This study rigorously defines lamin B1 loss as a marker of senescence in response to all classic signals of senescence, including DNA damage, oncogene activation, and replicative exhaustion. This decline is induced by activation of either the p53 or the pRb pathway and occurs in vivo in response to a senescence-inducing dose of radiation.
Abstract
Cellular senescence is a potent tumor-suppressive mechanism that arrests cell proliferation and has been linked to aging. However, studies of senescence have been impeded by the lack of simple, exclusive biomarkers of the senescent state. Senescent cells develop characteristic morphological changes, which include enlarged and often irregular nuclei and chromatin reorganization. Because alterations to the nuclear lamina can affect both nuclear morphology and gene expression, we examined the nuclear lamina of senescent cells. We show here than lamin B1 is lost from primary human and murine cell strains when they are induced to senesce by DNA damage, replicative exhaustion, or oncogene expression. Lamin B1 loss did not depend on the p38 mitogen-activated protein kinase, nuclear factor-κB, ataxia telangiectasia–mutated kinase, or reactive oxygen species signaling pathways, which are positive regulators of senescent phenotypes. However, activation of either the p53 or pRB tumor suppressor pathway was sufficient to induce lamin B1 loss. Lamin B1 declined at the mRNA level via a decrease in mRNA stability rather than by the caspase-mediated degradation seen during apoptosis. Last, lamin B1 protein and mRNA declined in mouse tissue after senescence was induced by irradiation. Our findings suggest that lamin B1 loss can serve as biomarker of senescence both in culture and in vivo.
INTRODUCTION
Cellular senescence is a potent tumor-suppressive mechanism that prevents the proliferation, essentially irreversibly, of cells that are at risk for malignant transformation. Many potentially oncogenic stimuli induce a senescence response. These stimuli include severe DNA damage, supraphysiological mitogenic signals, and acutely disrupted chromatin. They induce a senescence response by engaging either or both of two vital tumor suppressor pathways. These pathways, governed by the p53 and pRB tumor suppressor proteins, are crucial for implementing the senescence growth arrest (Campisi and d'Adda di Fagagna, 2007).
In addition to arresting growth, senescent cells adopt a complex phenotype. Salient features of this phenotype include development of a senescence-associated β-galactosidase (SA-βgal) activity (Dimri et al., 1995), which is often used to detect senescent cells in culture and in vivo. Senescent cells also show widespread changes in gene expression, leading in part to a senescence-associated secretory phenotype (SASP)—the robust secretion of numerous cytokines, growth factors, and proteases (Coppe et al., 2008). The SASP, which is proinflammatory (Freund et al., 2010), may explain a second process with which senescent cells have been linked: aging.
Senescent cells have been shown to accumulate with age in many mammalian tissues. They have also been shown to accumulate at sites of age-related pathology, both degenerative and preneoplastic (Parrinello et al., 2005; Campisi and d'Adda di Fagagna, 2007; Jeyapalan and Sedivy, 2008; Collado and Serrano, 2010). These studies have been aided by biomarkers such as SA-βgal activity, expression of the tumor suppressor protein p16, and characteristic DNA damage foci (Dimri et al., 1995; Krishnamurthy et al., 2004; Jeyapalan et al., 2007). However, because there are no senescence-specific biomarkers currently known, there is a need to identify additional robust markers that can be used to identify senescent cells both in culture and in vivo.
Senescent cells undergo striking morphological changes. Among these are an increase in average cell and nuclear size, an irregular nuclear envelope, and changes in chromosome condensation and distribution, with some chromosomes forming heterochromatic foci and larger chromosomes migrating toward the nuclear periphery (Narita et al., 2003; Mehta et al., 2007; Zhang et al., 2007). This nuclear rearrangement is correlated with gene expression changes, and recent work highlighted the important role of the nuclear envelope in regulating gene expression, particularly gene repression (Reddy et al., 2008).
The inner surface of the nuclear envelope is lined by a lamina, which contributes to the size, shape, and stability of the nucleus (Bridger et al., 2007; Dechat et al., 2008). The major structural proteins of the lamina are the nuclear lamins, which are type V intermediate filaments ranging in size from 60 to 80 kDa (Krohne and Benavente, 1986). The lamins are categorized as A type (lamin A, C) or B type (lamin B1, B2) based on their isoelectric points (Krohne and Benavente, 1986). The lamina is a dynamic structure because each time a cell enters mitosis, it is disassembled and then reassembled in the next cell cycle (Gerace and Blobel, 1980; Goldman et al., 2002).
Lamins A and C, which are alternatively spliced isoforms of the LMNA gene (Lin and Worman, 1993), are expressed primarily as cells commit to differentiation. Lamin A depletion does not perturb HeLa cell growth (Harborth et al., 2001), and mice that express lamin C but not lamin A appear normal (Fong et al., 2006). However, lamin A mutations that lead to incorrectly processed or misfolded lamin A are associated with a spectrum of diseases collectively termed “laminopathies.” Laminopathy pathologies include muscular dystrophy, cardiomyopathy, lipodystrophy, and an early-onset progeria (Hutchinson–Gilford progeria syndrome [HGPS]; Worman et al., 2010). HGPS is a caused by a misprocessed form of lamin A (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003). Fibroblasts from HGPS patients undergo more rapid senescence in culture than normal fibroblasts (Benson et al., 2010). Of interest, low-level expression of progerin in wild-type cells has been linked to normal aging (Scaffidi and Misteli, 2006).
The B-type lamins, on the other hand, arise from two different genes—LMNB1 and LMNB2. One or the other type B lamin may be sufficient for individual cell survival (Broers et al., 1997; Goldman et al., 2002), although recent reports suggest that embryonic stem cells, at least, can self-renew and retain their pluripotency without lamins (Kim et al., 2011). However, lamin B1 is required for proper organogenesis (Kim et al., 2011) and organismal survival: mice that lack a functional LMNB1 gene die minutes after birth, and fibroblasts from these mice have misshapen nuclei and undergo premature senescence in culture (Vergnes et al., 2004). Of interest, although the B and A lamins form separate networks, they interact and influence each other's expression and organization; in this context lamin B1 is particularly important (Shimi et al., 2008).
