Abstract
Hematopoietic stem cell transplantation is the most powerful treatment modality for a large number of hematopoietic malignancies, including leukemia. Successful hematopoietic recovery after transplantation depends on homing of hematopoietic stem cells to the bone marrow and subsequent lodging of those cells in specific niches in the bone marrow. Migration of hematopoietic stem cells to the bone marrow is a highly regulated process that requires correct regulation of the expression and activity of various molecules including chemoattractants, selectins and integrins. This review will discuss recent studies that have extended our understanding of the molecular mechanisms underlying adhesion, migration and bone marrow homing of hematopoietic stem cells.
Keywords: adhesion, bone marrow homing, hematopoietic stem cells, migration
Introduction
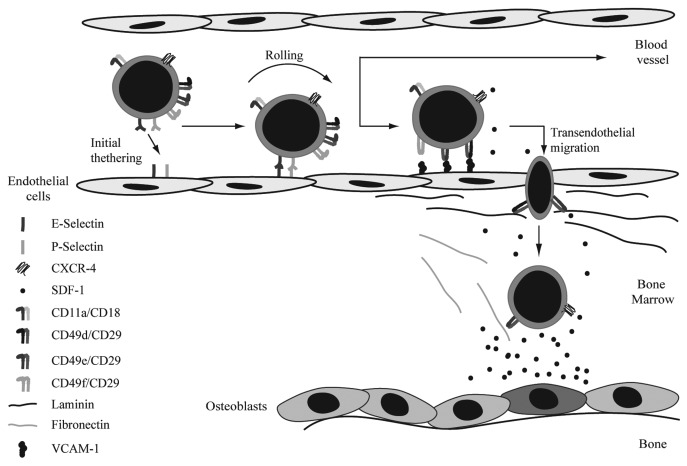
Bone marrow homing is a rapid, coordinated process in which circulating hematopoietic stem and progenitor cells actively enter the bone marrow after transplantation. Rolling and firm adhesion of those cells on endothelial cells in small marrow sinusoids is followed by trans-endothelial migration across the endothelium/extracellular matrix barrier (Fig. 1). Selectins are implicated in playing an important role in bone marrow homing of hematopoietic stem cells (HSCs) by regulating initial tethering and rolling of those cells along the endothelial wall of blood vessels. In addition, inhibition of VE-cadherin is thought to be important for transendothelial migration by disturbing the integrity of bone marrow derived endothelial cell monolayers. SDF-1 mediated integrin activation subsequently induces firm adhesion of the HSCs to the endothelial wall upon which firmly attached HSCs can transmigrate through the endothelial layer and basal lamina consisting of the integrin substrates fibronectin, collagen and laminin. Finally, HSCs anchor to their specialized niches within the bone marrow compartment near osteoblasts and initiate long-term repopulation. The importance of selectins, integrins, cadherins and chemoattractants in regulation of the different stages of bone marrow homing will be described below in more detail.
Figure 1. Homing of HSCs to the bone marrow. Initial tethering and rolling are the first steps in bone marrow homing. These processes are mediated by both E- and P-selectin. SDF-1 mediated integrin activation induces firm adhesion of the HSCs to the enodethelial wall. Firmly attached HSCs can subsequently transmigrate through the endothelial layer and basal lamina, consisting of fibronectin, collagen and laminin. Integrins involved in these steps are CD49d/CD29, CD49e/CD29 and CD49f/CD29. Finally, HSCs migrate toward the SDF-1 gradient to the osteoblasts. Chemoattractants involved in migration of HSCs.
Adhesion Molecules and Bone Marrow Homing
P- and E-selectin play an important role in rolling of hematopoietic stem cells
Selectins are implicated in playing an important role in bone marrow homing of hematopoietic stem and progenitor cells by regulating initial tethering and rolling of cells along the endothelial wall of blood vessels. It has, for example, been demonstrated that coating of a surface with immobilized P- or E-selectin is sufficient to induce rolling of human CD34+ hematopoietic progenitor cells (HPCs) under flow conditions.1 In addition, intravital microscopy in bone marrow sinoids and venules of mice deficient for individual selectins revealed that rolling of HPCs involves both P and E-selectin, but not L-selectin.2 To allow trans-endothelial migration to occur, firm adhesion of hematopoietic stem and progenitor cells to endothelial cells is required. In contrast to fluid-phase P- and E-selectin,1 adhesion of CD34+ HPCs to bone marrow derived endothelial cells under static conditions has been shown not to depend on E-selectin.3 Experiments performed to study the importance of E-selectin in trans-endothelial migration of human HPCs yielded contradictory results. Transwell experiments performed by Voermans et al., for example, suggested that E-selectin is not important for trans-endothelial migration.4 In contrast, Naiyer et al. have demonstrated in similar experiments that blocking of E-selectin with antibodies is sufficient to reduce trans-endothelial migration.3 Similarly, transplantation studies with mice deficient for both P-and E-selectin revealed that the recruitment of HPCs to the bone marrow does depend on selectins.5 Selectin ligands must be α1–3 fucosylated to form glycan determinants such as sialyl Lewis x [sLe(x)]. It has, for example, been demonstrated that inadequate α1–3 fucosylation of umbilical cord blood derived CD34+CD38-/low cells results in reduced binding to P- and E-selectin. In addition, treatment of human CD34+ cells with guanosine diphosphate GDP fucose and exogenous α1–3 fucosyltransferase VI improves rolling of those cells on P- and E-selectin and appears to be sufficient to enhance engraftment levels after transplantation in irradiated NOD/SCID mice.1
Since human CD34+ HPCs exhibit a stronger E-selectin binding capacity compared with mouse Lin-Sca-1+c-Kit+ (LSK) cells,6 it could be suggested that homing of human and mouse hematopoietic stem and progenitor cells are differentially regulated. Indeed, it has been shown that although the PSGL-1 glycoform CLA, CD43 and the CD44 glycoform HCELL, which are ligands for E-selectin, are all expressed on both mouse and human hematopoietic stem and progenitor cells,6,7 the interaction between E-selectin and CD44 only occurs in humans but not mice.6 A role for CD44 itself in regulation of bone marrow homing has also been determined. While shRNA mediated silencing of CD44 in human cells was shown to be sufficient to decrease E-selectin binding under physiologic shear conditions, enforced CD44 expression in mouse LSK cells conversely increased E-selectin adherence, resulting in improved bone marrow homing in vivo.6 In addition to a role in bone marrow homing, a role for CD44 in retention of HSCs in the bone marrow has also been suggested. Treatment of mice with blocking antibodies against CD44 has been shown to increase the number of committed progenitors in the peripheral blood.8
