Abstract
Background:
An inverse association between alcoholic beverage intake and risk of renal cell cancer has been suggested in recent studies.
Methods:
We examined the association between alcoholic beverages and renal cell cancer risk in a meta-analysis. We identified relevant studies by searching the database of PubMed, EMBASE, and MEDLINE published through August 2011. We combined the study-specific relative risks (RRs) using a random-effects model.
Results:
A total of 20 case–control studies, 3 cohort studies, and 1 pooled analysis of cohort studies were included in the meta-analysis. We observed that alcoholic beverage intake was associated with a lower risk of renal cell cancer in combined analysis of case–control and cohort studies; for total alcoholic beverage intake, combined RRs (95% confidence intervals) comparing top with bottom categories were 0.76 (0.68–0.85) in case–control studies, and 0.71 (0.63–0.78) in cohort studies (P for difference by study design=0.02). The inverse associations were observed for both men and women and for each specific type alcoholic beverage (beer, wine, and liquor). Also, we found that one drink per day of alcoholic beverage conferred the reduction in renal cell cancer risk, but further drinking above that level did not add benefit.
Conclusion:
The findings from our meta-analysis support the hypothesis that alcoholic beverage intake is inversely associated with a lower risk of renal cell cancer, with moderate consumption conferring the protection and higher consumption conferring no additional benefits.
Keywords: alcohol, renal cell cancer, meta-analysis
Incidence of renal cell cancer, the major type of kidney cancer, has increased worldwide (Mathew et al, 2002; Chow et al, 2010). Although smoking, obesity, and hypertension are known to be well-established risk factors for renal cell cancer (Chow et al, 2010), dietary modifiable risk factors for renal cell cancer have not been well defined. Alcoholic beverage has been widely consumed and its global consumption has been increasing in the last decades (World Health Organization, 2002). Merits and demerits of alcoholic beverage intake have been long explored because of its benefit for cardiovascular disease (Corrao et al, 2000; Fillmore et al, 2007; Ronksley et al, 2011) or carcinogenic effect (Kan et al, 2011). An International Review panel, sponsored by the World Cancer Research Fund (WCRF) and American Institute for Cancer Research, reported that alcoholic beverage intake increased the risk of cancers of the oral cavity, larynx, pharynx, oesophagus, liver, female breast, and colorectum (World Cancer Research Fund & American Institute for Cancer Research, 2007), but there was a limited evidence about the association between alcoholic beverage intake and kidney cancer.
As the WCRF reported the summary, further studies have examined the association between alcoholic beverage intake and kidney cancer (Greving et al, 2007; Hsu et al, 2007; Lee et al, 2007; Ozasa, 2007; Setiawan et al, 2007; Hu et al, 2008; Pelucchi et al, 2008; Kim et al, 2010; Lew et al, 2011; Allen et al, 2011). A few prospective studies and a pooled analysis of 12 prospective studies found an inverse association for alcoholic beverage intake. However, the associations for men and women were not consistent across studies, partly because of small sample size. Also, there was little evidence whether different types of specific alcoholic beverage including beer, wine, and liquor, confer similar effects.
To elucidate the role of alcoholic beverage intake in renal cell cancer, we investigated the association of total alcoholic beverage and specific alcoholic beverage intake in relation to risk of renal cell cancer in a comprehensive meta-analysis of cohort and case–control studies.
Materials and methods
Search strategy
We identified studies examining the association between alcoholic beverage intake and renal cell cancer by searching the database of PubMed, EMBASE, and MEDLINE published through August 2011. We used the following terms for the PubMed search: ((‘alcohol’ or ‘wine’ or ‘beer’ or ‘liquor’ or ‘ethanol’ or ‘spirit’) and (‘renal cell carcinoma’ or ‘kidney cancer’ or ‘renal cell cancer’ or ‘renal adenocarcinoma’ or ‘kidney adenocarcinoma’)). For the EMBASE and MEDLINE, we used the terms of (alcohol OR wine OR beer OR liquor OR ethanol) AND (carcinoma OR kidney cancer OR cancer OR kidney adenocarcinoma). We included human studies published in English language articles. In addition, we examined the references from the retrieved articles. We confirmed this meta-analysis according to the Meta-analysis of Observational Studies in Epidemiology guidelines (Stroup et al, 2000).
Selection criteria
Two authors (DY Song and S Song) independently assessed the eligibility criteria as follows; (1) case–control or cohort design, published as full-text manuscripts; (2) the exposure of interest was total alcoholic beverage or specific alcoholic beverage intake; (3) the endpoint of interest was renal cell, kidney cancer, renal, or kidney adenocarcinoma; and (4) relative risk (RR) estimates with 95% confidence intervals (CIs) for every category of alcoholic beverage intake or per unit increase in alcoholic beverage intake were reported. For one study (Wynder et al, 1974) that had not examined RRs and 95% CI, we calculated RRs and 95% CI based on the number of cases and controls. When there were multiple publications from the same study population, we only included the study that combined independent studies, examined alcoholic beverage intake as the main interest of exposure, or the study with the largest number of cases or more follow-up years. We did not include two studies (Ozasa, 2007; Kim et al, 2010) in which kidney cancer mortality was endpoint because mortality reflects both incidence and survival.