Here we demonstrate that lamin B1 loss occurs when human and mouse cells undergo senescence. Lamin B1 expression declined in human fibroblasts induced to senesce by diverse stimuli and in several fibroblast strains but did not decline in quiescent cells. This decrease was independent of the DNA damage response (DDR), p38 mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) activation, and reactive oxygen species (ROS), which are hallmarks of many senescent cells, but did occur in response to direct stimulation of either the p53 or pRB pathway. In addition, although lamins are cleaved by caspases during apoptosis, lamin B1 cleavage products were not evident in senescent cells, nor did caspase inhibition affect lamin B1 loss at senescence. Instead, lamin B1 mRNA levels declined at senescence via a decrease in lamin B1 mRNA stability. Finally, we detected a small but significant decrease in lamin B1 protein and mRNA in mouse liver after a senescence-inducing dose of ionizing radiation, consistent with the persistence of senescent cells in vivo after radiation (Le et al., 2010). Thus lamin B1 loss is a robust hallmark of senescence in culture and in vivo and serves as an easily detectable biomarker of the senescent state.
RESULTS
Lamin B1 loss is associated with multiple types of cellular senescence
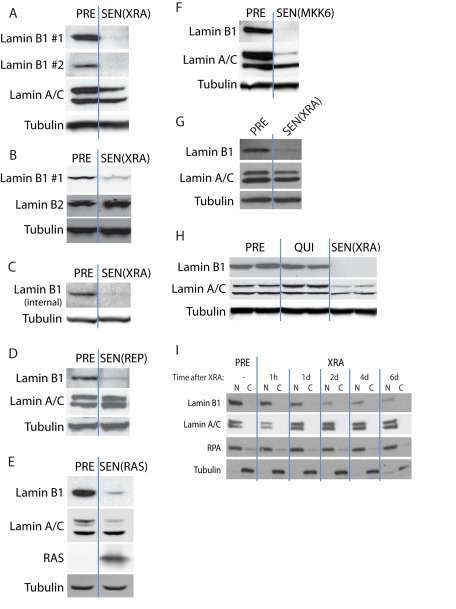
To determine whether lamin B1 expression changes in senescent cells, we examined presenescent (PRE) proliferating normal human fibroblasts (strain HCA2) and the same cells made senescent (SEN) by exposure to 10-Gy ionizing radiation (x-ray [XRA]; Supplemental Figure S1, A–C). We analyzed whole-cell lysates by Western blotting. Two independent lamin B1 antibodies showed that lamin B1, but not lamin A, C, or B2, protein levels declined markedly (>4-fold) in SEN(XRA) compared with PRE cells (Figure 1, A and B). Both lamin B1 antibodies recognize a C-terminal epitope in lamin B1. We therefore verified our findings using a third antibody that recognizes an internal lamin B1 epitope (Figure 1C). This third antibody verified that lamin B1 protein levels declined in senescent cells and in a different normal human fibroblast strain (BJ) (Figure 1C). Thus senescence-associated lamin B1 loss is not cell-strain specific or an antibody artifact. We used the first antibody (lamin B1 #1) for subsequent experiments, as it gave the strongest signal.
FIGURE 1:
Lamin B1 loss is associated with multiple types of cellular senescence. (A) Lamin B1 declines in DNA damage–induced senescence. HCA2 cells were mock irradiated (PRE) or irradiated (10-Gy x-rays) and allowed to senesce (SEN(XRA)). Whole-cell lysates were analyzed by Western blotting using either of two unrelated lamin B1 antibodies that recognize C-terminal epitopes. (B) Lamin B2 does not decline in DNA damage–induced senescence. HCA2 cells were mock irradiated (PRE) or irradiated and allowed to senesce (SEN(XRA)). Whole-cell lysates were analyzed by Western blotting. (C) Lamin B1 declines in SEN(XRA) cells. BJ cells were mock irradiated (PRE) or irradiated and allowed to senesce (SEN(XRA)). Whole-cell lysates were analyzed by Western blotting using a third lamin B1 antibody that recognizes an internal epitope. (D) Lamin B1 declines in replicative senescence. HCA2 cells were cultured until replicative senescence (SEN(REP); ∼70 population doublings). Whole-cell lysates were analyzed by Western blotting. (E) Lamin B1 declines in RAS-induced senescence. HCA2 cells were infected with a lentivirus lacking an insert (PRE) or expressing oncogenic RASV12 and allowed to senesce (SEN(RAS)). Whole-cell lysates were analyzed by Western blotting. (F) Lamin B1 declines in MKK6-induced senescence. HCA2 cells were infected with a lentivirus lacking an insert (PRE) or expressing a constitutively active MAP kinase kinase 6 mutant (MKK6EE) and allowed to senesce (SEN(MKK6). Whole-cell lysates were analyzed by Western blotting. (G) Lamin B1 declines in WI-38 cells after XRA. WI-38 cells were irradiated (SEN(XRA)) and allowed to senesce. PRE cells were mock irradiated. Whole-cell lysates were analyzed by Western blotting. (H) Lamin B1 does not decline in quiescent cells. HCA2 cells were cultured in media containing 10% serum (PRE), serum-free media for 48 h to induce quiescence (QUI), or irradiated and allowed to senesce (SEN(XRA)). Whole-cell lysates were analyzed by Western blotting. (I) Lamin B1 declines within 48 h after induction of senescence by irradiation. HCA2 cells were mock irradiated (PRE) or irradiated (XRA). Nuclear (N) and cytoplasmic (C) extracts collected at the indicated time points were analyzed by Western blotting. RPA serves as a loading control for the nuclear fraction; tubulin serves as a loading control for the cytoplasmic fraction.
A 10-Gy x-ray dose causes severe DNA damage that could reduce lamin B1 expression independent of the senescence program per se. We therefore examined cells that were cultured to replicative senescence (REP). Lamin B1, but not lamin A or C, declined sharply in these cells (Figure 1D). Senescence can also be driven by certain oncogenes, including an oncogenic form of H-RAS (RASV12; Serrano et al., 1997). We used a lentiviral vector to stably express RASV12 and allowed the cells to senesce (Supplemental Figure S1, A–C). Lamin B1 protein levels declined in these cells also, although in this case lamin A behaved similarly (Figure 1E). Lamin B1 and lamin A also declined in cells induced to senesce by expression of MKK6EE (Figure 1F), a constitutively active form of MAP kinase kinase 6 (MKK6; Raingeaud et al., 1996; Ishikawa, 2003). MKK6EE causes continual p38 MAPK activity, which induces senescence (Supplemental Figure S1, A–C; Freund et al., 2011). Finally, lamin B1 declined in a third cell strain, WI-38, that was induced to senesce by XRA (Figure 1G).
To determine whether lamin B1 loss was a consequence of arrested growth per se rather than senescence, we made cells quiescent (QUI) by incubating in serum-deficient medium for 48 h. QUI cells incorporated very little bromodeoxyuridine (BrdU; unpublished data), as expected. In contrast to SEN cells, QUI cells expressed lamin B1 to the same level as proliferating PRE cells (Figure 1H).