Integrins play an important role in HSC adhesion and transendothelial migration
Integrins are also implicated in playing an important role in regulation of bone marrow homing. In vitro studies with blocking antibodies have, for example, shown that both CD49d/CD29 (α4β1 or VLA-4) and CD11a/CD18 (αLβ2 or LFA-1), but not CD49e/CD29 (α5β1 or VLA-5), play an important role in adhesion of hematopoietic stem and progenitor cells to endothelial cells and subsequent trans-endothelial migration.4,9,10 Although inhibition of CD49e/CD29 alone was not sufficient to inhibit trans-endothelial migration, an additive effect was observed by a combination of antibodies directed against CD11a/CD18, CD49d/CD29 and CD49e/CD29.10 The importance of both CD49d/CD29 and CD49e/CD29 in directional migration through the basal lamina, which is composed of the extracellular matrix proteins laminin, collagen and fibronectin, has been examined utilizing a three dimensional extracellular matrix-like gel. These experiments showed that SDF-1-induced directional migration of CD34+ cells is dependent on both CD49d/CD29 and CD49e/CD29.10 In contrast, both adhesion of CD34+ HPCs to fibronectin10 and chemotaxis of peripheral blood CD34+ HPCs on recombinant fibronectin11 were found to be primarily dependent on CD49e/CD29. In vivo transplantation experiments were performed to study the role of CD49e/CD29 and CD49d/CD29 in bone marrow homing. Pre-treatment of HPCs with an antibody directed against CD49e/CD29, prior to transplantation, was sufficient to partially reduce homing of those cells to the bone marrow.11,12 Similarly, the migratory capacity of cells either deficient for CD49d13 or pretreated with CD49d antibodies8,11,14,15 has been shown to be impaired, resulting in delayed short-term engraftment.13 To investigate whether, in addition to CD49d/CD29, CD49d/ITGB7 (α4β7) could also be involved in bone marrow homing, either CD49d/ITGB7 (α4β7) or its substrate MadCam-1 were inhibited with blocking antibodies. These experiments resulted in a significant, but not complete, inhibition of bone marrow homing.16 Another integrin implicated in regulation of bone marrow homing is CD49f (α6). In contrast to CD49d that appears to be predominantly involved in bone marrow homing of bone marrow derived short-term repopulating HSCs, CD49f is thought to be important for homing of both short-term and long-term HSCs.15 However, similar experiments with fetal liver cells revealed that, in contrast to CD49d which appears to be important for homing of both hematopoietic stem and progenitor cells, CD49f is only important for homing of HPCs.17 In contrast, bone marrow homing was not affected in a more recent study in which mice were transplanted with mouse bone marrow derived hematopoietic stem and progenitor cells pretreated with blocking antibodies directed against CD49f.18 In addition, blocking the activity of CD49f on hematopoietic stem and progenitor cells obtained from human and primate bone marrow, but not from mobilized peripheral blood or cord blood, resulted in enhanced bone marrow homing.18 Although additional research is required to fully understand the role of CD49f, these studies suggest that CD49f regulates bone marrow homing of specific subsets of HPCs depending on the source of those cells.
Transplantation experiments were also performed to determine the role of CD11a and CD18 in migration of HSCs to the bone marrow. These experiments revealed that, in contrast to CD49d and CD49e, both CD11a8 and CD1814 are not involved in bone marrow homing. However, inhibition of CD49d/CD29 in CD18 deficient HSCs resulted in more dramatic reduction in bone marrow homing in comparison to inhibition of CD49d/CD29 in wild-type mice. This suggests that CD18 can contribute to bone marrow homing when the function of CD49d/CD29 is compromised.14 As described above, deletion of both P- and E-selectin in recipient mice significantly reduced bone marrow homing after transplantation of wild-type progenitors. Treatment of these mice with a blocking antibody against VCAM-1, thereby prohibiting interaction with CD49d/CD29, further reduced bone marrow homing after transplantation,5 suggesting that both selectins and integrins are required for optimal bone marrow homing. With blocking antibodies, a role for integrins in retention of HSCs in the bone marrow has also been investigated. Treatment of mice with blocking antibodies against either CD49d8 or CD49f15 revealed that only inhibition of CD49d is sufficient to induce mobilization of hematopoietic stem and progenitor cells to the peripheral blood, indicating that CD49d, but not CD49f, plays an important role in lodging of hematopoietic progenitors in the bone marrow.
VE-cadherin plays a role in regulation of the integrity of endothelial cell monolayers
A third group of proteins implicated in regulation of bone marrow homing are cadherins. Inhibition of VE-cadherin has, for example, been shown to enhance transendothelial migration of UCB derived CD34+ HPCs by reducing the integrity and enhancing the permeability of bone marrow derived endothelial cell monolayers. In addition, at the site of transmigration, CD34+ cells appear to induce a loss of VE-cadherin localization.19 The final step in bone marrow homing is anchoring of HSCs to their specialized niches within the bone marrow compartment. Based on studies in which high N-cadherin expression was observed at the junction between osteoblasts and HSCs,20 it has been suggested that N-cadherin could play a role in the interaction of both cell types in the bone marrow. However, the mRNA and protein expression of N-cadherin appears to be very low22-24 or absent in HSCs.21,25 To date, the role of N-cadherin in regulation of HSCs is therefore still controversial21 and requires further investigation.