Data extraction
We extracted from each article the following information: the first author’s last name, publication year, country in which the study was performed, study design, study period, participants’ age and sex, endpoint, exposure assessment, when available, and the number of cases and controls or person–years for each category of alcoholic beverage intake and covariates for adjustment in the analysis. When several estimates were reported, we used the estimates adjusted for the most number of covariates. The authors were contacted for additional information, when necessary. If studies reported the estimate of only specific type of alcoholic beverage (McLaughlin et al, 1984; Asal et al, 1988), we still included those estimates into our meta-analysis of total alcoholic beverage. For one study that reported different types of beer and wine (Greving et al, 2007), we used the estimate of strong beer and strong wine when we analysed the specific type of alcoholic beverages. The quality of each study was assessed by two independent authors (DY Song and S Song) using the Newcastle–Ottawa Scale (Wells et al, 2011) and then averaged. Discrepancies in >1 score between two authors were resolved by consensus.
Statistical analysis
We summarised the estimates across the studies using a random-effects model (DerSimonian and Laird, 1986). We compute the combined RRs and 95% CIs from the estimates reported in each study. We also examined the non-linearity of the relationship using restricted cubic splines (Durrleman and Simon, 1989; Greenland and Longnecker, 1992; Orsini et al, 2012) for studies that provided the number of participants or person–years and two or greater categories of alcoholic beverage intake or ethanol intake. As a result, we included 10 case–control (Wynder et al, 1974; McLaughlin et al, 1984; Asal et al, 1988; Yuan et al, 1998; Mattioli et al, 2002; Parker et al, 2002; Greving et al, 2007; Hsu et al, 2007; Hu et al, 2008; Pelucchi et al, 2008), 3 cohort (Setiawan et al, 2007; Allen et al, 2011; Lew et al, 2011), and 1 pooled analysis studies (Lee et al, 2007) in the spline analysis. To test for non-linearity, we compared the model fit including only the linear term with the model fit including the linear and cubic spline terms using the likelihood ratio test. We used the midpoint between the upper and lower levels in the categories. If the upper level for the highest category was open-ended, we assumed that the level had the same amplitude as the neighbourhood categories.
For the meta-analysis of specific beverages, we rescaled alcoholic beverage into gram (g) of ethanol per day using the conversion factors: 1 drink=15 g, 11.3 g of ethanol for a 4-oz (118 ml) glass of wine, 12.8 g for 12-oz (354 ml) one glass, bottle, or can for beer, and 14.0 g for one measure (45 ml) for liquor. We converted servings per day of alcoholic beverage to 15 g per day of ethanol.
A meta-regression analysis was used to investigate whether the association between alcoholic beverage and risk of renal cell cancer differed by study design (case–control and cohort studies), sex, smoking adjustment (yes, no), or hypertension adjustment (yes, no). Heterogeneity among studies was evaluated by using Q and I2 (Higgins and Thompson, 2002) statistics. The Egger’s regression asymmetry test was used to assess the publication bias (Egger et al, 1997). All analyses were conducted using Stata, version 10.1 (Stata Corp., College Station, TX, USA) and SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). P<0.05 (two-sided) was considered statistically significant.
Results
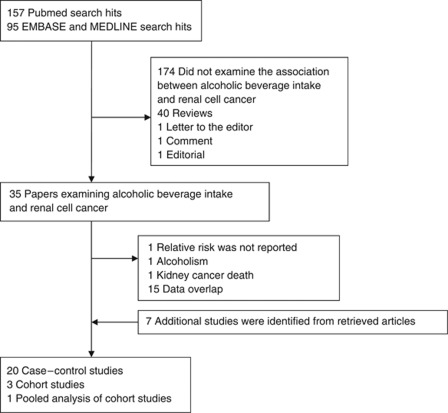
A total of 252 articles were extracted by querying PubMed, EMBASE, and MEDLINE through August 2011 (Figure 1). In all, 174 articles did not examine the association between alcoholic beverage intake and renal cell cancer, 40 were reviews, and 3 were letter, comment, or editorial. Out of 35 articles that examined the association between alcoholic beverage intake and renal cell cancer, 18 were excluded because of data overlap (n=15), the absence of RRs (n=1), assessment of alcoholism as exposure (n=1), and kidney cancer death (n=1). Seven additional studies were identified from the references of the retrieved articles. As a result, 20 case–control studies, 3 cohort studies, and 1 pooled analysis of cohort studies were included in this meta-analysis.
Figure 1.
Flowchart of publication selection for the meta-analysis of the association between alcoholic beverage intake and renal cell cancer.
Study characteristics such as country, study design, dates, age, alcohol assessment, unit of alcohol, amount of intake in the beverage for top and bottom categories, outcome/endpoint, number of cases or controls or cohort size, and potential confounders controlled are presented (Tables 1 and 2). A total of 13 819 incident renal cell cancer cases and 1537 incident kidney cancer cases were included in this meta-analysis. Three of twenty-four studies examined incidence of kidney cancer (Wynder et al, 1974; Hsu et al, 2007; Benedetti et al, 2009) and the others examined incidence of renal cell cancer (McLaughlin et al, 1984; Goodman et al, 1986; Asal et al, 1988; Brownson, 1988; Maclure and Willett, 1990; Benhamou et al, 1993; Kreiger et al, 1993; Hiatt et al, 1994; Muscat et al, 1995; Wolk et al, 1996; Boeing et al, 1997; Yuan et al, 1998; Mattioli et al, 2002; Parker et al, 2002; Greving et al, 2007; Lee et al, 2007; Setiawan et al, 2007; Hu et al, 2008; Pelucchi et al, 2008; Allen et al, 2011; Lew et al, 2011). All studies were conducted in the North America and Europe. The alcoholic beverage intake of participations in each study was assessed by using food frequency questionnaire (FFQ), interview, or self-administered questionnaire. The estimates for both men and women were reported in 17 studies. Specific alcoholic beverages were examined in 15 studies. A pooled analysis combined the original data from 12 prospective studies (Lee et al, 2007) conducted in the USA, Finland, Canada, the Netherlands, and Sweden and a multi-centre case–control study (Wolk et al, 1996) combined 4 case–control studies conducted in the Australia, Denmark, Sweden, and USA. Out of 24 studies, all studies adjusted for age, and 14 studies further adjusted for obesity. Smoking status was adjusted for 19 studies. Hypertension status was adjusted for seven studies. Nineteen of twenty-two studies that reported the amount of alcoholic beverage or ethanol as an exposure considered never or non-drinker as the reference and the other 3 studies selected the reference group of 0 or 1 drinks per day (Allen et al, 2011), low to 1 cup per week (Maclure and Willett, 1990), or <1 drink per week (Wolk et al, 1996). The ranges in the top category across studies were from 5 to 120 g per day.