The SASP and SA-βgal take 7–10 d to develop when cells are induced to senesce synchronously, for example, by XRA (Campisi and d'Adda di Fagagna, 2007; Rodier and Campisi, 2011). To determine whether lamin B1 loss followed similar kinetics, we analyzed nuclear extracts at varying intervals after XRA (Figure 1I). Whereas lamin B1 decline was slower than DDR activation, which occurs within the first hour (Freund et al., 2011), it was essentially complete 2 d after XRA, earlier than other senescence markers.
These data suggest that lamin B1 decline is part of the general senescence program, irrespective of the senescence inducer. It occurs earlier than expression of the SASP, SA-βgal, and the morphological change (unpublished data) and is not a consequence of the growth arrest per se. Thus lamin B1 decline may be useful as an early senescence–associated marker.
Lamin B1 loss is independent of p38 MAPK, NF-κB, ataxia telangiectasia–mutated kinase, and ROS signaling
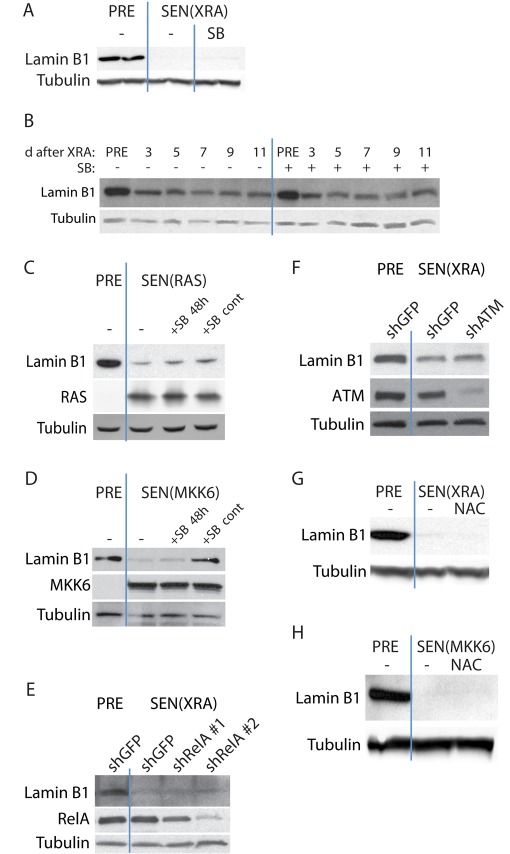
Several pathways have been identified that play causative roles in aspects of the senescence phenotype. The p38 MAPK pathway is important for both the senescence growth arrest and the SASP (Wang et al., 2002; Iwasa et al., 2003; Kwong et al., 2009; Freund et al., 2011). To determine whether p38 MAPK mediates lamin B1 decline, we inhibited p38 MAPK signaling with the well-characterized small molecule SB203580 (SB; Cuenda et al., 1995; Wilson et al., 1997; Young et al., 1997), which is effective at suppressing senescence-associated interleukin-6 (IL-6) secretion (Supplemental Figure S2A; Freund et al., 2011). When added to SEN(XRA) cells, 10 μM SB failed to reverse lamin B1 decline (Figure 2A). In addition, continuous treatment with SB, starting before XRA, failed to prevent or delay the decline in lamin B1 (Figure 2B).
FIGURE 2:
Lamin B1 loss at senescence is independent of p38 MAPK, NF-κB, ATM, and ROS signaling. (A) p38 MAPK inhibition does not reverse lamin B1 decline in DNA damage–induced senescence. The p38 MAPK inhibitor SB203580 (SB; 10 μM) was added to SEN(XRA) HCA2 cells for 48 h. Whole-cell lysates were analyzed by Western blotting. PRE cells were mock irradiated. (B) p38 MAPK inhibition does not prevent lamin B1 decline in DNA damage–induced senescence. SB was added to HCA2 cells before XRA. Cells were mock irradiated (PRE) or irradiated (XRA). Whole-cell lysates collected at the indicated time points were analyzed by Western blotting. SB was replaced daily. (C) p38 MAPK inhibition does not reverse or prevent RAS-induced lamin B1 decline. HCA2 cells were infected with a lentivirus expressing oncogenic RASV12 and allowed to senesce (SEN(RAS)). SB was not added (–), added 8 d after infection for 48 h (+SB 48 h), or added before infection and maintained for 10 d (+SB cont). Presenescent controls (PRE) were infected with an insertless vector. Whole-cell lysates were analyzed by Western blotting. (D) p38 MAPK inhibition prevents but does not reverse MKK6-induced lamin B1 decline. HCA2 cells were infected with a lentivirus expressing MKK6EE and allowed to senesce (SEN(MKK6)). SB was not added (–), added 8 d after infection for 48 h (+SB 48 h), or added before infection and maintained for 10 d (+SB cont). Presenescent controls (PRE) were infected with an insertless vector. Whole-cell lysates were analyzed by Western blotting. (E) RelA depletion does not prevent DNA damage–induced lamin B1 decline. HCA2 cells were infected with a lentivirus expressing either of two shRNAs against RelA (shRelA) or GFP (shGFP; control) and selected. Cells were then irradiated and allowed to senesce (SEN(XRA)). Presenescent controls (PRE) were mock irradiated. Whole-cell lysates were analyzed by Western blotting. (F) ATM depletion does not prevent DNA damage–induced lamin B1 decline. HCA2 cells were infected with a lentivirus expressing an shRNA against ATM (shATM) or GFP (shGFP; control) and selected. Cells were then irradiated and allowed to senesce (SEN(XRA)). Presenescent controls (PRE) were mock irradiated. Whole-cell lysates were analyzed by Western blotting. (G) ROS inhibition does not prevent DNA damage–induced lamin B1 decline. NAC (10 mM) was added to HCA2 cells before irradiation and maintained until whole-cell lysates were collected 10 d after XRA (SEN(XRA)). Presenescent controls (PRE) were mock irradiated. NAC was replaced daily. Whole-cell lysates were analyzed by Western blotting. (H) ROS inhibition does not prevent MKK6-induced lamin B1 decline. NAC (10 mM) was added to HCA2 cells before infection and continued until sample collection. Whole-cell lysates were collected 10 d after infection with a lentivirus expressing MKK6EE (SEN(MKK6)). Presenescent controls (PRE) were infected with a lentivirus lacking an insert. NAC was replaced daily. Whole-cell lysates were analyzed by Western blotting.