Chemoattractants and Migration of HSCs
Chemoattractants play an important role in directing migration of hematopoietic stem and progenitor cells to the bone marrow. Several studies have demonstrated that Stromal Cell Derived Factor 1 (SDF-1), also known as CXC chemokine ligand 12 (CXCL12)26 acts as a chemoattractant for hematopoietic stem and progenitor cells and is important for their trans-endothelial migration.3,27,28 Analysis of a large panel of CC and CXC chemokines indicated that, within that group, the only chemokine capable of inducing migration of murine hematopoietic stem and progenitor cells is SDF-1.29,30 Similarly, examination of a panel of chemokines and cytokines in trans-endothelial migration assays revealed that SDF-1 is also important for migration of human HPCs through a confluent layer of endothelial cells.30 However, to a lesser extent, also other chemokines and cytokines, including CXCL10 (IP-10), CCL2 (MCP-1), CCL5 (RANTES), SCF and IL-8 could also induce trans-endothelial migration.30 In addition, LTD4, a ligand for the G protein-coupled receptor CysLT(1), a mediator of the cysteinyl leukotriene family that is highly expressed in HPCs, has been demonstrated to upregulate integrin-dependent adhesion of HPCs31 and to induce chemotaxis and trans-endothelial migration in vitro.32 It has recently been demonstrated that, in addition to G protein coupled receptor recognizing chemokines, the proteolysis-resistant bioactive lipids sphingosine-1-phosphate and ceramide-1-phosphate act as chemoattractants for hematopoietic stem and progenitor cells during bone marrow homing.33 The role of SDF-1 in migration of hematopoietic stem and progenitor cells will be discussed below in more detail.
SDF-1 plays a critical role in bone marrow homing
SDF-1 is considered to be essential for migration of HSCs to the bone marrow.9,29,34 In the adult human bone marrow, SDF-1 was found to be expressed by endothelial cells and along the endosteum region in the bone marrow.10,35,36 To date, six different SDF-1 splicing variants have been described: α, β, γ, δ, ε and ϕ.37 From these six isoforms, SDF-1α appears to be the most abundantly expressed isoform. Whereas the expression of SDF-1β seems to be related to the vascular system, in humans and mice, SDF-1γ is primarily expressed in the heart.38,39 While SDF-1α consists of three exons, the other isoforms consist of a different fourth C-terminal exon. The N-terminal domain (aa 1–8) of SDF-1, which is present in all SDF-1 isoforms, is responsible for receptor binding and receptor activation.40 The C-terminal domain, which is different in all SDF-1 isoforms, is important for stabilization of the interaction with the receptor. To date, two seven-transmembrane domain, G-protein coupled, receptors for SDF-1 have been identified, CXCR4 (LESTR/fusin) and CXCR7, of which CXCR4 appears to be the most prominent.41,42 Mouse transplantation studies have been performed to investigate the importance of SDF-1 in migration of HSCs to the bone marrow. Upregulation of CXCR4 expression by incubation with hematopoietic cytokines (SCF and IL-6)43 or overexpression of CXCR4 by viral transduction44,45 was shown to be sufficient to enhance bone marrow homing of human CD34+ and CD34+CD38- cells in immune deficient mice.43,46,47 In addition, pre-treatment of human CD34+CD38-/low cells with a blocking antibody directed against CXCR4 impaired their capacity to home to the bone marrow of immune deficient recipient mice.43,46-48 Similarly, fetal liver hematopoietic stem and progenitor cells deficient for CXCR4 displayed a reduced bone marrow homing capacity compared with wild-type cells.49 In addition to migration to the bone marrow, SDF-1 also appears to play a critical role in retention of HSCs in the bone marrow. Enhancing the level of SDF-1 in plasma, but not bone marrow, utilizing adenoviral vectors50 or sulfated glycans51,52 was shown to induce mobilization of CXCR4 expressing hematopoietic stem and progenitor cells to the peripheral blood.50,52 In addition, treatment of mice or healthy human volunteers with AMD3100, a selective CXCR4 antagonist, also resulted in enhanced numbers of HSCs in peripheral blood, again suggesting a role for CXCR4 and SDF-1 in HSC retention in the bone marrow.53
In contrast to CXCR4 that is expressed by hematopoietic stem and progenitor cells,54 CXCR7 is only expressed at low levels in normal human hematopoietic stem and progenitor cells and does not appear to be important for migration of those cells.55 However, CXCR7 was found to be highly expressed in several human myeloid leukemic cell lines and is thought to play a role in adhesion and, to a lesser extent, also in migration of those cells.55