Table 1. Characteristics of case–control studies include in meta-analysis of renal cell cancer.
| First author, country (reference) | Study period | Age (years) | Alcohol assessment | Unit of alcohol | Top vs bottom | Outcome/endpoint | No. of cases | No. of controls or cohort size | Study quality a | Potential confounders |
|---|---|---|---|---|---|---|---|---|---|---|
| Wynder, USA (Wynder et al, 1974) | 1965–1973 | MF: 20–79, range | Interview | Unit per day | Total alcohol M: ⩾7 unit per day vs none-occasional F: ⩾3 unit per day vs none-occasional | Incidence of kidney adenocarcinoma | M: 129 F: 73 | M: 256 F: 138 | 6 | Age, sex, race, hospital, time of interview |
| McLaughlin, USA (McLaughlin et al, 1984) | 1974–1979 | M: 62, mean F: 66, mean | Interview | Bottles per week | Beer M: ⩾20 bottles per week vs never F: ⩾20 bottles per week vs never | Incidence of renal cell carcinoma | M: 307 F: 180 | M: 428 F: 268 | 6 | Age, cigarette smoking, BMI, phenacetin use, ethnicity, kidney infection, kidney stones, coffee, tea, beer and meat consumption, exposure to petroleum, tar, and pitch products |
| Goodman, USA (Goodman et al, 1986) | 1977–1983 | MF: 20–80, range | Interview | N/A | Total alcohol M: ever vs never F: ever vs never | Incidence of renal cell carcinoma | M: 182 F: 76 | M: 182 F: 76 | 6.5 | Age, sex, race, hospital, time of admission |
| Brownson, USA (Brownson, 1988) | 1984–1986 | N/A | N/A | N/A | Total alcohol M: ever drank vs never drank F: ever drank vs never drank | Incidence of renal cell carcinoma | M: 205 F: 121 | M: 615 F: 363 | 4.5 | Age, smoking, sex |
| Asal, USA (Asal et al, 1988) | 1981–1984 | MF: ⩾50 was >70% in controls | Interview | Wine: glasses per week Liquor: N/A | Wine M: >4 glasses per week vs never F: >3 glasses per week vs never Liquor M: use vs non-use | Incidence of renal cell carcinoma | M: 209 F: 106 | Hospital M: 208 Hospital F: 105 Population M: 195 Population F: 141 | 6 | Age, sex, race, hospital time of interview, weight |
| Maclure, USA (Maclure and Willett, 1990) | 1976–1983 | MF: ⩾30 | Interview | Cups per day | Beer, wine, and liquor MF: high intake (⩾2 cups per day) vs low intake (⩽1cup per week) | Incidence of renal adenocarcinoma | M: 135 F: 68 | M: 401 F: 204 | 4.5 | Age, sex, precinct of residence, smoking, energy |
| Benhamou, France (Benhamou et al, 1993) | 1987–1991 | Case M: 61.7, mean Case F: 61.3, mean Control M: 62.8, mean Control F: 62.5, mean | Questionnaire | g per day | Total alcohol M: high vs low F: high vs low | Incidence of renal cell carcinoma | M: 138 F: 58 | M: 235 F: 112 | 5 | Age, sex, hospital and interviewer |
| Kreiger, Canada (Kreiger et al, 1993) | 1986–1987 | MF: 25–69, range | Questionnaire | N/A | Total alcohol M: high vs low F: high vs low | Incidence of renal cell carcinoma | M: 252 F: 167 | M: 543 F: 592 | 5.5 | Age, sex, geographic region of residence, active cigarette smoking status, BMI |
| Hiatt, USA (Hiatt et al, 1994) | 1964–1989 | Case: 50.7, mean | N/A | N/A | Total alcohol M: ever vs never F: ever vs never | Incidence of renal cell carcinoma | M: 165 F: 87 | M: 166 F: 88 | 5 | Gender, year of multiphasic health check-up (MHC), age at MHC±1 year |
| Muscat, USA (Muscat et al, 1995) | 1977–1993 | Case M: 58.7, mean Case F: 59.3, mean Control M: 58.2, mean Control F: 59.4, mean | Questionnaire | Oz per day | Beer M: >7 oz per day vs never/occasionally F: ⩾1 oz per day vs never/occasionally Wine M: >4 oz per day vs never/occasionally F: >4 oz per day vs never/occasionally Liquor M: ⩾7 oz per day vs never/occasionally F: ⩾1 oz per day vs never/occasionally | Incidence of renal cell carcinoma | M: 543 F: 245 | M: 476 F: 244 | 7 | Age, education, and years of smoking, race, year of diagnosis |
| Wolk, Australia, Denmark, Sweden, and USA (Wolk et al, 1996)b | 1989–1991 | Case M: 62, mean Case F: 63, mean Control M: 62, mean Control F: 63, mean | Interviews | Total alcohol: drinks per week Beer, wine, and liquor: glasses per week | Total alcohol M: ⩾15 drinks per week vs <1 drinks per week F: ⩾10 drinks per week vs <1 drinks per week Beer M: ⩾6.3 glasses per week vs none F: ⩾1.3 glasses per week vs none Wine M: ⩾1.3 glasses per week vs none F: ⩾3.0 glasses per week vs none Liquor M: ⩾5.5 glasses per week vs none F: ⩾3.0 glasses per week vs none | Incidence of renal cell cancer | M: 698 F: 487 | M: 915 F: 611 | 6 | Age, sex, study centre, BMI, smoking, total calories |