Similarly, SB did not prevent lamin B1 decline in cells induced to senesce by RAS. We infected cells with RAS-expressing lentivirus and 8 d later added SB for 48 h (+SB 48 h). Alternatively, we continuously treated cells with SB for 12 d, starting 2 d before infection (+SB cont). In both cases, SB was replaced daily. Neither treatment regimen had any effect on lamin B1 decline (Figure 2C). In addition to verifying that lamin B1 decline does not depend on p38 MAPK signaling, the data suggest that lamin B1 decline does not require the senescence-associated growth arrest or morphological change, as continuous p38 MAPK inhibition prevents both these phenotypes in RAS-expressing cells (Iwasa et al., 2003; Kwong et al., 2009). We also gave SB to cells that express MKK6EE, which induces senescence via constitutive p38 MAPK activation (Figure 2D). SB did not reverse the decline in lamin B1 (+SB 48 h), suggesting that once lamin B1 has been lost, complete blockage of the senescence-inducing signal does not reverse that decline. However, continuous p38 MAPK inhibition starting before MKK6EE expression (+SB cont) prevented lamin B1 decline, verifying that blockage of senescence signals mitigates the decline in lamin B1 expression.
Lamin B1 loss was also independent of the SASP, which is regulated in large part by the transcription factor NF-κB (Freund et al., 2011). We suppressed NF-κB activity by using either of two unrelated short hairpin RNAs (shRNAs) against RelA, an NF-κB subunit required for NF-κB activity. The shRNAs efficiently reduced RelA levels (Figure 2E) and eliminated senescence-associated IL-6 secretion (Supplemental Figure S2B; Freund et al., 2011). NF-κB depletion did not prevent the decline of lamin B1 that occurred in cells induced to senesce by XRA (Figure 2E).
The DDR pathway, particularly activation of ataxia telangiectasia–mutated (ATM) kinase, is important for the senescence growth arrest and SASP (von Zglinicki, 2005; Mallette et al., 2007; Rodier et al., 2009; Kuilman et al., 2010). In addition, this pathway is regulated independently of p38 MAPK (Freund et al., 2011). To determine whether the DDR was required for lamin B1 decline, we used an shRNA against ATM and then induced senescence by XRA. Despite the efficient reduction in ATM levels and effective suppression of IL-6 (Supplemental Figure S2C), lamin B1 still declined markedly, similar to its behavior in normal senescent cells (Figure 2F).
Finally, reactive oxygen species (ROS) are elevated in senescent cells, and ROS signaling is believed to mediate the senescence growth arrest (Lu and Finkel, 2008; Jun and Lau, 2010; Passos et al., 2010; Rai et al., 2011). We inhibited ROS by continuously treating cells with 10 mM N-acetyl cysteine (NAC), starting 2 d before inducing senescence by XRA or MKK6EE expression. Although NAC effectively decreased ROS levels (Supplemental Figure S2D), NAC treatment had no effect on lamin B1 decline in either case (Figure 2, G and H).
We conclude that the senescence-associated decline in lamin B1 expression is independent of p38 MAPK, NF-κB, DDR, and ROS signaling.
Lamin B1 loss occurs upon activation of either the p53 or pRB pathway
Because all inducers of senescence examined thus far caused a sharp decline in lamin B1 expression, we asked whether activation of tumor suppressor pathways that implement the senescence growth arrest was sufficient to deplete cells of lamin B1. With rare exceptions (Olsen et al., 2002; Campisi and d'Adda di Fagagna, 2007, all senescence inducers identified activate the pRB and/or p53 tumor suppressor pathways, so we asked whether lamin B1 declined in response to direct activation of either of these pathways.
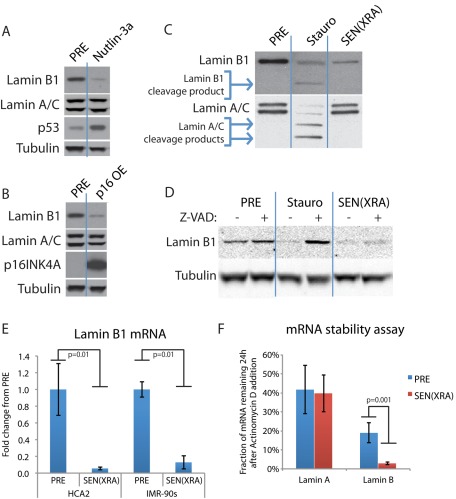
To activate p53, we used the small molecule MDM2-antagonist nutlin-3a, which causes an accumulation of p53 protein (Efeyan et al., 2007; Kumamoto et al., 2008). To activate the pRB pathway, we used a lentiviral vector to stably overexpress p16INK4a (Beausejour et al., 2003; Coppe et al., 2006), a tumor suppressor in its own right that activates pRB by preventing its phosphorylation (Ohtani et al., 2004). We collected whole-cell lysates after 4 d of continued treatment or expression, a time before the expression of other senescence markers. An increased level of either p53 or p16INK4a was sufficient to drive lamin B1 loss (Figure 3, A and B).
FIGURE 3:
p53 or p16 expression is sufficient to cause lamin B1 loss, which is regulated at the mRNA level. (A) p53 stabilization is sufficient to cause lamin B1 decline. HCA2 cells were treated with 5 μM nutlin-3a or vehicle (PRE). After 4 d of continuous treatment, whole-cell lysates were analyzed by Western blotting. (B) Ectopic p16INK4A expression is sufficient to cause lamin B1 decline. HCA2 cells were infected with a lentivirus lacking an insert (PRE) or expressing p16INK4A (p16INK4a OE) and selected. Four days after infection, whole-cell lysates were analyzed by Western blotting. (C) Lamin cleavage products are present in apoptotic but not senescent cells. HCA2 cells were treated with 500 nM staurosporine for 24 h to induce apoptosis (Stauro) or irradiated and collected 4 d later (SEN(XRA)). Presenescent controls (PRE) were mock irradiated. Whole-cell lysates were analyzed by Western blotting. (D) Caspase inhibition prevents staurosporine-induced lamin B1 degradation but not senescence-associated lamin B1 decline. HCA2 cells were treated with 500 nM staurosporine for 24 h (Stauro) or irradiated and collected 4 d later (SEN(XRA)). Presenescent controls (PRE) were mock irradiated. Where indicated, the pan-caspase inhibitor z-VAD-fmk (Z-VAD; 100 μM) was added starting before staurosporine/XRA and continuing until whole-cell lysates were collected. Z-VAD-fmk was replaced daily. (E) Lamin B1 mRNA declines in senescent cells. HCA2 and IMR-90 cells were mock irradiated (PRE) or treated with XRA. Total RNA was isolated 4 d later and analyzed by quantitative reverse transcription-PCR. The signal was normalized to tubulin. (F) Lamin B1 mRNA stability is decreased in senescent cells. HCA2 cells were mock irradiated (PRE) or treated with XRA. Three days later, actinomycin D was added (1 μg/ml final) to halt transcription. After 24 h, total RNA was isolated and analyzed by quantitative RT-PCR. Transcript levels immediately before actinomycin D addition were set to 100%.