Negative regulation of SDF-1 activity
Proteolytic enzymes have been implicated in negatively regulating migration of HSCs by cleaving and inactivating SDF-1.52,56,57 Matrix metalloproteinases (MMP) 2/9 mediated cleavage of SDF-1 at the Ser4-Leu5 bond, has for example, been demonstrated to result in a reduced binding capacity of SDF-1 for CXCR-4 and a diminished chemoattractant activity for hematopoietic stem and progenitor cells.58,59 A second class of SDF-1 proteases include the carboxypeptidases M and N.60,61 Carboxypeptidase M, which is expressed by both stromal cells and CD34+ HPCs,60,62 is a membrane bound zinc-dependent peptidase that cleaves C-terminal basic residues. Carboxypeptidase M mediated cleavage of SDF-1 results in reduced chemotactic activity of hematopoietic stem and progenitor cells, which can be rescued by addition of the carboxypeptidase inhibitor DL-2-mercaptomethyl-3-guanidino-ethylthiopropanoic acid.60 Carboxypeptidase N, which is present in human serum and plasma,61 specifically cleaves SDF-1 at Lysine 68 resulting in reduced SDF-1 activity and inhibition of SDF-1 mediated induction of migration of HPCs.61 Another membrane-bound protein involved in negatively regulating the activity of SDF-1 is CD26 (DPPIV).63 CD26 is expressed by a small number of umbilical cord blood derived CD34+CXCR4+ cells. SDF-1α and SDF-1β appear to be differentially processed by CD26.64 Whereas SDF-1α (1–68) undergoes processing at both the C- and N-terminal regions to produce SDF-1α (3–67), SDF-1β (1–72) is processed only at the N-terminus resulting in SDF-1β (3–72).64 This differential processing suggests that these SDF-1 isoforms may not only have overlapping, but also unique roles. Migration experiments revealed that inhibition of endogenous CD26 activity is sufficient to enhance the migration of CD34+ cells toward SDF-1, suggesting that CD26 abrogates SDF-1 induced migration of HPCs.63,65,66 In addition, inhibition of CD26 with peptides such as diprotin A (Ile-Pro-Ile) or Val-Pyr enhanced homing and engraftment of both limited numbers of mouse bone marrow HSCs in lethally irradiated congenic mice67 and human CD34+ cells in immune deficient mice.68-70 Furthermore, pretreatment of lethally irradiated congenic recipient mice with diprotin A enhanced the engraftment of non-treated mouse bone marrow cells.71
Other proteolytic enzymes implicated in negatively regulating SDF-1 activity include neutrophil elastase,72,73 cathepsin G72,73 and cathepsin K.57 The importance of proteolytic enzymes for retention of HSCs in the bone marrow has also been investigated. An accumulation of various proteolytic enzymes including MMP-9, neutrophil elastase and cathepsin G or K72,73 has, for example, been observed in mouse bone marrow upon G-CSF administration, which correlated with a gradually decrease in SDF-1 in the bone marrow.72 Similarly, an enhanced SDF-1 plasma level was shown to result in upregulation of MMP-9 in the bone marrow and mobilization of hematopoietic stem and progenitor cells.56 In contrast, a high level of SDF-1 in plasma was not sufficient to induce mobilization of HPCs in MMP-9 deficient mice.56
Molecular mechanisms underlying SDF-1 mediated regulation of migration
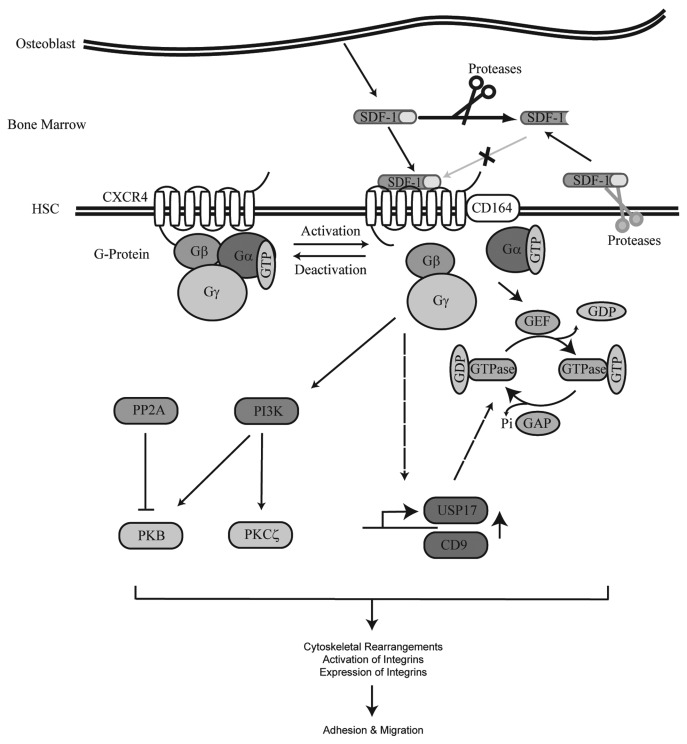
To understand the molecular mechanisms underlying migration of hematopoietic stem and progenitor cells, research has focused on identifying the downstream effectors of CXCR4 (Fig. 2). SDF-1 has, for example, been demonstrated to induce the activity of the integrins CD11a/CD1810 and CD49/CD2910,74 on CD34+ cells which allow interactions with their substrates ICAM-1 and VCAM-1, respectively.
Figure 2. SDF-1/CXCR4 signaling cascade. SDF-1 is produced by bone lining osteoblasts in the bone marrow. Upon binding of SDF-1 to the G protein coupled receptor CXCR4, CD164 is recruited to the receptor and a downstream signaling cascade is activated. SDF-1 stimulation results in the activation of multiple signal transduction molecules. Proteins activated by SDF-1 that have been demonstrated to play an important role in HSC migration include PI3K and the GTPases Rac, Rho and Cdc42. In addition, activation of CXCR4 also results in upregulation of USP17 and CD9 at the transcriptional level. USP17 is involved in the translocation of Rac, Rho and Cdc42 to G-Proteins resulting in activation of these GTPases. Activation of CXCR4 eventually leads to cytoskeletal rearrangements, activation of integrins and migration of HSCs. SDF-1 can be cleaved by both extracellular and membrane-bound proteases thereby prohibiting activation of CXCR4.