| Boeing, Germany (Boeing et al, 1997) | 1989–1991 | N/A | Interview | N/A | Total alcohol, beer, wine, and liquor MF: highest vs none | Incidence of renal cell carcinoma | M: 185 F: 92 | M: 192 F: 94 | 7 | Age, gender, educational status, tobacco smoking |
| Yuan, USA (Yuan et al, 1998) | 1986–1994 | MF: 25–74, range | Questionnaire | Drinks per week | Total alcohol MF: ⩾43 drinks per week vs none | Incidence of renal cell carcinoma | MF: 1203 | MF: 1201 | 6 | Sex, date of birth (within 5 years), ethnicity, neighbourhood of residence, education and BMI, history of hypertension, number of cigarettes per day, current smoking status, total grams of analgesics consumed over lifetime, regular use of amphetamines |
| Parker, USA (Parker et al, 2002) | 1986–1989 | Case: 68, mean Control: 64, mean | FFQ | Total alcohol: g per week Beer: 12-ounce can per week Wine: 8-ounce glass per week Liquor: 1-ounce shot per week | Total alcohol M: >35 g per week vs none F: >35 g per week vs none Beer M: >1 vs non-drinker of beer F: >1 vs non-drinker of beer Wine M: >0.5 vs non-drinker of wine F: >0.5 vs non-drinker of wine Liquor M: >1 vs non-drinker of liquor F: >1 vs non-drinker of liquor | Incidence of renal cell carcinoma | M: 261 F: 145 | M: 1598 F: 831 | 6 | Age, pack-years of smoking, history of hypertension, history of bladder infection, family history of kidney cancer, exercise, consumption of red meat, consumption of fruit (men) Age, pack-years of smoking, history of hypertension, BMI, family history of kidney cancer, consumption of red meat, fruit and vegetables (women) |
| Mattioli, Italy (Mattioli et al, 2002) | 1987–1994 | All | Questionnaire | g per day | Total alcohol M: >48 g per day vs 0 g per day F: >12 g per day vs 0 g per day | Incidence of renal cell cancer | M: 165 F: 52 | M: 165 F: 52 | 7 | Age, gender, birthplace, residence, BMI, smoking, consumption of coffee, phenacetin and/or of diuretics, and meat |
| Hsu, Russia, Romania, Poland, and Czech Republic (Hsu et al, 2007) | 1999–2003 | MF: 20–79, range | FFQ | g per week | Total alcohol MF: ⩾137.5 g per week vs none Beer MF: ⩾49.0 g per week vs none Wine MF: ⩾23.0 g per week vs none Liquor MF: ⩾157.0 g per week vs none | Incidence of kidney cancer | M: 622 F: 443 | M: 973 F: 536 | 7 | Age, country, gender, tobacco pack–years of smoking, education, BMI, hypertension medication use, and tertiles of total vegetable, total white meat, and red meat consumption |
| Greving, Sweden (Greving et al, 2007) | 1996–1998 | MF: 20–79, range Case: 64.3, mean Control: 64.4, mean | Questionnaire | Total alcohol: g per month Beer, wine, and liquor: glasses per month | Total alcohol MF: >620 g per month vs non-users alcohol Strong beer MF: >8 glasses per month vs non-user strong beer Strong wine MF: >2 glasses per month vs non-user strong wine Liquor MF: >9 glasses per month vs non-user liquor | Incidence of renal cell cancer | MF: 855 | MF: 1204 | 6 | Age, sex, BMI, cigarette smoking, the other six beverages |
| Pelucchi, Italia (Pelucchi et al, 2008) | 1985–2004 | MF: 22–79, range | Interview | Total alcohol, and wine: drinks per day Beer, and liquor: N/A | Total alcohol MF: >8 drinks per day vs non-drinkers Beer MF: drinkers vs non-drinkers Wine MF: >8 drinks per day vs non-drinkers liquor MF: drinkers vs non-drinkers | Incidence of renal cell cancer | M: 730 F: 385 | M: 1741 F: 841 | 7 | Age, sex, study, study centre, education, smoking habits, BMI, family history of renal cancer |
| Hu, Canada (Hu et al, 2008) | 1994–1997 | MF: 20–76, range | FFQ | g per day | Total alcohol M: ⩾30 g per day vs non-drinkers F: ⩾20 g per day vs non-drinkers Beer, wine, and liquor M: >10.68 g per day vs non-drinkers F: >6 g per day vs non-drinkers | Incidence of renal cell carcinoma | M: 617 F: 521 | M: 2547 F: 2492 | 5 | Age, province, education, BMI, total consumption meat, total consumption of vegetables and fruit, pack-years of smoking |
| Benedetti, Canada (Benedetti et al, 2009) | Early 1980s | Case: 58.3 mean age | Interview | Drinks per week | Total alcohol M: ⩾7 drinks per week vs never drinkers | Incidence of kidney cancer | M: 156 | M: 507 | 6.5 | Age, smoking status, cigarette-year, respondent status, ethnicity, census tract income, years of schooling, and time since quitting |
Abbreviations: BMI=body mass index; F=women; FFQ=food frequency questionnaire; M=men; MF=men and women; N/A: not available.
