scholar]
- Talamonti MS, Roh MS, Curley SA, Gallick G (1993) Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest 91: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh D, Ayeni TA, Rubatt JM, Adams DJ, Grace L, Starr MD, Barry WT, Berchuck A, Murphy SK, Secord AA (2011) Dasatinib (BMS-35482) has synergistic activity with paclitaxel and carboplatin in ovarian cancer cells. Gynecol Oncol 121: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaeybroeck S, Kelly DM, Kyula J, Stokesberry S, Fennell DA, Johnston PG, Longley DB (2008) Src and ADAM-17-mediated shedding of transforming growth factor-α is a mechanism of acute resistance to TRAIL. Cancer Res 68: 8312–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DL, Iida M, Dunn EF (2009) The role of Src in solid tumors. Oncologist 14: 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, Bast RC (2003) Activated Src protein tyrosine kinase is overexpressed in late-stage human ovarian cancers 1. Gynecol Oncol 88: 73–79 [DOI] [PubMed] [Google Scholar]
- Zou D, Yoon HS, Anjomshoaa A, Perez D, Fukuzawa R, Guilford P, Humar B (2009) Increased levels of active c-Src distinguish invasive from in situ lobular lesions. Breast Cancer Res 11: R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.