Small guanosine triphosphatases (GTPases) that belong to the Ras superfamily of GTPases, including Rho, Rac and Cdc42 can be activated by SDF-1.75-78 An important mediator of SDF-1 induced HPC migration is Rho.79-81 It has, for example, been demonstrated that SDF-1 mediated release of intracellular Ca2+ stores requires activation of Rho, but not Rac or Cdc42.82 In addition, overexpresssion of dominant negative RhoA by retroviral transduction in mouse cells resulted in decreased migration of HPCs toward SDF-1 and reduced integrin-mediated adhesion.82 Furthermore, overexpression of RhoH, a GTPase deficient type of Rho,83 in hematopoietic stem and progenitor cells resulted in impaired activation of Rac GTPases, defective actin polymerization and impaired chemotaxis. In contrast, inhibition of RhoH expression in these cells conversely stimulated SDF-1-induced migration in vitro.84
It has been demonstrated that SDF-1 induced chemo-attraction in vitro is, at least in part, also mediated by Rac.76,77,85 Deletion of Rac2 was shown to be sufficient to enhance SDF-1 induced migration of hematopoietic stem and progenitor cells.86 However, in Rac2 deficient cells, the activity of Cdc42 and Rac1 was enhanced, suggesting a compensatory role of Cdc42 and Rac1 in migration.86 In addition, analysis of Rac2 deficient mice revealed that Rac2 is essential for lodging of HPCs in the bone marrow.86 The hematopoietic-specific guanine nucleotide exchange factor Vav1 is an upstream regulator of Rac activity. Deletion of Vav1 in HSCs has been demonstrated to result in impaired responses to SDF1, deregulated Rac/Cdc42 activation, a reduction in in vitro migration and impaired localization in the bone marrow after transplantation.87 Recently, R-Ras, a negative upstream regulator of Rac activity has been identified that is inhibited by SDF-1.87 Deletion of R-Ras in hematopoietic stem and progenitor cells resulted in increased directional migration which could be reversed by inhibition of Rac.87 Furthermore, R-Ras deficient mice showed enhanced responsiveness to G-CSF for progenitor cell mobilization and exhibited decreased bone marrow homing.87 Finally, a role for Rap1, another GTPase, in regulation of SDF-1 induced migration has also been suggested. It has, for example, been demonstrated that Epac1, a nucleotide exchange protein for the GTPase Rap1, which is directly activated by cAMP, improves the adhesive and migratory capacity CD34+ HPCs.88
CD164 (Endolyn), a type I integral transmembrane silomucin,89,90 which is recruited to CXCR4 upon SDF-1 stimulation91 was shown to play an important role in SDF-1 mediated migration of human CD133+ hematopoietic stem and progenitor cells.91 Both siRNA mediated knockdown of CD164 or inhibition of CD164 with the 103B2 mAb resulted in a specific reduction in migration of CD133+ cells toward SDF-1.91 Knock-down of CD164 resulted in a significant reduction in SDF-1 mediated activation of PI3K and PKCζ.91 Both PI3K and PKCζ92 have been implicated in playing an important role in SDF-1 mediated migration of CD34+ cells. The role of PI3K in regulation of bone marrow homing will be discussed below.
SDF-1 not only regulates the activity of downstream effectors, but can also regulate the expression of specific target genes. A rapid increase in expression of the ubiquitin-specific protease 17 (USP17) has, for example, been observed in peripheral blood mononuclear, Jurkat and HeLa cells stimulated with SDF-1.93 While inhibition of USP17 in HPCs was shown to be sufficient to decrease migration of these cells toward SDF-1, overexpression of USP17 conversely resulted in enhanced migration.93 shRNA mediated inhibition of USP17 expression prohibited the transport of RAC1, Cdc42 and RhoA to the plasma membrane upon SDF-1 stimulation and resulted in decreased polymerization of actin and tubulin and reduced membrane ruffling.93 Since CXCR4 levels were not affected by inhibition or overexpression of USP17, it is likely that USP17 modulates CXCR4 signaling. Another SDF-1 responsive gene is CD9, a member of the tetraspanin superfamily.94,95 Inhibition of CD9, utilizing a neutralizing antibody, resulted in enhanced adhesion of progenitors to fibronectin and human umbilical vein endothelial cells and reduced transendothelial migration toward a SDF-1 gradient.95 Transplantation experiments revealed that pre-treatment of human CD34+ cells with a neutralizing CD9 antibody prior to transplantation results in reduced bone marrow homing.95
The PI3K/PKB Signaling Module and Bone Marrow Homing
It has been demonstrated that SDF-1 induces the activity of Phosphatidylinositol-3-Kinase (PI3K) and its downstream effector Protein Kinase B (PKB/c-Akt)96 in leukemic cell lines.97 A role for this signaling module in mediating SDF-1 induced migration of HSCs was therefore suggested. However, Protein Phosphatase 2A was shown to positively regulate SDF-1 induced migration of human HPCs by inhibition of PKB activity.98 Similarly, inhibition of PKB activity in human CD34+ cells for over 24 h resulted in a reduced capacity to adhere to bone marrow derived stromal cells and an induction of their basal migratory capacity.99 Transwell migration experiments through a confluent layer of human umbilical vein endothelial cells revealed that the observed reduction in firm adhesion does not ameliorate the induced migratory capacity of CD34+ cells pre-treated with a PKB inhibitor.99 Furthermore, ectopic expression of constitutively active PKB in CD34+ cells conversely induced firm adhesion and reduced the basal level of migration.99 Although it cannot be excluded that transient activation of PI3K/PKB by SDF-1 is important for induction of migration, these studies indicate that prolonged activation of PKB activity is detrimental for migration of hematopoietic stem and progenitor cells. In agreement with this, it has been demonstrated that deletion of SHIP (SH2-containing inositol-5′-phosphatase), a negative regulator of PI3K,100 in HSCs is sufficient to impair their ability to home to the bone marrow and spleen.101 In addition, deletion of Phosphate and tensin homolog (PTEN), another critical negative regulator of PI3K signaling102 in HSCs diminished their bone marrow homing capacity when these cells were transplanted into non-irradiated recipients where vacant niches are limited.103 Constitutive activation of PKB in human HPCs cells was, similar to deletion of SHIP, shown to be sufficient to significantly inhibit migration of these cells to the bone marrow and spleen of recipient mice.99 In addition, transient inhibition of PKB activity in human HPCs prior to transplantation was shown to conversely improve bone marrow homing.99 However, ectopic expression of constitutively active PKB in mouse HPCs only modestly impaired bone marrow homing, 18 h after transplantation.104 Together, these studies demonstrated that correct regulation of PI3K/PKB is essential for migration of hematopoietic stem and progenitor cells to the bone marrow after transplantation, which is a prerequisite for optimal engraftment and hematopoietic recovery.99,101,104
The molecular mechanisms underlying PKB mediated regulation of migration and bone marrow homing are, thus far, incompletely understood. As described above, the capacity of HSCs to migrate to the bone marrow after transplantation depends on chemokines, integrins and selectins. A reduced CXCR4 expression was observed in SHIP deficient HSCs, suggesting that activation of PI3K impairs their response to SDF-1.103 In addition, in NIH 3T3 fibroblasts, an important role for PKB and its downstream effector GSK-3 in recycling of the CD49e/CD29 and CD51/CD61 (αvβ3) integrins to the membrane has been demonstrated.105 Furthermore, ectopic expression of PKB in human hematopoietic stem and progenitor cells has been demonstrated to enhance the level of CD49d, while inhibition of PKB activity conversely reduces the expression of both CD49d and CD18.99 Although it is evident that integrins play an important role in adhesion and migration of cells, the importance of these molecules in PKB mediated inhibition of migration remains to be investigated.