Senescence-associated lamin B1 loss is regulated at the mRNA level
The decision as to whether a cell undergoes senescence or apoptosis depends on only a few factors, such as p53 phosphorylation and PTEN status (Bargonetti and Manfredi, 2002; Campisi and d'Adda di Fagagna, 2007; Lane et al., 2010; Lee et al., 2010). During apoptosis, cellular structural proteins are degraded, largely by caspases, and the nuclear lamins are caspase targets (Neamati et al., 1995; Rao et al., 1996; Kivinen et al., 2005). Senescent cells often have disrupted nuclei (Mehta et al., 2007), raising the possibility that the lamin B1 decline seen at senescence is a result of caspase-mediated degradation.
Lamin cleavage by caspases can be visualized by Western blotting (Rao et al., 1996; Gajdusek et al., 2001; Kivinen et al., 2005). We therefore asked whether lamin B1 cleavage products were detectable in senescent cells. As a positive control, we induced apoptosis with staurosporine (500 nM; Kivinen et al., 2005). Staurosporine effectively induced apoptosis, including characteristic lamin B1 and lamin A/C cleavage products; however, no such cleavage products were detectable in senescent cells (Figure 3C).
Because lamin cleavage products can disappear 24 h after apoptosis induction (Gajdusek et al., 2001), we treated cells with z-VAD-fmk, an irreversible pan-caspase inhibitor that blocks caspase degradation of lamins during apoptosis (Kivinen et al., 2005). Although 100 μM z-VAD-fmk prevented staurosporine-induced lamin B1 degradation, z-VAD-fmk administered before XRA and for 3 d thereafter did not prevent XRA-induced lamin B1 loss (Figure 3D). We conclude that lamin B1 is not degraded by caspase cleavage during senescence.
We next asked whether lamin B1 declined due to a decrease in mRNA level. Quantitative PCR showed that lamin B1 mRNA decreased significantly (p = 0.01) within 2 d after XRA in two cell strains (Figure 3E). Combining this result with the inability of caspase-inhibition to prevent lamin B1 decline, we conclude that lamin B1 is regulated at the mRNA level during senescence rather than posttranslationally by caspase cleavage. Of interest, the decline in lamin B1 mRNA was at least partially due to decreased lamin B1 mRNA stability. Twenty-four hours after the addition of actinomycin D to halt transcription, we measured the amount of remaining lamin mRNA. Whereas lamin A mRNA declined equally in presenescent and senescent cells, lamin B1 mRNA declined significantly (p = 0.001) more rapidly in senescent cells (Figure 3F), after accounting for differences in initial (pre–actinomycin D) mRNA level. This finding suggests that the lamin B1 mRNA, and subsequent protein, decline in senescent cells is due at least in part to reduced lamin B1 mRNA stability.
Lamin B1 loss occurs in vivo after a senescence-inducing dose of ionizing radiation
To determine whether senescence-associated lamin B1 loss occurs in vivo, we examined irradiated mice. We first verified that lamin B1 was lost in mouse embryonic fibroblasts (MEFs) in culture. MEFs were derived from four different mice and irradiated to induce senescence. Senescent MEFs had significantly less lamin B1 than did control MEFs (Supplemental Figure S3A), suggesting that lamin B1 loss also serves as a biomarker of murine cellular senescence.
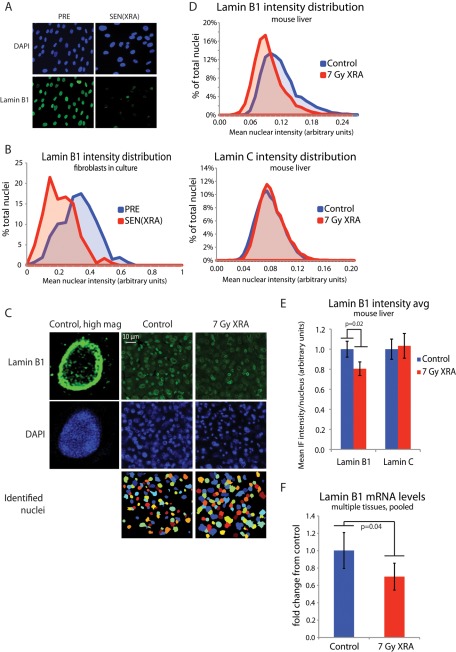
To quantify lamin B1 levels in individual cells in vivo, we used immunofluorescence. Identification of senescent cells in their native tissue environment is a challenge, and immunofluorescence (as opposed to flow cytometry, in which cells must be dissociated) offers the most information. We validated this approach in human fibroblasts, using an antibody that specifically detects lamin B1 by immunofluorescence (Shimi et al., 2008). Visual inspection showed that lamin B1 declined in senescent compared with control cells (Figure 4A), resulting in a marked shift in the histogram of lamin B1 intensity (Figure 4B). When the data were averaged for each condition, there was a highly significant decrease in average lamin B1 intensity in the senescent cells (Supplemental Figure S3B). Neither an antibody that specifically detects lamin B2 nor an antibody that detects both lamin B1 and B2 showed a significant alteration in intensity between senescent and control cells (Supplemental Figure S3C).
FIGURE 4:
Lamin B1 loss occurs in vivo after acute DNA damage. (A) Representative images of lamin B1 immunofluorescence. HCA2 cells were mock irradiated (PRE) or irradiated (XRA) and allowed to senesce (SEN(XRA)). Cells were fixed and immunostained for lamin B1. (B) Validation of lamin B1 quantitation via immunofluorescence: histogram of nuclear intensity. HCA2 cells were treated as in A. Lamin B1 intensity was measured across each identified nuclear area using Cell Profiler. p < 0.0001 (C) Representative images of lamin B1 staining in mouse liver. Mice were irradiated (7-Gy XRA) or untreated (control). Twelve weeks later, livers were harvested, sectioned, and immunostained for lamin B1. Lamin B1 intensity was measured across each identified nuclear area. (D) Lamin B1 but not lamin C nuclear intensity declines in mouse liver after irradiation: histogram of nuclear lamin intensity. Mice were irradiated or untreated (control). Twelve weeks later, livers were harvested, sectioned, and immunostained for lamin B1 (top) and lamin C (bottom). Nuclear staining was quantitated with Cell Profiler. For both control and XRA conditions, bin populations were calculated by averaging the relative contribution from each of four mice. (E) Lamin B1 but not lamin C nuclear intensity declines in mouse liver after irradiation: average nuclear lamin intensity. Data from D are presented as average of the mean lamin intensity from four mice for both control and XRA conditions. p = 0.02 for lamin B1, p = 0.71 for lamin C. (F) Lamin B1 mRNA declines in vivo after irradiation. Mouse liver, lung, kidney, and skin were harvested from control mice and mice 12 wk after irradiation. mRNA was purified and lamin B1 mRNA levels determined by quantitative PCR, normalized to tubulin. To generate a single lamin B1 mRNA value from four organs for each mouse, each organ was weighted equally (average control lamin B1 level of each organ set to 1) and pooled for each mouse. Whole-mouse pools were then averaged for each condition: control, n = 4; 7-Gy XRA, n = 5.