Study quality was judged based on the Newcastle–Ottawa Scale (range, 1–9 stars).
Multiple studies included.
Table 2. Characteristics of cohort and pooled analysis studies include in meta-analysis of renal cell cancer.
| First author, country (reference) | Study period | Age (years) | Alcohol assessment | Unit of alcohol | Top vs bottom | Outcome/endpoint | No. of cases | No. of controls or cohort size | Study quality a | Potential confounders |
|---|---|---|---|---|---|---|---|---|---|---|
| Lee, Finland, USA, Canada, The Netherlands, and Sweden (Lee et al, 2007)b | 1980–2004 | MF: 15–107, range | FFQ | g per day | Total alcohol M: ⩾15.0 g per day vs non-drinker F: ⩾15.0 g per day vs non-drinker Beer, wine, and liquor M: ⩾5.0 g per day vs non-drinker F: ⩾5.0 g per day vs non-drinker | Incidence of renal cell cancer | M: 711 F: 719 | M: 229 575 F: 530 469 | 8 | Age, history of hypertension, BMI, pack-years of smoking, combination of parity and age at first birth, total energy intake |
| Setiawan, USA (Setiawan et al, 2007) | 1993–2002 | M: 59.3, mean F: 58.8, mean | Questionnaire | g per day | Total alcohol M: ⩾10.9 g per day vs none F: ⩾3.3 g per day vs none | Incidence of renal cell cancer | M: 220 F: 127 | M: 75 162 F: 85 964 | 8 | Age, ethnicity, smoking, hypertension, physical activity |
| Lew, USA (Lew et al, 2011) | 1995–2006 | MF: 50–71, range | FFQ | g per day | Total alcohol M: ⩾30 g per day vs 0 g per day F: ⩾30 g per day vs 0 g per day Beer, wine, and liquor M: ⩾15 g per day vs 0 g per day F: ⩾5 g per day vs 0 g per day | Incidence of renal cell cancer | M: 1348 F: 466 | M: 293 666 F: 198 721 | 8 | Age, race, BMI, marital status, education, vigorous physical activity, smoking, history of hypertension, intakes of protein and total energy excluding energy from alcohol |
| Allen, UK (Allen et al, 2011) | 1996–2007 | F: 59, mean | FFQ | Drinks per day | Total alchol F: ⩾2 drinks per day vs 0 or 1drinks per day | Incidence of renal cell carcinoma | F:588 | F:779 369 | 8 | Age, socioeconomic status, BMI, smoking, use of menopausal hormone therapy, treatment for high blood pressure |
Abbreviations: BMI=body mass index; F=women; FFQ=food frequency questionnaire; M=men; MF=men and women; N/A: not available.
Study quality was judged based on the Newcastle–Ottawa Scale (range, 1–9 stars).
Multiple studies included.
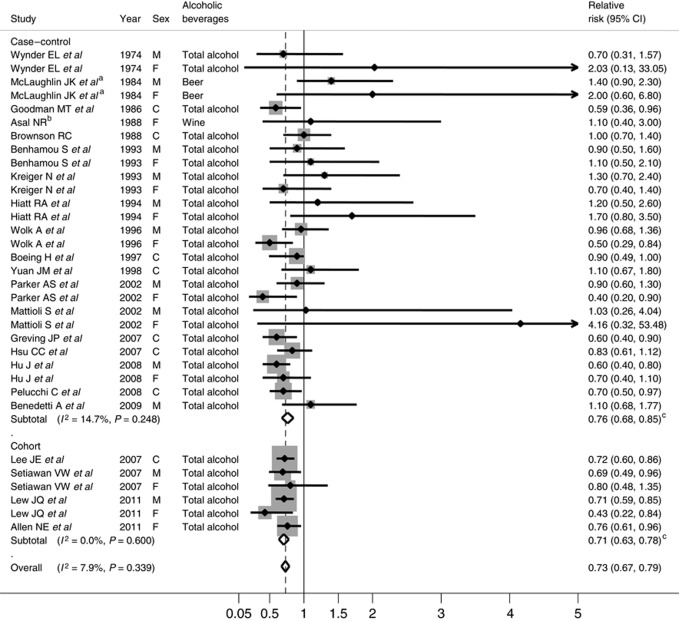
We found a decreased risk of renal cell cancer with alcoholic beverage intake; combined RR (95% CI) comparing top with bottom category was 0.73 (0.67–0.79) for total alcoholic beverage intake (Figure 2). There was no significant heterogeneity across the studies (P for heterogeneity=0.34). A stronger inverse association was observed in cohort studies compared with case–control studies (P for difference=0.02); combined RRs (95% CIs) were 0.76 (0.68–0.85) for case–control studies and 0.71 (0.63–0.78) for cohort studies. When we examined the association by study period (before or after 2000), we found a stronger inverse association for recent studies; comparing top with bottom category, RRs were 0.85 (95% CI, 0.72–0.98; P for heterogeneity=0.32) for earlier studies and 0.70 (95% CI, 0.64–0.76; P for heterogeneity=0.63) for recent studies.
Figure 2.
Combined RRs of renal cell cancer for total alcoholic beverage, comparing top with bottom category. McLaughlin et ala examined only beer consumption, Asalb examined only wine consumption. M, F, and C represented male, female, and combined gender, respectively. cP for difference by study design was 0.02.
When we examined specific alcoholic beverages (Table 3), we found that intakes of all three beverage types significantly lowered risk of renal cell cancer. Notably, these inverse associations for each type of alcoholic beverage were observed in both case–control and cohort studies, and the magnitude of the association was similar across three types of alcoholic beverages. There was no evidence of publication bias based on the Egger’s test for beer, wine, or liquor (P>0.19).