Conclusion and Future Perspectives
Bone marrow homing is a coordinated multistep process. While initial tethering and rolling of hematopoietic stem and progenitor cells along the endothelial wall of blood vessels are primarily regulated by specific selectins, various integrins have been shown to be involved in regulation of the next stages in this process; firm adhesion to the endothelial wall and trans-endothelial migration. Directional migration toward the hematopoietic stem cell niche in the bone marrow requires a chemokine gradient. Although SDF-1 appears to be the most prominent chemokine involved in this process, it has recently been demonstrated that proteolysis-resistant bioactive lipids can also act as chemoattractants for HSCs. The balance between migration of HSCs to and from the bone marrow is not only regulated by the level of SDF-1 in the bone marrow, but also by a variety of proteolytic enzymes that negatively regulate the activity of SDF-1. The molecular mechanism underlying SDF-1 mediated regulation of HSC migration has been investigated extensively and revealed that multiple signaling molecules, including several GTPases and PI3K, are activated upon SDF-1 stimulation. Although PI3K and its downstream effector PKB are activated upon SDF-1 stimulation, recent studies, however, have demonstrated that this signaling module plays a critical role in negatively regulating migration of HSCs and bone marrow homing.
Correct regulation of bone marrow homing process is a prerequisite for optimal hematopoietic recovery after HSC transplantation. Research has therefore focused on improving current stem cell transplantation regimes both either circumventing or enhancing bone marrow homing. Phase I/II clinical trials have, for example, been initiated to circumvent bone marrow homing by intra bone injection of UCB cells (NCT00696046; NCT01332006; NCT00295880) in patients. Thus far, only the latter trial has been terminated. However, the results of this trial did not reveal any improvement in the rate of engraftment as compared with historical results.106 Alternatively, optimization of bone marrow homing may be achieved by improving initial tethering and rolling of HSCs to endothelial cells or enhancing chemoattractant induced migration. Preclinical studies in mouse models have revealed that both enhanced fucosylation of CD34+ cells1 and inhibition of CD26,67-71 resulting in enhanced SDF-1 activity, is sufficient to enhance bone marrow homing and to induce engraftment levels. Phase I/II clinical trials have therefore recently been initiated to investigate whether enhanced fucosylation (NCT01471067) or CD26 peptidase inhibition using Sitagliptin (NCT00862719) would be sufficient to improve stem cell transplantation regimes in patients with hematological malignancies.
Acknowledgments
M.B. was supported by ZonMW (Veni 916.76.13.).
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/18975
References
- 1.Xia L, McDaniel JM, Yago T, Doeden A, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–6. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 2.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–74. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naiyer AJ, Jo DY, Ahn J, Mohle R, Peichev M, Lam G, et al. Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood. 1999;94:4011–9. [PubMed] [Google Scholar]
- 4.Voermans C, Rood PM, Hordijk PL, Gerritsen WR, van der Schoot CE. Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells. 2000;18:435–43. doi: 10.1634/stemcells.18-6-435. [DOI] [PubMed] [Google Scholar]
- 5.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–8. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merzaban JS, Burdick MM, Gadhoum SZ, Dagia NM, Chu JT, Fuhlbrigge RC, et al. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood. 2011;118:1774–83. doi: 10.1182/blood-2010-11-320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–86. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 9.Imai K, Kobayashi M, Wang J, Ohiro Y, Hamada J, Cho Y, et al. Selective transendothelial migration of hematopoietic progenitor cells: a role in homing of progenitor cells. Blood. 1999;93:149–56. [PubMed] [Google Scholar]
- 10.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–96. [PubMed] [Google Scholar]
- 11.Carstanjen D, Gross A, Kosova N, Fichtner I, Salama A. The alpha4beta1 and alpha5beta1 integrins mediate engraftment of granulocyte-colony-stimulating factor-mobilized human hematopoietic progenitor cells. Transfusion. 2005;45:1192–200. doi: 10.1111/j.1537-2995.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 12.Wierenga PK, Weersing E, Dontje B, de Haan G, van Os R. Differential role for very late antigen-5 in mobilization and homing of hematopoietic stem cells. Bone Marrow Transplant. 2006;38:789–97. doi: 10.1038/sj.bmt.1705534. [DOI] [PubMed] [Google Scholar]
- 13.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–60. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–11. doi: 10.1182/blood.V98.8.2403. [DOI] [PubMed] [Google Scholar]
- 15.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–10. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 16.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104:2020–6. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- 17.Qian H, Georges-Labouesse E, Nystrom A, Domogatskaya A, Tryggvason K, Jacobsen SE, et al. Distinct roles of integrins alpha6 and alpha4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood. 2007;110:2399–407. doi: 10.1182/blood-2006-10-051276. [DOI] [PubMed] [Google Scholar]
- 18.Bonig H, Priestley GV, Wohlfahrt M, Kiem HP, Papayannopoulou T. Blockade of alpha6-integrin reveals diversity in homing patterns among human, baboon, and murine cells. Stem Cells Dev. 2009;18:839–44. doi: 10.1089/scd.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, et al. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–96. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Zon LI. Resolving the controversy about N-cadherin and hematopoietic stem cells. Cell Stem Cell. 2010;6:199–202. doi: 10.1016/j.stem.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Haug JS, He XC, Grindley JC, Wunderlich JP, Gaudenz K, Ross JT, et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell. 2008;2:367–79. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Hosokawa K, Arai F, Yoshihara H, Nakamura Y, Gomei Y, Iwasaki H, et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–83. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Hembree M, Yin T, et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell. 2010;6:194–8. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–17. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–3. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 27.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass TJ, Lund TC, Patrinostro X, Tolar J, Bowman TV, Zon LI, et al. Stromal cell-derived factor-1 and hematopoietic cell homing in an adult zebrafish model of hematopoietic cell transplantation. Blood. 2011;118:766–74. doi: 10.1182/blood-2011-01-328476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liesveld JL, Rosell K, Panoskaltsis N, Belanger T, Harbol A, Abboud CN. Response of human CD34+ cells to CXC, CC, and CX3C chemokines: implications for cell migration and activation. J Hematother Stem Cell Res. 2001;10:643–55. doi: 10.1089/152581601753193850. [DOI] [PubMed] [Google Scholar]