We performed a similar immunofluorescence analysis on liver sections from untreated mice (control) and mice that had been irradiated 12 wk previously with 7 Gy (7-Gy XRA). We chose liver because previous reports showed an accumulation of senescent cells in this tissue (Paradis et al., 2001; Xue et al., 2007; Krizhanovsky et al., 2008; Wang et al., 2009; Le et al., 2010), and liver showed low background staining, allowing for accurate quantitation. To determine the lamin B1 expression level, we quantified the lamin B1 and lamin C staining intensity in thousands of nuclei from control (n = 4) and irradiated mice (n = 4; Figure 4C). Lamin B1, but not lamin C, showed a shift to lower intensity in irradiated livers (Figure 4D) in which senescent cells persist (Le et al., 2010). The overlapping distributions of staining intensity did not allow us to confidently score individual nuclei as “lamin B1 positive” or “lamin B1 negative” in irradiated tissue, although future optimization may allow for a single-cell assay. When the mean lamin intensities of livers from each condition were averaged, lamin B1 but not lamin C showed a significant decrease (p = 0.02) in average intensity in the livers from irradiated mice (Figure 4E).
Given the decrease in lamin B1 protein after ionizing radiation, we asked whether lamin B1 mRNA declined in liver and other tissues 12 wk after irradiation. We analyzed mRNA mouse liver, kidney, skin, and lung before and after XRA, normalized to tubulin, and weighted each organ equally to calculate an average lamin B1 level in each animal. Animals from each condition were then averaged. Irradiated mice showed significantly increased p16INK4a mRNA levels, suggesting the presence of senescent cells across multiple tissues (Supplemental Figure S3D). Irradiated mice also had a significantly lower lamin B1 mRNA level than untreated mice (control; Figure 4F), demonstrating that lamin B1 protein and mRNA decline in vivo in response to a senescence-inducing dose of ionizing radiation.
DISCUSSION
We show here that lamin B1 declines precipitously when cells are induced to senesce by multiple means. We also show that lamin B1 decline is a hallmark of senescence in multiple human and murine fibroblast cell strains and after acute DNA damage that results in the persistence of senescent cells in vivo (Le et al., 2010). Lamin A, although depleted in cells induced to senesce by oncogenic RAS or MKK6EE expression, was not depleted in cells induced to senesce by DNA damage or replicative exhaustion. Furthermore, neither lamin B2 nor lamin C declined, regardless of the senescence inducer. We conclude that a decline in lamin B1, but not lamin B2, lamin A, or lamin C, may be general biomarker of senescence.
Lamin B1 decline occurred rapidly (2 d) after exposure to a senescence-inducing stimulus and was not a general feature of cell cycle arrest, as quiescent cells retained lamin B1 at levels equal to those of proliferating cells. Of interest, lamin B1 loss was not prevented by inhibition of pathways that control many other aspects of senescence, such as the SASP. Thus lamin B1 decline was independent of the p38 MAPK/NF-κB pathway, the DDR pathway, and ROS signaling. However, lamin B1 decline was strongly induced by either direct p53 activation or direct p16INK4a expression. Because almost all senescence inducers strongly depend on either the p53 or pRB/p16INK4a pathways, lamin B1 loss might serve as a general senescence marker in other contexts and cell types. One exception might be oxidative stress, which can induce some features of senescence (e.g., arrested growth, SA-βgal expression) but not all features (e.g., loss of c-FOS inducibility, the SASP; Parrinello et al., 2003; Coppe et al., 2010). A recent report (Barascu et al., 2012) showed that oxidative stress induces senescence via an increase in lamin B1 expression, suggesting that some senescent cells do not express the full complement of senescence markers. However, the relationship between lamin B1 and oxidative stress bears further investigation, as another recent report demonstrates that depletion of lamin B1 via shRNAs leads to senescence via decreased ROS (Shimi et al., 2011).
Lamin B1 decline at senescence was distinct from the caspase-mediated lamin degradation seen at apoptosis, as no lamin cleavage products were evident in senescent cells. Furthermore, caspase inhibition did not prevent the senescence-associated decline in lamin B1 protein levels. Instead, lamin B1 loss was due to an early decline in mRNA level. This decline was at least partially mediated by a decrease in lamin B1 mRNA stability. Given that lamin A mRNA stability was unaffected, we hypothesize that lamin B1 mRNA is specifically destabilized as part of the senescence program by an as-yet-unidentified mechanism.
A number of senescence markers have been used to identify senescent cells in vivo, although no single marker is completely specific (Campisi and d'Adda di Fagagna, 2007; Kuilman et al., 2010). For example, growth arrest, although a necessary condition for senescence, is not sufficient to define the state—many cells in vivo are terminally differentiated or quiescent (Kuilman et al., 2010). p16INK4a is highly correlated with senescence and age (Zindy et al., 1997; Krishnamurthy et al., 2004; Ohtani et al., 2010), although some tumor cells express high levels of p16INK4a. Moreover, p16INK4a expression is not necessary for senescence, as some cells senesce after DNA damage via activation of the p53 pathway alone (Beausejour et al., 2003). In addition, the most widely used senescence marker, SA-βgal, is neither necessary nor sufficient for senescence (Dimri et al., 1995; Lee et al., 2006) and is both labile and difficult to detect in vivo. We report here that lamin B1 declines in mouse tissues with persistent senescent cells and therefore may be useful in both research and clinical applications. We found a small but significant decrease in lamin B1 mRNA and protein in mouse tissues that had been irradiated 12 wk previously, consistent with a small population of persistent senescent cells (Le et al., 2010). The radiation regimen to which we subjected the mice is similar to that experienced by human patients undergoing radiotherapy for cancer. Optimization of lamin B1 measurement may generate a robust, single-cell test for senescent cells in vivo, giving researchers and clinicians a tool with which to accurately assess the relative contribution of senescent cells in tissues of patients with a variety of conditions, including cancer, diabetes, chronic inflammation, and age-related disease.