Table 3. Combined RR of (95% CI) renal cell cancer for specific alcoholic beverages, comparing top with bottom category.
| Beverages | Bottom | Top | P for |
|---|---|---|---|
| (no. of study) | RR | RR (95% CI) | heterogeneity |
| Case–control study (n=12) | |||
| Beer | 1.00 | 0.81 (0.70–0.91) | 0.26 |
| Wine | 1.00 | 0.75 (0.59–0.91) | <0.001 |
| Liquor | 1.00 | 0.76 (0.66–0.87) | 0.12 |
| Cohort study (n=2) | |||
| Beer | 1.00 | 0.75 (0.55–0.95) | 0.16 |
| Wine | 1.00 | 0.81 (0.65–0.97) | 0.17 |
| Liquor | 1.00 | 0.87 (0.77–0.97) | 0.99 |
Abbreviations: CI=confidence interval; RR=relative risk.
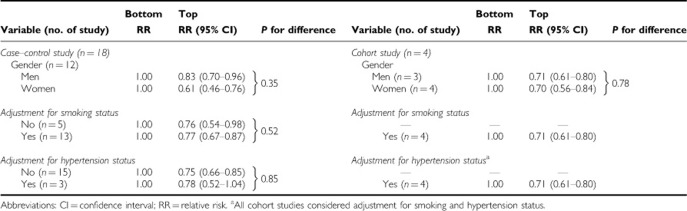
When we examined whether the associations differed by gender, adjustment for smoking status, or adjustment for hypertension status, the associations did not vary by these factors (Table 4).
Table 4. Combined RR (95% CI) of renal cell cancer for the associations by gender, study design, and smoking status.
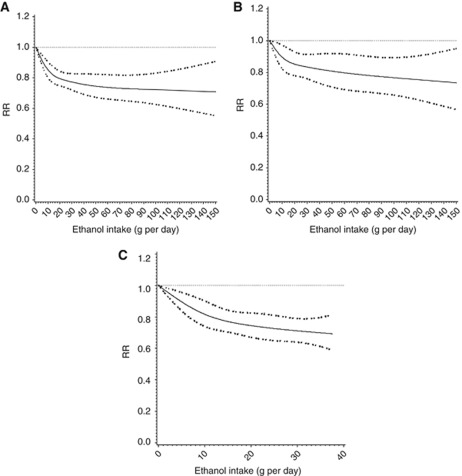
When we examined for non-linearity of the association using the regression cubic spline, we observed a significant non-linearity for overall association between ethanol intake and renal cell cancer. The degree of decline in the risk of renal cell cancer appeared to be attenuated above ∼15 g per day (P for non-linearity<0.001) (Figure 3A). We also found a significant or suggestive non-linearity for case–control and cohort studies (P-values for non-linearity=0.03 for case–control studies and 0.10 for cohort studies; Figures 3B and C).
Figure 3.
(A) Combined RR (95% CI) of renal cell cancer and test for the non-linearity of the association using the regression cubic spline. (B) Combined RR (95% CI) of renal cell cancer and test for the non-linearity of the association using the regression cubic spline in case–control studies. (C) Combined RR (95% CI) of renal cell cancer and test for the non-linearity of the association using the regression cubic spline in cohort studies.
Discussion
We observed that alcoholic beverage intake was inversely associated with risk of renal cell cancer in this comprehensive meta-analysis. The inverse association was stronger for cohort studies compared with case–control studies. The inverse associations were consistent across specific alcoholic beverages, suggesting that ethanol per se is most likely the responsible factor. We also found that alcoholic beverage intake lowered risk of renal cell cancer for both men and women. Notably, our spline analysis showed that ∼15 g per day of ethanol intake could pose a decrease in renal cell cancer risk, but additional drinking did not confer further benefit in the prevention of renal cell cancer.
The magnitude of the association was stronger for cohort studies than case–control studies. Recall bias or selection bias in case–control studies may attenuate the association between alcoholic beverage intake and renal cell cancer risk because alcoholic beverage could be overestimated among cases or underestimated among controls. A stronger inverse association for recent studies compared with earlier studies that we observed also could be partly explained by recall or selection bias because all the studies published before 2000 were case–control studies. Also, our meta-analysis of specific type of alcoholic beverage supports the evidence that the benefits associated with alcoholic beverage intake were similar for beer, wine, and liquor.
The possible mechanism by which alcoholic beverage intake reduces the risk of renal cell cancer could be beneficial changes in insulin sensitivity or vasculature. Light to moderate alcohol consumption has been associated with improved insulin sensitivity (Facchini et al, 1994; Davies et al, 2002; Joosten et al, 2008). Increased risk of renal cell cancer among diabetic (Lindblad et al, 1999; Joh et al, 2011) or obese individuals (Chow et al, 2000; Adams et al, 2008) may suggest that factors contributing to the development of insulin resistance are important determinants for renal cell cancer risk. Alcoholic beverage intake may increase high-density lipoprotein cholesterol levels or decrease blood clotting (Booyse et al, 2007) in blood vessels surrounding kidney, a vascular-rich organ.
Our meta-analysis had some limitations. Residual confounding by un-adjustment for confounding factors or inadequate measurement of covariates cannot be ruled out, although the significant inverse association was observed for those adjusted for smoking status and hypertension. Measurement error in assessment of alcoholic beverage intake could attenuate or de-attenuate the association, but the consistent significant inverse associations by different study design, specific types of alcoholic beverage, and gender may not support the possibility that measurement error fully explained our findings in this meta-analysis. The wider CIs observed at heavy alcoholic beverage intake in our spline analysis warrants further studies because of the limited number of renal cell cancer cases for heavy drinking.