- 31.Boehmler AM, Drost A, Jaggy L, Seitz G, Wiesner T, Denzlinger C, et al. The CysLT1 ligand leukotriene D4 supports alpha4beta1- and alpha5beta1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J Immunol. 2009;182:6789–98. doi: 10.4049/jimmunol.0801525. [DOI] [PubMed] [Google Scholar]
- 32.Bautz F, Denzlinger C, Kanz L, Mohle R. Chemotaxis and transendothelial migration of CD34(+) hematopoietic progenitor cells induced by the inflammatory mediator leukotriene D4 are mediated by the 7-transmembrane receptor CysLT1. Blood. 2001;97:3433–40. doi: 10.1182/blood.V97.11.3433. [DOI] [PubMed] [Google Scholar]
- 33.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106–16. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–9. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maekawa T, Ishii T. Chemokine/receptor dynamics in the regulation of hematopoiesis. Intern Med. 2000;39:90–100. doi: 10.2169/internalmedicine.39.90. [DOI] [PubMed] [Google Scholar]
- 37.Janowski M. Functional diversity of SDF-1 splicing variants. Cell Adh Migr. 2009;3:243–9. doi: 10.4161/cam.3.3.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, et al. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–9. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/A:1012808525370. [DOI] [PubMed] [Google Scholar]
- 40.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heesen M, Berman MA, Hopken UE, Gerard NP, Dorf ME. Alternate splicing of mouse fusin/CXC chemokine receptor-4: stromal cell-derived factor-1alpha is a ligand for both CXC chemokine receptor-4 isoforms. J Immunol. 1997;158:3561–4. [PubMed] [Google Scholar]
- 42.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–7. [PubMed] [Google Scholar]
- 43.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 44.Brenner S, Whiting-Theobald N, Kawai T, Linton GF, Rudikoff AG, Choi U, et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–33. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 45.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 46.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–91. doi: 10.1182/blood.V97.10.3283. [DOI] [PubMed] [Google Scholar]
- 47.Kollet O, Petit I, Kahn J, Samira S, Dar A, Peled A, et al. Human CD34(+)CXCR4(-) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100:2778–86. doi: 10.1182/blood-2002-02-0564. [DOI] [PubMed] [Google Scholar]
- 48.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 49.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–60. doi: 10.1182/blood.V97.11.3354. [DOI] [PubMed] [Google Scholar]
- 51.Sweeney EA, Priestley GV, Nakamoto B, Collins RG, Beaudet AL, Papayannopoulou T. Mobilization of stem/progenitor cells by sulfated polysaccharides does not require selectin presence. Proc Natl Acad Sci USA. 2000;97:6544–9. doi: 10.1073/pnas.97.12.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002;99:44–51. doi: 10.1182/blood.V99.1.44. [DOI] [PubMed] [Google Scholar]
- 53.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 55.Tarnowski M, Liu R, Wysoczynski M, Ratajczak J, Kucia M, Ratajczak MZ. CXCR7: a new SDF-1-binding receptor in contrast to normal CD34(+) progenitors is functional and is expressed at higher level in human malignant hematopoietic cells. Eur J Haematol. 2010;85:472–83. doi: 10.1111/j.1600-0609.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- 56.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/S0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 58.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 59.Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–10. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- 60.Marquez-Curtis L, Jalili A, Deiteren K, Shirvaikar N, Lambeir AM, Janowska-Wieczorek A. Carboxypeptidase M expressed by human bone marrow cells cleaves the C-terminal lysine of stromal cell-derived factor-1alpha: another player in hematopoietic stem/progenitor cell mobilization? Stem Cells. 2008;26:1211–20. doi: 10.1634/stemcells.2007-0725. [DOI] [PubMed] [Google Scholar]
- 61.Davis DA, Singer KE, De La Luz Sierra M, Narazaki M, Yang F, Fales HM, et al. Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation. Blood. 2005;105:4561–8. doi: 10.1182/blood-2004-12-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skidgel RA, Erdos EG. Cellular carboxypeptidases. Immunol Rev. 1998;161:129–41. doi: 10.1111/j.1600-065X.1998.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 63.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–8. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 64.De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, et al. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103:2452–9. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 65.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–6. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 66.Christopherson KW, 2nd, Uralil SE, Porecha NK, Zabriskie RC, Kidd SM, Ramin SM. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34+CD38- human cord blood hematopoietic cells. Exp Hematol. 2006;34:1060–8. doi: 10.1016/j.exphem.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 68.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–54. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- 69.Christopherson KW, 2nd, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage- human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 2007;16:355–60. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- 70.Kawai T, Choi U, Liu PC, Whiting-Theobald NL, Linton GF, Malech HL. Diprotin A infusion into nonobese diabetic/severe combined immunodeficiency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem Cells Dev. 2007;16:361–70. doi: 10.1089/scd.2007.9997. [DOI] [PubMed] [Google Scholar]