MATERIALS AND METHODS
Cell culture
Primary human fibroblasts (HCA2, WI-38, IMR-90, BJ strains) were obtained and cultured as described (Coppe et al., 2008). Unless noted otherwise, “fibroblast” or “cells” in the text and legends refer to HCA2 fibroblasts. PRE HCA2 cells completed <35 population doublings and had a 24-h BrdU labeling index of >60%. Cells were made replicatively senescent by repeated subculture, as described (Dimri et al., 1995; Krtolica et al., 2001). For DNA damage–induced senescence, cells were grown to confluence, exposed to 10-Gy x-rays, and, unless noted otherwise, analyzed 8–10 d later; PRE cells were mock irradiated. For oncogene-induced senescence, cells were infected with a lentivirus expressing RASV12 (Beausejour et al., 2003; Coppe et al., 2008) and analyzed 8–10 d later.
Chemical inhibitors and inducers
Cells were given 10 μM SB203580 (559395; Calbiochem, La Jolla, CA) or 10 mM NAC (A7250; Sigma-Aldrich, St. Louis, MO) dissolved in water with daily replenishment. Cells were treated with 100 μM z-VAD-fmk (FMK001; R&D Systems, Minneapolis, MN) dissolved in dimethyl sulfoxide with daily replenishment. Staurosporine (500 nM) was from Sigma-Aldrich (S4400).
Vectors, viruses, and infections
MKK6EE, genetic suppressor element 22, and RASV12 were subcloned into Gateway destination vector 670-1, as described (Beausejour et al., 2003; Campeau et al., 2009). Infection with an insertless vector was used as a control. Lentiviral vectors encoding shRNAs against green fluorescent protein (GFP; RHS4459) and RelA (TRCN0000014686, TRCN0000014687) were from Open Biosystems (Thermo Biosystems, Huntsville, AL). Lentiviral vectors encoding ATM shRNA (Rodier et al., 2009) and virus production were as described (Naldini et al., 1996; Beausejour et al., 2003).
Immunofluorescence
Cells were cultured on glass chamber slides, fixed with Formalin for 10 min, and permeabilized with 0.1% Triton in phosphate-buffered saline (PBS). Slides were blocked for 30 min at room temperature with 1% bovine serum albumin (BSA) and 4% normal donkey serum in PBS, washed, and incubated with primary antibody for 1 h at room temperature in 1% BSA in PBS. Slides were washed and incubated with Alexa Fluor (Alexa 350, 488, 594; Molecular Probes, Invitrogen, Carlsbad, CA) secondary antibodies in 1% BSA in PBS at 1:750 for 45 min. 4′,6-Diamidino-2-phenylindole (DAPI) was used to label DNA. Slides were washed and mounted with Vectashield (H1000; Vector Laboratories, Burlingame, CA). Primary antibodies were as described (Shimi et al., 2008) and used at the following dilutions: lamin B1, 1:1000; lamin B2, 1:500; lamin B1 and B2 (M-20), 1:500; lamin C (321-11), 1:3000. Images were quantitated using CellProfiler, an open-access image analysis program (www.cellprofiler.org).
Mouse livers were harvested, snap frozen, sectioned, mounted on slides, and then fixed and permeabilized with ice-cold methanol/acetone (50/50) for 5 min at −20°C. Slides were washed and blocked for 1 h at room temperature with 1% BSA and 5% normal goat serum in PBS, washed, and incubated with primary and secondary antibodies as described for cell culture. Slides were washed and mounted with ProLong Gold antifade reagent with DAPI (P36931; Invitrogen). Images were quantitated using CellProfiler.
Western blot analysis
Cells were washed with warm PBS, lysed and scraped in either denaturing (1% SDS, 10 mM Tris) or nondenaturing (Cell Lysis Buffer, 9803; Cell Signaling, Beverly, MA) buffer containing inhibitors of proteases (P8340, 1:200; Sigma-Aldrich) and phosphatases (200 mM imidazole, 100 mM sodium fluoride, 115 mM sodium molybdate, 100 mM sodium orthovanadate, 400 mM sodium tartrate, 1:100). Lysates were needle sheared, clarified by centrifugation, subjected to SDS–PAGE using 4–12% Bis-Tris gels, and transferred to polyvinylidene fluoride membranes. Membranes were blocked and incubated overnight at 4°C with primary antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA; NF-κB p65, SC-8008, 1:1000; MKK6, SC-1992, 1:1000; p53, SC-126, 1:1000; lamin B1 [C-20], SC-6216, 1:1000; lamin B1 [S-20], SC-30264, 1:1000), Abcam (Cambridge, MA; ATM, AB32420, 1:2000; lamin B1, AB16048, 1:000), Sigma-Aldrich (tubulin, T-5168, 1:4000), BD Transduction Laboratories (Lexington, KY; RAS, R02120-050, 1:1000), or BD Biosciences (San Diego, CA; p16INK4a, 554070, 1:1000; lamin A/C, 612162, 1:4000). Membranes were washed and incubated with horseradish peroxidase (1:5000; Cell Signaling) or IR-dye (1:20,000; LI-COR Biosciences, Lincoln, NE)–conjugated secondary antibodies for 45 min at room temperature and washed, and signals were detected by enhanced chemiluminescence or LI-COR Odyssey, respectively.
Quantitative reverse transcription-PCR
RNA was isolated using the RNeasy Mini Kit (74104; Qiagen, Valencia, CA) and cDNA generated with iScript cDNA Synthesis Kit (170-8891; Bio-Rad, Hercules, CA). mRNA levels were determined using the UPL system (Roche, Indianapolis, IN). Human primers (probes #31, #17, and #58, respectively) were as follows: LMNB1, 3′: aagcagctggagtggttgtt; LMNB1, 5′: ttggatgctcttggggttc; LMNA, 3′: agcaaagtgcgtgaggagtt; LMNA, 5′: tcaggtcaccctccttcttg; tubulin, 3′: cttcgtctccgccatcag; tubulin, 5′: ttgccaatctggacacca. Mouse LMNB1 primers were as follows (probe #15): 3′: gggaagtttattcgcttgaaga; 5′: atctcccagcctcccatt.
Enzyme-linked immunosorbent assays and conditioned media
Enzyme-linked immunosorbent assay (ELISA) kits to detect IL-6 (D6050) were from R&D Systems. Conditioned media were prepared by washing with serum-free DMEM and incubating in serum-free DMEM for 24 h. All ELISA data were normalized to cell number.
Statistical analyses
Except where indicated, statistical significance was evaluated using a two-tailed Student's t test and assumption of equal variance. Statistical significance between binary assays (i.e., positive and negative scores) was evaluated using a chi-squared test. Error bars denote SDs.