In conclusion, we found that alcoholic beverage intake lowered the risk of renal cell cancer with the greatest reduction at the moderate level, but suggesting the evidence that drinking >15 g per day of ethanol does not confer additional benefit for prevention in renal cell cancer risk. Also, reduction of risk was not restricted to any specific type of alcoholic beverages. Moderate alcohol drinking may confer health benefits in the overall survival (Di Castelnuovo et al, 2006), cardiovascular disease (Rimm et al, 1996), and overall health status among elderly (Sun et al, 2011). However, drinking guidelines for men and women from various countries generally limit alcohol drinking to 1–2 standard units of drink (ICAP, 2009) because excessive drinking is associated with increased the risk of birth defects, injury, hypertension, stroke, type 2 diabetes, cancers of oral cavity and pharynx, oesophagus and larynx, stomach, colon and rectum, liver, breast, and ovary (Bagnardi et al, 2001; Reynolds et al, 2003; Baliunas et al, 2009; Taylor et al, 2009; Rehm et al, 2010). Along with the potential for health benefit and risks associated with alcohol consumption, our finding provides the evidence that light to moderate alcohol drinking is enough to reduce renal cell cancer risk without additional benefit above the level of one drink.
Acknowledgments
This work was supported by the Sookmyung Women’s University Research Grant (2010) and the Brain Korea 21 (BK 21) Project from the Ministry of Education and Human Resources Development, Republic of Korea. We thank Dr Setiawan for the data provision.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, Chow WH (2008) Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol 168: 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NE, Balkwill A, Beral V, Green J, Reeves G (2011) Fluid intake and incidence of renal cell carcinoma in UK women. Br J Cancer 104: 1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asal NR, Risser DR, Kadamani S, Geyer JR, Lee ET, Cherng N (1988) Risk factors in renal cell carcinoma: I. Methodology, demographics, tobacco, beverage use, and obesity. Cancer Detect Prev 11: 359–377 [PubMed] [Google Scholar]
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G (2001) A meta-analysis of alcohol drinking and cancer risk. Br J Cancer 85: 1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J (2009) Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 32: 2123–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A, Parent ME, Siemiatycki J (2009) Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal. Cancer Detect Prev 32: 352–362 [DOI] [PubMed] [Google Scholar]
- Benhamou S, Lenfant MH, Ory-Paoletti C, Flamant R (1993) Risk factors for renal-cell carcinoma in a French case-control study. Int J Cancer 55: 32–36 [DOI] [PubMed] [Google Scholar]
- Boeing H, Schlehofer B, Wahrendorf J (1997) Diet, obesity and risk for renal cell carcinoma: results from a case control-study in Germany. Z Ernahrungswiss 36: 3–11 [DOI] [PubMed] [Google Scholar]
- Booyse FM, Pan W, Grenett HE, Parks DA, Darley-Usmar VM, Bradley KM, Tabengwa EM (2007) Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Ann Epidemiol 17: S24–S31 [DOI] [PubMed] [Google Scholar]
- Brownson RC (1988) A case-control study of renal cell carcinoma in relation to occupation, smoking, and alcohol consumption. Arch Environ Health 43: 238–241 [DOI] [PubMed] [Google Scholar]
- Chow WH, Dong LM, Devesa SS (2010) Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WH, Gridley G, Fraumeni JF, Jarvholm B (2000) Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 343: 1305–1311 [DOI] [PubMed] [Google Scholar]
- Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K (2000) Alcohol and coronary heart disease: a meta-analysis. Addiction 95: 1505–1523 [DOI] [PubMed] [Google Scholar]
- Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR (2002) Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 287: 2559–2562 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled Clin Trials 7: 177–188 [DOI] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G (2006) Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 166: 2437–2445 [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8: 551–561 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini F, Chen YD, Reaven GM (1994) Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care 17: 115–119 [DOI] [PubMed] [Google Scholar]
- Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, Kerr W (2007) Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol 17: S16–S23 [DOI] [PubMed] [Google Scholar]
- Goodman MT, Morgenstern H, Wynder EL (1986) A case-control study of factors affecting the development of renal cell cancer. Am J Epidemiol 124: 926–941 [DOI] [PubMed] [Google Scholar]
- Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135: 1301–1309 [DOI] [PubMed] [Google Scholar]
- Greving JP, Lee JE, Wolk A, Lukkien C, Lindblad P, Bergstrom A (2007) Alcoholic beverages and risk of renal cell cancer. Br J Cancer 97: 429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt RA, Tolan K, Quesenberry CP (1994) Renal cell carcinoma and thiazide use: a historical, case-control study (California, USA). Cancer Causes Control 5: 319–325 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558 [DOI] [PubMed] [Google Scholar]
- Hsu CC, Chow WH, Boffetta P, Moore L, Zaridze D, Anush M, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Brennan P (2007) Dietary risk factors for kidney cancer in eastern and central Europe. Am J Epidemiol 166: 62–70 [DOI] [PubMed] [Google Scholar]
- Hu J, Chen Y, Mao Y, Desmeules M, Mery L (2008) Alcohol drinking and renal cell carcinoma in Canadian men and women. Cancer Detect Prev 32: 7–14 [DOI] [PubMed] [Google Scholar]
- ICAP (2009) Blue Book. International Center on Alcohol Policies. http://www.icap.org/LinkClick.aspx?fileticket=iSBBbvI8INI%3d&tabid=179 (accesed 12 March 2012). Washington, DC.