- 71.Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 72.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 73.Le´vesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–9. doi: 10.1016/S0301-472X(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 74.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, Albella B, Blaya C, Wright N, et al. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29:345–55. doi: 10.1016/S0301-472X(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 75.Fuhler GM, Drayer AL, Olthof SG, Schuringa JJ, Coffer PJ, Vellenga E. Reduced activation of protein kinase B, Rac, and F-actin polymerization contributes to an impairment of stromal cell derived factor-1 induced migration of CD34+ cells from patients with myelodysplasia. Blood. 2008;111:359–68. doi: 10.1182/blood-2006-11-060632. [DOI] [PubMed] [Google Scholar]
- 76.del Pozo MA, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–20. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 77.Shirvaikar N, Marquez-Curtis LA, Ratajczak MZ, Janowska-Wieczorek A. Hyaluronic acid and thrombin upregulate MT1-MMP through PI3K and Rac-1 signaling and prime the homing-related responses of cord blood hematopoietic stem/progenitor cells. Stem Cells Dev. 2011;20:19–30. doi: 10.1089/scd.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–91. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 79.Ghiaur G, Lee A, Bailey J, Cancelas JA, Zheng Y, Williams DA. Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood. 2006;108:2087–94. doi: 10.1182/blood-2006-02-001560. [DOI] [PubMed] [Google Scholar]
- 80.Göttig S, Mobest D, Ruster B, Grace B, Winter S, Seifried E, et al. Role of the monomeric GTPase Rho in hematopoietic progenitor cell migration and transplantation. Eur J Immunol. 2006;36:180–9. doi: 10.1002/eji.200525607. [DOI] [PubMed] [Google Scholar]
- 81.Bug G, Rossmanith T, Henschler R, Kunz-Schughart LA, Schroder B, Kampfmann M, et al. Rho family small GTPases control migration of hematopoietic progenitor cells into multicellular spheroids of bone marrow stroma cells. J Leukoc Biol. 2002;72:837–45. [PubMed] [Google Scholar]
- 82.Henschler R, Piiper A, Bistrian R, Mobest D. SDF-1alpha-induced intracellular calcium transient involves Rho GTPase signalling and is required for migration of hematopoietic progenitor cells. Biochem Biophys Res Commun. 2003;311:1067–71. doi: 10.1016/j.bbrc.2003.10.112. [DOI] [PubMed] [Google Scholar]
- 83.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 84.Gu Y, Jasti AC, Jansen M, Siefring JE. RhoH, a hematopoietic-specific Rho GTPase, regulates proliferation, survival, migration, and engraftment of hematopoietic progenitor cells. Blood. 2005;105:1467–75. doi: 10.1182/blood-2004-04-1604. [DOI] [PubMed] [Google Scholar]
- 85.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–8. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 86.Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, et al. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001;98:5614–8. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez-Aguilera A, Lee YJ, Lo Celso C, Ferraro F, Brumme K, Mondal S, et al. Guanine nucleotide exchange factor Vav1 regulates perivascular homing and bone marrow retention of hematopoietic stem and progenitor cells. Proc Natl Acad Sci USA. 2011;108:9607–12. doi: 10.1073/pnas.1102018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111:2640–6. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 89.Chan JY, Lee-Prudhoe JE, Jorgensen B, Ihrke G, Doyonnas R, Zannettino AC, et al. Relationship between novel isoforms, functionally important domains, and subcellular distribution of CD164/endolyn. J Biol Chem. 2001;276:2139–52. doi: 10.1074/jbc.M007965200. [DOI] [PubMed] [Google Scholar]
- 90.Zannettino AC, Buhring HJ, Niutta S, Watt SM, Benton MA, Simmons PJ. The sialomucin CD164 (MGC-24v) is an adhesive glycoprotein expressed by human hematopoietic progenitors and bone marrow stromal cells that serves as a potent negative regulator of hematopoiesis. Blood. 1998;92:2613–28. [PubMed] [Google Scholar]
- 91.Forde S, Tye BJ, Newey SE, Roubelakis M, Smythe J, McGuckin CP, et al. Endolyn (CD164) modulates the CXCL12-mediated migration of umbilical cord blood CD133+ cells. Blood. 2007;109:1825–33. doi: 10.1182/blood-2006-05-023028. [DOI] [PubMed] [Google Scholar]
- 92.Petit I, Goichberg P, Spiegel A, Peled A, Brodie C, Seger R, et al. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest. 2005;115:168–76. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de la Vega M, Kelvin AA, Dunican DJ, McFarlane C, Burrows JF, Jaworski J, et al. The deubiquitinating enzyme USP17 is essential for GTPase subcellular localization and cell motility. Nat Commun. 2011;2:259. doi: 10.1038/ncomms1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boucheix C, Benoit P, Frachet P, Billard M, Worthington RE, Gagnon J, et al. Molecular cloning of the CD9 antigen. A new family of cell surface proteins. J Biol Chem. 1991;266:117–22. [PubMed] [Google Scholar]
- 95.Leung KT, Chan KY, Ng PC, Lau TK, Chiu WM, Tsang KS, et al. The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood. 2011;117:1840–50. doi: 10.1182/blood-2010-04-281329. [DOI] [PubMed] [Google Scholar]
- 96.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 97.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, et al. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 98.Basu S, Ray NT, Atkinson SJ, Broxmeyer HE. Protein phosphatase 2A plays an important role in stromal cell-derived factor-1/CXC chemokine ligand 12-mediated migration and adhesion of CD34+ cells. J Immunol. 2007;179:3075–85. doi: 10.4049/jimmunol.179.5.3075. [DOI] [PubMed] [Google Scholar]
- 99.Buitenhuis M, van der Linden E, Ulfman LH, Hofhuis FM, Bierings MB, Coffer PJ. Protein kinase B (PKB/c-akt) regulates homing of hematopoietic progenitors through modulation of their adhesive and migratory properties. Blood. 2010;116:2373–84. doi: 10.1182/blood-2009-10-250258. [DOI] [PubMed] [Google Scholar]
- 100.Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, et al. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–93. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Desponts C, Hazen AL, Paraiso KH, Kerr WG. SHIP deficiency enhances HSC proliferation and survival but compromises homing and repopulation. Blood. 2006;107:4338–45. doi: 10.1182/blood-2005-12-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–22. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 104.Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–15. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol Cell Biol. 2004;24:1505–15. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, McKenna D, Chong SY, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 2009;43:935–40. doi: 10.1038/bmt.2008.417. [DOI] [PMC free article] [PubMed] [Google Scholar]