Supplementary Material
Acknowledgments
We thank Robert Goldman for the anti-lamin antibodies used for immunofluorescence and Eisuke Nishida (Tokyo University, Tokyo, Japan) for the pSRalpha-myc-MKK6-EE vector. This work was supported by National Institutes of Health Research Grants AG09909 and AG017242 (J.C.) and a National Science Foundation Graduate Research Fellowship (A.F.).
Abbreviations used:
- ATM
ataxia telangiectasia-mutated
- BrdU
bromodeoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- DDR
DNA damage response
- ELISA
enzyme-linked immunosorbent assay
- HGPS
Hutchinson-Gilford progeria syndrome
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- NAC
N-acetyl cysteine
- OE
overexpression
- PRE
presenescent
- QUI
quiescent
- REP
replicative
- ROS
reactive oxygen species
- SA-βgal
senescence-associated beta-galactosidase
- SASP
senescence-associated secretory phenotype
- SB
SB203580
- SEN
senescent
- shRNA
short hairpin RNA
- XRA
x-ray
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-10-0884) on April 11, 2012.
REFERENCES
- Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31:1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargonetti J, Manfredi JJ. Multiple roles of the tumor suppressor p53. Curr Opin Oncol. 2002;14:86–91. doi: 10.1097/00001622-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci. 2010;123:2605–2612. doi: 10.1242/jcs.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger JM, Foeger N, Kill IR, Herrmann H. The nuclear lamina. Both a structural framework and a platform for genome organization. FEBS J. 2007;274:1354–1361. doi: 10.1111/j.1742-4658.2007.05694.x. [DOI] [PubMed] [Google Scholar]
- Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, Raymond Y, Ramaekers FC. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol. 1997;107:505–517. doi: 10.1007/s004180050138. [DOI] [PubMed] [Google Scholar]
- Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK, Kaufman PD. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One. 2009;4:e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin K, Desprez PY, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Ortega-Molina A, Velasco-Miguel S, Herranz D, Vassilev LT, Serrano M. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 2007;67:7350–7357. doi: 10.1158/0008-5472.CAN-07-0200. [DOI] [PubMed] [Google Scholar]
- Eriksson M, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong LG, et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Orjalo A, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–248. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil CK, Campisi J. p38 MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C, Onoda K, London S, Johnson M, Morrison R, Mayberg M. Early molecular changes in irradiated aortic endothelium. J Cell Physiol. 2001;188:8–23. doi: 10.1002/jcp.1091. [DOI] [PubMed] [Google Scholar]
- Gerace L, Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19:277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–547. doi: 10.1101/gad.960502. [DOI] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- Ishikawa F. Cellular senescence, an unpopular yet trustworthy tumor suppressor mechanism. Cancer Sci. 2003;94:944–947. doi: 10.1111/j.1349-7006.2003.tb01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivinen K, Kallajoki M, Taimen P. Caspase-3 is required in the apoptotic disintegration of the nuclear matrix. Exp Cell Res. 2005;311:62–73. doi: 10.1016/j.yexcr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G, Benavente R. The nuclear lamins. A multigene family of proteins in evolution and differentiation. Exp Cell Res. 1986;162:1–10. doi: 10.1016/0014-4827(86)90421-0. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez P-Y, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto K, Spillare EA, Fujita K, Horikawa I, Yamashita T, Appella E, Nagashima M, Takenoshita S, Yokota J, Harris CC. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–3203. doi: 10.1158/0008-5472.CAN-07-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J, Hong L, Liao R, Deng Q, Han J, Sun P. p38alpha and p38gamma mediate oncogenic Ras-induced senescence through differential mechanisms. J Biol Chem. 2009;284:11237–11246. doi: 10.1074/jbc.M808327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP, Verma C, Fang CC. The p53 inducing drug dosage may determine quiescence or senescence. Aging (Albany NY) 2010;2:748. doi: 10.18632/aging.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le ON, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J, Laverdière C, Kokta V, Haddad E, Beauséjour CM. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010;9:398–409. doi: 10.1111/j.1474-9726.2010.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Kim BC, Park MJ, Lee YS, Kim YN, Lee BL, Lee JS. PTEN status switches cell fate between premature senescence and apoptosis in glioma exposed to ionizing radiation. Cell Death Differ. 2010;18:666–677. doi: 10.1038/cdd.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallette FA, Gaumont-Leclerc M-F, Ferbeyre G. The DNA damage signaling pathway is a critical mediator of oncogene-induced senescence. Genes Dev. 2007;21:43–48. doi: 10.1101/gad.1487307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta IS, Figgitt M, Clements CS, Kill IR, Bridger JM. Alterations to nuclear architecture and genome behavior in senescent cells. Ann NY Acad Sci. 2007;1100:250–263. doi: 10.1196/annals.1395.027. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Neamati N, Fernandez A, Wright S, Kiefer J, McConkey DJ. Degradation of lamin B1 precedes oligonucleosomal DNA fragmentation in apoptotic thymocytes and isolated thymocyte nuclei. J Immunol. 1995;154:3788–3795. [PubMed] [Google Scholar]
- Ohtani N, Yamakoshi K, Takahashi A, Hara E. The p16INK4a-RB pathway: molecular link between cellular senescence and tumor suppression. J Med Invest. 2004;51:146–153. doi: 10.2152/jmi.51.146. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Yamakoshi K, Takahashi A, Hara E. Real-time in vivo imaging of p16 gene expression: a new approach to study senescence stress signaling in living animals. Cell Div. 2010;5:1. doi: 10.1186/1747-1028-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CL, Gardie B, Yaswen P, Stampfer MR. Raf-1-induced growth arrest in human mammary epithelial cells is p16-independent and is overcome in immortal cells during conversion. Oncogene. 2002;21:6328–6339. doi: 10.1038/sj.onc.1205780. [DOI] [PubMed] [Google Scholar]
- Paradis V, Youssef N, Dargere D, Ba N, Bonvoust F, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Coppe J-P, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P, Young JJ, Burton DG, Giribaldi MG, Onder TT, Weinberg RA. Enhanced elimination of oxidized guanine nucleotides inhibits oncogenic RAS-induced DNA damage and premature senescence. Oncogene. 2011;30:1489–1496. doi: 10.1038/onc.2010.520. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shimi T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki TV. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KP, McCaffrey PG, Hsiao K, Pazhanisamy S, Galullo V, Bemis GW, Fitzgibbon MJ, Caron PR, Murcko MA, Su MS. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem Biol. 1997;4:423–431. doi: 10.1016/s1074-5521(97)90194-0. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PR, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.