- Joh HK, Willett WC, Cho E (2011) Type 2 diabetes and the risk of renal cell cancer in women. Diabetes Care 34: 1552–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten MM, Beulens JW, Kersten S, Hendriks HF (2008) Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia 51: 1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan HP, Huang YQ, Tan YF, Zhou J (2011) Meta-analysis of alcohol consumption and risk of extrahepatic bile system cancer. Hepatol Res 41: 746–753 [DOI] [PubMed] [Google Scholar]
- Kim MK, Ko MJ, Han JT (2010) Alcohol consumption and mortality from all-cause and cancers among 1.34 million Koreans: the results from the Korea national health insurance corporation's health examinee cohort in 2000. Cancer Causes Control 21: 2295–2302 [DOI] [PubMed] [Google Scholar]
- Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA (1993) Risk factors for renal cell carcinoma: results of a population-based case-control study. Cancer Causes Control 4: 101–110 [DOI] [PubMed] [Google Scholar]
- Lee JE, Hunter DJ, Spiegelman D, Adami HO, Albanes D, Bernstein L, van den Brandt PA, Buring JE, Cho E, Folsom AR, Freudenheim JL, Giovannucci E, Graham S, Horn-Ross PL, Leitzmann MF, McCullough ML, Miller AB, Parker AS, Rodriguez C, Rohan TE, Schatzkin A, Schouten LJ, Virtanen M, Willett WC, Wolk A, Zhang SM, Smith-Warner SA (2007) Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst 99: 801–810 [DOI] [PubMed] [Google Scholar]
- Lew JQ, Chow WH, Hollenbeck AR, Schatzkin A, Park Y (2011) Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br J Cancer 104: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad P, Chow WH, Chan J, Bergstrom A, Wolk A, Gridley G, McLaughlin JK, Nyren O, Adami HO (1999) The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 42: 107–112 [DOI] [PubMed] [Google Scholar]
- Maclure M, Willett W (1990) A case-control study of diet and risk of renal adenocarcinoma. Epidemiology 1: 430–440 [DOI] [PubMed] [Google Scholar]
- Mathew A, Devesa SS, Fraumeni JF, Chow WH (2002) Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev 11: 171–178 [DOI] [PubMed] [Google Scholar]
- Mattioli S, Truffelli D, Baldasseroni A, Risi A, Marchesini B, Giacomini C, Bacchini P, Violante FS, Buiatti E (2002) Occupational risk factors for renal cell cancer: a case--control study in northern Italy. J Occup Environ Med 44: 1028–1036 [DOI] [PubMed] [Google Scholar]
- McLaughlin JK, Mandel JS, Blot WJ, Schuman LM, Mehl ES, Fraumeni JF (1984) A population-based case-control study of renal cell carcinoma. J Natl Cancer Inst 72: 275–284 [PubMed] [Google Scholar]
- Muscat JE, Hoffmann D, Wynder EL (1995) The epidemiology of renal cell carcinoma. A second look. Cancer 75: 2552–2557 [DOI] [PubMed] [Google Scholar]
- Orsini N, Ruifeng L, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and non-linear dose-response relationships: examples, an evaluation of approximations, and software. Am J Epidemiol 175: 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozasa K (2007) Alcohol use and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 8(Suppl:): 81–88 [PubMed] [Google Scholar]
- Parker AS, Cerhan JR, Lynch CF, Ershow AG, Cantor KP (2002) Gender, alcohol consumption, and renal cell carcinoma. Am J Epidemiol 155: 455–462 [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Galeone C, Montella M, Polesel J, Crispo A, Talamini R, Negri E, Ramazzotti V, Grimaldi M, Franceschi S, La Vecchia C (2008) Alcohol consumption and renal cell cancer risk in two Italian case-control studies. Ann Oncol 19: 1003–1008 [DOI] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B (2010) The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 105: 817–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J (2003) Alcohol consumption and risk of stroke: a meta-analysis. JAMA 289: 579–588 [DOI] [PubMed] [Google Scholar]
- Rimm EB, Klatsky A, Grobbee D, Stampfer MJ (1996) Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. BMJ 312: 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA (2011) Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 342: d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE (2007) Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol 166: 932–940 [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012 [DOI] [PubMed] [Google Scholar]
- Sun Q, Townsend MK, Okereke OI, Rimm EB, Hu FB, Stampfer MJ, Grodstein F (2011) Alcohol consumption at midlife and successful ageing in women: a prospective cohort analysis in the nurses' health study. PLoS Med 8: e1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B, Irving HM, Baliunas D, Roerecke M, Patra J, Mohapatra S, Rehm J (2009) Alcohol and hypertension: gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction 104: 1981–1990 [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle–Ottawa Scle (NOS) for assessing the quality of nonrandomised sutdies in meta-analysis, Dept of Epidemiology and Community Medicine, University of Ottawa: Ottawa, Canada, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 10 February 2011).
- Wolk A, Gridley G, Niwa S, Lindblad P, McCredie M, Mellemgaard A, Mandel JS, Wahrendorf J, McLaughlin JK, Adami HO (1996) International renal cell cancer study. VII. Role of diet. Int J Cancer 65: 67–73 [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund, American Institute for Cancer Research (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. American Institute for Cancer Research: Washington, DC [Google Scholar]
- World Health Organization (2002) The World Health Report 2002 - Reducing Risks, Promoting Healthy Life. World Health Organization: Geneva, Switzerland [Google Scholar]
- Wynder EL, Mabuchi K, Whitmore WF (1974) Epidemiology of adenocarcinoma of the kidney. J Natl Cancer Inst 53: 1619–1634 [PubMed] [Google Scholar]
- Yuan JM, Gago-Dominguez M, Castelao JE, Hankin JH, Ross RK, Yu MC (1998) Cruciferous vegetables in relation to renal cell carcinoma. Int J Cancer 77: 211–216 [DOI] [PubMed] [Google Scholar]